Unlocking the Power of Late-Evening Snacks: Practical Ready-to-Prescribe Chart Menu for Patients with Cirrhosis
Abstract
:1. Introduction
2. Data Sources and Searches
3. Impact of Malnutrition in Patients with Liver Cirrhosis
4. Nutritional Requirements in Liver Cirrhosis Patient
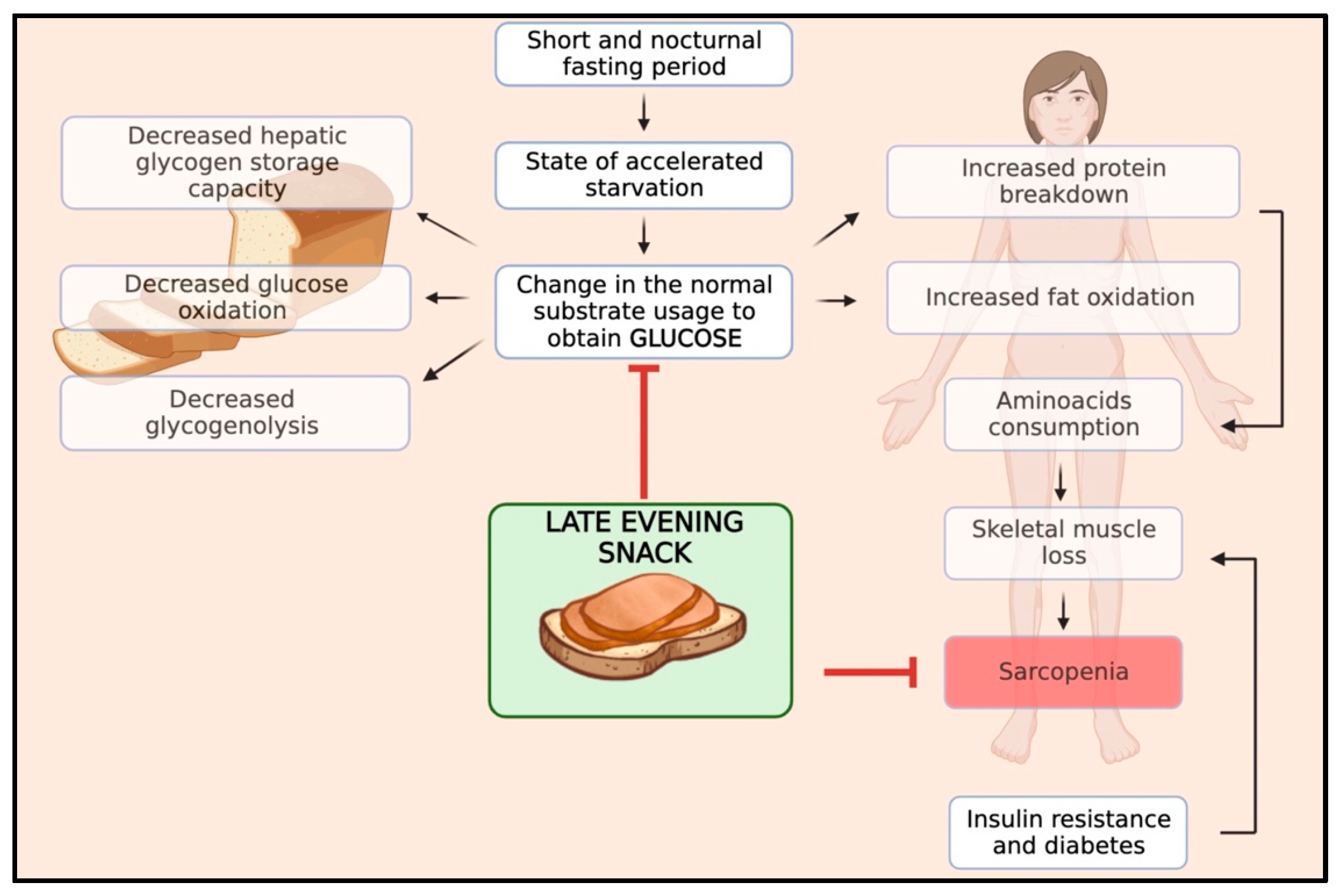
5. Rationale and Pathophysiological Principles of the Late-Evening Snack (LES)
6. Evidence of an LES in Patients with ACLD
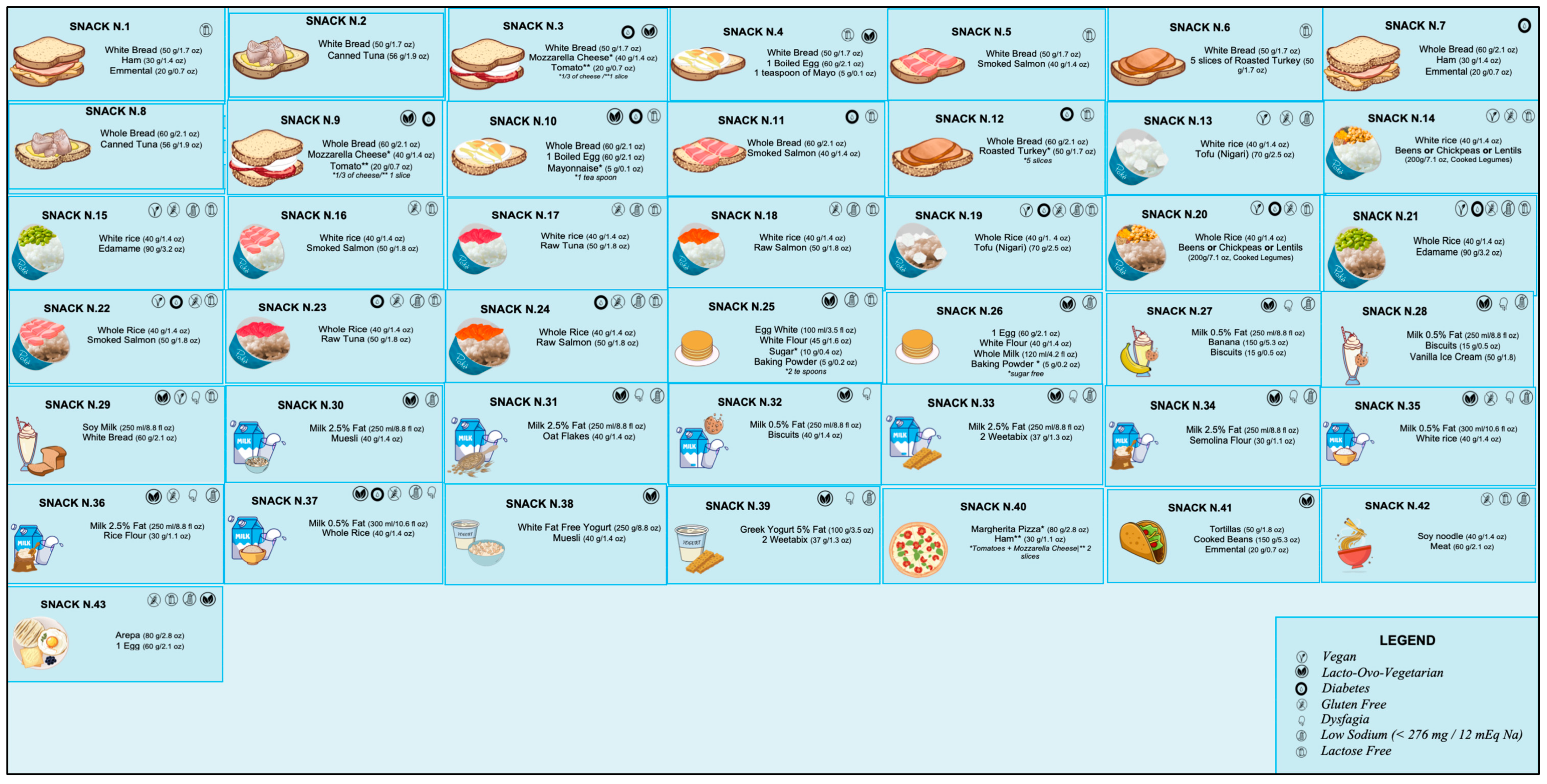
7. Nutritional Composition of the Late-Evening Snack (LES)
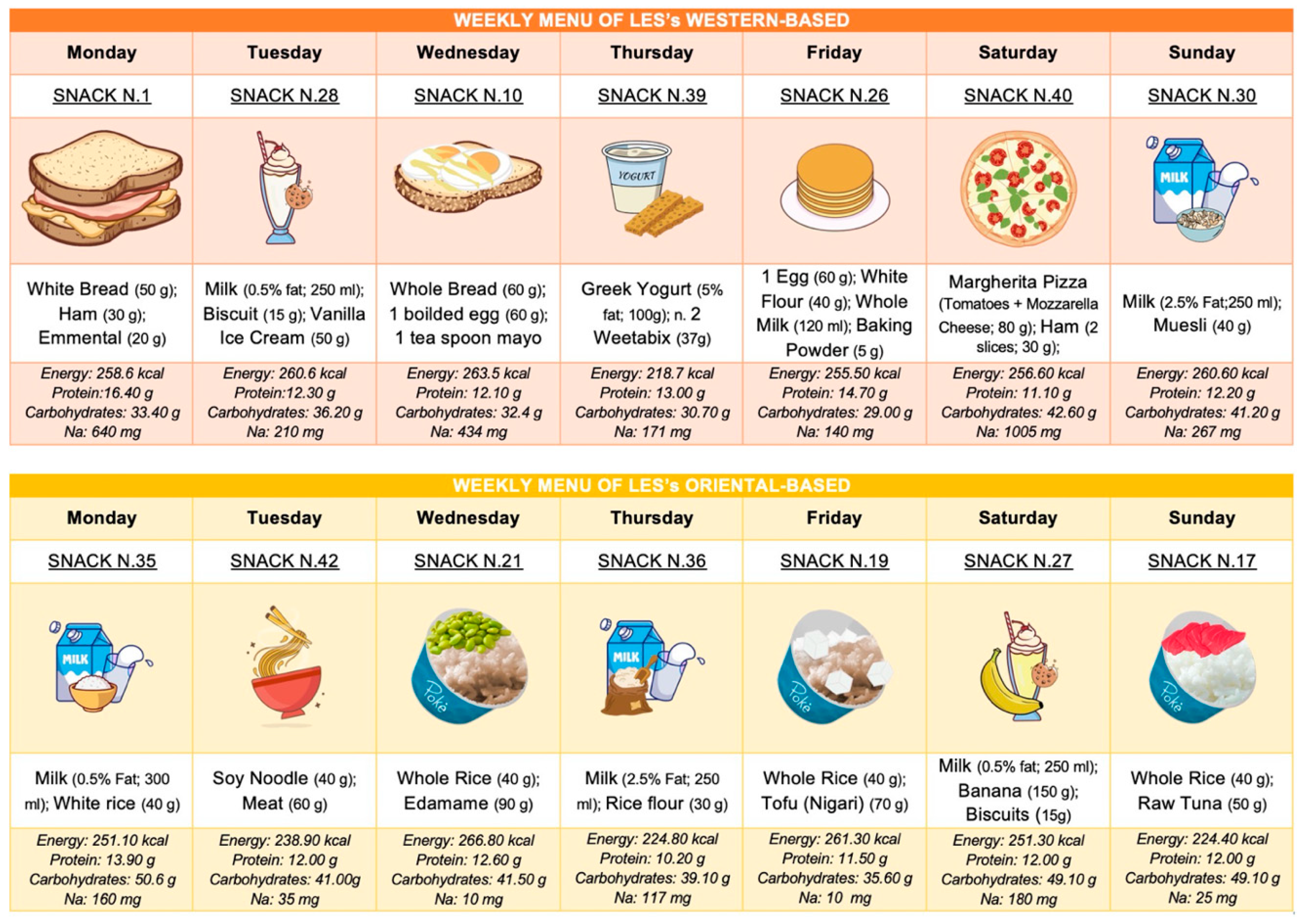
8. Practical Approach to a Late-Evening Snack (LES) for ACLD
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ginès, P.; Krag, A.; Abraldes, J.G.; Solà, E.; Fabrellas, N.; Kamath, P.S. Liver Cirrhosis. Lancet 2021, 398, 1359–1376. [Google Scholar] [CrossRef]
- D’Amico, G.; Morabito, A.; D’Amico, M.; Pasta, L.; Malizia, G.; Rebora, P.; Valsecchi, M.G. Clinical States of Cirrhosis and Competing Risks. J. Hepatol. 2018, 68, 563–576. [Google Scholar] [CrossRef] [Green Version]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN Guidelines on Definitions and Terminology of Clinical Nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM Criteria for the Diagnosis of Malnutrition—A Consensus Report from the Global Clinical Nutrition Community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.M.; et al. Diagnostic Criteria for Malnutrition—An ESPenteral Nutrition Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Traub, J.; Reiss, L.; Aliwa, B.; Stadlbauer, V. Malnutrition in Patients with Liver Cirrhosis. Nutrients 2021, 13, 540. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, A.; Toori, K.U.; Shaikh, J.I. To Determine Correlation between Biochemical Parameters of Nutritional Status with Disease Severity in HCV Related Liver Cirrhosis. Pak. J. Med. Sci. 2018, 34, 154. [Google Scholar] [CrossRef]
- Naqvi, I.H.; Mahmood, K.; Salekeen, S.; Akhter, S.T. Determining the Frequency and Severity of Malnutrition and Correlating It with the Severity of Liver Cirrhosis. Turk. J. Gastroenterol. 2013, 24, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Maharshi, S.; Sharma, B.C.; Srivastava, S. Malnutrition in Cirrhosis Increases Morbidity and Mortality: Malnutrition in Cirrhosis. J. Gastroenterol. Hepatol. 2015, 30, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Huisman, E.J.; Trip, E.J.; Siersema, P.D.; van Hoek, B.; van Erpecum, K.J. Protein Energy Malnutrition Predicts Complications in Liver Cirrhosis. Eur. J. Gastroenterol. Hepatol. 2011, 23, 982–989. [Google Scholar] [CrossRef]
- Lindqvist, C.; Majeed, A.; Wahlin, S. Body Composition Assessed by Dual-Energy X-Ray Absorptiometry Predicts Early Infectious Complications after Liver Transplantation. J. Hum. Nutr. Diet. 2017, 30, 284–291. [Google Scholar] [CrossRef]
- Ruiz-Margáin, A.; Macías-Rodríguez, R.U.; Ampuero, J.; Cubero, F.J.; Chi-Cervera, L.; Ríos-Torres, S.L.; Duarte-Rojo, A.; Espinosa-Cuevas, Á.; Romero-Gómez, M.; Torre, A. Low Phase Angle Is Associated with the Development of Hepatic Encephalopathy in Patients with Cirrhosis. World J. Gastroenterol. 2016, 22, 10064. [Google Scholar] [CrossRef]
- Aby, E.S.; Saab, S. Frailty, Sarcopenia, and Malnutrition in Cirrhotic Patients. Clin. Liver Dis. 2019, 23, 589–605. [Google Scholar] [CrossRef]
- Marasco, G.; Dajti, E.; Ravaioli, F.; Brocchi, S.; Rossini, B.; Alemanni, L.V.; Peta, G.; Bartalena, L.; Golfieri, R.; Festi, D.; et al. Clinical Impact of Sarcopenia Assessment in Patients with Liver Cirrhosis. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; Zanetto, A.; Piano, S.; Heimbach, J.K.; Dasarathy, S. Liver Transplantation in the Patient with Physical Frailty. J. Hepatol. 2023, 78, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tandon, P.; Montano-Loza, A.J.; Lai, J.C.; Dasarathy, S.; Merli, M. Sarcopenia and Frailty in Decompensated Cirrhosis. J. Hepatol. 2021, 75, S147–S162. [Google Scholar] [CrossRef] [PubMed]
- Dajti, E.; Renzulli, M.; Ravaioli, F.; Marasco, G.; Milandri, M.; Rossini, B.; Colecchia, L.; Alemanni, L.V.; Ferrarese, A.; Tamè, M.; et al. Sarcopenia Predicts Ascitic Decompensation and Mortality Independently of Portal Hypertension Status in Patients with Advanced Chronic Liver Disease Outside the Liver Transplantation Setting. Dig. Liver Dis. 2022, 54, S46. [Google Scholar] [CrossRef]
- Marasco, G.; Dajti, E.; Serenari, M.; Alemanni, L.V.; Ravaioli, F.; Ravaioli, M.; Vestito, A.; Vara, G.; Festi, D.; Golfieri, R.; et al. Sarcopenia Predicts Major Complications after Resection for Primary Hepatocellular Carcinoma in Compensated Cirrhosis. Cancers 2022, 14, 1935. [Google Scholar] [CrossRef]
- Tantai, X.; Liu, Y.; Yeo, Y.H.; Praktiknjo, M.; Mauro, E.; Hamaguchi, Y.; Engelmann, C.; Zhang, P.; Jeong, J.Y.; van Vugt, J.L.A.; et al. Effect of Sarcopenia on Survival in Patients with Cirrhosis: A Meta-Analysis. J. Hepatol. 2022, 76, 588–599. [Google Scholar] [CrossRef]
- Lai, J.C.; Tandon, P.; Bernal, W.; Tapper, E.B.; Ekong, U.; Dasarathy, S.; Carey, E.J. Malnutrition, Frailty, and Sarcopenia in Patients With Cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 74, 1611–1644. [Google Scholar] [CrossRef] [PubMed]
- Vasques, J.; Guerreiro, C.S.; Sousa, J.; Pinto, M.; Cortez-Pinto, H. Nutritional Support in Cirrhotic Patients with Sarcopenia. Clin. Nutr. ESPEN 2019, 33, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Casciola, R.; Leoni, L.; Cuffari, B.; Pecchini, M.; Menozzi, R.; Colecchia, A.; Ravaioli, F. Creatine Supplementation to Improve Sarcopenia in Chronic Liver Disease: Facts and Perspectives. Nutrients 2023, 15, 863. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.E.; Wong, V.W.-S.; Rinella, M. Non-Alcoholic Fatty Liver Disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Clin. Nutr. 2022, 41, 990–1000. [Google Scholar] [CrossRef]
- Dhariwal, S.; Roy, A.; Taneja, S.; Bansal, A.; Gorsi, U.; Singh, S.; De, A.; Verma, N.; Premkumar, M.; Duseja, A.; et al. Assessment of Sarcopenia Using Muscle Ultrasound in Patients With Cirrhosis and Sarcopenic Obesity (AMUSE STUDY). J. Clin. Gastroenterol. 2022. publish ahead of print. [Google Scholar] [CrossRef]
- Ha, N.B.; Fan, B.; Shui, A.M.; Huang, C.-Y.; Brandman, D.; Lai, J.C. CT-Quantified Sarcopenic Visceral Obesity Is Associated with Poor Transplant Waitlist Mortality in Patients with Cirrhosis. Liver Transpl. 2023. publish ahead of print. [Google Scholar] [CrossRef]
- Ravaioli, F.; De Maria, N.; Di Marco, L.; Pivetti, A.; Casciola, R.; Ceraso, C.; Frassanito, G.; Pambianco, M.; Pecchini, M.; Sicuro, C.; et al. From Listing to Recovery: A Review of Nutritional Status Assessment and Management in Liver Transplant Patients. Nutrients 2023, 15, 2778. [Google Scholar] [CrossRef]
- Ebadi, M.; Burra, P.; Zanetto, A.; Montano-Loza, A.J. Current Treatment Strategies and Future Possibilities for Sarcopenia in Cirrhosis. J. Hepatol. 2023, 78, 889–892. [Google Scholar] [CrossRef]
- West, J.; Gow, P.J.; Testro, A.; Chapman, B.; Sinclair, M. Exercise Physiology in Cirrhosis and the Potential Benefits of Exercise Interventions: A Review. J. Gastroenterol. Hepatol. 2021, 36, 2687–2705. [Google Scholar] [CrossRef]
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Bischoff, S.C. ESPEN Guideline on Clinical Nutrition in Liver Disease. Clin. Nutr. 2019, 38, 485–521. [Google Scholar] [CrossRef] [Green Version]
- Kondrup, J.; Müller, M.J. Energy and Protein Requirements of Patients with Chronic Liver Disease. J. Hepatol. 1997, 27, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Berzigotti, A.; Zelber-Sagi, S.; Dasarathy, S.; Montagnese, S.; Genton, L.; Plauth, M.; Parés, A. EASL Clinical Practice Guidelines on Nutrition in Chronic Liver Disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amodio, P.; Bemeur, C.; Butterworth, R.; Cordoba, J.; Kato, A.; Montagnese, S.; Uribe, M.; Vilstrup, H.; Morgan, M.Y. The Nutritional Management of Hepatic Encephalopathy in Patients with Cirrhosis: International Society for Hepatic Encephalopathy and Nitrogen Metabolism Consensus. Hepatology 2013, 58, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.C. Nutrition and Muscle in Cirrhosis. J. Clin. Exp. Hepatol. 2017, 7, 340–357. [Google Scholar] [CrossRef]
- Ravaioli, F.; Pivetti, A.; Di Marco, L.; Chrysanthi, C.; Frassanito, G.; Pambianco, M.; Sicuro, C.; Gualandi, N.; Guasconi, T.; Pecchini, M.; et al. Role of Vitamin D in Liver Disease and Complications of Advanced Chronic Liver Disease. Int. J. Mol. Sci. 2022, 23, 9016. [Google Scholar] [CrossRef]
- McCullough, A.J.; Mullen, K.D.; Kalhan, S.C. Body Cell Mass and Leucine Metabolism in Cirrhosis. Gastroenterology 1992, 102, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- McCullough, A.J.; Mullen, K.D.; Kalhan, S.C. Defective Nonoxidative Leucine Degradation and Endogenous Leucine Flux in Cirrhosis during an Amino Acid Infusion. Hepatology 1998, 28, 1357–1364. [Google Scholar] [CrossRef]
- Owen, O.E.; Reichle, F.A.; Mozzoli, M.A.; Kreulen, T.; Patel, M.S.; Elfenbein, I.B.; Golsorkhi, M.; Chang, K.H.; Rao, N.S.; Sue, H.S.; et al. Hepatic, Gut, and Renal Substrate Flux Rates in Patients with Hepatic Cirrhosis. J. Clin. Investig. 1981, 68, 240–252. [Google Scholar] [CrossRef] [Green Version]
- Brosnan, J. Comments on Metabolic Needs for Glucose and the Role of Gluconeogenesis. Eur. J. Clin. Nutr. 1999, 53, s107–s111. [Google Scholar] [CrossRef] [Green Version]
- Tsien, C.D.; McCullough, A.J.; Dasarathy, S. Late Evening Snack: Exploiting a Period of Anabolic Opportunity in Cirrhosis: Evening Snack for Cirrhotic Sarcopenia. J. Gastroenterol. Hepatol. 2012, 27, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Swart, G.R.; Zillikens, M.C.; Van Vuure, J.K.; Van Den Berg, J.W. Effect of a Late Evening Meal on Nitrogen Balance in Patients with Cirrhosis of the Liver. BMJ 1989, 299, 1202–1203. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Tian, Z.; Jiang, N.; Ding, X.; Mao, T.; Jing, X. Effects of Late Evening Snack on Cirrhotic Patients: A Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2018, 2018, 9189062. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Michitaka, K.; Kiguchi, D.; Izumoto, H.; Ueki, H.; Kaneto, M.; Kitahata, S.; Aibiki, T.; Okudaira, T.; Tomida, H.; et al. Efficacy of Branched-Chain Amino Acid Supplementation and Walking Exercise for Preventing Sarcopenia in Patients with Liver Cirrhosis. Eur. J. Gastroenterol. Hepatol. 2017, 29, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, L.; Kuo, H.; Fang, Y.; Lee, H. Significant Effects of Late Evening Snack on Liver Functions in Patients with Liver Cirrhosis: A Meta-analysis of Randomized Controlled Trials. J. Gastroenterol. Hepatol. 2019, 34, 1143–1152. [Google Scholar] [CrossRef]
- Zillikens, M.C.; Van Den Berg, J.W.O.; Wattimena, J.L.D.; Rietveld, T.; Swart, G.R. Nocturnal Oral Glucose Supplementation. J. Hepatol. 1993, 17, 377–383. [Google Scholar] [CrossRef]
- Verboeket-van De Venne, W.P.; Westerterp, K.R.; Van Hoek, B.; Swart, G.R. Energy Expenditure and Substrate Metabolism in Patients with Cirrhosis of the Liver: Effects of the Pattern of Food Intake. Gut 1995, 36, 110–116. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.-K.; Chao, Y.-C.; Tang, H.-S.; Lang, H.-F.; Hsu, C.-T. Effects of Extra-Carbohydrate Supplementation in the Late Evening on Energy Expenditure and Substrate Oxidation in Patients With Liver Cirrhosis. JPEN J. Parenter. Enter. Nutr. 1997, 21, 96–99. [Google Scholar] [CrossRef]
- Miwa, Y. Improvement of Fuel Metabolism by Nocturnal Energy Supplementation in Patients with Liver Cirrhosis. Hepatol. Res. 2000, 18, 184–189. [Google Scholar] [CrossRef]
- Yamauchi, M. Effect of Oral Branched Chain Amino Acid Supplementation in the Late Evening on the Nutritional State of Patients with Liver Cirrhosis. Hepatol. Res. 2001, 21, 199–204. [Google Scholar] [CrossRef]
- Nakaya, Y.; Harada, N.; Kakui, S.; Okada, K.; Takahashi, A.; Inoi, J.; Ito, S. Severe Catabolic State after Prolonged Fasting in Cirrhotic Patients: Effect of Oral Branched-Chain Amino-Acid-Enriched Nutrient Mixture. J. Gastroenterol. 2002, 37, 531–536. [Google Scholar] [CrossRef]
- Fukushima, H.; Miwa, Y.; Ida, E.; Kuriyama, S.; Toda, K.; Shimomura, Y.; Sugiyama, A.; Sugihara, J.; Tomita, E.; Moriwaki, H. Nocturnal Branched-Chain Amino Acid Administration Improves Protein Metabolism in Patients with Liver Cirrhosis: Comparison with Daytime Administration. JPEN J. Parenter. Enter. Nutr. 2003, 27, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Sako, K. Branched-Chain Amino Acids Supplements in the Late Evening Decrease the Frequency of Muscle Cramps with Advanced Hepatic Cirrhosis. Hepatol. Res. 2003, 26, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Sakaida, I.; Tsuchiya, M.; Okamoto, M.; Okita, K. Late Evening Snack and the Change of Blood Glucose Level in Patients with Liver Cirrhosis. Hepatol. Res. 2004, 30, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka-Okumura, H.; Nakamura, T.; Takeuchi, H.; Miyake, H.; Katayama, T.; Arai, H.; Taketani, Y.; Fujii, M.; Shimada, M.; Takeda, E. Effect of Late Evening Snack with Rice Ball on Energy Metabolism in Liver Cirrhosis. Eur. J. Clin. Nutr. 2006, 60, 1067–1072. [Google Scholar] [CrossRef] [Green Version]
- Plank, L.D.; Gane, E.J.; Peng, S.; Muthu, C.; Mathur, S.; Gillanders, L.; McIlroy, K.; Donaghy, A.J.; McCall, J.L. Nocturnal Nutritional Supplementation Improves Total Body Protein Status of Patients with Liver Cirrhosis: A Randomized 12-Month Trial. Hepatology 2008, 48, 557–566. [Google Scholar] [CrossRef]
- Nakaya, Y.; Okita, K.; Suzuki, K.; Moriwaki, H.; Kato, A.; Miwa, Y.; Shiraishi, K.; Okuda, H.; Onji, M.; Kanazawa, H.; et al. BCAA-Enriched Snack Improves Nutritional State of Cirrhosis. Nutrition 2007, 23, 113–120. [Google Scholar] [CrossRef]
- Takeshita, S.; Ichikawa, T.; Nakao, K.; Miyaaki, H.; Shibata, H.; Matsuzaki, T.; Muraoka, T.; Honda, T.; Otani, M.; Akiyama, M.; et al. A Snack Enriched with Oral Branched-Chain Amino Acids Prevents a Fall in Albumin in Patients with Liver Cirrhosis Undergoing Chemoembolization for Hepatocellular Carcinoma. Nutr. Res. 2009, 29, 89–93. [Google Scholar] [CrossRef]
- Ichikawa, T.; Naota, T.; Miyaaki, H.; Miuma, S.; Isomoto, H.; Takeshima, F.; Nakao, K. Effect of an Oral Branched Chain Amino Acid-Enriched Snack in Cirrhotic Patients with Sleep Disturbance: Effect of BCAA for LC with Sleep Disturbance. Hepatol. Res. 2010, 40, 971–978. [Google Scholar] [CrossRef] [Green Version]
- Harima, Y.; Yamasaki, T.; Hamabe, S.; Saeki, I.; Okita, K.; Terai, S.; Sakaida, I. Effect of a Late Evening Snack Using Branched-Chain Amino Acid-Enriched Nutrients in Patients Undergoing Hepatic Arterial Infusion Chemotherapy for Advanced Hepatocellular Carcinoma: Effects of LES on Advanced HCC during HAIC. Hepatol. Res. 2010, 40, 574–584. [Google Scholar] [CrossRef]
- Yamanaka-Okumura, H.; Nakamura, T.; Miyake, H.; Takeuchi, H.; Katayama, T.; Morine, Y.; Imura, S.; Shimada, M.; Takeda, E. Effect of Long-Term Late-Evening Snack on Health-Related Quality of Life in Cirrhotic Patients: Long-Term Nutritional Intervention. Hepatol. Res. 2010, 40, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, H.; Ushio, A.; Miyamoto, Y.; Sawara, K.; Oikawa, K.; Kasai, K.; Endo, R.; Takikawa, Y.; Kato, A.; Suzuki, K. Effects of Branched-Chain Amino Acid-Enriched Nutrient for Patients with Hepatocellular Carcinoma Following Radiofrequency Ablation: A One-Year Prospective Trial: Effect of BCAA for HCC Patients after RFA. J. Gastroenterol. Hepatol. 2010, 25, 1550–1555. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, P.; Castaldo, G.; Tarantino, L.; Bracigliano, A.; Perrella, A.; Perrella, O.; Fiorentino, F.; Vecchione, R.; D’ Angelo, S. Preservation of Nutritional-Status in Patients with Refractory Ascites Due to Hepatic Cirrhosis Who Are Undergoing Repeated Paracentesis: Nutritional-Support in RA. J. Gastroenterol. Hepatol. 2012, 27, 813–822. [Google Scholar] [CrossRef]
- Morihara, D.; Iwata, K.; Hanano, T.; Kunimoto, H.; Kuno, S.; Fukunaga, A.; Yotsumoto, K.; Takata, K.; Tanaka, T.; Sakurai, K.; et al. Late-Evening Snack with Branched-Chain Amino Acids Improves Liver Function after Radiofrequency Ablation for Hepatocellular Carcinoma: LES with BCAA Improves Liver Function. Hepatol. Res. 2012, 42, 658–667. [Google Scholar] [CrossRef]
- Hidaka, H.; Nakazawa, T.; Kutsukake, S.; Yamazaki, Y.; Aoki, I.; Nakano, S.; Asaba, N.; Minamino, T.; Takada, J.; Tanaka, Y.; et al. The Efficacy of Nocturnal Administration of Branched-Chain Amino Acid Granules to Improve Quality of Life in Patients with Cirrhosis. J. Gastroenterol. 2013, 48, 269–276. [Google Scholar] [CrossRef]
- Nojiri, S.; Fujiwara, K.; Shinkai, N.; Iio, E.; Joh, T. Effects of Branched-Chain Amino Acid Supplementation after Radiofrequency Ablation for Hepatocellular Carcinoma: A Randomized Trial. Nutrition 2017, 33, 20–27. [Google Scholar] [CrossRef]
- Hou, W.; Lv, Z.; Yang, J.; Wu, J.; Wang, Z.; Meng, Q. Long-Term Carbohydrate-Containing Late-Evening Snack Significantly Improves the Ratio of Branched Chain Amino Acids to Aromatic Amino Acids in Adults with Liver Cirrhosis Due to Hepatitis B. BioMed Res. Int. 2021, 2021, 1074565. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, J.; Li, J.; Wang, Z.-Y.; Meng, Q.-H. Late Evening Snack and Oral Amino Acid Capsules Improved Respiratory Quotient and Fischer Ratio in Patients with Alcoholic Liver Cirrhosis. Ann. Hepatol. 2023, 28, 100750. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Ivancovsky-Wajcman, D.; Rabinowich, L.; Bentov, I.; Deutsch, L. Nutritional Evaluation and Treatment of the Cirrhotic Patient. Clin. Liver Dis. 2021, 25, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Gnagnarella, P.; Parpinel, M. Food Composition Database for Epidemiological Studies in Italy. 2022. Available online: http://www.bda-ieo.it/wordpress/en/ (accessed on 11 July 2023).
- Consiglio per la ricerca in agricoltura e l’analisi dell’economia agraria (CREA). Tabelle Di Composizione Degli Alimenti (CREA). 2019. Available online: https://www.crea.gov.it/-/tabella-di-composizione-degli-alimenti (accessed on 11 July 2023).
- U.S. Department of Agriculture, Agricultural Research Service. Food Data Central. 2019. Available online: https://fdc.nal.usda.gov/ (accessed on 11 July 2023).
| Authors | Year | Region | n. Cases | n. Controls | Study Type | Major Findings |
|---|---|---|---|---|---|---|
| Swart et al. [42] | 1989 | NL | 9 | . | RCT (unblinded) | LES improves efficiency of nitrogen balance in LC |
| Zillikens et al. [46] | 1993 | NL | 8 | 8 | Crossover | LES improves nitrogen balance during the night in LC but not in HE |
| Verboeket-van De Venne et al. [47] | 1995 | NL | 8 | 23 | Crossover | LES reduces episodes of protein catabolism during the day in LC |
| Chang et al. [48] | 1997 | China | 16 | 8 | Crossover | LES effectively diminishes fat and protein oxidation in LC |
| Miwa et al. [49] | 2000 | Japan | 12 | 14 | Crossover | LES corrects abnormal metabolism and prevents malnutrition in LC. |
| Yamauchi et al. [50] | 2001 | Japan | 14 | . | Crossover | LES improves protein catabolism and lipolysis in LC. |
| Nakaya et al. [51] | 2002 | Japan | 7 | 5 | Crossover | LES based on BCAA mixture or carbohydrate-rich snacks can reduce fat oxidation in LC |
| Fukushima et al. [52] | 2003 | Japan | 12 | 12 | RCT | LES by BCAA administration improved serum albumin levels in LC more than daytime BCAA administration |
| Sako et al. [53] | 2003 | Japan | 8 | . | Crossover | LES with BCAA supplements provides relief from muscle cramping, |
| Sakaida et al. [54] | 2004 | Japan | 11 | . | Crossover | LES improves glucose intolerance in hospitalized LC. |
| Yamanaka-Okumura et al. [55] | 2006 | Japan | 21 | 26 | Crossover | LES with a 200-kcal rice ball improves the nutritional metabolism LC |
| Gheorghe et al.[56] | 2005 | Romania | 122 | . | . | LES improves HE |
| Nakaya et al. [57] | 2007 | Japan | 19 | 19 | RCT | Long-term oral supplementation with BCAA mixture as LES improves albumin levels and energy metabolism in LC |
| Plank et al. [56] | 2008 | NZ | 51 | 52 | RCT | LES leads to significant protein improvement, equivalent to 2 kg of lean tissue over 12 months. |
| Takeshita et al. [58] | 2009 | Japan | 28 | 28 | RCT | LES with BCAA supplement prevents liver function failure after TACE in LC patients with HCC |
| Ichikawa et al. [59] | 2010 | Japan | 12 | 9 | RCT (unblinded) | LES with BCAA improves sleep quality for LC without HE |
| Harima et al. [60] | 2010 | Japan | 13 | 10 | RCT | LES with BCAA-enriched nutrients may improve energy metabolism and glucose tolerance in LC with HCC |
| Yamanaka-Okumura et al. [61] | 2010 | Japan | 15 | 24 | RCT (unblinded) | LES may help maintain higher HRQOL in LC. |
| Kuroda et al. [62] | 2010 | Japan | 20 | 15 | RCT | LES with BCAA-enriched nutrients after RFA therapy for HCC improves nutritional status and quality of life of LC for 1-years |
| Sorrentino et al. [63] | 2012 | Italy | 40 | 40 | RCT | LES reduces the rate of refractory ascites in LC |
| Morihara et al. [64] | 2012 | Japan | 10 | 10 | RCT | LES with BCAA supplementation improves liver functioning and cognitive performance in LC patients who have undergone RFA for HCC. |
| Hidaka et al. [65] | 2013 | Japan | 16 | 21 | RCT | LES with BCAA administration reduced leg cramps in LC but did not improve quality of life. |
| Nojiri et al. [66] | 2017 | Japan | 25 | 26 | RCT | LES with EN formula (Aminoleban) improves HE in LC after RFA and reduce the risks for HCC recurrence. |
| Hiraoka et al. [44] | 2017 | Japan | 33 | . | Crossover | LES with BCAA and walking exercise improves muscle volume and strength in LC |
| Hou et al. [67] | 2021 | China | 43 | 43 | RCT | LES with carbohydrate-predominant significantly increases BCAA and decreases ammonia and glutamine after 6 months of supplementation. |
| Zhao et al. [68] | 2023 | China | 63 | 28 | RCT | LES increases the respiratory quotient in alcoholic LC. LES and BCAA were more effective than LES alone in improving serum isoleucine and the Fischer ratio. |
| Energy (kcal) | Proteins (g) | Complex Carbohydrates (g) | Sodium (mg) |
|---|---|---|---|
| 246 (200–275) | 15 (11.5–18) | 40 (25–55) | 550 (10–1085) |
| Brand | Name | Size (cc) | Energy (kcal) | Protein (g) | Fat (g) | Total CHOT(g) | MonoCHOT | DiCHOT | OligoCHOT | PoliCHOT |
|---|---|---|---|---|---|---|---|---|---|---|
| Nutricia | Cubitan© | 200 | 256 | 18.00 | 7.00 | 28.40 | X | X | X | |
| Nutricia | Diasip© | 200 | 208 | 9.80 | 7.60 | 23.40 | X | X | X | X |
| Nutricia | Forticare© | 125 | 200 | 11.00 | 6.60 | 23.90 | X | X | X | |
| Nutricia | Fortimel/12 measuring spoon© | 69 | 300 | 15.00 | 10.50 | 30.30 | X | X | X | |
| Nutricia | Fortimel Compact Protein© | 125 | 300 | 18.00 | 11.80 | 30.50 | X | X | X | |
| Nutricia | Nutilis Complete Stage 1© | 125 | 300 | 12.00 | 11.70 | 36.40 | X | X | ||
| Nutricia | Nutridrink Compact© | 125 | 300 | 12.00 | 11.70 | 37.10 | X | X | ||
| Abbott | Ensure Compact© | 125 | 300 | 12.30 | 11.70 | 35.90 | X | X | ||
| Abbott | Ensure Compact Protein HMB© | 125 | 300 | 18.00 | 12.50 | 28.00 | X | X | ||
| Abbott | Ensure Plus Drink© | 200 | 300 | 12.50 | 9.80 | 40.40 | X | X | ||
| Abbott | Glucerna SR© | 220 | 205 | 9.40 | 7.70 | 23.90 | X | X | ||
| Abbott | Prosure© | 220 | 280 | 14.60 | 5.60 | 40.30 | X | X | ||
| Nestlè | Resource Repair© | 200 | 254 | 18.00 | 6.00 | 32.00 | X | X | X | |
| Nestlè | Resource Ultra© | 125 | 281 | 17.50 | 11.00 | 28.00 | X | X | X | |
| Nestlè | Resource Energy© | 200 | 302 | 11.20 | 10.00 | 42.00 | X | X | X | |
| Nestlè | Resource Drink© | 200 | 250 | 18.80 | 7.00 | 28.00 | X | X | ||
| Nestlè | Meritene Crème© | 125 | 212 | 12.00 | 9.10 | 20.00 | X | X | X | |
| Fresenius Kabi | Fresubin Protein Energy Drink© | 200 | 300 | 20.00 | 13.40 | 24.80 | X | X | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leoni, L.; Valoriani, F.; Barbieri, R.; Pambianco, M.; Vinciguerra, M.; Sicuro, C.; Colecchia, A.; Menozzi, R.; Ravaioli, F. Unlocking the Power of Late-Evening Snacks: Practical Ready-to-Prescribe Chart Menu for Patients with Cirrhosis. Nutrients 2023, 15, 3471. https://doi.org/10.3390/nu15153471
Leoni L, Valoriani F, Barbieri R, Pambianco M, Vinciguerra M, Sicuro C, Colecchia A, Menozzi R, Ravaioli F. Unlocking the Power of Late-Evening Snacks: Practical Ready-to-Prescribe Chart Menu for Patients with Cirrhosis. Nutrients. 2023; 15(15):3471. https://doi.org/10.3390/nu15153471
Chicago/Turabian StyleLeoni, Laura, Filippo Valoriani, Riccardo Barbieri, Martina Pambianco, Martina Vinciguerra, Chiara Sicuro, Antonio Colecchia, Renata Menozzi, and Federico Ravaioli. 2023. "Unlocking the Power of Late-Evening Snacks: Practical Ready-to-Prescribe Chart Menu for Patients with Cirrhosis" Nutrients 15, no. 15: 3471. https://doi.org/10.3390/nu15153471
APA StyleLeoni, L., Valoriani, F., Barbieri, R., Pambianco, M., Vinciguerra, M., Sicuro, C., Colecchia, A., Menozzi, R., & Ravaioli, F. (2023). Unlocking the Power of Late-Evening Snacks: Practical Ready-to-Prescribe Chart Menu for Patients with Cirrhosis. Nutrients, 15(15), 3471. https://doi.org/10.3390/nu15153471







