The Therapeutic Potential of Natural Dietary Flavonoids against SARS-CoV-2 Infection
Abstract
1. Introduction
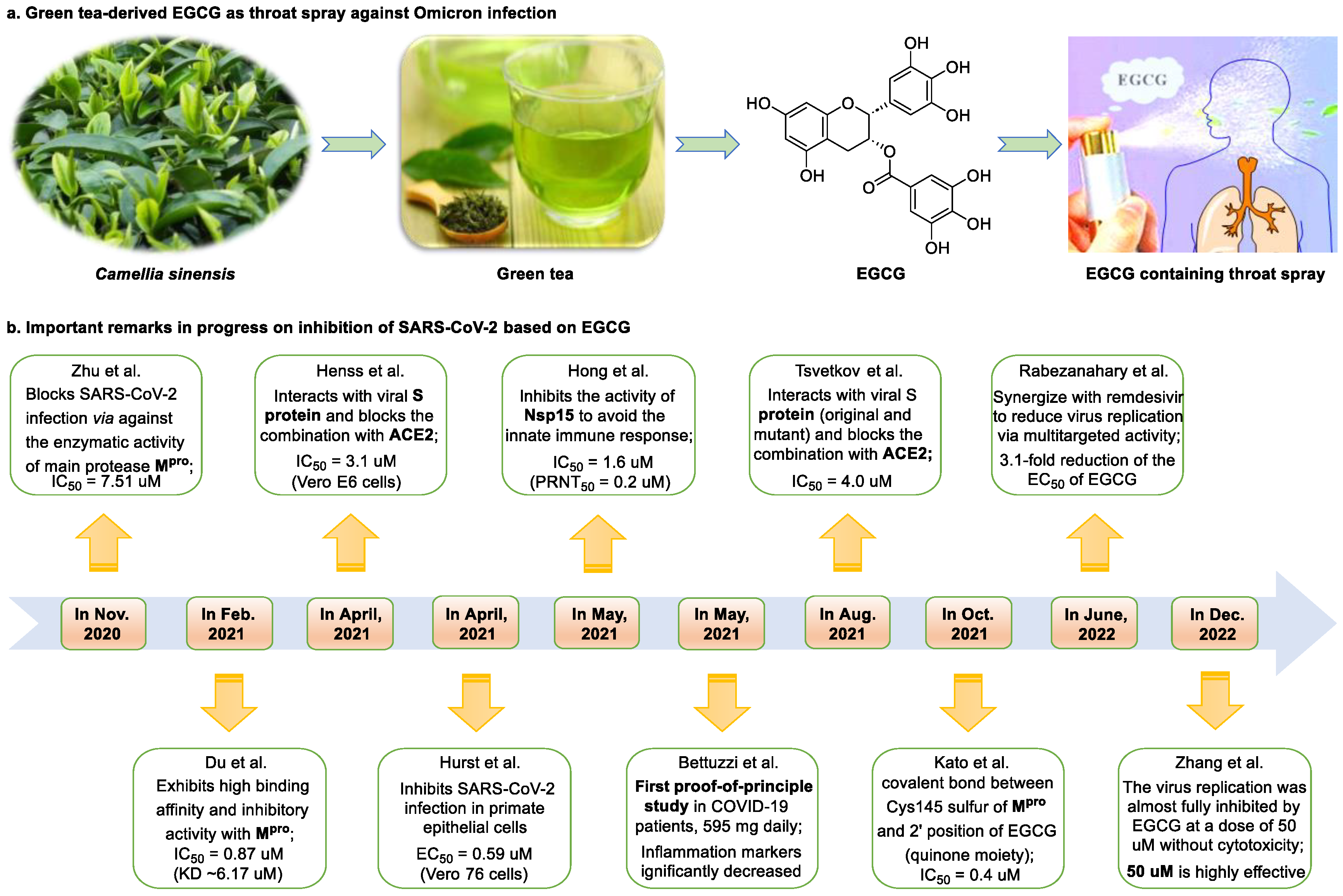
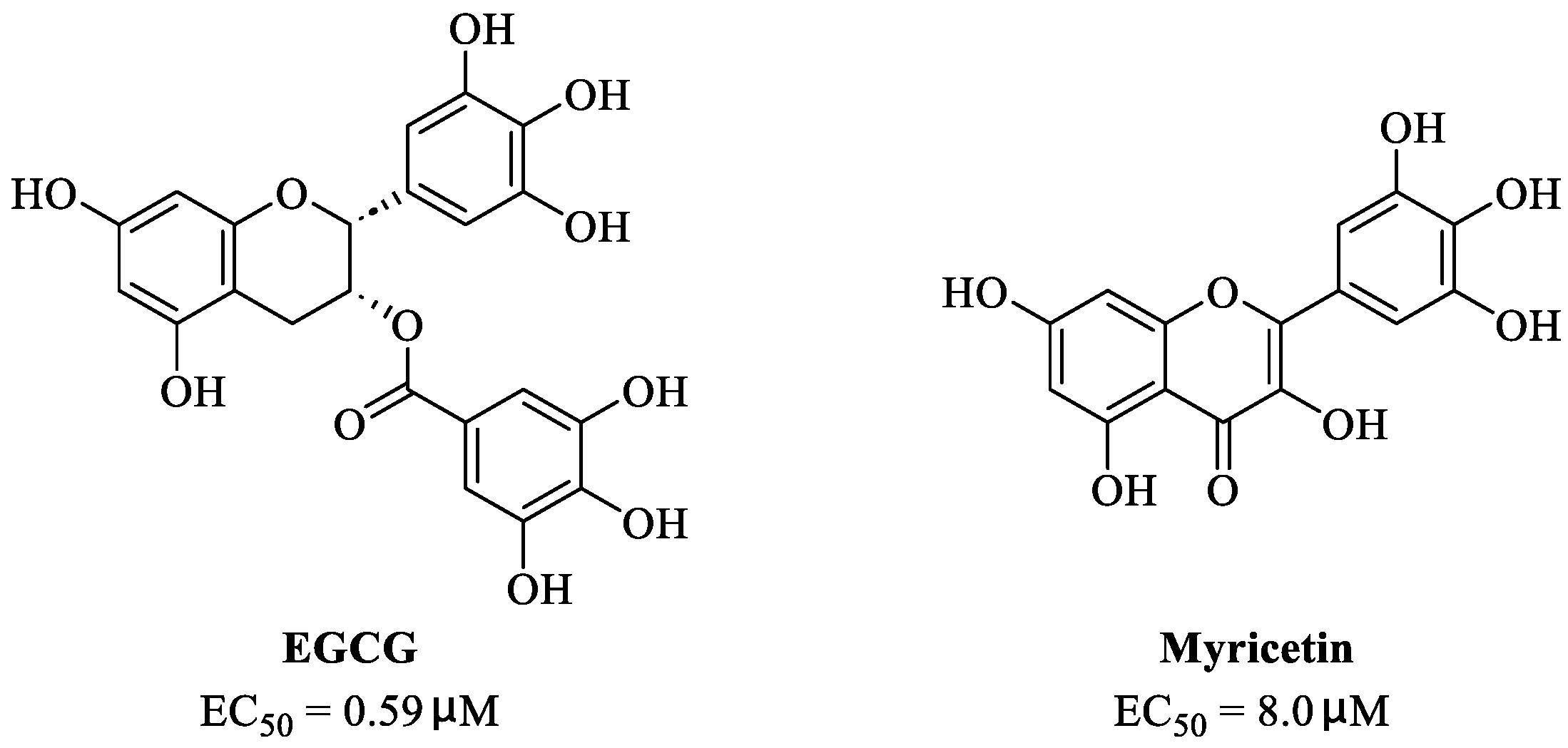
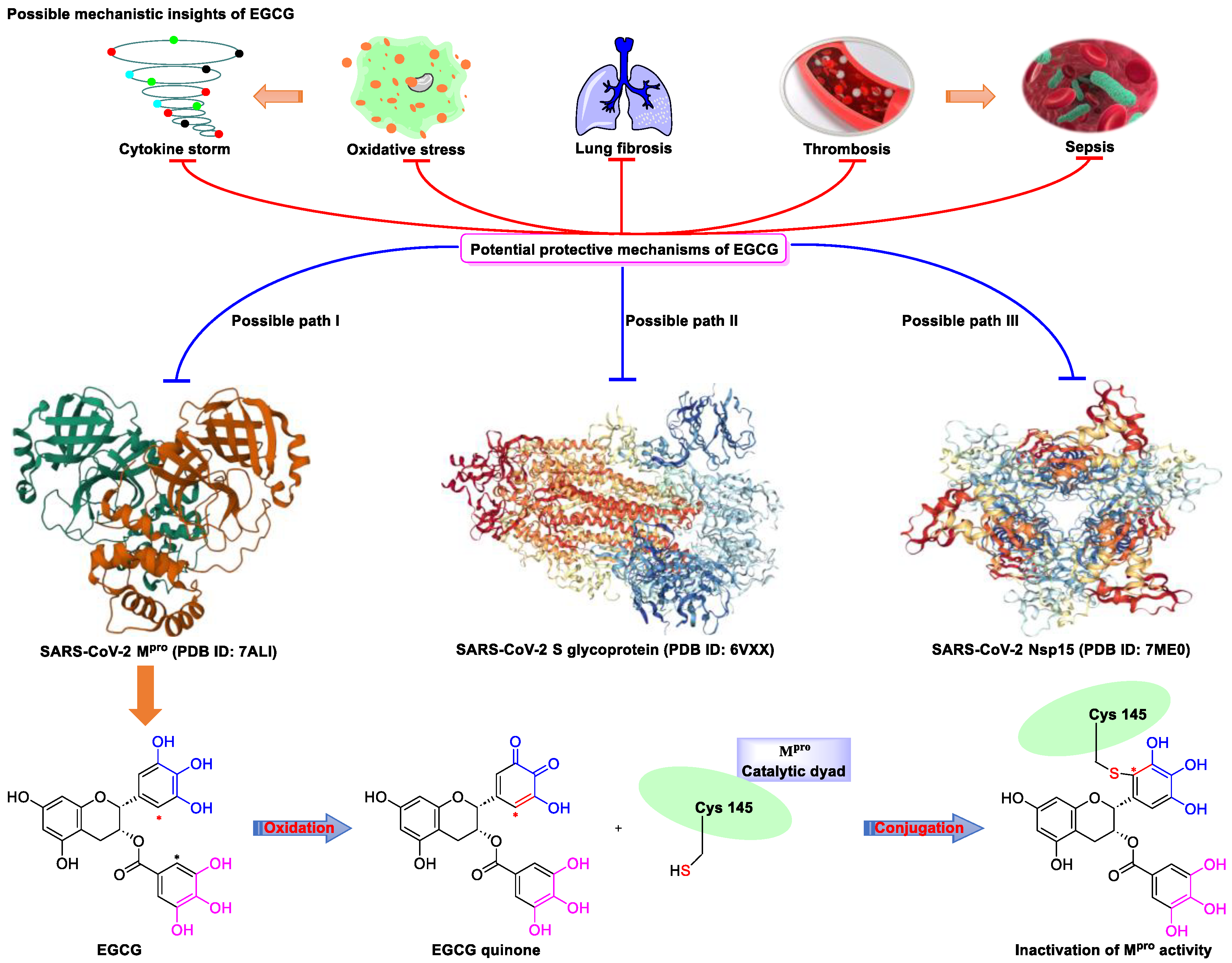
2. Epigallocatechin 3-Gallate—A Green Tea-Derived, Multitargeting, Anti-SARS-CoV-2 Therapeutic Candidate
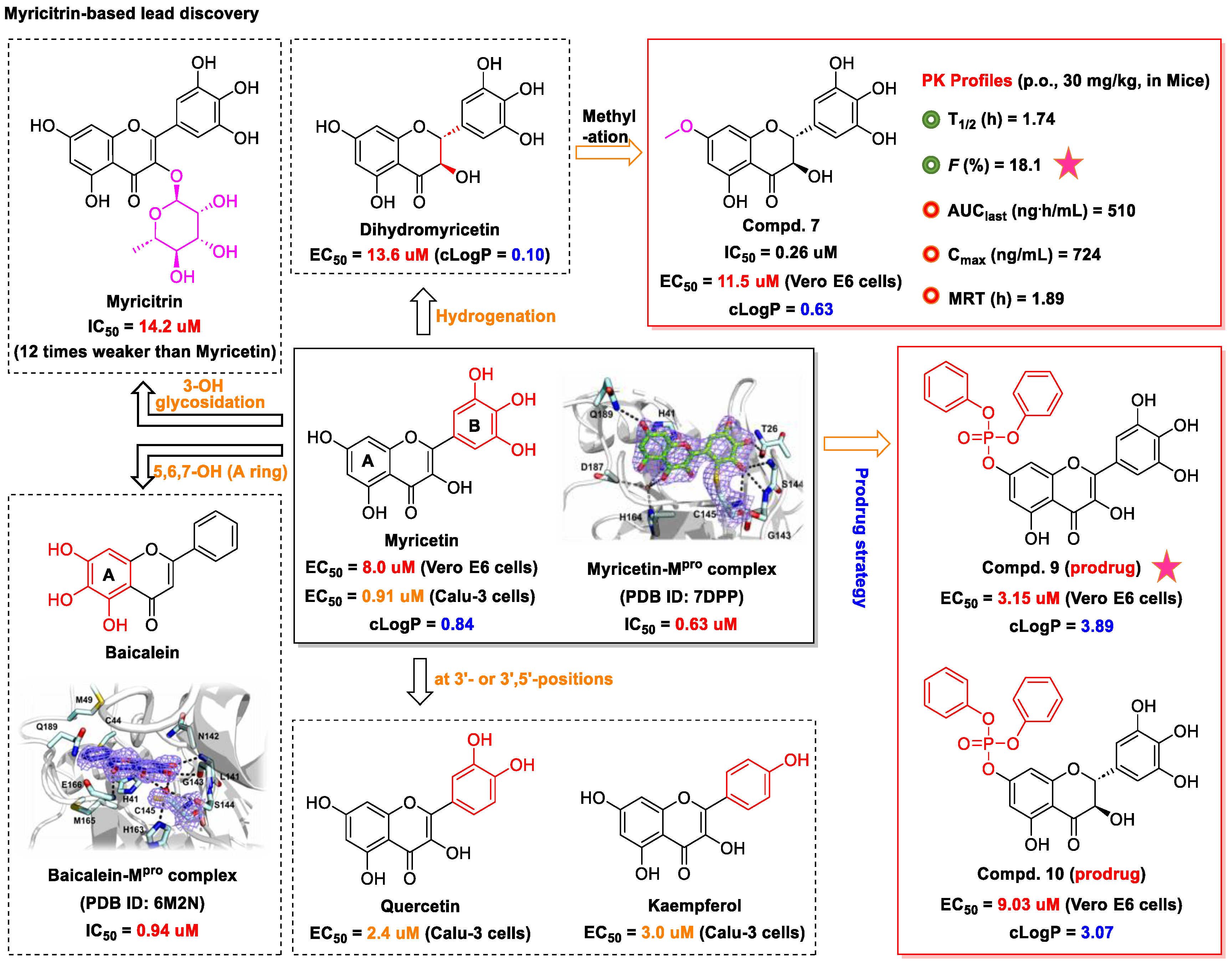
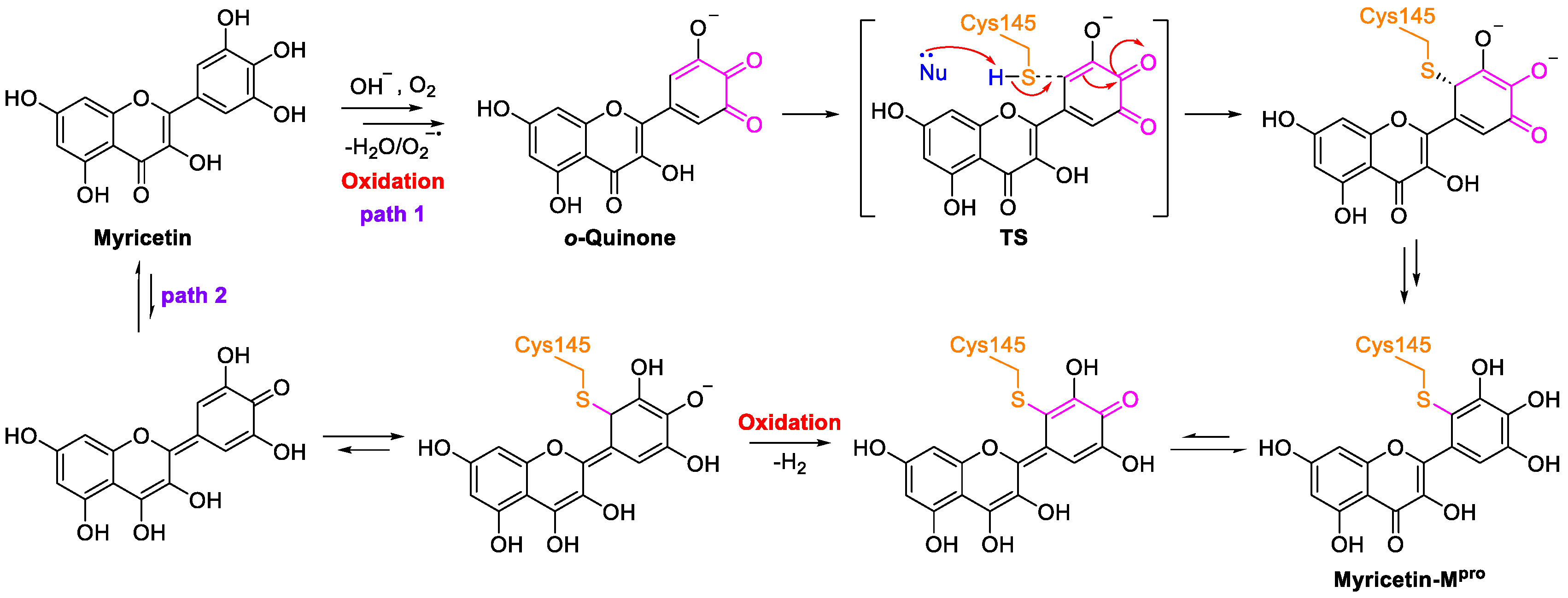
3. Myricetin—A Waxberry-Derived Covalent Mpro Inhibitor Suitable for Lead Optimization
4. Other Anti-SARS-CoV-2 Natural Dietary Flavonoids in Development for Treating SARS-CoV-2 Infection
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petersen, E.; Koopmans, M.; Go, U.; Hamer, D.H.; Petrosillo, N.; Castelli, F.; Storgaard, M.; Khalili, S.A.; Simonsen, L. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect. Dis. 2020, 20, e238–e244. [Google Scholar] [CrossRef] [PubMed]
- Toussi, S.S.; Hammond, J.L.; Gerstenberger, B.S.; Anderson, A.S. Therapeutics for COVID-19. Nat. Microbiol. 2023, 8, 771–786. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, N.; Yang, L.; Song, X.Q. Bioactive natural products in COVID-19 therapy. Front. Pharmacol. 2022, 13, 926507. [Google Scholar] [CrossRef] [PubMed]
- Wahl, A.; Gralinski, L.E.; Johnson, C.E.; Yao, W.; Kovarova, M.; Dinnon, K.H., III; Liu, H.; Madden, V.J.; Krzystek, H.M.; De, C.; et al. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature 2021, 591, 451–457. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z. Bench-to-bedside: Innovation of small molecule anti-SARS-CoV-2 drugs in China. Eur. J. Med. Chem. 2023, 257, 115503. [Google Scholar] [CrossRef]
- Levin, M.J.; Ustianowski, A.; De Wit, S.; Launay, O.; Avila, M.; Templeton, A.; Yuan, Y.; Seegobin, S.; Ellery, A.; Levinson, D.J.; et al. Intramuscular AZD7442 (tixagevimab–cilgavimab) for prevention of COVID-19. N. Engl. J. Med. 2022, 386, 2188–2200. [Google Scholar] [CrossRef]
- Connors, M.; Graham, B.S.; Lane, H.C.; Fauci, A.S. SARS-CoV-2 vaccines: Much accomplished, much to learn. Ann. Intern. Med. 2021, 174, 687–690. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L.; Song, X.Q. Oral GS-441524 derivatives: Next-generation inhibitors of SARS-CoV-2 RNA-dependent RNA polymerase. Front. Immunol. 2022, 13, 1015355. [Google Scholar] [CrossRef]
- Bushman, M.; Kahn, R.; Taylor, B.P.; Lipsitch, M.; Hanage, W.P. Population impact of SARS-CoV-2 variants with enhanced transmissibility and/or partial immune escape. Cell 2021, 184, 6229–6242. [Google Scholar] [CrossRef]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; Peacock, S.J.; Barclay, W.S.; de Silva, T.; Towers, G.J.; Robertson, D.L. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef]
- Uriu, K.; Ito, J.; Zahradnik, J.; Fujita, S.; Kosugi, Y.; Schreiber, G.; The Genotype to Phenotype Japan (G2P-Japan) Consortium; Sato, K. Enhanced transmissibility, infectivity and immune resistance of the SARS-CoV-2 Omicron XBB. 1.5 variant. Lancet Infect. Dis. 2023, 23, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, L. Post-acute sequelae of SARS-CoV-2 infection: A neglected public health issue. Front. Public Health 2022, 10, 908757. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar]
- Wang, Z.; Yang, L. Turning the tide: Natural products and natural-product-inspired chemicals as potential counters to SARS-CoV-2 infection. Front. Pharmacol. 2020, 11, 1013. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L. Chinese herbal medicine: Fighting SARS-CoV-2 infection on all fronts. J. Ethnopharmacol. 2021, 270, 113869. [Google Scholar]
- Banerjee, A.; Somasundaram, I.; Das, D.; Jain Manoj, S.; Banu, H.; Mitta Suresh, P.; Paul, S.; Bisgin, A.; Zhang, H.; Sun, X.F.; et al. Functional Foods: A promising strategy for restoring gut microbiota diversity impacted by SARS-CoV-2 variants. Nutrients 2023, 15, 2631. [Google Scholar] [CrossRef] [PubMed]
- Hurst, B.L.; Dickinson, D.; Hsu, S. Epigallocatechin-3-gallate (EGCG) inhibits SARS-CoV-2 infection in primate epithelial cells: (A short communication). Microbiol. Infect. Dis. 2021, 5, 1–6. [Google Scholar] [CrossRef]
- Su, H.; Yao, S.; Zhao, W.; Zhang, Y.; Liu, J.; Shao, Q.; Wang, Q.; Li, M.; Xie, H.; Shang, W.; et al. Identification of pyrogallol as a warhead in design of covalent inhibitors for the SARS-CoV-2 3CL protease. Nat. Commun. 2021, 12, 3623. [Google Scholar] [CrossRef]
- Wang, L.; Li, P.; Feng, K. EGCG adjuvant chemotherapy: Current status and future perspectives. Eur. J. Med. Chem. 2023, 250, 115197. [Google Scholar] [CrossRef]
- Cai, Y.; Yu, S.S.; He, Y.; Bi, X.Y.; Gao, S.; Yan, T.D.; Zheng, G.D.; Chen, T.T.; Ye, J.T.; Liu, P.Q. EGCG inhibits pressure overload-induced cardiac hypertrophy via the PSMB5/Nmnat2/SIRT6-dependent signalling pathways. Acta Physiol. 2021, 231, e13602. [Google Scholar] [CrossRef]
- Wen, J.J.; Li, M.Z.; Chen, C.H.; Hong, T.; Yang, J.R.; Huang, X.J.; Geng, F.; Hu, J.L.; Nie, S.P. Tea polyphenol and epigallocatechin gallate ameliorate hyperlipidemia via regulating liver metabolism and remodeling gut microbiota. Food Chem. 2023, 404, 134591. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Wang, I.H.; Rajesh, R. Use of leptin-conjugated phosphatidic acid liposomes with resveratrol and epigallocatechin gallate to protect dopaminergic neurons against apoptosis for Parkinson’s disease therapy. Acta Biomater. 2021, 119, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Cheong, Y.; Kim, M.; Ahn, J.; Oh, H.; Lim, J.; Chae, W.; Yang, S.W.; Kim, M.S.; Yu, J.E.; Byun, S.; et al. Epigallocatechin-3-gallate as a novel vaccine adjuvant. Front. Immunol. 2021, 12, 4803. [Google Scholar] [CrossRef]
- Hara, Y. Tea catechins and their applications as supplements and pharmaceutics. Pharmacol. Res. 2011, 64, 100–104. [Google Scholar] [CrossRef]
- Menegazzi, M.; Campagnari, R.; Bertoldi, M.; Crupi, R.; Di Paola, R.; Cuzzocrea, S. Protective effect of epigallocatechin-3-gallate (EGCG) in diseases with uncontrolled immune activation: Could such a scenario be helpful to counteract COVID-19? Int. J. Mol. Sci. 2020, 21, 5171. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.M.; Batista, M.N.; Braga, A.C.S.; Nogueira, M.L.; Rahal, P. The green tea molecule EGCG inhibits Zika virus entry. Virology 2016, 496, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.Y.; Zhao, K.J.; Wang, J.B.; Ma, Z.J.; Xiao, X.H. Green tea polyphenol, epigallocatechin-3-gallate, possesses the antiviral activity necessary to fight against the hepatitis B virus replication in vitro. J. Zhejiang Univ.-Sci. B 2014, 15, 533–539. [Google Scholar] [CrossRef]
- Wang, C.Y.; Hour, M.J.; Lai, H.C.; Chen, C.H.; Chang, P.J.; Huang, S.H.; Lin, C.W. Epigallocatechin-3-gallate inhibits the early stages of Japanese encephalitis virus infection. Virus Res. 2018, 253, 140–146. [Google Scholar] [CrossRef]
- LeBlanc, E.V.; Colpitts, C.C. The green tea catechin EGCG provides proof-of-concept for a pan-coronavirus attachment inhibitor. Sci. Rep. 2022, 12, 12899. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, S.; Dinda, M. Therapeutic potential of green tea catechin, (-)-epigallocatechin-3-O-gallate (EGCG) in SARS-CoV-2 infection: Major interactions with host/virus proteases. Phytomed. Plus 2022, 3, 100402. [Google Scholar]
- Wang, Z.; Yang, L.; Zhao, X.E. Co-crystallization and structure determination: An effective direction for anti-SARS-CoV-2 drug discovery. Comput. Struct. Biotechnol. J. 2021, 19, 4684–4701. [Google Scholar]
- Brier, L.; Hassan, H.; Hanoulle, X.; Landry, V.; Moschidi, D.; Desmarets, L.; Rouillé, Y.; Dumont, J.; Herledan, A.; Warenghem, S.; et al. Novel dithiocarbamates selectively inhibit 3CL protease of SARS-CoV-2 and other coronaviruses. Eur. J. Med. Chem. 2023, 250, 115186. [Google Scholar] [CrossRef]
- Pang, X.; Xu, W.; Liu, Y.; Li, H.; Chen, L. The research progress of SARS-CoV-2 main protease inhibitors from 2020 to 2022. Eur. J. Med. Chem. 2023, 257, 115491. [Google Scholar] [CrossRef] [PubMed]
- Du, A.; Zheng, R.; Disoma, C.; Li, S.; Chen, Z.; Li, S.; Liu, P.; Zhou, Y.; Shen, Y.; Liu, S.; et al. Epigallocatechin-3-gallate, an active ingredient of Traditional Chinese Medicines, inhibits the 3CLpro activity of SARS-CoV-2. Int. J. Biol. Macromol. 2021, 176, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xie, D.Y. Docking characterization and in vitro inhibitory activity of flavan-3-ols and dimeric proanthocyanidins against the main protease activity of SARS-CoV-2. Front. Plant Sci. 2020, 11, 1884. [Google Scholar] [CrossRef]
- Ngwe Tun, M.M.; Luvai, E.; Nwe, K.M.; Toume, K.; Mizukami, S.; Hirayama, K.; Komatsu, K.; Morita, K. Anti-SARS-CoV-2 activity of various PET-bottled Japanese green teas and tea compounds in vitro. Arch. Virol. 2022, 167, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Higashiyama, A.; Takaoka, E.; Nishikawa, M.; Ikushiro, S. Food phytochemicals, epigallocatechin gallate and myricetin, covalently bind to the active site of the coronavirus main protease in vitro. Adv. Redox Res. 2021, 3, 100021. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, V.; Varizhuk, A.; Kozlovskaya, L.; Shtro, A.; Lebedeva, O.; Komissarov, A.; Vedekhina, T.; Manuvera, V.; Zubkova, O.; Eremeev, A.; et al. EGCG as an anti-SARS-CoV-2 agent: Preventive versus therapeutic potential against original and mutant virus. Biochimie 2021, 191, 27–32. [Google Scholar] [CrossRef]
- Henss, L.; Auste, A.; Schürmann, C.; Schmidt, C.; von Rhein, C.; Mühlebach, M.D.; Schnierle, B.S. The green tea catechin epigallocatechin gallate inhibits SARS-CoV-2 infection. J. Gen. Virol. 2021, 102, 1574. [Google Scholar] [CrossRef]
- Hong, S.; Seo, S.H.; Woo, S.J.; Kwon, Y.; Song, M.; Ha, N.C. Epigallocatechin gallate inhibits the uridylate-specific endoribonuclease Nsp15 and efficiently neutralizes the SARS-CoV-2 strain. J. Agric. Food Chem. 2021, 69, 5948–5954. [Google Scholar] [CrossRef]
- Rabezanahary, H.; Badr, A.; Checkmahomed, L.; Pageau, K.; Desjardins, Y.; Baz, M. Epigallocatechin Gallate and Isoquercetin synergize with Remdesivir to reduce SARS-CoV-2 replication in vitro. Front. Virol. 2022, 2, 62. [Google Scholar] [CrossRef]
- Bettuzzi, S.; Gabba, L.; Cataldo, S. Efficacy of a polyphenolic, standardized green tea extract for the treatment of COVID-19 syndrome: A proof-of-principle study. COVID 2021, 1, 2–12. [Google Scholar] [CrossRef]
- Zhang, Z.; Hao, M.; Zhang, X.; He, Y.; Chen, X.; Taylor, E.W.; Zhang, J. Potential of green tea EGCG in neutralizing SARS-CoV-2 Omicron variant with greater tropism toward the upper respiratory tract. Trends Food Sci. Technol. 2023, 132, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Pillon, M.C.; Frazier, M.N.; Dillard, L.B.; Williams, J.G.; Kocaman, S.; Krahn, J.M.; Perera, L.; Hayne, C.K.; Gordon, J.; Stewart, Z.D.; et al. Cryo-EM structures of the SARS-CoV-2 endoribonuclease Nsp15 reveal insight into nuclease specificity and dynamics. Nat. Commun. 2021, 12, 636. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z. Natural products, alone or in combination with FDA-approved drugs, to treat COVID-19 and lung cancer. Biomedicines 2021, 9, 689. [Google Scholar] [CrossRef]
- Tian, D.; Sun, Y.; Xu, H.; Ye, Q. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J. Med. Virol. 2022, 94, 2376–2383. [Google Scholar] [CrossRef]
- Yang, C.S.; Lee, M.J.; Chen, L. Human salivary tea catechin levels and catechin esterase activities: Implication in human cancer prevention studies. Cancer Epidem. Biomar. Prev. 1999, 8, 83–89. [Google Scholar]
- Furushima, D.; Otake, Y.; Koike, N.; Onishi, S.; Mori, T.; Ota, N.; Yamada, H. Investigation of the oral retention of tea catechins in humans: An exploratory interventional study. Nutrients 2021, 13, 3024. [Google Scholar] [CrossRef]
- Wu, Y.; Cui, J. (−)-Epigallocatechin-3-gallate provides neuroprotection via AMPK activation against traumatic brain injury in a mouse model. N-S Arch. Pharmacol. 2020, 393, 2209–2220. [Google Scholar] [CrossRef]
- Wang, Y.; Jian, S.; Li, W.; Zhao, L.; Ye, G.; Shi, F.; Li, L.; Zou, Y.; Song, X.; Zhao, X.; et al. Epigallocatechin-3-gallate ameliorates liver injury secondary to Pseudomonas aeruginosa pneumonia. Int. Immunopharmacol. 2022, 112, 109239. [Google Scholar] [CrossRef]
- Nan, J.; Nan, C.; Ye, J.; Qian, L.; Geng, Y.; Xing, D.; Rahman, M.S.U.; Huang, M. EGCG protects cardiomyocytes against hypoxia-reperfusion injury through inhibition of OMA1 activation. J. Cell Sci. 2019, 132, jcs220871. [Google Scholar]
- Zhang, Z.; Zhang, X.; Bi, K.; He, Y.; Yan, W.; Yang, C.S.; Zhang, J. Potential protective mechanisms of green tea polyphenol EGCG against COVID-19. Trends Food Sci. Technol. 2021, 114, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Pervin, M.; Unno, K.; Takagaki, A.; Isemura, M.; Nakamura, Y. Function of green tea catechins in the brain: Epigallocatechin gallate and its metabolites. Int. J. Mol. Sci. 2019, 20, 3630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jiang, X.; Xiao, L.; Lu, Y.; Sang, S.; Lv, L.; Dong, W. Mechanistic studies of inhibition on acrolein by myricetin. Food Chem. 2020, 323, 126788. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, R.C.; Ye, L.; Baek, N.; Teixeira, G.H.; O’Keefe, S.F. Vine tea (Ampelopsis grossedentata): A review of chemical composition, functional properties, and potential food applications. J. Funct. Foods 2021, 76, 104317. [Google Scholar] [CrossRef]
- Li, X.; Yu, H.; Liang, L.; Bi, Z.; Wang, Y.; Gao, S.; Wang, M.; Li, H.; Miao, Y.; Deng, R.; et al. Myricetin ameliorates bleomycin-induced pulmonary fibrosis in mice by inhibiting TGF-β signaling via targeting HSP90β. Biochem. Pharmacol. 2020, 178, 114097. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Wang, L.; Wang, T.; Fan, L.; Chen, L.; Xiang, H.; Shi, Y.; Wang, D. Myricetin protects mice against MRSA-related lethal pneumonia by targeting ClpP. Biochem. Pharmacol. 2021, 192, 114753. [Google Scholar] [CrossRef]
- Wu, S.; Yue, Y.; Peng, A.; Zhang, L.; Xiang, J.; Cao, X.; Ding, H.; Yin, S. Myricetin ameliorates brain injury and neurological deficits via Nrf2 activation after experimental stroke in middle-aged rats. Food Funct. 2016, 7, 2624–2634. [Google Scholar] [CrossRef]
- Bhat RA, H.; Rehman, S.; Tandel, R.S.; Dash, P.; Bhandari, A.; Ganie, P.A.; Shah, T.K.; Pant, K.; Yousuf, D.J.; Bhat, I.A.; et al. Immunomodulatory and antimicrobial potential of ethanolic extract of Himalayan Myrica esculanta in Oncorhynchus mykiss: Molecular modelling with Aeromonas hydrophila functional proteins. Aquaculture 2021, 533, 736213. [Google Scholar] [CrossRef]
- Yao, Q.; Li, S.; Li, X.; Wang, F.; Tu, C. Myricetin modulates macrophage polarization and mitigates liver inflammation and fibrosis in a murine model of nonalcoholic steatohepatitis. Front. Med. 2020, 7, 71. [Google Scholar] [CrossRef]
- Daino, G.L.; Frau, A.; Sanna, C.; Rigano, D.; Distinto, S.; Madau, V.; Esposito, F.; Fanunza, E.; Bianco, G.; Taglialatela-Scafati, O.; et al. Identification of myricetin as an ebola virus VP35–double-stranded RNA interaction inhibitor through a novel fluorescence-based assay. Biochemistry 2018, 57, 6367–6378. [Google Scholar] [CrossRef]
- Zinzula, L.; Mereu, A.M.; Orsini, M.; Seeleitner, C.; Bracher, A.; Nagy, I.; Baumeister, W. Ebola and Marburg virus VP35 coiled-coil validated as antiviral target by tripartite split-GFP complementation. iScience 2022, 25, 105354. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Fang, C.; He, H.; Song, X.; Zhao, X.; Zou, Y.; Li, L.; Jia, R.; Yin, Z. Myricetin exerts its antiviral activity against infectious bronchitis virus by inhibiting the deubiquitinating activity of papain-like protease. Poultry Sci. 2022, 101, 101626. [Google Scholar] [CrossRef]
- Ortega, J.T.; Suárez, A.I.; Serrano, M.L.; Baptista, J.; Pujol, F.H.; Rangel, H.R. The role of the glycosyl moiety of myricetin derivatives in anti-HIV-1 activity in vitro. AIDS Res. Ther. 2017, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, S.; Shin, D.H.; Kim, M.S. Inhibition of African swine fever virus protease by myricetin and myricitrin. J. Enzym. Inhib. Med. Chem. 2020, 35, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Ning, K.; Wang, X.; Wang, J.; Cheng, F.; Ganaie, S.S.; Tavis, J.E.; Qiu, J. Establishment of a replicon reporter of the emerging tick-borne bourbon virus and use it for evaluation of antivirals. Front. Microbiol. 2020, 11, 572631. [Google Scholar] [CrossRef]
- Li, W.; Xu, C.; Hao, C.; Zhang, Y.; Wang, Z.; Wang, S.; Wang, W. Inhibition of herpes simplex virus by myricetin through targeting viral gD protein and cellular EGFR/PI3K/Akt pathway. Antivir. Res. 2020, 177, 104714. [Google Scholar] [CrossRef] [PubMed]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Corona, A.; Wycisk, K.; Talarico, C.; Manelfi, C.; Milia, J.; Cannalire, R.; Esposito, F.; Gribbon, P.; Zaliani, A.; Iaconis, D.; et al. Natural compounds inhibit SARS-CoV-2 nsp13 unwinding and ATPase enzyme activities. ACS Pharmacol. Transl. Sci. 2022, 5, 226–239. [Google Scholar] [CrossRef]
- Xiao, T.; Cui, M.; Zheng, C.; Wang, M.; Sun, R.; Gao, D.; Bao, J.; Ren, S.; Yang, B.; Lin, J.; et al. Myricetin inhibits SARS-CoV-2 viral replication by targeting Mpro and ameliorates pulmonary inflammation. Front. Pharmacol. 2021, 12, 669642. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.Y.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Kuzikov, M.; Costanzi, E.; Reinshagen, J.; Esposito, F.; Vangeel, L.; Wolf, M.; Ellinger, B.; Claussen, C.; Geisslinger, G.; Corona, A.; et al. Identification of inhibitors of SARS-CoV-2 3CL-pro enzymatic activity using a small molecule in vitro repurposing screen. ACS Pharmacol. Transl. Sci. 2021, 4, 1096–1110. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hong, C.; Yao, Y.; Shen, H.; Ji, G.; Li, G.; Xie, Y. Development of a pharmaceutical cocrystal with solution crystallization technology: Preparation, characterization, and evaluation of myricetin-proline cocrystals. Eur. J. Pharm. Biopharm. 2016, 107, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Meng, H.; Xin, L.; Xia, M.; Shen, H.; Li, G.; Xie, Y. Self-nanoemulsifying drug delivery systems of myricetin: Formulation development, characterization, and in vitro and in vivo evaluation. Colloid. Surface. B 2017, 160, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Chaves, O.A.; Fintelman-Rodrigues, N.; Wang, X.; Sacramento, C.Q.; Temerozo, J.R.; Ferreira, A.C.; Mattos, M.; Pereira-Dutra, F.; Bozza, P.T.; Castro-Faria-Neto, H.C.; et al. Commercially available flavonols are better SARS-CoV-2 inhibitors than isoflavone and flavones. Viruses 2022, 14, 1458. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhu, G.H.; Zhang, Y.N.; Hu, Q.; Wang, H.N.; Yu, H.N.; Qin, X.Y.; Guan, X.Q.; Xiang, Y.W.; Tang, H.; et al. Flavonoids in Ampelopsis grossedentata as covalent inhibitors of SARS-CoV-2 3CLpro: Inhibition potentials, covalent binding sites and inhibitory mechanisms. Int. J. Biol. Macromol. 2021, 187, 976–987. [Google Scholar] [CrossRef]
- Morak-Młodawska, B.; Jeleń, M.; Martula, E.; Korlacki, R. Study of lipophilicity and ADME properties of 1,9-diazaphenothiazines with anticancer action. Int. J. Mol. Sci. 2023, 24, 6970. [Google Scholar] [CrossRef]
- Johnson, T.W.; Gallego, R.A.; Edwards, M.P. Lipophilic efficiency as an important metric in drug design. J. Med. Chem. 2018, 61, 6401–6420. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L. Broad-spectrum prodrugs with anti-SARS-CoV-2 activities: Strategies, benefits, and challenges. J. Med. Virol. 2022, 94, 1373–1390. [Google Scholar] [CrossRef]
- Walther, R.; Rautio, J.; Zelikin, A.N. Prodrugs in medicinal chemistry and enzyme prodrug therapies. Adv. Drug Deliv. Rev. 2017, 118, 65–77. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, W.; Sun, J.; Ou, G.; Zhong, N.S.; Liu, Z. Exploring the potential pharmacological mechanism of hesperidin and glucosyl hesperidin against COVID-19 based on bioinformatics analyses and antiviral assays. Am. J. Chin. Med. 2022, 50, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Bellavite, P.; Donzelli, A. Hesperidin and SARS-CoV-2: New light on the healthy function of citrus fruits. Antioxidants 2020, 9, 742. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.C.; Lu, H.F.; Hsu, N.Y.; Chang, T.Y.; Chin, Y.F.; Liu, P.C.; Lo, J.M.; Wu, Y.B.; Yang, J.M.; Huang, C. Ugonin J acts as a SARS-CoV-2 3C-like protease inhibitor and exhibits anti-inflammatory properties. Front. Pharmacol. 2021, 12, 720018. [Google Scholar] [CrossRef]
- Klein, C.F.; Petek, B.J.; Moulson, N.; Baggish, A.L.; Churchill, T.W.; Harmon, K.G.; Kliethermes, S.A.; Patel, M.R.; Drezner, J.A. Non-COVID-19 cardiovascular pathology from return-to-play screening in college athletes after COVID-19. Heart, 2023; in press. [Google Scholar] [CrossRef]
- Mahmud, S.; Biswas, S.; Paul, G.K.; Mita, M.A.; Promi, M.M.; Afrose, S.; Afrose, S.; Hasan, M.R.; Zaman, S.; Uddin, M.S.; et al. Plant-based phytochemical screening by targeting main protease of SARS-CoV-2 to design effective potent inhibitors. Biology 2021, 10, 589. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, M.; Borkotoky, M.; Borchetia, S.; Chowdhury, P.; Mahanta, S.; Barooah, A.K. Black tea bioactives as inhibitors of multiple targets of SARS-CoV-2 (3CLpro, PLpro and RdRp): A virtual screening and molecular dynamic simulation study. J. Biomol. Struct. Dyn. 2022, 40, 7143–7166. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Ko, M.; Lee, J.; Choi, I.; Byun, S.Y.; Park, S.; Shum, D.; Kim, S. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob. Agents Chemother. 2020, 64, e00819-20. [Google Scholar] [CrossRef] [PubMed]
- Kousar, K.; Majeed, A.; Yasmin, F.; Hussain, W.; Rasool, N. Phytochemicals from selective plants have promising potential against SARS-CoV-2: Investigation and corroboration through molecular docking, MD simulations, and quantum computations. BioMed Res. Int. 2020, 2020, 6237160. [Google Scholar] [CrossRef]
- Xiao, T.; Cui, M.; Zheng, C.; Zhang, P.; Ren, S.; Bao, J.; Gao, D.; Sun, R.; Wang, M.; Lin, J.; et al. Both baicalein and gallocatechin gallate effectively inhibit SARS-CoV-2 replication by targeting Mpro and sepsis in Mice. Inflammation 2022, 45, 1076–1088. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Zhu, G.H.; Liu, W.; Xiong, Y.; Hu, Q.; Zhuang, X.Y.; Jia, G.H.; Zhang, W.D.; Ge, G.B. Discovery and characterization of the covalent SARS-CoV-2 3CLpro inhibitors from Ginkgo biloba extract via integrating chemoproteomic and biochemical approaches. Phytomedicine 2023, 114, 154796. [Google Scholar] [CrossRef]
- Abdallah, H.M.; El-Halawany, A.M.; Sirwi, A.; El-Araby, A.M.; Mohamed, G.A.; Ibrahim, S.R.; Koshak, A.E.; Asfour, H.Z.; Awan, Z.A.; A. Elfaky, M. Repurposing of some natural product isolates as SARS-CoV-2 main protease inhibitors via in vitro cell free and cell-based antiviral assessments and molecular modeling approaches. Pharmaceuticals 2021, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Boufissiou, A.; Abdalla, M.; Sharaf, M.; Al-Resayes, S.I.; Imededdine, K.; Alam, M.; Yagi, S.; Azam, M.; Yousfi, M. In-Silico investigation of phenolic compounds from leaves of Phillyrea angustifolia L. as a potential inhibitor against the SARS-CoV-2 main protease (Mpro PDB ID: 5R83) using a virtual screening method. J. Saudi Chem. Soc. 2022, 26, 101473. [Google Scholar] [CrossRef]
- Aleebrahim-Dehkordi, E.; Ghoshouni, H.; Koochaki, P.; Esmaili-Dehkordi, M.; Aleebrahim, E.; Chichagi, F.; Jafari, A.; Hanaei, S.; Heidari-Soureshjani, E.; Rezaei, N. Targeting the vital non-structural proteins (NSP12, NSP7, NSP8 and NSP3) from SARS-CoV-2 and inhibition of RNA polymerase by natural bioactive compound naringenin as a promising drug candidate against COVID-19. J. Mol. Struct. 2023, 1287, 135642. [Google Scholar] [CrossRef]
- Liu, S.Y.; Wang, W.; Ke, J.P.; Zhang, P.; Chu, G.X.; Bao, G.H. Discovery of Camellia sinensis catechins as SARS-CoV-2 3CL protease inhibitors through molecular docking, intra and extra cellular assays. Phytomedicine 2022, 96, 153853. [Google Scholar] [CrossRef] [PubMed]
- Kandeil, A.; Mostafa, A.; Kutkat, O.; Moatasim, Y.; Al-Karmalawy, A.A.; Rashad, A.A.; Kayed, A.E.; Kayed, A.E.; El-Shesheny, R.; Kayali, G.; et al. Bioactive polyphenolic compounds showing strong antiviral activities against severe acute respiratory syndrome coronavirus 2. Pathogens 2021, 10, 758. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, L.; Cui, S.; Liang, Y.; Zhang, X. Synthesis and anti-hypertensive effects of the twin drug of nicotinic acid and quercetin tetramethyl ether. Molecules 2014, 19, 4791–4801. [Google Scholar] [CrossRef]
- Yang, M.; Lin, L.; Scartelli, C.; Chen, D.Y.; Patel, A.; Bekendam, R.; Sun, L.; Saeed, M.; Flaumenhaft, R. Inhibition of SARS-CoV-2 viral replication and in vivo thrombus formation by a novel plant flavonoid. Blood 2021, 138, 3144. [Google Scholar] [CrossRef]
- Shahhamzehei, N.; Abdelfatah, S.; Efferth, T. In silico and in vitro identification of pan-coronaviral main protease inhibitors from a large natural product library. Pharmaceuticals 2022, 15, 308. [Google Scholar] [CrossRef]
- Zhu, J.; Yan, H.; Shi, M.; Zhang, M.; Lu, J.; Wang, J.; Chen, L.; Wang, Y.; Li, L.; Miao, L.; et al. Luteolin inhibits spike protein of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) binding to angiotensin-converting enzyme 2. Phytother. Res. 2023; in press. [Google Scholar] [CrossRef]
- Xie, Y.Z.; Peng, C.W.; Su, Z.Q.; Huang, H.T.; Liu, X.H.; Zhan, S.F.; Huang, X.F. A practical strategy for exploring the pharmacological mechanism of luteolin against COVID-19/asthma comorbidity: Findings of system pharmacology and bioinformatics analysis. Front. Immunol. 2022, 12, 769011. [Google Scholar] [CrossRef]
- Spiegel, M.; Ciardullo, G.; Marino, T.; Russo, N. Computational investigation on the antioxidant activities and on the Mpro SARS-CoV-2 non-covalent inhibition of isorhamnetin. Front. Chem. 2023, 11, 1122880. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ye, F.; Sun, Q.; Liang, H.; Li, C.; Li, S.; Lu, R.; Huang, B.; Tan, W.; Lai, L. Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. J. Enzym. Inhib. Med. Chem. 2021, 36, 497–503. [Google Scholar] [CrossRef]
- Zandi, K.; Musall, K.; Oo, A.; Cao, D.; Liang, B.; Hassandarvish, P.; Lan, S.; Slack, R.L.; Kirby, K.A.; Bassit, L.; et al. Baicalein and baicalin inhibit SARS-CoV-2 RNA-dependent-RNA polymerase. Microorganisms 2021, 9, 893. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Lee, J.; Jeon, S.; Kim, S.; Min, J.S.; Kwon, S. Natural polyphenols, 1, 2, 3, 4, 6-O-pentagalloyglucose and proanthocyanidins, as broad-spectrum anticoronaviral inhibitors targeting Mpro and RdRp of SARS-CoV-2. Biomedicines 2022, 10, 1170. [Google Scholar] [CrossRef]
- Chen, H.F.; Wang, W.J.; Chen, C.Y.; Chang, W.C.; Hsueh, P.R.; Peng, S.L.; Wu, C.S.; Chen, Y.; Huang, H.Y.; Shen, W.J. The natural tannins oligomeric proanthocyanidins and punicalagin are potent inhibitors of infection by SARS-CoV-2 in vitro. bioRxiv, 2023; preprint. [Google Scholar] [CrossRef]
- Chauhan, M.; Bhardwaj, V.K.; Kumar, A.; Kumar, V.; Kumar, P.; Enayathullah, M.G.; Thomas, J.; George, J.; Kumar, B.K.; Purohit, R.; et al. Theaflavin 3-gallate inhibits the main protease (Mpro) of SARS-CoV-2 and reduces its count in vitro. Sci. Rep. 2022, 12, 13146. [Google Scholar] [CrossRef]
- Tsai, M.S.; Yang, Y.H.; Lin, Y.S.; Chang, G.H.; Hsu, C.M.; Yeh, R.A.; Shu, L.H.; Cheng, Y.C.; Liu, H.T.; Wu, Y.H.; et al. GB-2 blocking the interaction between ACE2 and wild type and mutation of spike protein of SARS-CoV-2. Biomed. Pharmacother. 2021, 142, 112011. [Google Scholar] [CrossRef] [PubMed]
- Wahab, G.A.; Aboelmaaty, W.S.; Lahloub, M.F.; Sallam, A. In vitro and in silico studies of SARS-CoV-2 main protease Mpro inhibitors isolated from Helichrysum bracteatum. RSC Adv. 2022, 12, 18412–18424. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Heng, W.; Wang, Y.; Qiu, J.; Wei, X.; Peng, S.; Saleem, S.; Khan, M.; Ali, S.S.; Wei, D.Q. In silico and in vitro evaluation of kaempferol as a potential inhibitor of the SARS-CoV-2 main protease (3CLpro). Phytother. Res. 2021, 35, 2841. [Google Scholar] [CrossRef]
- Youssef, F.S.; Altyar, A.E.; Omar, A.M.; Ashour, M.L. Phytoconstituents, in vitro anti-infective activity of Buddleja indica Lam., and in silico evaluation of its SARS-CoV-2 inhibitory potential. Front. Pharmacol. 2021, 12, 619373. [Google Scholar] [CrossRef]
- Li, L.; Ma, L.; Hu, Y.; Li, X.; Yu, M.; Shang, H.; Zou, Z. Natural biflavones are potent inhibitors against SARS-CoV-2 papain-like protease. Phytochemistry 2022, 193, 112984. [Google Scholar] [CrossRef]
- Miroshnychenko, K.V.; Shestopalova, A.V. Combined use of the hepatitis C drugs and amentoflavone could interfere with binding of the spike glycoprotein of SARS-CoV-2 to ACE2: The results of a molecular simulation study. J. Biomol. Struct. Dyn. 2022, 40, 8672–8686. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.K.; Rehman, M.U.; Katyal, A.; Rajvanshi, K.; Kannan, M.; Garg, M.; Murugesan, S.; Deepa, P.R. In silico anti-viral assessment of phytoconstituents in a traditional (Siddha Medicine) polyherbal formulation—Targeting Mpro and pan-coronavirus post-fusion Spike protein. J. Tradit. Complement. Med. 2023; in press. [Google Scholar] [CrossRef]
- Bouback, T.A.; Aljohani, A.M.; Albeshri, A.; Al-Talhi, H.; Moatasim, Y.; GabAllah, M.; Badierah, R.; Albiheyri, R.; Al-Sarraj, F.; Ali, M.A. Antiviral activity of Humulus lupulus (HOP) aqueous extract against MERS-CoV and SARS-CoV-2: In-vitro and in-silico study. Biotechnol. Biotechnol. Equip. 2023, 37, 167–179. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sharma, D.; Sharma, A.K.; Jaiswal, S.; Sharma, N.; Borah, S.; Kaur, G. Flavan-based phytoconstituents inhibit Mpro, a SARS-CoV-2 molecular target, in silico. J. Biomol. Struct. Dyn. 2022, 40, 11545–11559. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Zhang, M.; Xue, H.; Yu, R.; Bao, Y.O.; Kuang, Y.; Chai, Y.; Ma, W.; Wang, J.; Shi, X.; et al. Schaftoside inhibits 3CLpro and PLpro of SARS-CoV-2 virus and regulates immune response and inflammation of host cells for the treatment of COVID-19. Acta Pharm. Sin. B 2022, 12, 4154–4164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, W.; Hu, Y.; Ding, T.; Zafar, M.M.; Jia, X.; Zhang, L.; Ren, M.; Li, F.; Wang, W. Cotton flower metabolites inhibit SARS-CoV-2 main protease. FEBS Open Bio 2022, 12, 1886–1895. [Google Scholar] [CrossRef]
- Vicidomini, C.; Roviello, V.; Roviello, G.N. In silico investigation on the interaction of chiral phytochemicals from opuntia ficus-indica with SARS-CoV-2 Mpro. Symmetry 2021, 13, 1041. [Google Scholar] [CrossRef]
- Chaves, O.A.; Lima, C.R.; Fintelman-Rodrigues, N.; Sacramento, C.Q.; de Freitas, C.S.; Vazquez, L.; Temerozo, J.R.; Rocha, M.E.N.; Dias, S.S.G.; Carels, N.; et al. Agathisflavone, a natural biflavonoid that inhibits SARS-CoV-2 replication by targeting its proteases. Int. J. Biol. Macromol. 2022, 222, 1015–1026. [Google Scholar] [CrossRef]
- Farhat, A.; Ben Hlima, H.; Khemakhem, B.; Ben Halima, Y.; Michaud, P.; Abdelkafi, S.; Fendri, I. Apigenin analogues as SARS-CoV-2 main protease inhibitors: In-silico screening approach. Bioengineered 2022, 13, 3350–3361. [Google Scholar] [CrossRef]
- Ngwe Tun, M.M.; Toume, K.; Luvai, E.; Nwe, K.M.; Mizukami, S.; Hirayama, K.; Komatsu, K.; Morita, K. The discovery of herbal drugs and natural compounds as inhibitors of SARS-CoV-2 infection in vitro. J. Nat. Med. 2022, 76, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, S.; Kim, D.Y.; Kim, M.S.; Shin, D.H. Flavonoids with inhibitory activity against SARS-CoV-2 3CLpro. J. Enzym. Inhib. Med. Chem. 2020, 35, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Peng, Y.; Yao, H.; Wang, Y.; Li, J.; Yang, Y.; Lin, Z. Punicalagin as an allosteric NSP13 helicase inhibitor potently suppresses SARS-CoV-2 replication in vitro. Antivir. Res. 2022, 206, 105389. [Google Scholar] [CrossRef] [PubMed]
| No. | Name | Species | Structure | EC50 or IC50 (μM) | Target or Mechanism | Refs. |
|---|---|---|---|---|---|---|
| 1 | Hesperidin | Citrus sinensis | 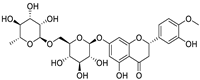 | 13.25 | ACE2, M, S, and RBD proteins | [81,82] |
| 2 | Ugonin J | Helminthostachys zeylanica | 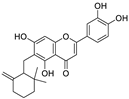 | 2.38 | Mpro | [83] |
| 3 | Epicatechin-3-O-gallate | Camellia sinensis var. sinensis |  | 5.21 | Mpro | [84,85] |
| 4 | Catechin-3-O-gallate | Senegalia catechu | 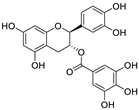 | 2.98 | Mpro | [84] |
| 5 | Procyanidin B2 | Punica granatum |  | 75.3 | Mpro | [84,86] |
| 6 | Osajin | Maclura pomifera | 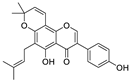 | 3.87 | N protein, nsp16, and nsp13 | [87,88] |
| 7 | (+)-Gallocatechin | Musa Cavendish | 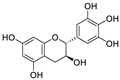 | 13.14 | Mpro | [89,90] |
| 8 | Apigenin-7-O-glucoside | Achillea millefolium L. |  | 0.074 | Mpro | [91,92] |
| 9 | Naringenin | Citrus reticulata | 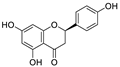 | 0.092 | Mpro, NSP12, NSP7, NSP8, and NSP3 | [91,93] |
| 10 | etc-pyrrolidinone C and D | Camellia sinensis |  | 0.90 | Mpro | [94] |
| 11 | (−)-epicatechin 3-O-caffeoate | Camellia sinensis | 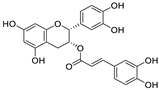 | 1.58 | Mpro | [94] |
| 12 | Quercetin | Citrus reticulata Blanco | 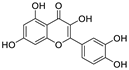 | 18.2 | Mpro | [95,96] |
| 13 | 3,8′-biapigenin | Forsythia suspensa | 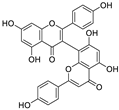 | 13.0 | Mpro, protein disulfide isomerase | [97] |
| 14 | PGHG | Penthorum chinense Pursh | 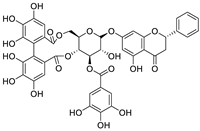 | 6.5 | Mpro, protein disulfide isomerase | [97] |
| 15 | Luteolin | Taraxacum antungense Kitag | 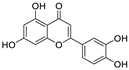 | 11.81 | Mpro, RBD-ACE2 | [98,99,100] |
| 16 | Isorhamnetin | Sea buckthorn | 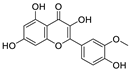 | 8.42/2.51 | Mpro | [98,101] |
| 17 | Baicalein | Scutellaria baicalensis Georgi | 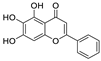 | 0.39 | Mpro, RdRp | [102,103] |
| 18 | Scutellarein | Scutellaria baicalensis Georgi | 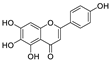 | 5.8 | Mpro | [102] |
| 19 | Proanthocyanidin | Grape seed | 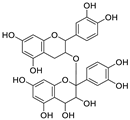 | 25.90/21.02 | Mpro, and RdRp | [104,105] |
| 20 | Theaflavin 3-gallate | Black tea | 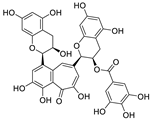 | 18.48 | Mpro, S protein | [106,107] |
| 21 | Theaflavin | Black tea | 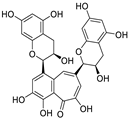 | 22.22 | Mpro | [106] |
| 22 | 3′,5′,5,7-tetrahydroxy-6-methoxyflavanone | Helichrysum bracteatum | 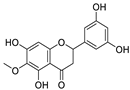 | 5.565 | Mpro | [108] |
| 23 | Kaempferol | Canavalia ensiformis L. | 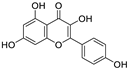 | 34.46 | Mpro, PLpro | [109,110] |
| 24 | Amentoflavone | Nandina domestica | 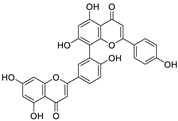 | 13.0 | PLpro, RBD-ACE2 | [111,112] |
| 25 | Scutellarein | Scutellaria baicalensis | 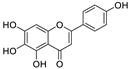 | 5.80 | Mpro | [102,113] |
| 26 | Epicatechin gallate | Fagopyrum esculentum | 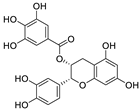 | 12.5 | Mpro | [36,114,115] |
| 27 | Schaftoside | Prosopis alba cotyledons | 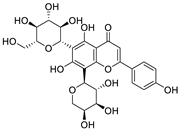 | 11.83 | Mpro and PLpro | [116] |
| 28 | Astilbin | Smilax glabra Roxb. | 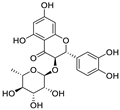 | 7.92 | Mpro | [117] |
| 29 | Astragalin | Nelumbo nucifera | 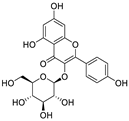 | 0.13 | Mpro | [117,118] |
| 30 | Apigenin | Apium Graveolens L. | 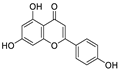 | 5.21 | Mpro | [119,120] |
| 31 | Baicalin | Scutellaria baicalensis | 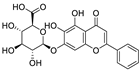 | 8.8 | RdRp and Mpro | [121,122] |
| 32 | Rhodiosin | Rhodiola rosea | 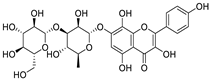 | 0.48 | NSP13 | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Yang, L. The Therapeutic Potential of Natural Dietary Flavonoids against SARS-CoV-2 Infection. Nutrients 2023, 15, 3443. https://doi.org/10.3390/nu15153443
Wang Z, Yang L. The Therapeutic Potential of Natural Dietary Flavonoids against SARS-CoV-2 Infection. Nutrients. 2023; 15(15):3443. https://doi.org/10.3390/nu15153443
Chicago/Turabian StyleWang, Zhonglei, and Liyan Yang. 2023. "The Therapeutic Potential of Natural Dietary Flavonoids against SARS-CoV-2 Infection" Nutrients 15, no. 15: 3443. https://doi.org/10.3390/nu15153443
APA StyleWang, Z., & Yang, L. (2023). The Therapeutic Potential of Natural Dietary Flavonoids against SARS-CoV-2 Infection. Nutrients, 15(15), 3443. https://doi.org/10.3390/nu15153443





