TT Genotype of TLR4 rs1928295 Is a Risk Factor of Overweight/Obesity in Han Chinese Children Aged 7–12 Years and Can Interact with Dietary Patterns to Affect the Incidence of Central Obesity and Lipid Profile, Systolic Blood Pressure Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Population
2.2. Anthropometric Assessments
2.3. Diet Assessment
2.4. Laboratory Assays
2.5. Genotyping
2.6. Statistical Analysis
3. Results
3.1. Association between TLR4 rs1928295 Polymorphism and the Incidence Rate of Overweight/Obesity
3.2. Comparison of Obesity Related Indicators between the CC + CT and TT Genotype of TLR4 rs1928295 Polymorphism
3.3. The Relationship between Indicators Related to Obesity, Macronutrients Intake, and TLR4 rs1928295 Polymorphism
- (1)
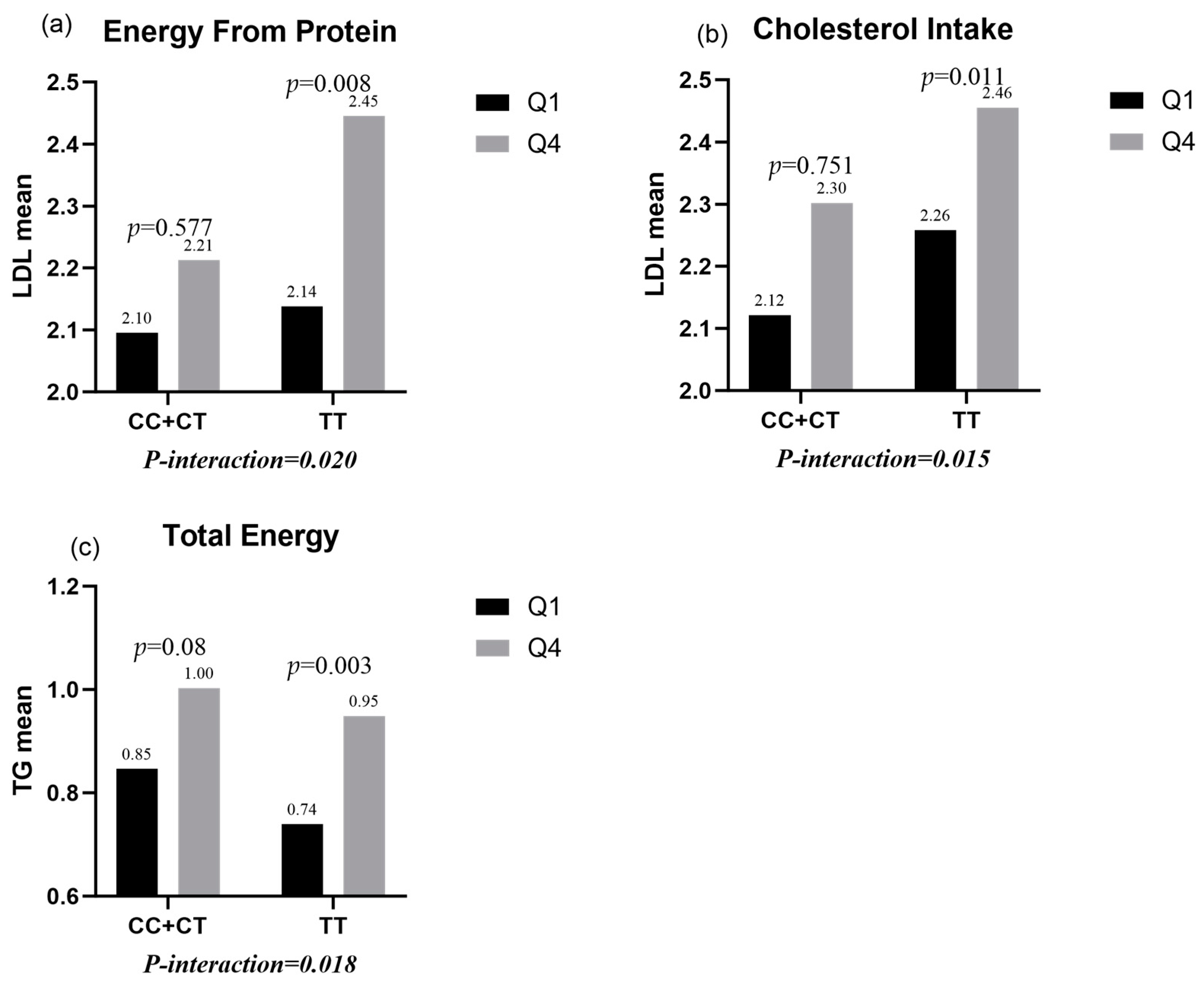
- Protein intake and cholesterol intake had significant interactions with TLR4 rs1928295 polymorphism in male children (p-interaction = 0.02 and 0.015). In this way, protein intake and cholesterol intake in the highest quartile group significantly increased LDL levels compared with protein intake and cholesterol intake in the lowest quartile group (p = 0.008 and 0.011) for the TT genotype, but there was no statistical difference in the CC/CT genotypes (p = 0.577 and 0.751) (Figure 1a,b).
- (2)
- There were significant interactions between total energy intake and the TLR4 rs1928295 polymorphism in female children (p-interaction = 0.018). In this way, TG levels were significantly increased in the TT genotype (p = 0.003) for total energy intake in the highest quartile group compared to total energy intake in the lowest quartile group, while there was no statistical difference in the CC/CT genotypes (p = 0.08) (Figure 1c).
3.4. The Relationship between Children’s Dietary Patterns and Obesity-Related Indices
3.4.1. The Relationship between Male Children’s Dietary Patterns and Obesity-Related Indicators
3.4.2. The Relationship between Female Children’s Dietary Patterns and Obesity-Related Indicators
3.5. Association of TLR4 rs1928295 Polymorphisms with Energy Intake and Dietary Patterns
- (1)
- TLR4 rs1928295 polymorphism and AFDP dietary pattern of male students had significant interaction with SP (p = 0.044), among which, the male students carrying the TT genotype who were most inclined to AFDP dietary pattern had higher SP (p = 0.0333). There was no significant difference between the male patients with the CC/CT genotype (p = 0.8323) (Figure 2a).
- (2)
- TLR4 rs1928295 polymorphism had a significant interaction with AFDP dietary pattern in male students (p = 0.017), among which, the male students with the TT genotype who were most inclined to AFDP dietary pattern had a higher LDL (p = 0.0541). There was no significant difference in the male patients with the CC/CT genotype (p = 0.1496) (Figure 2b).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Zhao, L.; Gao, L.; Pan, A.; Xue, H. Health policy and public health implications of obesity in China. Lancet Diabetes Endocrinol. 2021, 9, 446–461. [Google Scholar] [CrossRef]
- Zhang, N.; Ma, G.S. Interpretation of Report on Childhood Obesity in China. Acta Nutr. Sin. 2017, 39, 530–534. [Google Scholar] [CrossRef]
- Yılmaz, B.; Gezmen Karadağ, M. The current review of adolescent obesity: The role of genetic factors. J. Pediatr. Endocrinol. Metab. JPEM 2021, 34, 151–162. [Google Scholar] [CrossRef]
- Vettori, A.; Pompucci, G.; Paolini, B.; Del Ciondolo, I.; Bressan, S.; Dundar, M.; Kenanoğlu, S.; Unfer, V.; Bertelli, M. Genetic background, nutrition and obesity: A review. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1751–1761. [Google Scholar] [CrossRef] [PubMed]
- Crovesy, L.; Rosado, E.L. Interaction between genes involved in energy intake regulation and diet in obesity. Nutrition 2019, 67–68, 110547. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.A.; Primo, D.; Izaola, O.; Gómez, E.; Bachiller, R. Serum Lipid and Adiponectin Improvements after a Mediterranean Dietary Pattern in Non-G-Allele Carriers of the Variant rs3774261. Lifestyle Genom. 2020, 13, 164–171. [Google Scholar] [CrossRef]
- de Luis, D.; Aller, R.; Izaola, O.; Primo, D. Role of the rs10401670 variant in the resistin gene on the metabolic response after weight loss secondary to a high-fat hypocaloric diet with a Mediterranean pattern. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2022, 35, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Chupeerach, C.; Tapanee, P.; On-Nom, N.; Temviriyanukul, P.; Chantong, B.; Reeder, N.; Adegoye, G.A.; Tolar-Peterson, T. The influence of TAS2R38 bitter taste gene polymorphisms on obesity risk in three racially diverse groups. BioMedicine 2021, 11, 43–49. [Google Scholar] [CrossRef]
- Lee, J.Y.; Ye, J.; Gao, Z.; Youn, H.S.; Lee, W.H.; Zhao, L.; Sizemore, N.; Hwang, D.H. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J. Biol. Chem. 2003, 278, 37041–37051. [Google Scholar] [CrossRef]
- Tramullas, M.; Finger, B.C.; Dinan, T.G.; Cryan, J.F. Obesity Takes Its Toll on Visceral Pain: High-Fat Diet Induces Toll-Like Receptor 4-Dependent Visceral Hypersensitivity. PLoS ONE 2016, 11, e0155367. [Google Scholar] [CrossRef]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Raghuraman, S.; Donkin, I.; Versteyhe, S.; Barrès, R.; Simar, D. The Emerging Role of Epigenetics in Inflammation and Immunometabolism. Trends Endocrinol. Metab. TEM 2016, 27, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Benomar, Y.; Taouis, M. Molecular Mechanisms Underlying Obesity-Induced Hypothalamic Inflammation and Insulin Resistance: Pivotal Role of Resistin/TLR4 Pathways. Front. Endocrinol. 2019, 10, 140. [Google Scholar] [CrossRef]
- Schneider, S.; Hoppmann, P.; Koch, W.; Kemmner, S.; Schmaderer, C.; Renders, L.; Kastrati, A.; Laugwitz, K.L.; Heemann, U.; Baumann, M. Obesity-associated hypertension is ameliorated in patients with TLR4 single nucleotide polymorphism (SNP) rs4986790. J. Inflamm. 2015, 12, 57. [Google Scholar] [CrossRef]
- Schneider, S.; Koch, W.; Hoppmann, P.; Ubrich, R.; Kemmner, S.; Steinlechner, E.; Heemann, U.; Laugwitz, K.L.; Kastrati, A.; Baumann, M. Association of Toll-like receptor 4 polymorphism with age-dependent systolic blood pressure increase in patients with coronary artery disease. Immun. Ageing I A 2015, 12, 4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharif, E.; Al-Wakeel, M.; Mohamed, A.; Kerkadi, A.; Rizk, N. TLR4 Receptor D299G/T399I Haplotype Polymorphism Is Associated with Insulin Resistance in Obese Female Subjects. Genes 2020, 11, 814. [Google Scholar] [CrossRef]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, G.; Shen, H.; Li, W.; Mao, J.; Pan, Y. Association of TLR4 gene polymorphisms with childhood Henoch-Schönlein purpura in a Chinese population. Rheumatol. Int. 2017, 37, 1909–1915. [Google Scholar] [CrossRef]
- Zhao, J.; Han, X.; Xue, L.; Zhu, K.; Liu, H.; Xie, A. Association of TLR4 gene polymorphisms with sporadic Parkinson’s disease in a Han Chinese population. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2015, 36, 1659–1665. [Google Scholar] [CrossRef]
- Liu, L. Correlation between Polymorphism of TLR2 and TLR4 Gene in gout in Xinjiang Uyghur Male. Master’s Thesis, Xinjiang Medical University, Urumqi, China, 2019. [Google Scholar]
- Zhu, T.F.; Liu, X.M.; Yao, Y.C.; Li, Y.F. Relevance analysis on TLR4polymorphism with lung function and body mass index in Han population from North China. J. Jilin Univ. Med. Ed. 2010, 36, 687–690. [Google Scholar] [CrossRef]
- National Health Commission of the People’s Republic of China: Screening for Overweight and Obesity in School-Age Children and Adolescents. 2018. Available online: http://www.nhc.gov.cn/ewebeditor/uploadfile/2018/03/20180330094031236.pdf (accessed on 24 January 2023).
- Zhou, D.; Yang, M.; Yuan, Z.P.; Zhang, D.D.; Liang, L.; Wang, C.L.; Zhang, S.; Zhu, H.H.; Lai, M.D.; Zhu, Y.M. Waist-to-Height Ratio: A simple, effective and practical screening tool for childhood obesity and metabolic syndrome. Prev. Med. 2014, 67, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Xue, K.; Guo, H.W.; Yang, Y.H. LMX1B rs10733682 Polymorphism Interacts with Macronutrients, Dietary Patterns on the Risk of Obesity in Han Chinese Girls. Nutrients 2020, 12, 1227. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.C.; Huang, Y.; Lo, K.; Huang, Y.Q.; Chen, J.Y.; Feng, Y.Q. Quotient of Waist Circumference and Body Mass Index: A Valuable Indicator for the High-Risk Phenotype of Obesity. Front. Endocrinol. 2021, 12, 697437. [Google Scholar] [CrossRef]
- Stadler, J.T.; Lackner, S.; Mörkl, S.; Trakaki, A.; Scharnagl, H.; Borenich, A.; Wonisch, W.; Mangge, H.; Zelzer, S.; Meier-Allard, N.; et al. Obesity Affects HDL Metabolism, Composition and Subclass Distribution. Biomedicines 2021, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Chaffin, M.; Wade, K.H.; Zahid, S.; Brancale, J.; Xia, R.; Distefano, M.; Senol-Cosar, O.; Haas, M.E.; Bick, A.; et al. Polygenic Prediction of Weight and Obesity Trajectories from Birth to Adulthood. Cell 2019, 177, 587–596.e589. [Google Scholar] [CrossRef]
- Doaei, S.; Kalantari, N.; Izadi, P.; Salonurmi, T.; Jarrahi, A.M.; Rafieifar, S.; Azizi Tabesh, G.; Rahimzadeh, G.; Gholamalizadeh, M.; Goodarzi, M.O. Interactions between macro-nutrients’ intake, FTO and IRX3 gene expression, and FTO genotype in obese and overweight male adolescents. Adipocyte 2019, 8, 386–391. [Google Scholar] [CrossRef]
- Mirzababaei, A.; Daneshzad, E.; Shiraseb, F.; Pourreza, S.; Setayesh, L.; Clark, C.C.T.; Tangestani, H.; Abaj, F.; Yarizadeh, H.; Mirzaei, K. Variants of the cry 1 gene may influence the effect of fat intake on resting metabolic rate in women with overweight of obesity: A cross-sectional study. BMC Endocr. Disord. 2021, 21, 196. [Google Scholar] [CrossRef]
- Segovia-Siapco, G.; Khayef, G.; Pribis, P.; Oda, K.; Haddad, E.; Sabaté, J. Animal Protein Intake Is Associated with General Adiposity in Adolescents: The Teen Food and Development Study. Nutrients 2019, 12, 110. [Google Scholar] [CrossRef]
- Sánchez-Tapia, M.; Moreno-Vicencio, D.; Ordáz-Nava, G.; Guevara-Cruz, M.; Granados-Portillo, O.; Vargas-Castillo, A.; Torres, N.; Tovar, A.R. Antibiotic Treatment Reduces the Health Benefits of Soy Protein. Mol. Nutr. Food Res. 2020, 64, e2000532. [Google Scholar] [CrossRef]
- Dor, C.; Stark, A.H.; Dichtiar, R.; Keinan-Boker, L.; Sinai, T. Non-Dairy Animal Protein Consumption Is Positively Associated with Overweight and Obesity in Israeli Adolescents. Foods 2022, 11, 2072. [Google Scholar] [CrossRef]
- Li, X.F.; Jiang, Z.Q.; Ma, L.L.; Xiong, J.Y. Effect of soybean egg white on lipid substitution. Chin. J. Prev. Med. 2008, 42, 599–601. [Google Scholar] [CrossRef]
- Jenkins, D. Counterpoint: Soy protein. J. Clin. Lipidol. 2017, 11, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Efficacy and safety of lowering dietary intake of fat and cholesterol in children with elevated low-density lipoprotein cholesterol. The Dietary Intervention Study in Children (DISC). The Writing Group for the DISC Collaborative Research Group. JAMA 1995, 273, 1429–1435. [CrossRef] [PubMed]
- Tyrovolas, S.; Pounis, G.; Zeimbekis, A.; Antonopoulou, M.; Bountziouka, V.; Gotsis, E.; Metallinos, G.; Polystipioti, A.; Polychronopoulos, E.; Lionis, C.; et al. Associations of energy intake and type 2 diabetes with hypertryglyceridemia in older adults living in the Mediterranean islands: The MEDIS study. J. Nutr. Elder. 2010, 29, 72–86. [Google Scholar] [CrossRef]
- Liberali, R.; Kupek, E.; Assis, M.A.A. Dietary Patterns and Childhood Obesity Risk: A Systematic Review. Child. Obes. 2020, 16, 70–85. [Google Scholar] [CrossRef]
- Shatwan, I.M.; Alhinai, E.A.; Alawadhi, B.; Surendran, S.; Aljefree, N.M.; Almoraie, N.M. High Adherence to the Mediterranean Diet Is Associated with a Reduced Risk of Obesity among Adults in Gulf Countries. Nutrients 2021, 13, 995. [Google Scholar] [CrossRef]
- Lassale, C.; Fitó, M.; Morales-Suárez-Varela, M.; Moya, A.; Gómez, S.F.; Schröder, H. Mediterranean diet and adiposity in children and adolescents: A systematic review. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2022, 23 (Suppl. S1), e13381. [Google Scholar] [CrossRef]
- Pretorius, R.A.; Palmer, D.J. High-Fiber Diet during Pregnancy Characterized by More Fruit and Vegetable Consumption. Nutrients 2020, 13, 35. [Google Scholar] [CrossRef]
- Ledoux, T.A.; Hingle, M.D.; Baranowski, T. Relationship of fruit and vegetable intake with adiposity: A systematic review. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2011, 12, e143–e150. [Google Scholar] [CrossRef]
- Hosseini-Esfahani, F.; Koochakpoor, G.; Daneshpour, M.S.; Mirmiran, P.; Sedaghati-Khayat, B.; Azizi, F. The interaction of fat mass and obesity associated gene polymorphisms and dietary fiber intake in relation to obesity phenotypes. Sci. Rep. 2017, 7, 18057. [Google Scholar] [CrossRef]
- Lisboa, J.V.C.; Ribeiro, M.R.; Luna, R.C.P.; Lima, R.P.A.; Nascimento, R.; Monteiro, M.; Lima, K.Q.F.; Fechine, C.; Oliveira, N.F.P.; Persuhn, D.C.; et al. Food Intervention with Folate Reduces TNF-α and Interleukin Levels in Overweight and Obese Women with the MTHFR C677T Polymorphism: A Randomized Trial. Nutrients 2020, 12, 361. [Google Scholar] [CrossRef]
- Siroma, T.K.; Machate, D.J.; Zorgetto-Pinheiro, V.A.; Figueiredo, P.S.; Marcelino, G.; Hiane, P.A.; Bogo, D.; Pott, A.; Cury, E.R.J.; Guimarães, R.C.A.; et al. Polyphenols and ω-3 PUFAs: Beneficial Outcomes to Obesity and Its Related Metabolic Diseases. Front. Nutr. 2021, 8, 781622. [Google Scholar] [CrossRef]
- Jantan, I.; Haque, M.A.; Arshad, L.; Harikrishnan, H.; Septama, A.W.; Mohamed-Hussein, Z.A. Dietary polyphenols suppress chronic inflammation by modulation of multiple inflammation-associated cell signaling pathways. J. Nutr. Biochem. 2021, 93, 108634. [Google Scholar] [CrossRef] [PubMed]
- Azam, S.; Jakaria, M.; Kim, I.S.; Kim, J.; Haque, M.E.; Choi, D.K. Regulation of Toll-Like Receptor (TLR) Signaling Pathway by Polyphenols in the Treatment of Age-Linked Neurodegenerative Diseases: Focus on TLR4 Signaling. Front. Immunol. 2019, 10, 1000. [Google Scholar] [CrossRef] [PubMed]
- Rahimifard, M.; Maqbool, F.; Moeini-Nodeh, S.; Niaz, K.; Abdollahi, M.; Braidy, N.; Nabavi, S.M.; Nabavi, S.F. Targeting the TLR4 signaling pathway by polyphenols: A novel therapeutic strategy for neuroinflammation. Ageing Res. Rev. 2017, 36, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Barth, S.W.; Koch, T.C.; Watzl, B.; Dietrich, H.; Will, F.; Bub, A. Moderate effects of apple juice consumption on obesity-related markers in obese men: Impact of diet-gene interaction on body fat content. Eur. J. Nutr. 2012, 51, 841–850. [Google Scholar] [CrossRef]
- Duchen, K.; Faresjö, Å.O.; Klingberg, S.; Faresjö, T.; Ludvigsson, J. Fatty fish intake in mothers during pregnancy and in their children in relation to the development of obesity and overweight in childhood: The prospective ABIS study. Obes. Sci. Pract. 2020, 6, 57–69. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; Mera, R.M.; Recalde, B.Y. Intracranial atherosclerosis and oily fish intake. A population study in frequent fish consumers living in rural Ecuador. Int. J. Stroke Off. J. Int. Stroke Soc. 2020, 15, Np4–Np5. [Google Scholar] [CrossRef]
- Jia, L.; Vianna, C.R.; Fukuda, M.; Berglund, E.D.; Liu, C.; Tao, C.; Sun, K.; Liu, T.; Harper, M.J.; Lee, C.E.; et al. Hepatocyte Toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nat. Commun. 2014, 5, 3878. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Differential Effects of Red Meat/Refined Grain Diet and Dairy/Chicken/Nuts/Whole Grain Diet on Glucose, Insulin and Triglyceride in a Randomized Crossover Study. Nutrients 2016, 8, 687. [Google Scholar] [CrossRef]
- Fretts, A.M.; Follis, J.L.; Nettleton, J.A.; Lemaitre, R.N.; Ngwa, J.S.; Wojczynski, M.K.; Kalafati, I.P.; Varga, T.V.; Frazier-Wood, A.C.; Houston, D.K.; et al. Consumption of meat is associated with higher fasting glucose and insulin concentrations regardless of glucose and insulin genetic risk scores: A meta-analysis of 50,345 Caucasians. Am. J. Clin. Nutr. 2015, 102, 1266–1278. [Google Scholar] [CrossRef]
- Berg, J.; Seyedsadjadi, N.; Grant, R. Saturated Fatty Acid Intake Is Associated With Increased Inflammation, Conversion of Kynurenine to Tryptophan, and Delta-9 Desaturase Activity in Healthy Humans. Int. J. Tryptophan Res. IJTR 2020, 13, 1178646920981946. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Q.; Wang, L. Appetite Regulation of TLR4-Induced Inflammatory Signaling. Front. Endocrinol. 2021, 12, 777997. [Google Scholar] [CrossRef] [PubMed]
- Garver, W.S.; Newman, S.B.; Gonzales-Pacheco, D.M.; Castillo, J.J.; Jelinek, D.; Heidenreich, R.A.; Orlando, R.A. The genetics of childhood obesity and interaction with dietary macronutrients. Genes Nutr. 2013, 8, 271–287. [Google Scholar] [CrossRef]
- Feingold, K.R. The effect of diet on cardiovascular disease and lipid and lipoprotein levels. In Endotext [Internet]; MDText.com, Inc.: Portland, OR, USA, 2021. [Google Scholar]
- Wróblewska, J.; Klocek, M.; Czarnecka, D. Consumption of polyunsaturated fatty acids by young hypertensive adults. Prz. Lek. 2016, 73, 382–387. [Google Scholar]
- Xu, X.P.; Li, J.; Fan, Y.W.; Deng, Z.Y. Effect of cooking method on fatty acid composition in muscle fat of pork. J. Chin. Inst. Food Sci. Technol. 2020, 20, 196–203. [Google Scholar] [CrossRef]
- Wu, T.; Sonoda, S.; Liu, H. Unprocessed red meat intakes are associated with increased inflammation, triglycerides and HDL cholesterol in past smokers. Nutr. Diet. J. Dietit. Assoc. Aust. 2020, 77, 182–188. [Google Scholar] [CrossRef]
- Satija, A.; Hu, F.B.; Bowen, L.; Bharathi, A.V.; Vaz, M.; Prabhakaran, D.; Reddy, K.S.; Ben-Shlomo, Y.; Davey Smith, G.; Kinra, S.; et al. Dietary patterns in India and their association with obesity and central obesity. Public Health Nutr. 2015, 18, 3031–3041. [Google Scholar] [CrossRef]
- Byrne, A.; Makadia, S.; Sutherland, A.; Miller, M. Optimizing Non-Pharmacologic Management of Hypertriglyceridemia. Arch. Med. Res. 2017, 48, 483–487. [Google Scholar] [CrossRef]
- Foster, G.D.; Shantz, K.L.; Vander Veur, S.S.; Oliver, T.L.; Lent, M.R.; Virus, A.; Szapary, P.O.; Rader, D.J.; Zemel, B.S.; Gilden-Tsai, A. A randomized trial of the effects of an almond-enriched, hypocaloric diet in the treatment of obesity. Am. J. Clin. Nutr. 2012, 96, 249–254. [Google Scholar] [CrossRef]
- Sharma, S.P.; Chung, H.J.; Kim, H.J.; Hong, S.T. Paradoxical Effects of Fruit on Obesity. Nutrients 2016, 8, 633. [Google Scholar] [CrossRef]
- Su, J.; Li, Q.; Mao, P.; Peng, H.; Han, H.; Wiley, J.; Guo, J.; Chen, J.L. Does the Association of Sedentary Time or Fruit/Vegetable Intake with Central Obesity Depend on Menopausal Status among Women? Int. J. Environ. Res. Public Health 2022, 19, 83. [Google Scholar] [CrossRef] [PubMed]
- Keogh, J.B.; Clifton, P.M. Energy Intake and Satiety Responses of Eggs for Breakfast in Overweight and Obese Adults-A Crossover Study. Int. J. Environ. Res. Public Health 2020, 17, 5583. [Google Scholar] [CrossRef]
- Kral, T.V.; Bannon, A.L.; Chittams, J.; Moore, R.H. Comparison of the satiating properties of egg- versus cereal grain-based breakfasts for appetite and energy intake control in children. Eat. Behav. 2016, 20, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shang, D.J. Research progress of TLR4 signaling pathway and its targeted drugs. Chin. J. Cell Mol. Immunol. 2021, 37, 657–662. [Google Scholar] [CrossRef]
- Xia, J. The mechanisms of Toil-like receptor 4 in metabolic inflammatory response in childhood obesity. Int. J. Pediatr. 2015, 42, 152–155. [Google Scholar] [CrossRef]
- Vitseva, O.I.; Tanriverdi, K.; Tchkonia, T.T.; Kirkland, J.L.; McDonnell, M.E.; Apovian, C.M.; Freedman, J.; Gokce, N. Inducible Toll-like receptor and NF-kappaB regulatory pathway expression in human adipose tissue. Obes. Silver Spring Md. 2008, 16, 932–937. [Google Scholar] [CrossRef]
- De Loera-Rodriguez, C.O.; Delgado-Rizo, V.; Alvarado-Navarro, A.; Agraz-Cibrian, J.M.; Segura-Ortega, J.E.; Fafutis-Morris, M. Over-expression of TLR4-CD14, pro-inflammatory cytokines, metabolic markers and NEFAs in obese non-diabetic Mexicans. J. Inflamm. 2014, 11, 39. [Google Scholar] [CrossRef][Green Version]
- Zatterale, F.; Raciti, G.A.; Prevenzano, I.; Leone, A.; Campitelli, M.; De Rosa, V.; Beguinot, F.; Parrillo, L. Epigenetic Reprogramming of the Inflammatory Response in Obesity and Type 2 Diabetes. Biomolecules 2022, 12, 982. [Google Scholar] [CrossRef]
- Tsukumo, D.M.; Carvalho-Filho, M.A.; Carvalheira, J.B.; Prada, P.O.; Hirabara, S.M.; Schenka, A.A.; Araújo, E.P.; Vassallo, J.; Curi, R.; Velloso, L.A.; et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 2007, 56, 1986–1998. [Google Scholar] [CrossRef]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef]
- Suganami, T.; Tanimoto-Koyama, K.; Nishida, J.; Itoh, M.; Yuan, X.; Mizuarai, S.; Kotani, H.; Yamaoka, S.; Miyake, K.; Aoe, S.; et al. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Schaeffler, A.; Gross, P.; Buettner, R.; Bollheimer, C.; Buechler, C.; Neumeier, M.; Kopp, A.; Schoelmerich, J.; Falk, W. Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-kappaB pathway in adipocytes links nutritional signalling with innate immunity. Immunology 2009, 126, 233–245. [Google Scholar] [CrossRef]
- Vanderwall, C.; Eickhoff, J.; Randall Clark, R.; Carrel, A.L. BMI z-score in obese children is a poor predictor of adiposity changes over time. BMC Pediatr. 2018, 18, 187. [Google Scholar] [CrossRef] [PubMed]
- Chinese Nutrition Society Obesity Prevention and Control Section; Chinese Nutrition Society Clinical Nutrition Section; Chinese Preventive Medicine Association Behavioral Health Section; Chinese Preventive Medicine Association Sports and Health Section. Expert Consensus on Obesity Prevention and Treatment in China. Chin. Prev. Med. 2022, 43, 609–626. [Google Scholar] [CrossRef]
- Dong, Y.; Bai, L.; Cai, R.; Zhou, J.; Ding, W. Visceral adiposity index performed better than traditional adiposity indicators in predicting unhealthy metabolic phenotype among Chinese children and adolescents. Sci. Rep. 2021, 11, 23850. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.M.; Gu, H.Z.; Yuan, H.M. Correlation of Blood Glucose and Lipid Metabolism Characteristics with Precocious Puberty in Obese Children. Chin. Community Dr. 2022, 38, 138–140. [Google Scholar]
- Li, Y.Y. Pubertal Progression of Girls and its Influencing Factors. Master’s Thesis, Chongqing Medical University, Chongqing, China, 2021. [Google Scholar]

| Variables | Male | Female | ||||
|---|---|---|---|---|---|---|
| CC + CT (n = 230) | TT (n = 170) | p-Value 1 | CC + CT (n = 223) | TT (n = 175) | p-Value 1 | |
| BMI | 17.33 ± 2.97 | 17.60 ± 2.96 | 0.608 | 16.42 ± 2.50 | 16.63 ± 2.64 | 0.272 |
| WC | 58.26 ± 7.85 | 59.28 ± 8.65 | 0.057 | 55.14 ± 6.71 | 55.60 ± 7.42 | 0.09 |
| WHtR | 0.43 ± 0.05 | 0.43 ± 0.05 | 0.472 | 0.41 ± 0.04 | 0.41 ± 0.05 | 0.306 |
| SP (mmHg) | 99.50 ± 9.72 | 101.12 ± 9.64 | 0.562 | 99.26 ± 9.89 | 99.16 ± 9.33 | 0.249 |
| DP (mmHg) | 61.83 ± 6.19 | 62.12 ± 6.54 | 0.423 | 61.76 ± 6.88 | 61.55 ± 5.64 | 0.014 |
| TC (mM) | 3.84 ± 0.76 | 3.82 ± 0.81 | 0.546 | 3.93 ± 0.85 | 3.80 ± 0.84 | 0.56 |
| TG (mM) | 0.84 ± 0.53 | 0.81 ± 0.63 | 0.62 | 0.86 ± 0.49 | 0.84 ± 0.42 | 0.265 |
| HDL (mM) | 1.46 ± 0.29 | 1.41 ± 0.26 | 0.147 | 1.41 ± 0.24 | 1.39 ± 0.28 | 0.006 |
| LDL (mM) | 2.24 ± 0.58 | 2.27 ± 0.64 | 0.903 | 2.33 ± 0.68 | 2.27 ± 0.62 | 0.56 |
| FBG (mM) | 4.73 ± 0.44 | 4.75 ± 0.44 | 0.799 | 4.64 ± 0.40 | 4.64 ± 0.36 | 0.321 |
| Food Species | Dietary Patterns | |||
|---|---|---|---|---|
| HBDP | NDDP | AFDP | HWDP | |
| Vegetables | 0.758 | |||
| Fruits | 0.580 | |||
| Fish | 0.708 | |||
| Egg | 0.476 | |||
| Dairy | ||||
| Rice | −0.723 | |||
| Wheat | 0.726 | |||
| Potato | 0.484 | |||
| Nut | 0.769 | |||
| Pork | 0.708 | |||
| Poultry meat | 0.402 | |||
| Cakes and pastries | 0.684 | |||
| Candies | 0.444 | |||
| Beans and their products | ||||
| Characteristic root | 1.820 | 1.430 | 1.393 | 1.382 |
| Variance (%) | 12.997 | 10.216 | 9.953 | 9.630 |
| Variance of involvement (%) = 43.039 | ||||
| Variables | HBDP | P 3 | P′ 4 | NDDP | P 3 | P′ 4 | AFDP | P 3 | P′ 4 | HWDP | P 3 | P′ 4 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (n = 100) | Q4 (n = 100) | Q1 (n = 100) | Q4 (n = 100) | Q1 (n = 100) | Q4 (n = 100) | Q1 (n = 100) | Q4 (n = 100) | |||||||||
| Age (year) | 9.41 ± 0.94 | 9.35 ± 1.00 | 0.699 | 9.58 ± 0.84 | 9.131 ± 0.87 | <0.001 | 9.20 ± 0.95 | 9.50 ± 0.89 | 0.023 | 9.24 ± 0.93 | 9.41 ± 0.88 | 0.193 | ||||
| Overweight/obesity (%) 1 | 16.50% | 10.50% | 0.056 | 15.50% | 13.50% | 0.533 | 13.50% | 16.50% | 0.355 | 13.00% | 13.50% | 0.873 | ||||
| Central obesity (%) 1 | 12.00% | 6.50% | 0.045 | 10.00% | 7.00% | 0.259 | 8.50% | 10.00% | 0.585 | 9.50% | 8.50% | 0.713 | ||||
| SP (mmHg) 2 | 100.36 ± 9.09 | 100.32 ± 10.01 | 0.976 | 0.952 | 100.45 ± 9.52 | 99.76 ± 10.07 | 0.619 | 0.614 | 99.89 ± 10.08 | 102.18 ± 10.41 | 0.116 | 0.247 | 100.98 ± 9.78 | 99.72 ± 8.88 | 0.341 | 0.205 |
| DP (mmHg) 2 | 61.80 ± 6.34 | 61.46 ± 5.84 | 0.694 | 0.730 | 62.34 ± 5.85 | 62.40 ± 6.35 | 0.945 | 0.422 | 62.58 ± 0.54 | 62.11 ± 6.25 | 0.604 | 0.483 | 62.19 ± 6.35 | 61.97 ± 6.51 | 0.809 | 0.713 |
| TCH (mM) 2 | 3.89 ± 0.67 | 3.76 ± 0.95 | 0.281 | 0.277 | 3.81 ± 0.79 | 3.77 ± 0.83 | 0.757 | 0.999 | 3.79 ± 0.68 | 3.82 ± 0.80 | 0.821 | 0.932 | 3.90 ± 0.63 | 3.72 ± 0.81 | 0.080 | 0.062 |
| TG (mM) 2 | 0.76 ± 0.43 | 1.02 ± 0.80 | 0.005 | 0.003 | 0.84 ± 0.57 | 0.92 ± 0.73 | 0.439 | 0.246 | 0.80 ± 0.46 | 0.98 ± 0.84 | 0.073 | 0.136 | 0.79 ± 0.46 | 0.83 ± 0.53 | 0.510 | 0.566 |
| HDL (mM) 2 | 1.42 ± 0.25 | 1.43 ± 0.32 | 0.780 | 0.786 | 1.42 ± 0.26 | 1.46 ± 0.26 | 0.302 | 0.260 | 1.44 ± 0.27 | 1.45 ± 0.27 | 0.800 | 0.720 | 1.45 ± 0.27 | 1.44 ± 0.28 | 0.866 | 0.873 |
| LDL (mM) 2 | 2.26 ± 0.54 | 2.28 ± 0.66 | 0.810 | 0.815 | 2.24 ± 0.68 | 2.24 ± 0.59 | 0.973 | 0.778 | 2.14 ± 0.50 | 2.35 ± 0.65 | 0.014 | 0.016 | 2.25 ± 0.60 | 2.20 ± 0.54 | 0.515 | 0.393 |
| FBG (mM) 2 | 4.83 ± 0.37 | 4.75 ± 0.46 | 0.184 | 0.201 | 4.76 ± 0.44 | 4.71 ± 0.45 | 0.419 | 0.952 | 4.65 ± 0.42 | 4.80 ± 0.44 | 0.016 | 0.047 | 4.81 ± 0.43 | 4.65 ± 0.44 | 0.010 | 0.004 |
| Food Species | Dietary Patterns | |||
|---|---|---|---|---|
| NPDP | DPDP | EFDP | VFDP | |
| Vegetables | 0.678 | |||
| Fruits | 0.665 | |||
| Fish | 0.593 | 0.748 | ||
| Egg | 0.703 | |||
| Dairy | ||||
| Rice | ||||
| Wheat | 0.573 | |||
| Potato | ||||
| Nut | 0.718 | 0.388 | ||
| Pork | 0.757 | |||
| Poultry meat | 0.529 | 0.387 | ||
| Cakes and pastries | 0.785 | |||
| Candies | 0.649 | |||
| Beans and their products | ||||
| Characteristic root | 1.618 | 1.580 | 1.462 | 1.420 |
| Variance (%) | 11.555 | 11.285 | 10.441 | 10.145 |
| Variance of involvement (%) = 43.425 | ||||
| Variables | NPDP | P 3 | P′ 4 | DPDP | P 3 | P′ 4 | EFDP | P 3 | P′ 4 | VFDP | P 3 | P′ 4 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (n = 100) | Q4 (n = 99) | Q1 (n = 100) | Q4 (n = 100) | Q1 (n = 100) | Q4 (n = 99) | Q1 (n = 100) | Q4 (n = 99) | |||||||||
| Age (year) | 9.09 ± 0.95 | 9.35 ± 0.83 | 0.044 | 9.09 ± 0.95 | 9.25 ± 0.93 | 0.228 | 9.15 ± 0.90 | 9.06 ± 0.91 | 0.498 | 9.16 ± 0.80 | 9.27 ± 0.91 | 0.337 | ||||
| Overweight/obesity (%) 1 | 9.50% | 10.10% | 0.831 | 7.50% | 9.00% | 0.568 | 13.00% | 8.00% | 0.083 | 8.50% | 9.50% | 0.688 | ||||
| Central obesity (%) 1 | 10.10% | 7.00% | 0.272 | 6.50% | 8.00% | 0.547 | 10.00% | 4.50% | 0.027 | 3.50% | 8.50% | 0.028 | ||||
| SP (mmHg) 2 | 97.42 ± 8.63 | 99.69 ± 10.22 | 0.092 | 0.244 | 98.54 ± 9.40 | 101.10 ± 10.30 | 0.068 | 0.102 | 100.79 ± 10.69 | 98.75 ± 10.00 | 0.165 | 0.191 | 99.65 ± 9.75 | 98.41 ± 8.84 | 0.350 | 0.268 |
| DP (mmHg) 2 | 60.88 ± 5.92 | 62.12 ± 6.84 | 0.173 | 0.386 | 61.43 ± 6.54 | 62.54 ± 6.21 | 0.220 | 0.281 | 62.68 ± 6.30 | 61.57 ± 6.25 | 0.212 | 0.237 | 62.00 ± 6.37 | 61.70 ± 6.35 | 0.737 | 0.619 |
| TCH (mM) 2 | 3.84 ± 0.72 | 3.72 ± 0.86 | 0.286 | 0.336 | 3.87 ± 0.86 | 3.92 ± 0.89 | 0.693 | 0.613 | 3.91 ± 0.93 | 3.86 ± 0.93 | 0.657 | 0.643 | 3.91 ± 0.95 | 3.87 ± 0.79 | 0.781 | 0.817 |
| TG (mM) 2 | 0.82 ± 0.41 | 0.95 ± 0.50 | 0.036 | 0.053 | 0.89 ± 0.53 | 0.89 ± 0.45 | 0.984 | 0.924 | 0.85 ± 0.45 | 0.91 ± 0.48 | 0.343 | 0.36 | 0.82 ± 0.42 | 0.82 ± 0.46 | 0.952 | 0.958 |
| HDL (mM) 2 | 1.37 ± 0.28 | 1.41 ± 0.25 | 0.311 | 0.309 | 1.39 ± 0.27 | 1.40 ± 0.27 | 0.887 | 0.862 | 1.39 ± 0.26 | 1.43 ± 0.27 | 0.327 | 0.307 | 1.44 ± 0.26 | 1.37 ± 0.26 | 0.048 | 0.055 |
| LDL (mM) 2 | 2.29 ± 0.58 | 2.27 ± 0.62 | 0.888 | 0.981 | 2.25 ± 0.66 | 2.39 ± 0.72 | 0.157 | 0.115 | 2.31 ± 0.67 | 2.38 ± 0.74 | 0.527 | 0.577 | 2.41 ± 0.72 | 2.27 ± 0.62 | 0.152 | 0.161 |
| FBG (mM) 2 | 4.66 ± 0.40 | 4.64 ± 0.38 | 0.752 | 0.542 | 4.63 ± 0.35 | 4.71 ± 0.37 | 0.148 | 0.223 | 4.70 ± 0.43 | 4.60 ± 0.40 | 0.094 | 0.116 | 4.56 ± 0.37 | 4.65 ± 0.40 | 0.097 | 0.146 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Q.; Qian, B.; Xue, K.; Guo, H.; Liang, R.; Wu, J.; Wu, Q.; Zhou, G. TT Genotype of TLR4 rs1928295 Is a Risk Factor of Overweight/Obesity in Han Chinese Children Aged 7–12 Years and Can Interact with Dietary Patterns to Affect the Incidence of Central Obesity and Lipid Profile, Systolic Blood Pressure Levels. Nutrients 2023, 15, 3441. https://doi.org/10.3390/nu15153441
Zhu Q, Qian B, Xue K, Guo H, Liang R, Wu J, Wu Q, Zhou G. TT Genotype of TLR4 rs1928295 Is a Risk Factor of Overweight/Obesity in Han Chinese Children Aged 7–12 Years and Can Interact with Dietary Patterns to Affect the Incidence of Central Obesity and Lipid Profile, Systolic Blood Pressure Levels. Nutrients. 2023; 15(15):3441. https://doi.org/10.3390/nu15153441
Chicago/Turabian StyleZhu, Qi, Ben Qian, Kun Xue, Hongwei Guo, Rui Liang, Jinlong Wu, Qisu Wu, and Geyi Zhou. 2023. "TT Genotype of TLR4 rs1928295 Is a Risk Factor of Overweight/Obesity in Han Chinese Children Aged 7–12 Years and Can Interact with Dietary Patterns to Affect the Incidence of Central Obesity and Lipid Profile, Systolic Blood Pressure Levels" Nutrients 15, no. 15: 3441. https://doi.org/10.3390/nu15153441
APA StyleZhu, Q., Qian, B., Xue, K., Guo, H., Liang, R., Wu, J., Wu, Q., & Zhou, G. (2023). TT Genotype of TLR4 rs1928295 Is a Risk Factor of Overweight/Obesity in Han Chinese Children Aged 7–12 Years and Can Interact with Dietary Patterns to Affect the Incidence of Central Obesity and Lipid Profile, Systolic Blood Pressure Levels. Nutrients, 15(15), 3441. https://doi.org/10.3390/nu15153441






