Abstract
Taking into account previous data that sustain a relationship between vitamin D deficiency and higher H. pylori infection positivity rates, this review aims to assess the influence of vitamin D deficiency and/or insufficiency upon the prevalence of H. pylori infection and its eradication success. Three major databases were searched for articles that analyzed a relationship between vitamin D status and H. pylori infection. The literature search retrieved a total of 37 reports, after the article selection process. Hypovitaminosis D emerged as a potential risk factor for H. pylori infection, given the higher prevalence of vitamin D deficiency and/or insufficiency among H. pylori-positive subjects. Furthermore, the same type of micronutrient deficiency has been directly linked to H. pylori eradication failure. An inverse linear relationship between vitamin D status and gastric cancer risk exists, but the additional involvement of H. pylori in this correlation is still in question. The potential benefit of oral supplements in enhancing the success of classical therapeutic regimens of H. pylori still requires future research. Future population-based studies from larger geographical areas are warranted to address this subject in more depth.
1. Introduction
Vitamin D represents one of the essential micronutrients in the human body, the main function of which is to ensure calcium hemostasis and bone mineralization [1]. Available from both exogenous—namely dietary—and endogenous—synthetized after exposure to sunlight—sources, vitamin D is initially present in an inactivated form, either as ergocalciferol or as cholecalciferol [2]. The synthesis of the active form of vitamin D, calcitriol, requires two hydroxylations, which take place in the liver and kidney [3,4]. Calcitriol binds to vitamin D receptors (VDRs), which are found throughout the entire human body and present numerous binding sites as well. Hence, vitamin D regulates multiple biologic processes [3]. The omnipresence of VDRs seems to be one of the key elements through which vitamin D metabolites take part in immune defense. The attachment of pathogen-associated molecular patterns (PAMPs) to toll-like receptors (TLRs), which are activated inside the macrophages as a result of lipopolysacharide (LPS) recognition, triggers an increase in the number of VDRs and consequently enhances the conversion of circulating 25-hydroxyvitamin D into its active form, calcitriol [5,6]. Vitamin D is also involved in T-cell antigen receptor maturation and mediates T-cell immune response [7]. Moreover, 25-hydroxyvitamin D sufficiency is mandatory for normal macrophage function and adequate monocyte production of cathelicidin, an antimicrobial peptide, with an antiviral effect [5,8]. The active involvement of vitamin D in immune processes was certified by multiple studies which successfully proved that there is a link between vitamin D deficiency or insufficiency and the incidence of various infections. Hence, infections of the respiratory and digestive systems and the urinary tract, as well as systemic infections, have been linked to low levels of vitamin D and seem to be prevented by vitamin D supplementation [9,10,11,12,13,14].
Helicobacter pylori (H. pylori), a spiral-shaped Gram negative bacterium, is ubiquitously spread, infecting more than half of the world’s population [15]. H. pylori represents a major causative factor of peptic ulcer disease, an important precursor lesion of gastric cancer, and possesses virulence factors such as cytotoxin-associated gene A (CagA) and vacuolating cytotoxin A, as well as various outer membrane proteins which have been directly linked to the activation of pathways involved in aberrant cell proliferation and gastric carcinogenesis [16,17]. Hence, continuous efforts have been made to eradicate symptomatic H. pylori infection, but not without treatment-associated side effects [18]. The increase in antibiotic resistance patterns and H. pylori eradication failure represents a threat to human health, which can lead to a higher incidence of gastric cancer [19]. Vitamin D deficiency has been regarded as one of the potential risk factors for H. pylori therapeutic failure, with studies proposing its supplementation as an adjuvant to standard medication [20,21]. Moreover, H. pylori positivity rates seem to be higher among populations with low serum vitamin D levels [22,23]. Thus, starting from an association between vitamin D deficiency and various types of infections, a link between vitamin D and H. pylori infection has been found that constitutes grounds for further research.
This review aims to highlight the association between vitamin D deficiency and/or insufficiency and the prevalence of H. pylori infection, its complications, and its eradication success.
2. Materials and Methods
Web of Science, Scopus, and PubMed databases were searched for articles published until 15 April 2023 that analyzed a relationship between vitamin D status and H. pylori infection. Search terms included “Helicobacter pylori” OR “Helicobacter” AND “vitamin D” OR “cholecalciferol” OR “calcitriol”. The inclusion criteria were human-based population studies, literature data published in the English language, and research articles that analyzed a correlation between serum vitamin D levels and H. pylori incidence and therapeutic management. Papers in-extenso were included, consisting of randomized controlled trials (RCTs), prospective cohort studies, retrospective cross-sectional studies, and longitudinal studies. We excluded studies that did not meet our article objective, case reports, editorials, review articles, and meta-analyses, as well as non-English records, articles without available abstracts, and duplicates/triplicates. Furthermore, abstracts and proceeding papers were excluded from our search results due to the insufficiently detailed information regarding the characteristics of the study population included, diagnostic methods applied for H. pylori infection, and the eradication regimen used.
The Rayyan web app aided in the article selection process. Initially, duplicates and triplicates were removed, and afterwards each of the authors examined the records’ titles and their abstracts in order to exclude irrelevant articles. Authors SM and KAM assessed the full-length text of each manuscript to ensure its compliance with inclusion criteria. Potential disagreements between authors were debated and discussed by all authors, who afterwards mutually agreed with the inclusion of each individual record within this review.
3. Results
The search retrieved a total of 465 records, which further translated into 319 results after the exclusion of duplicates. The article selection process has been represented in more detail in Figure 1, which complies to the PRISMA 2020 statement and its revised flow diagrams [24]. Four non-English language articles and one article without an available abstract were initially excluded. After exclusion of experimental studies, meta-analyses, review type articles, one case report and one editorial, the final selection pool consisted of 37 articles, the primary or secondary objective of which was to assess a correlation between vitamin D and H. pylori infection.
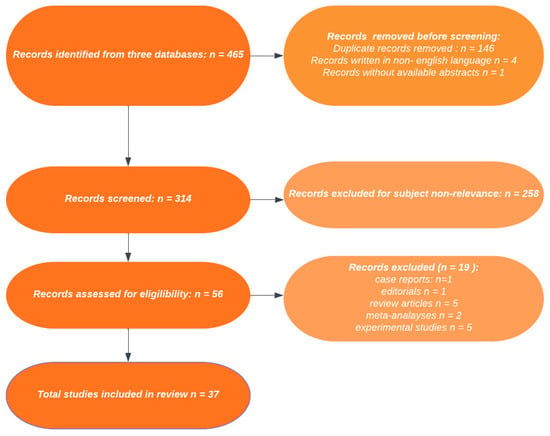
Figure 1.
PRISMA flowchart for assessment of eligible studies.
3.1. Vitamin D Status: A Possible Risk Factor for H. pylori Infection?
Among various dietary habits, decreased vitamin D intake has emerged as a possible concern related to H. pylori infection [25]. Vitamin D deficiency, namely serum levels below 20 ng/mL, poses an increased risk for H. pylori infection according to two studies conducted on Turkish and Lebanese adult populations [26,27]. Moreover, an inverse linear correlation was established between vitamin D sufficiency and H. pylori infection prevalence, which suggested that adequate vitamin D levels might offer protection against H. pylori [26]. Furthermore, one study conducted in Bahrain highlighted that, for each unit decrease in serum vitamin D level, the risk of H. pylori infection increases by 1.1 [25]. In a study conducted on an older population with sarcopenia, an inverse relationship between vitamin D deficiency and H. pylori was proposed, suggesting that H. pylori might actually be the risk factor for vitamin D level decrease [28]. However, Bashir et al. proved on a limited population sample that an 8-week supplementation regimen of high oral vitamin D3 doses can significantly decrease H. pylori colonization of the gastric mucosa. Therefore, vitamin D intake is probably influencing the prevalence of this particular infection [29].
Shafrir et al. found an inverse association between vitamin D serum levels and H. pylori infection and concluded that those individuals with vitamin D values below 20 ng/mL stand the best chances of acquiring this particular bacterial infection [23]. However, Han et al. reported significantly lower serum vitamin D levels among their study group infected with H. pylori, despite including a significant number of patients with levels under 20 ng/mL among the control group as well [30]. Hence, a certain lower limit of serum vitamin D that increases the risk of H. pylori infection was put into question. Other authors proposed that vitamin D insufficiency, rather than deficiency, is the major risk factor for H. pylori infection [31]. In contrast, one study conducted in Iraq on obese women identified significant differences in terms of H. pylori serum antibody positivity only in patients with vitamin D deficiency, while the presence/absence rate of antibodies directed against the same bacterial infection was not influenced by vitamin D insufficiency [32].
Several studies failed to identify an association between serum vitamin D levels and H. pylori infection. One study that analyzed the impact of concomitant vitamin D deficiency and H. pylori infection upon the prevalence of metabolic syndrome concluded that vitamin D levels were similar among the enrolled individuals, independent of H. pylori infectious status. The authors reported that those subjects with both H. pylori infection and vitamin D deficiency were the most susceptible to developing metabolic syndrome [33]. Furthermore, among the study participants, who were obese and undergoing bariatric surgery, preoperative nutritional deficiencies such as hypoproteinemia, hypoalbuminemia, hypocalcemia, hypomagnesemia, zinc, copper, folate, or vitamin B12 or vitamin D deficits were encountered independently of H. pylori infectious status, confirmed through analysis of gastric mucosa specimens [34]. Contradictorily, another study conducted on obese individuals revealed that vitamin D levels might be higher in the study group infected with H. pylori. However, these differences did not reach statistical significance [35].
H. pylori co-infection in the setting of other infectious diseases or chronic conditions seems to be additionally associated with vitamin D deficiency. One study conducted on 800 dengue fever patients, divided into two equal groups based on H. pylori co-infection status, revealed that those individuals who were positive for the infection presented significantly lower vitamin D serum levels than dengue fever controls [36]. In patients with end-stage renal failure undergoing hemodialysis, a linear positive relationship between vitamin D levels and H. pylori immunoglobulin G (IgG) serum antibodies was discovered [37]. Moreover, Bener et al. revealed that, in patients with type 2 diabetes mellitus and high serum titers of H. pylori specific antibodies, vitamin D levels were significantly lower than in healthy controls [38]. Similarly, in patients with type 1 diabetes and a positive H. pylori stool antigen test, significantly lower values of vitamin D levels were reported [39].
In children, results have been scarce, contradictory, and did not match those obtained in adult populations. Agin et al. found an association between lower vitamin D levels and peptic ulcer in children, but concluded that vitamin D levels did not significantly impact H. pylori infection rates within their pediatric study group [40]. Moreover, Urganci et al. concluded that, in children with chronic gastritis, parameters of bone metabolism, such as serum vitamin D, magnesium, calcium, phosphorus or parathormone levels, are similar, regardless of gastro-duodenal H. pylori colonization [41]. One study suggested that vitamin D deficiency might represent a risk factor for H. pylori infection in infants and toddlers. This study assessed H. pylori infection based on serum antibody positivity, which cannot distinguish present from past H. pylori infection [42]. Another study, in which a histology based-diagnosis of H. pylori was performed, revealed that vitamin D levels are significantly higher in children with with non-H. pylori gastro-duodenal conditions, as opposed to infected individuals. Moreover, the same study identified an inverse association between vitamin D levels and CagA positive H. pylori strains, which are known to increase gastric cancer risk even further [43]. This finding was not confirmed within the general population of an adult study subgroup who took part in the National Health and Nutrition Examination Survey (NHANES) III, which described a lack of correlation between vitamin D status and CagA seropositivity. However, when separately analyzing a population sample of non-Hispanic whites, the authors identified the same vitamin D deficiency-Cag A seropositivity association as in the aforementioned pediatric study [44]. The studies described within this chapter, which analyzed the relationship between vitamin D serum levels and H. pylori, have been depicted in Table 1.

Table 1.
Characteristics of clinical studies that assessed the impact of vitamin D deficiency upon H. pylori infection.
3.2. Vitamin D and Its Influence on H. pylori Eradication
Vitamin D deficiency has been regarded as a risk factor for H. pylori eradication failure [20,45]. The studies investigating this topic have been summarized in Table 2. Differences in the eradication rates of H. pylori positive patients have been linked to vitamin D sufficiency, which translated into serum levels exceeding at least 20 ng/mL. Significantly lower mean serum vitamin D levels have been reported among patients with therapeutic failure [45]. Through their study, Shatla et al. also emphasized that vitamin D sufficiency is one of the key factors for H. pylori eradication success [20]. This theory was tested using a classical clarithromycin-based triple eradication therapy of 14 days duration [20]. Magsi et al. also reported an even higher, ascending discrepancy in vitamin D levels between H. pylori infected patients that responded to the same therapeutic regimen and those who did not [46]. Similar findings were reported by Almani et al. in their study, in which the identical, classical therapeutic scheme failed to eradicate H. pylori infection in more than 80% of patients with vitamin D levels below 30 ng/mL [47]. However, even when considering a modified, novel therapeutic approach consisting of bismuth potassium citrate, proton pump inhibitors (PPIs), amoxicillin, tetracycline, furazolidone, or levofloxacin, vitamin D levels under 20 ng/mL qualified as an independent risk factor for H. pylori eradication failure [48]. Therefore, the therapeutic hypothesis of adding vitamin D3 in conjunction with classical eradication schemes emerged. El Shahawy et al. are the first to support the addition of oral vitamin D3 supplements to a classical regimen consisting of PPPIs, amoxicillin, and clarithromycin, and to report superior eradication rates of H. pylori [49].

Table 2.
Characteristics of clinical studies that assessed the impact of vitamin D deficiency upon H. pylori eradication.
A lower vitamin D serum level of 10 ng/mL was proposed as referential for its impact on H. pylori eradication. In the study of Yildirim et al., over 80% of the patients in whom H. pylori eradication attempt was unsuccessful had serum vitamin D levels below 10 ng/mL [21]. Another study confirmed the very low H. pylori eradication rates in patients with vitamin D levels under 10 ng/mL and the best therapeutic success rates for populations with vitamin D serum values ranging between 30 and 34.9 ng/mL [23]. This 10 ng/mL threshold might represent the lowest necessary vitamin D serum level for H. pylori eradication success [30].
As previously described, lower vitamin D levels have been found in patients with diabetes and H. pylori infection/positive serum antibodies [38,39]. However, Huang et al. were the only ones who evaluated how vitamin D deficiency interferes with H. pylori therapeutic success in patients with type 2 diabetes mellitus. Vitamin D levels under 20 ng/mL significantly negatively impacted H. pylori eradication, whereas levels ranging between 20 and 30 ng/mL did not influence therapeutic success. Furthermore, the authors acknowledged that vitamin D deficit might also have enhanced dyslipidemia in their study cohort, which, together with prolonged evolution of the glycemic imbalance, could have also contributed to eradication failure [50].
In children, data on the role of vitamin D in H. pylori eradication rates are currently limited to a few studies. Zhang et al. found the lowest vitamin D serum values among a pediatric population with H. pylori recurrence, despite adequate treatment. Within their study, significantly higher values of the same parameter were found in those patients in whom bacterial eradication was successful and in healthy controls, but each of the analyzed study groups had a low percentage of vitamin D-deficient and -insufficient individuals [51]. Sorokman et al. found that vitamin D levels of over 20 ng/mL are more likely to ensure eradication success. Their study provided no information related to vitamin D supplementation amount and status, which is a routine approach at pediatric ages [43]. In fact, literature data regarding vitamin D intake/supplementation and H. pylori eradication success are still very limited. Only one population-based study has managed so far to identify a correlation between a higher vitamin D intake, positively correlated with a higher dietary fish intake, and efficacy of H. pylori eradication [52].
3.3. VDR and H. pylori Infection
H. pylori induces increased expression of VDRs, which have been shown to play an important role in innate immunity and to render antimicrobial activity. One study conducted on adult patients showed that cells of gastric mucosa specimens harvested from H. pylori-infected patients presented a significant elevation in VDR expression, as opposed to healthy controls [53]. Starting from the theory of possible interference of VDRs with H. pylori infection, recent research tried to evaluate the impact of VDR gene’s polymorphisms as well. Martins et al. found a differential distribution of BsmI genotypes of the VDR gene within a study population, divided based on H. pylori positivity within gastric mucosa biopsy specimens. The other three polymorphisms studied—namely FokI, ApaI, and TaqI—presented no significant genotype distribution variation among the two study groups [54]. However, in another study, a significant correlation between the Fokl and Apal polymorphisms of the VDR gene and H. pylori infection was found [55].
3.4. Vitamin D and Gastric Cancer
Vitamin D intake seems to decrease gastric cancer risk, as reflected through a study conducted on a Vietnamese population. The same study also revealed that vitamin D intake is inversely associated with both positive and negative IgG serum antibody titers directed against H. pylori [56]. Thus, this study concluded that vitamin D supplementation can protect against gastric cancer, but failed to establish a relationship between daily vitamin D oral intake and H. pylori infection [56]. The possible link between hypovitaminosis D and increased cancer risk is further strengthened by Antico et al., who comparatively assessed vitamin D status between four different study groups: one group with atrophic gastritis type A, one group with H. pylori gastritis, a group of patients diagnosed with non-specific lymphocytic gastritis, and a control group of healthy subjects. The study group of patients with atrophic, autoimmune-induced gastritis presented the lowest vitamin D levels, followed by the H. pylori gastritis group, which also exhibited significantly lower values of serum vitamin D than the other two study groups [57]. Contrary to previous findings, other authors claimed that a higher intake of vitamin D accounts for an increase in gastric cancer risk, even in patients without serological traces of H. pylori infection [58].
4. Discussion
One meta-analysis of 48 studies pointed out that H. pylori infection has been associated with a decrease in serum levels of various vitamins, including vitamin B12, vitamin C, and vitamin D. Higher serum vitamin D and vitamin B12 levels were warranted to improve H. pylori eradication rates, whereas vitamin C supplementation helped achieve the same result [59]. This meta-analysis revealed some shortcomings of the analyzed studies, which included limited geographical research areas—as a significant number of studies were conducted on Turkish populations—as well as controversy surrounding adequate vitamin D supplementation [59]. One other meta-analysis, limited to 10 articles, only focused on the impact of vitamin D status upon H. pylori infection. Despite the heterogenicity of the included studies, the authors concluded that lower vitamin D levels might account for a higher prevalence of H. pylori infection and might negatively influence bacterial eradication [22]. Similar to this meta-analysis, our review included only those studies that analyzed the manner in which vitamin D impacts H. pylori infection occurrence and its subsequent eradication. As seen in Table 1 and Table 2, and further detailed in the results section, most of the clinical studies included in this review confirm an inverse relationship between serum vitamin D levels and H. pylori infection and eradication rates. However, despite the important number of relevant studies on this topic, their heterogeneity cannot be neglected and hinders the performance of a meta-analysis. Thus, H. pylori detection methods vary greatly, whereas the data were mostly limited to Asian studies, which might significantly impact the interpretation of results due to the miscellaneous dietary and nutrition patterns across the globe. Moreover, a handful of studies found no significant relationship between vitamin D and H. pylori, and very few of the studies included in this review assessed the importance of vitamin D supplementation [33,34,35,49,52]. The main population benefitting from regular vitamin D supplementation belongs to the pediatric segment. However, the limited number of pediatric studies performed on this subject did not offer any information regarding the regularity, amount, and/or adequacy of the intake of vitamin D supplements within the analyzed study populations [40,41,42,43,51]. Hence, future prospective studies and randomized controlled trials are required to clarify the relationship between vitamin D serum levels, its supplementation, and H. pylori, and to evaluate whether the addition of vitamin D3 to therapeutic regimens influences bacterial eradication. Cofounding factors such as age, interfering in the relationship between vitamin D supplementation and H. pylori prevalence and eradication, are also subject to future debate, given the lack of regular vitamin D intake among adult populations and the possible inverse relationship between H. pylori and vitamin D deficiency, as proposed by Bahși et al. [28].
Our review excluded experimental data, due to the significant abundance of clinical data and the limited number of studies that included animal populations. However, some important aspects can be retained from experimental studies as well, which might aid future clinical research. After artificial infection of the stomach with H. pylori in mice, the administration of oral vitamin D3 produced a significant decrease in colonization rates, as well as an upregulation of VDRs [60,61]. The mouse model proved that anti-H. pylori activity of vitamin D3 is further enhanced by the activation of the VDR-Cathelicidin antimicrobial peptide (CAMP) pathway [60,61]. Another in vitro study showed that vitamin D3 supplementation triggers a restoration of lysosomal degradation through the activation of the protein disulfide-isomerase A3 (PDIA3) receptor, which promotes calcium release from lysosomes, lysosomal acidification and, consequently, the elimination of H. pylori through the autolysosomal pathway [62]. Furthermore, the synthetical production of indene compounds derived from vitamin D led to selective antibacterial action against H. pylori [63,64]. More specifically, vitamin D decomposition products, such as vitamin D3 decomposition product 1 (VDP1), detain an anti-H. pylori action through induction of the bacteria’s cell membrane structure collapse [65]. A synergistic effect of the addition of 1α, 25-dihydroxyvitamin D3 to the standard quadruple therapy in the eradication of H. pylori has been proposed, after the compound was proven to protect against H.pylori-induced apoptosis of gastric epithelial cells [66]. This theory is further supported by the important tumor suppressor role of the vitamin D3 upregulated protein 1 (VDUP1), which was shown to protect against gastric carcinogenesis [67]. Thus, in light of these recently surfaced experimental data, future population-based studies are required to shine a light upon the role of vitamin D3 supplementation in preventing H. pylori infection and in facilitating its eradication. Pediatric studies could provide more insight into this matter, given that vitamin D supplements are routinely administered in children [68]. The data available so far for pediatric ages did not provide any information regarding the amount of vitamin D supplementation that the enrolled populations were receiving [40,41,42,43,51].
The link between vitamin D intake and gastric cancer risk, while taking into consideration the presence/absence of H. pylori infection, still requires more research. Two review articles clearly underlined an inverse correlation between vitamin D serum levels and gastric cancer risk, morbidity, and mortality, but most of the studies included in these articles did not perform a separate analysis on H. pylori infection status [69,70]. As previously reported in the results section, patients with gastric cancer and atrophic gastritis, infected with H. pylori, might present lower vitamin D serum levels, but research on this matter needs to be expanded [4,57]. Furthermore, the clear mechanisms through which vitamin D offers protection against gastric cancer, possibly through VDR interaction, still require elucidation [69,70].
A few previous reviews also provided some important take-home messages on the role of vitamin D in H. pylori infection. The bacteriolytic action of hydrophobic lipid structures, such as indene compounds, vitamin D, and 3-carbonyl steroids, has been recently highlighted [71]. Among multiple micronutrients that can influence H. pylori’s survival, pathogenicity, and eradication, vitamin D distinguished itself as an important regulator of host immune response [72]. Hence, one literature survey (Golpour et al.) proposed potential antibiotic-independent approaches for H. pylori treatment based on vitamin D supplements [73]. However, these review articles had a narrative structure and referred to fewer population-based studies than the present work.
5. Conclusions
This comprehensive review highlights the impact of vitamin D deficiency and/or insufficiency upon an ascending prevalence of H. pylori infection and its eradication failure. Vitamin D-deficient subjects might be more prone to developing H. pylori infection, but the influence of H. pylori infection upon vitamin D status is still puzzling. The role of vitamin D intake is still in question, as most of the reviewed studies failed to analyze whether the addition of oral supplements influences the performance of current therapeutic regimens. Thus, the screening of vitamin D levels in subjects with H. pylori infection might establish the need for its supplementation in ensuring therapeutic success. Vitamin D deficit might be connected to an increase in gastric cancer risk, but the additional influence of H. pylori in this linear relationship is still unclear. Furthermore, the currently available data are hindered by the enrollment of populations from restricted geographical regions, as well as by the heterogenicity of H. pylori infection detection methods and the reduced number of pediatric populations included so far. Future population-based studies and randomized clinical trials from larger geographical areas are warranted to address this subject in more depth and to analyze the synergistic effect of vitamin D supplementation and classical therapeutic schemes.
Author Contributions
M.O.S. and C.O.M. conceptualized and designed the study and conducted literature search and drafted the initial manuscript. M.O.S. and C.O.M. reviewed and revised the manuscript. A.L. and A.M.K. participated in collecting literature data and helped in drafting the manuscript tables. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carmeliet, G.; Dermauw, V.; Bouillon, R. Vitamin D Signaling in Calcium and Bone Homeostasis: A Delicate Balance. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Muntingh, G.L. Vitamin D—The Vitamin Hormone. S. Afr. Fam. Pract. 2016, 58, 32–36. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Nguyen, T. Vitamin D and Vitamin D Analogs. J. Nurse Pract. 2016, 12, 208–209. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like Receptor Triggering of a Vitamin D-Mediated Human Antimicrobial Response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T.; Stenger, S.; Tang, D.H.; Modlin, R.L. Cutting Edge: Vitamin D-Mediated Human Antimicrobial Activity against Mycobacterium Tuberculosis Is Dependent on the Induction of Cathelicidin. J. Immunol. 2007, 179, 2060–2063. [Google Scholar] [CrossRef]
- von Essen, M.R.; Kongsbak, M.; Schjerling, P.; Olgaard, K.; Odum, N.; Geisler, C. Vitamin D Controls T Cell Antigen Receptor Signaling and Activation of Human T Cells. Nat. Immunol. 2010, 11, 344–349. [Google Scholar] [CrossRef]
- Ismailova, A.; White, J.H. Vitamin D, Infections and Immunity. Rev. Endocr. Metab. Disord. 2022, 23, 265–277. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; Goodall, E.C.; et al. Vitamin D Supplementation to Prevent Acute Respiratory Infections: Individual Participant Data Meta-Analysis. Health Technol. Assess. 2019, 23, 1–44. [Google Scholar] [CrossRef]
- Panfili, F.M.; Roversi, M.; D’Argenio, P.; Rossi, P.; Cappa, M.; Fintini, D. Possible Role of Vitamin D in COVID-19 Infection in Pediatric Population. J. Endocrinol. Investig. 2021, 44, 27–35. [Google Scholar] [CrossRef]
- Uwaezuoke, S.N.; Odimegwu, C.L.; Mbanefo, N.R.; Eneh, C.I.; Arodiwe, I.O.; Muoneke, U.V.; Ogbuka, F.N.; Ndiokwelu, C.O.; Akwue, A.T. Vitamin D3 Supplementation as an Adjunct in the Management of Childhood Infectious Diarrhea: A Systematic Review. BMC Infect. Dis. 2023, 23, 159. [Google Scholar] [CrossRef] [PubMed]
- Thornton, K.A.; Marín, C.; Mora-Plazas, M.; Villamor, E. Vitamin D Deficiency Associated with Increased Incidence of Gastrointestinal and Ear Infections in School-Age Children. Pediatr. Infect. Dis. J. 2013, 32, 585. [Google Scholar] [CrossRef] [PubMed]
- Muntean, C.; Săsăran, M. Vitamin D Status and Its Role in First-Time and Recurrent Urinary Tract Infections in Children: A Case-Control Study. Children 2021, 8, 419. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Zhang, X.; Ying, J.; Zhou, Y.; Li, X.; Mu, D.; Qu, Y. Association between Vitamin D Status and Sepsis in Children: A Meta-Analysis of Observational Studies. Clin. Nutr. 2020, 39, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Waskito, L.A.; Yamaoka, Y. The Story of Helicobacter Pylori: Depicting Human Migrations from the Phylogeography. Adv. Exp. Med. Biol. 2019, 1149, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Alipour, M. Molecular Mechanism of Helicobacter Pylori-Induced Gastric Cancer. J. Gastrointest. Cancer 2021, 52, 23–30. [Google Scholar] [CrossRef]
- den Hollander, W.J.; Sostres, C.; Kuipers, E.J.; Lanas, A. Helicobacter Pylori and Nonmalignant Diseases. Helicobacter 2013, 18, 24–27. [Google Scholar] [CrossRef]
- Du, L.-J.; Chen, B.-R.; Kim, J.J.; Kim, S.; Shen, J.-H.; Dai, N. Helicobacter Pylori Eradication Therapy for Functional Dyspepsia: Systematic Review and Meta-Analysis. World J. Gastroenterol. 2016, 22, 3486–3495. [Google Scholar] [CrossRef]
- Tshibangu-Kabamba, E.; Yamaoka, Y. Helicobacter Pylori Infection and Antibiotic Resistance—From Biology to Clinical Implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 613–629. [Google Scholar] [CrossRef]
- Shatla, M.M.; Faisal, A.S.; El-Readi, M.Z. Is Vitamin D Deficiency a Risk Factor for Helicobacter Pylori Eradication Failure? Clin. Lab. 2021, 67. [Google Scholar] [CrossRef]
- Yildirim, O.; Yildirim, T.; Seckin, Y.; Osanmaz, P.; Bilgic, Y.; Mete, R. The Influence of Vitamin D Deficiency on Eradication Rates of Helicobacter Pylori. Adv. Clin. Exp. Med. 2017, 26, 1377–1381. [Google Scholar] [CrossRef]
- Yang, L.; He, X.; Li, L.; Lu, C. Effect of Vitamin D on Helicobacter Pylori Infection and Eradication: A Meta-Analysis. Helicobacter 2019, 24, e12655. [Google Scholar] [CrossRef] [PubMed]
- Shafrir, A.; Shauly-Aharonov, M.; Katz, L.H.; Paltiel, O.; Pickman, Y.; Ackerman, Z. The Association between Serum Vitamin D Levels and Helicobacter Pylori Presence and Eradication. Nutrients 2021, 13, 278. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Habbash, F.; Alalwan, T.A.; Perna, S.; Ahmed, N.; Sharif, O.; Al Sayyad, A.; Gasparri, C.; Ferraris, C.; Rondanelli, M. Association between Dietary Habits and Helicobacter Pylori Infection among Bahraini Adults. Nutrients 2022, 14, 4215. [Google Scholar] [CrossRef] [PubMed]
- Mut Surmeli, D.; Surmeli, Z.G.; Bahsi, R.; Turgut, T.; Selvi Oztorun, H.; Atmis, V.; Varli, M.; Aras, S. Vitamin D Deficiency and Risk of Helicobacter Pylori Infection in Older Adults: A Cross-Sectional Study. Aging Clin. Exp. Res. 2019, 31, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Assaad, S.; Chaaban, R.; Tannous, F.; Costanian, C. Dietary Habits and Helicobacter Pylori Infection: A Cross Sectional Study at a Lebanese Hospital. BMC Gastroenterol. 2018, 18, 48. [Google Scholar] [CrossRef] [PubMed]
- Bahşi, R.; Atmiş, V.; Mut Sürmeli, D.; Öztorun, H.S.; Turgut, T.; Coşarderelioğlu, Ç.; Yalçin, A.; Aras, S.; Varli, M. Effect of Helicobacter Pylori Infection on Vitamin D Levels in Old Patients with Sarcopenia. Adv. Dig. Med. 2022, 9, 98–102. [Google Scholar] [CrossRef]
- Bashir, M.; Prietl, B.; Tauschmann, M.; Mautner, S.I.; Kump, P.K.; Treiber, G.; Wurm, P.; Gorkiewicz, G.; Högenauer, C.; Pieber, T.R. Effects of High Doses of Vitamin D3 on Mucosa-Associated Gut Microbiome Vary between Regions of the Human Gastrointestinal Tract. Eur. J. Nutr. 2016, 55, 1479–1489. [Google Scholar] [CrossRef]
- Han, C.; Ni, Z.; Yuan, T.; Zhang, J.; Wang, C.; Wang, X.; Ning, H.B.; Liu, J.; Sun, N.; Liu, C.F.; et al. Influence of Serum Vitamin D Level on Helicobacter Pylori Eradication: A Multi-Center, Observational, Prospective and Cohort Study. J. Dig. Dis. 2019, 20, 421–426. [Google Scholar] [CrossRef]
- Assaad, S.; Costanian, C.; Jaffal, L.; Tannous, F.; Stathopoulou, M.G.; El Shamieh, S. Association of TLR4 Polymorphisms, Expression, and Vitamin D with Helicobacter Pylori Infection. J. Pers. Med. 2019, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, Z.J.; Rasool, K.H.; Ahmed, M.A. Relationship between Helicobacter Pylori Infections and Vitamin D Level and Lipid Profile in Some Obese Iraqi Women. Casp. J. Environ. Sci. 2021, 19, 801–807. [Google Scholar] [CrossRef]
- Chen, L.-W.; Chien, C.-Y.; Hsieh, C.-W.; Chang, L.-C.; Huang, M.-H.; Huang, W.-Y.; Kuo, S.-F.; Chien, C.-H.; Lin, C.-L.; Chien, R.-N. The Associations Between Helicobacter Pylori Infection, Serum Vitamin D, and Metabolic Syndrome: A Community-Based Study. Medicine 2016, 95, e3616. [Google Scholar] [CrossRef]
- Gerig, R.; Ernst, B.; Wilms, B.; Thurnheer, M.; Schultes, B. Preoperative Nutritional Deficiencies in Severely Obese Bariatric Candidates Are Not Linked to Gastric Helicobacter Pylori Infection. Obes. Surg. 2013, 23, 698–702. [Google Scholar] [CrossRef]
- Mihalache, L.; Gavril, R.; Arhire, L.I.; Niţă, O.; Gherasim, A.; Oprescu, A.C.; Lapuste, C.; Constantinescu, D.; Padureanu, S.S.; Danciu, M.; et al. Nutritional Biomarkers in Patients with Obesity-the Relation between Helicobacter Pylori Infection and Micronutrients. Rev. Chim. 2016, 67, 2413–2416. [Google Scholar]
- Mirza, W.A.; Zhang, K.; Zhang, R.; Duan, G.; Khan, M.S.N.; Ni, P. Vitamin D Deficiency in Dengue Fever Patients’ Coinfected with H. pylori in Pakistan. A Case-Control Study. Front. Public Health 2022, 10, 1035560. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; Baradaran, A. The Influence of Serum 25-Hydroxy Vitamin D Levels on Helicobacter Pylori Infections in Patients with End-Stage Renal Failure on Regular Hemodialysis. Saudi J. Kidney Dis. Transpl. 2007, 18, 215–219. [Google Scholar] [PubMed]
- Bener, A.; Ağan, A.F.; Al-Hamaq, A.O.A.A.; Barisik, C.C.; Öztürk, M.; Ömer, A. Prevalence of Helicobacter Pylori Infection among Type 2 Diabetes Mellitus. Adv. Biomed. Res. 2020, 9, 27. [Google Scholar] [CrossRef]
- Zawada, A.E.; Naskręt, D.; Piłaciński, S.; Adamska, A.; Grzymisławski, M.; Eder, P.; Grzelka-Woźniak, A.; Zozulińska-Ziółkiewicz, D.; Dobrowolska, A. Helicobacter Pylori Infection Is Associated with Increased Accumulation of Advanced Glycation End Products in the Skin in Patients with Type 1 Diabetes: A Preliminary Study. Adv. Clin. Exp. Med. 2023, 32. [Google Scholar] [CrossRef]
- Agin, M.; Tas, S. The Relationship between Vitamin D Deficiency and the Frequency of Helicobacter Pylori and Peptic Ulcer in Childhood. Ann. Clin. Anal. Med. 2021, 12, 563–566. [Google Scholar] [CrossRef]
- Urganci, N.; Kalyoncu, D. Assessment of Bone Metabolism and Bone Mineral Density in Children with Helicobacter Pylori Infection. Med. J. Bakirkoy 2020, 16, 343–348. [Google Scholar] [CrossRef]
- Gao, T.; Zhao, M.; Zhang, C.; Wang, P.; Zhou, W.; Tan, S.; Zhao, L. Association of Helicobacter Pylori Infection with Vitamin D Deficiency in Infants and Toddlers. Am. J. Trop. Med. Hyg. 2020, 102, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Sorokman, T.; Chernei, N.; Sokolnyk, S.; Sokolnyk, I.; Popelyuk, N.; Shvygar, L. Efficacy of Eradication Therapy in Children with H. Pylori-Associated Diseases Depending on Levels of Nitric Oxide and Vitamin D. Med. Sci. 2020, 24, 1895–1903. [Google Scholar]
- Kuang, W.-M.; Ren, Y.-J.; Chen, X.; Luo, Q.; Chen, W.; Pan, H.-G.; Li, R.-L.; Hu, L. Association between Serum Vitamin D Levels and Helicobacter Pylori Cytotoxic-Associated Gene A Seropositivity: A Cross-Sectional Study in US Adults from NHANES III. BMJ Open 2022, 12, e058164. [Google Scholar] [CrossRef]
- El Shahawy, M.S.; Hemida, M.H.; El Metwaly, I.; Shady, Z.M. The Effect of Vitamin D Deficiency on Eradication Rates of Helicobacter Pylori Infection. JGH Open 2018, 2, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Magsi, I.; Hussain Keerio, S.; Kumar, C.; Talpur, A.S.; Shahzeen, F.; Mushtaq Abbasi, Z.; Lohano, M.; Kumar, V.; Rizwan, A. Response of Helicobacter Pylori Eradication Treatment in Patients with Normal and Below-Normal Serum Vitamin D Levels. Cureus 2021, 13, e14777. [Google Scholar] [CrossRef] [PubMed]
- Almani, S.A.; Nazia, S.; Shah, M.I.; Kumar, S. Response of H. pylori Eradication Treatment in Patients with Normal and Below Normal Serum Vitamin D Levels. J. Liaquat Univ. Med. Health Sci. 2020, 19, 242–246. [Google Scholar]
- Lan, Q.-L.; Sun, H.-Y.; Ye, Y.; Wang, Y.; Liu, Y.; Weng, X.-J. Factors Affect the Eradication Rate of Helicobacter Pylori by Modified Quadruple Therapy: A Prospective Cohort Study. Infect. Drug Resist. 2022, 15, 2339–2345. [Google Scholar] [CrossRef] [PubMed]
- El Shahawy, M.S.; Shady, Z.M.; Gaafar, A. Influence of Adding Vitamin D3 to Standard Clarithromycin-Based Triple Therapy on the Eradication Rates of Helicobacter Pylori Infection. Arab. J. Gastroenterol. 2021, 22, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yan, S.; Chen, C.; Ye, S. Effect of 25-Hydroxyvitamin D on Helicobacter Pylori Eradication in Patients with Type 2 Diabetes. Wien. Klin. Wochenschr. 2019, 131, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, B.; Guo, X.; Zhang, S. Analysis of Eradication, Recurrence and Levels of 25-Hydroxyvitamin D3 and Interleukin-1β in Paediatric Patients with Helicobacter Pylori Infection-Related Gastritis. Pak. J. Med. Sci. 2020, 36, 1377–1381. [Google Scholar] [CrossRef]
- Ikezaki, H.; Furusyo, N.; Jacques, P.F.; Shimizu, M.; Murata, M.; Schaefer, E.J.; Urita, Y.; Hayashi, J. Higher Dietary Cholesterol and ω-3 Fatty Acid Intakes Are Associated with a Lower Success Rate of Helicobacter Pylori Eradication Therapy in Japan. Am. J. Clin. Nutr. 2017, 106, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chen, W.; Zhu, H.; Chen, Y.; Wan, X.; Yang, N.; Xu, S.; Yu, C.; Chen, L. Helicobacter Pylori Induces Increased Expression of the Vitamin d Receptor in Immune Responses. Helicobacter 2014, 19, 37–47. [Google Scholar] [CrossRef]
- Martins, D.d.J.; Matos, G.C.; Loiola, R.S.; D’Annibale, V.; Corvelo, T. Relationship of Vitamin D Receptor Gene Polymorphisms in Helicobacter Pylori Gastric Patients. Clin. Exp. Gastroenterol. 2018, 11, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.; Moussa, S.; Shaheen, M.M.; Abd-Elsalam, S.; Ahmed, R.; Mostafa, S.M.; Fouad, A.; Alegaily, H.S.; Megahed, S.A.; Abo-Amer, Y.E. Association Between Vitamin D Receptor Gene Polymorphisms and Infection. Open Biomark. J. 2020, 10, 8–14. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Huynh, N.N.Y.; Nguyen, D.D.; Ta, N.H.; Van Nguyen, T.; Dang, H.T.; Le, N.T. Vitamin D Intake and Gastric Cancer in Viet Nam: A Case-Control Study. BMC Cancer 2022, 22, 838. [Google Scholar] [CrossRef] [PubMed]
- Antico, A.; Tozzoli, R.; Giavarina, D.; Tonutti, E.; Bizzaro, N. Hypovitaminosis D as Predisposing Factor for Atrophic Type A Gastritis: A Case-Control Study and Review of the Literature on the Interaction of Vitamin D with the Immune System. Clin. Rev. Allergy Immunol. 2012, 42, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Toorang, F.; Narmcheshm, S.; Sasanfar, B.; Amini, N.; Hadji, M.; Mortazavi, M.; Zendehdel, K. Vitamins and Stomach Cancer: A Hospital Based Case-Control Study in Iran. J. Nutr. Food Secur. 2022, 7, 474–483. [Google Scholar] [CrossRef]
- Cai, X.; Li, X.; Jin, Y.; Zhang, M.; Xu, Y.; Liang, C.; Weng, Y.; Yu, W.; Li, X. Vitamins and Helicobacter Pylori: An Updated Comprehensive Meta-Analysis and Systematic Review. Front. Nutr. 2022, 8, 781333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, C.; Zhang, L.; Yu, J.; Yuan, W.; Li, L. Vitamin D3 Eradicates Helicobacter Pylori by Inducing VDR-CAMP Signaling. Front. Microbiol. 2022, 13, 1033201. [Google Scholar] [CrossRef]
- Zhou, A.; Li, L.; Zhao, G.; Min, L.; Liu, S.; Zhu, S.; Guo, Q.; Liu, C.; Zhang, S.; Li, P. Vitamin D3 Inhibits Helicobacter Pylori Infection by Activating the VitD3/VDR-CAMP Pathway in Mice. Front. Cell. Infect. Microbiol. 2020, 10, 566730. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, L.; Li, M.X.; Shen, J.; Liu, X.D.; Xiao, Z.G.; Wu, D.L.; Ho, I.H.T.; Wu, J.C.Y.; Cheung, C.K.Y.; et al. Vitamin D3 Activates the Autolysosomal Degradation Function against Helicobacter Pylori through the PDIA3 Receptor in Gastric Epithelial Cells. Autophagy 2019, 15, 707–725. [Google Scholar] [CrossRef]
- Wanibuchi, K.; Hosoda, K.; Ihara, M.; Tajiri, K.; Sakai, Y.; Masui, H.; Takahashi, T.; Hirai, Y.; Shimomura, H. Indene Compounds Synthetically Derived from Vitamin D Have Selective Antibacterial Action on Helicobacter Pylori. Lipids 2018, 53, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Wanibuchi, K.; Takezawa, M.; Hosoda, K.; Amgalanbaatar, A.; Tajiri, K.; Koizumi, Y.; Niitsu, S.; Masui, H.; Sakai, Y.; Shoji, M.; et al. Antibacterial Effect of Indene on Helicobacter Pylori Correlates with Specific Interaction between Its Compound and Dimyristoyl-Phosphatidylethanolamine. Chem. Phys. Lipids 2020, 227, 104871. [Google Scholar] [CrossRef]
- Hosoda, K.; Shimomura, H.; Wanibuchi, K.; Masui, H.; Amgalanbaatar, A.; Hayashi, S.; Takahashi, T.; Hirai, Y. Identification and Characterization of a Vitamin D₃ Decomposition Product Bactericidal against Helicobacter Pylori. Sci. Rep. 2015, 5, 8860. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wan, D.; Zhong, Y.; Xu, X. 1α, 25-Dihydroxyvitamin D3 Protects Gastric Mucosa Epithelial Cells against Helicobacter Pylori-Infected Apoptosis through a Vitamin D Receptor-Dependent c-Raf/MEK/ERK Pathway. Pharm. Biol. 2022, 60, 801–809. [Google Scholar] [CrossRef]
- Kwon, H.-J.; Won, Y.-S.; Nam, K.-T.; Yoon, Y.-D.; Jee, H.; Yoon, W.-K.; Nam, K.-H.; Kang, J.-S.; Han, S.-U.; Choi, I.-P.; et al. Vitamin D₃ Upregulated Protein 1 Deficiency Promotes N-Methyl-N-Nitrosourea and Helicobacter Pylori-Induced Gastric Carcinogenesis in Mice. Gut 2012, 61, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, N.; Fatima, A.; Gillani, S.W.; Kaddour, N.; Banoori, R.; Elshafie, R.M.; Rathore, H.A. Evaluation of Vitamin D Supplementation Intake among Children; Cross-Sectional Observational Study. F1000Research 2022, 11, 1456. [Google Scholar] [CrossRef]
- Du, C.; Yang, S.; Zhao, X.; Dong, H. Pathogenic Roles of Alterations in Vitamin D and Vitamin D Receptor in Gastric Tumorigenesis. Oncotarget 2017, 8, 29474–29486. [Google Scholar] [CrossRef]
- Parizadeh, S.M.; Ghandehari, M.; Jafarzadeh-Esfehani, R.; Parizadeh, S.M.; Hassanian, S.M.; Ghayour-Mobarhan, M.; Ferns, G.A.; Avan, A. The Relationship Between Vitamin D Status and Risk of Gastric Cancer. Nutr. Cancer 2020, 72, 15–23. [Google Scholar]
- Shimomura, H.; Wanibuchi, K.; Hosoda, K.; Amgalanbaatar, A.; Masui, H.; Takahashi, T.; Hirai, Y. Unique Responses of Helicobacter Pylori to Exogenous Hydrophobic Compounds. Chem. Phys. Lipids 2020, 229, 104908. [Google Scholar] [CrossRef]
- Nabavi-Rad, A.; Azizi, M.; Jamshidizadeh, S.; Sadeghi, A.; Aghdaei, H.A.; Yadegar, A.; Zali, M.R. The Effects of Vitamins and Micronutrients on Helicobacter Pylori Pathogenicity, Survival, and Eradication: A Crosstalk between Micronutrients and Immune System. J. Immunol. Res. 2022, 2022, e4713684. [Google Scholar] [CrossRef]
- Golpour, A.; Bereswill, S.; Heimesaat, M.M. Antimicrobial and Immune-Modulatory Effects of Vitamin D Provide Promising Antibiotics-Independent Approaches to Tackle Bacterial Infections—Lessons Learnt from a Literature Survey. Eur. J. Microbiol. Immunol. 2019, 9, 80–87. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).