Sulforaphane Combined with Vitamin D Induces Cytotoxicity Mediated by Oxidative Stress, DNA Damage, Autophagy, and JNK/MAPK Pathway Modulation in Human Prostate Tumor Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Agents and Reagents
2.2. Cell Culture Conditions
2.3. Selection of Sulforaphane and Vitamin D Concentrations
2.4. Cell Viability Assay
2.5. Oxidative Stress Assay
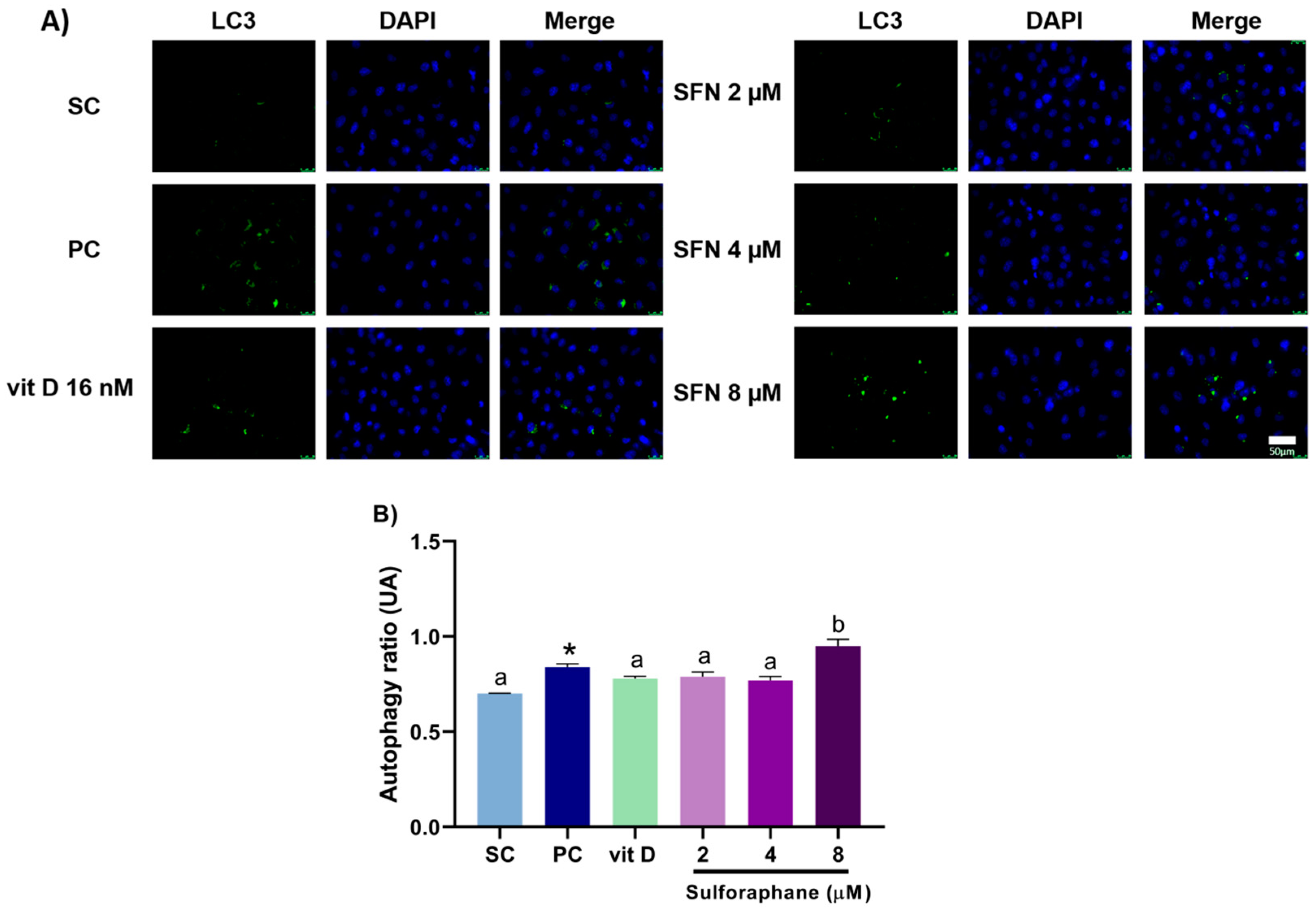
2.6. Autophagy Assay
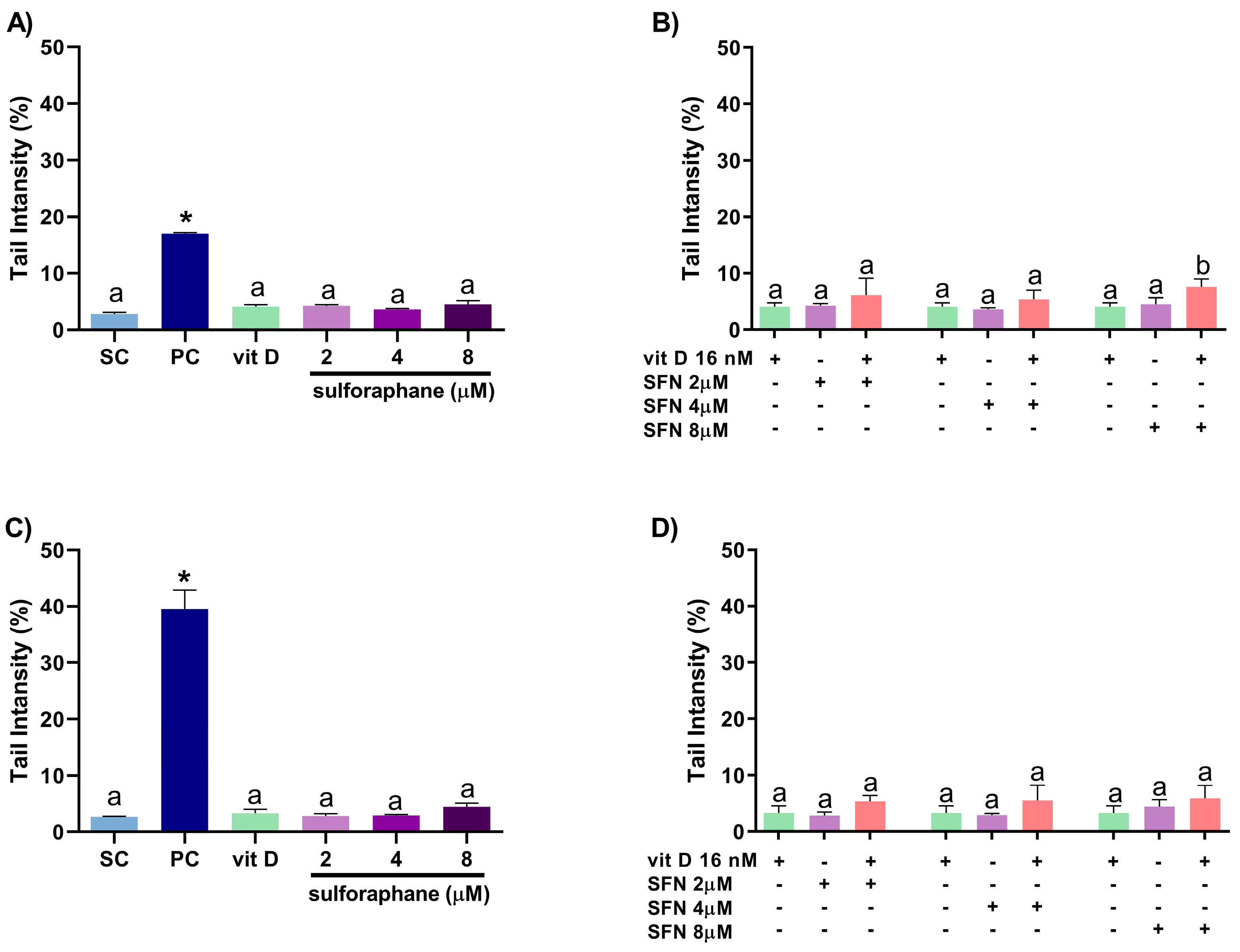
2.7. Comet Assay
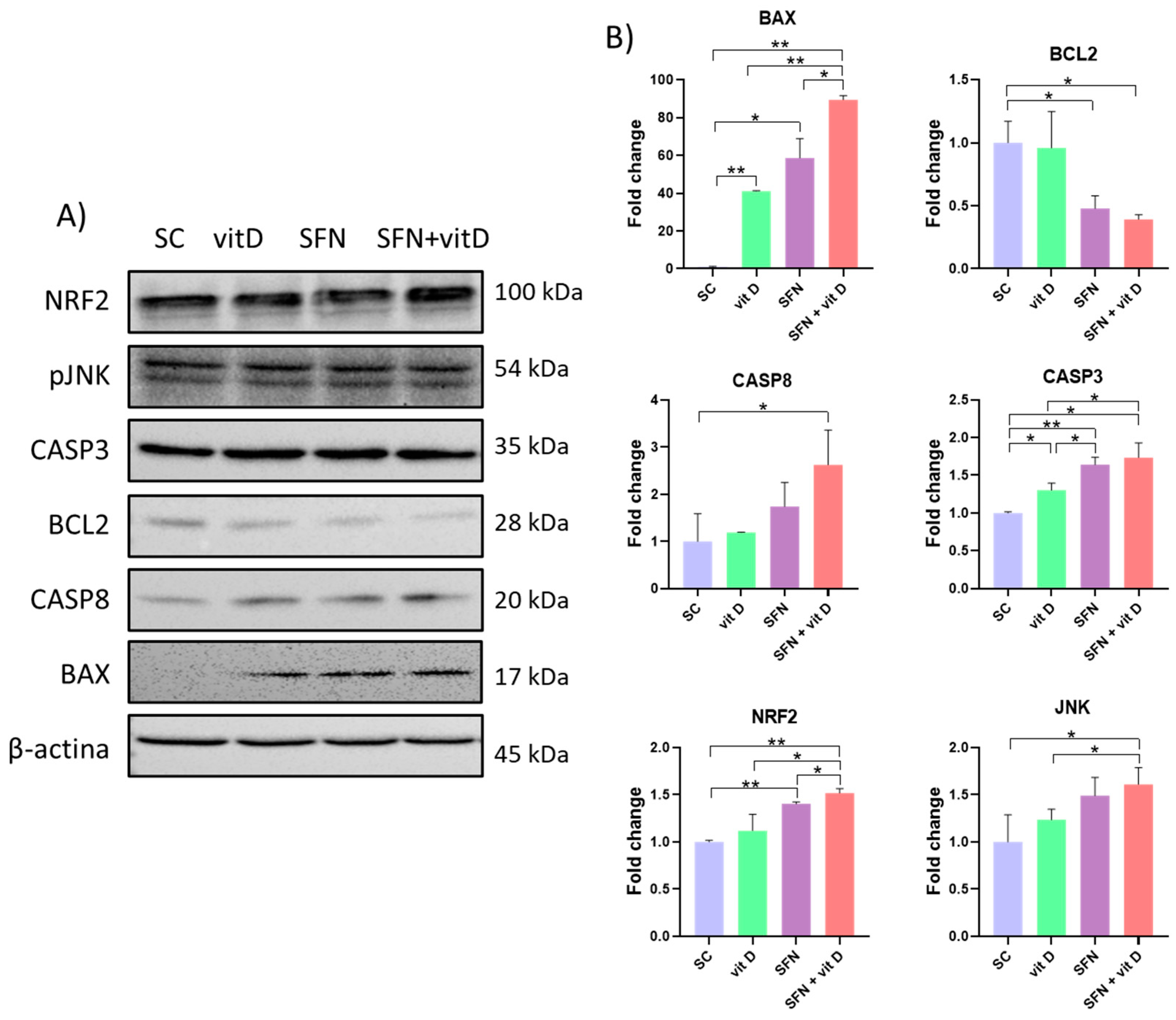
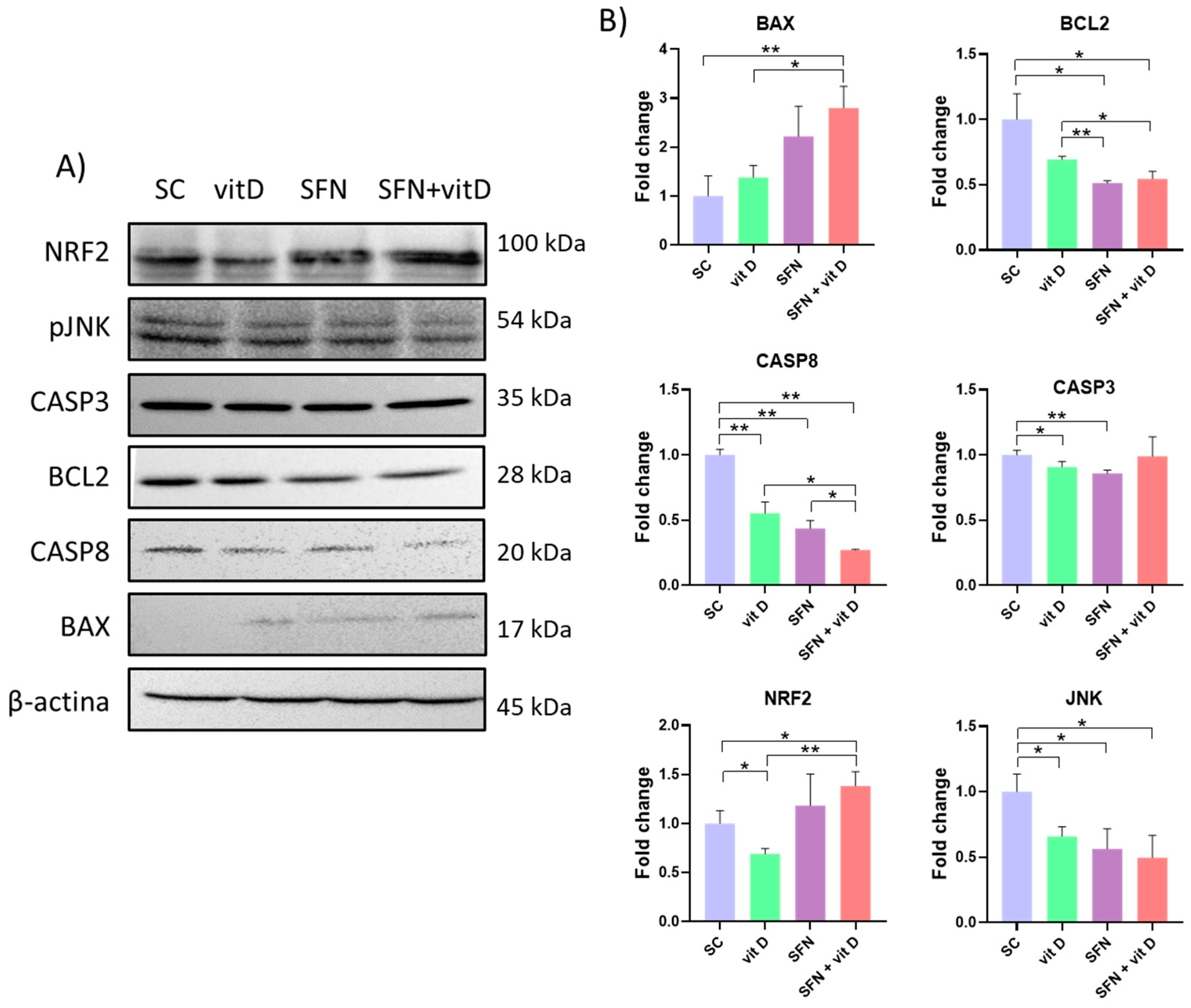
2.8. Protein Extraction and Western Blot Assay
2.9. Prediction of Interactions
2.10. Statistical Analysis
3. Results
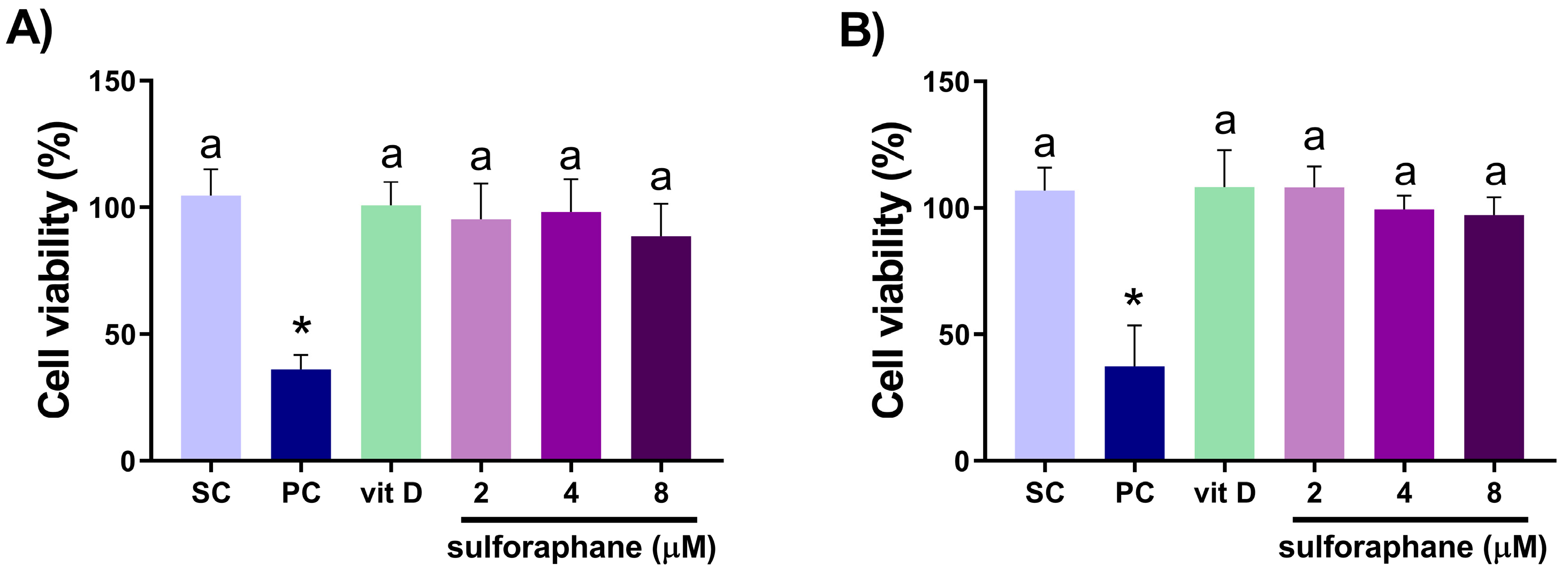
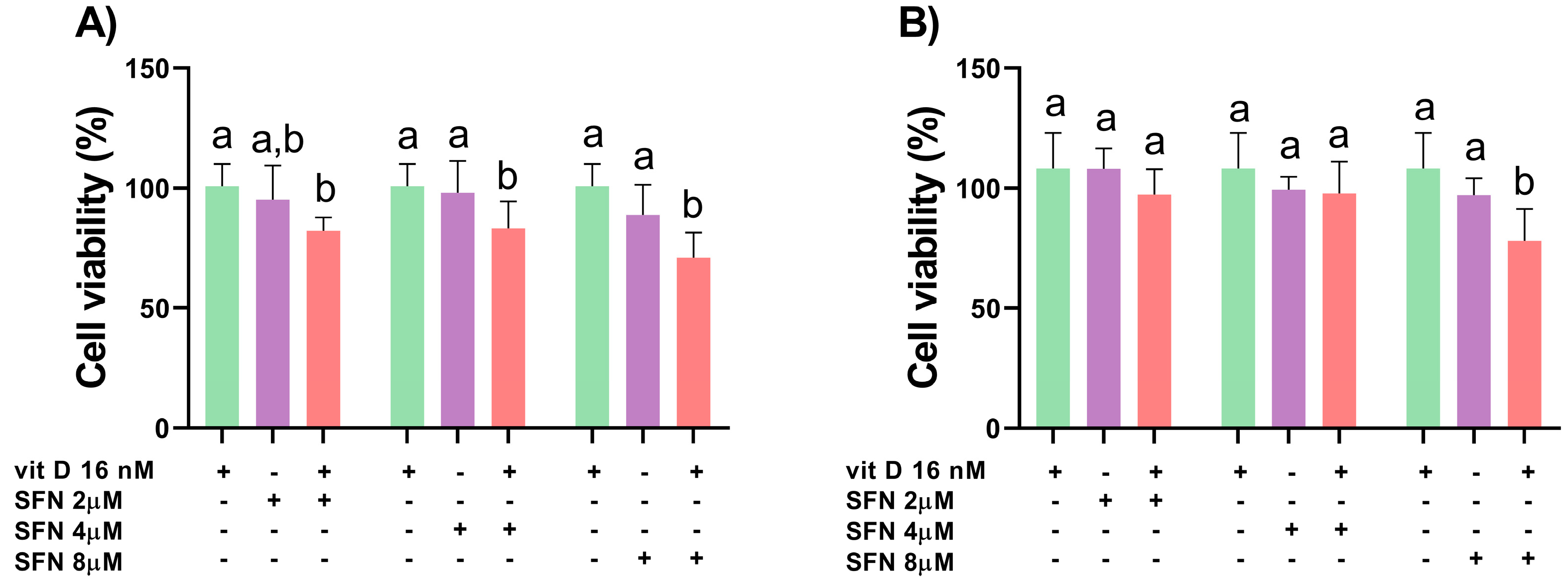
3.1. Analysis of Cell Viability
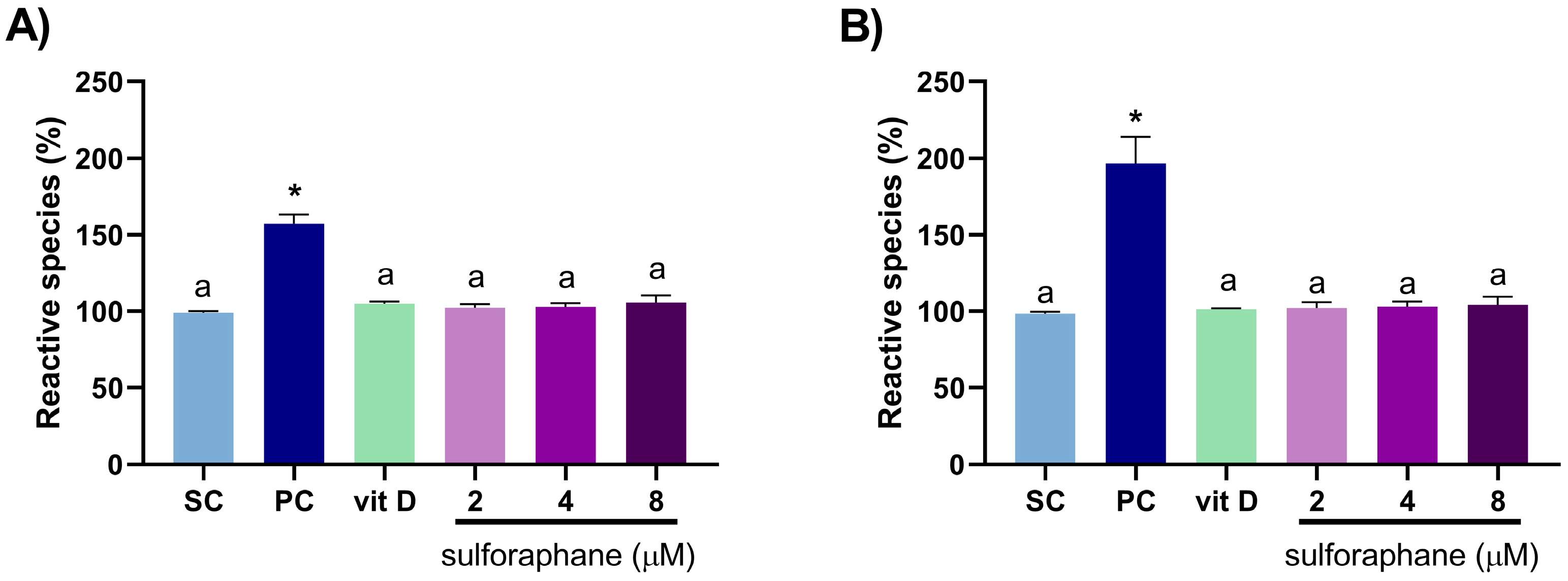
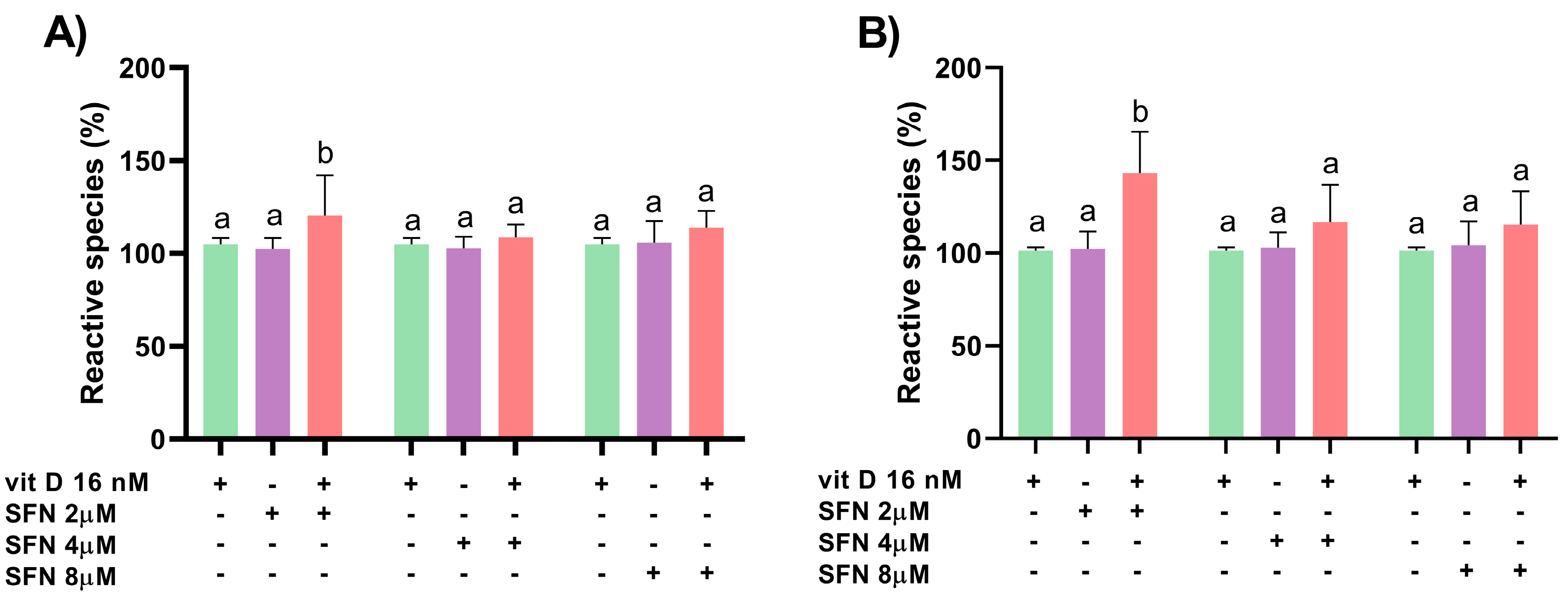
3.2. Induction of Oxidative Stress
3.3. Induction of Autophagy
3.4. Induction of DNA Damage
3.5. Analysis of Protein Expression
3.6. Analysis of Interactions between Compounds and Analyzed Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bleyer, A.; Spreafico, F.; Barr, R. Prostate Cancer in Young Men: An Emerging Young Adult and Older Adolescent Challenge. Cancer 2020, 126, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Kallifatidis, G.; Hoy, J.; Lokeshwar, B.L. Bioactive Natural Products for Chemoprevention and Treatment of Castration-Resistant Prostate Cancer. Semin. Cancer Biol. 2016, 40–41, 160–169. [Google Scholar] [CrossRef] [PubMed]
- George, B.P.; Chandran, R.; Abrahamse, H. Role of Phytochemicals in Cancer Chemoprevention: Insights. Antioxidants 2021, 10, 1455. [Google Scholar] [CrossRef]
- Chhabra, G.; Singh, C.K.; Ndiaye, M.A.; Fedorowicz, S.; Molot, A.; Ahmad, N. Prostate Cancer Chemoprevention by Natural Agents: Clinical Evidence and Potential Implications. Cancer Lett. 2018, 422, 9–18. [Google Scholar] [CrossRef]
- Calvani, M.; Pasha, A.; Favre, C. Nutraceutical Boom in Cancer: Inside the Labyrinth of Reactive Oxygen Species. Int. J. Mol. Sci. 2020, 21, 1936. [Google Scholar] [CrossRef]
- Reed, D.; Raina, K.; Agarwal, R. Nutraceuticals in Prostate Cancer Therapeutic Strategies and Their Neo-Adjuvant Use in Diverse Populations. Npj Precis. Oncol. 2018, 2, 15. [Google Scholar] [CrossRef]
- Ullah, M.F.; Ahmad, A.; Bhat, S.H.; Abuduhier, F.M.; Mustafa, S.K.; Usmani, S. Diet-Derived Small Molecules (Nutraceuticals) Inhibit Cellular Proliferation by Interfering with Key Oncogenic Pathways: An Overview of Experimental Evidence in Cancer Chemoprevention. Biol. Futura 2022, 73, 55–69. [Google Scholar] [CrossRef]
- Abbaoui, B.; Lucas, C.R.; Riedl, K.M.; Clinton, S.K.; Mortazavi, A. Cruciferous Vegetables, Isothiocyanates, and Bladder Cancer Prevention. Mol. Nutr. Food Res. 2018, 62, e1800079. [Google Scholar] [CrossRef]
- Russo, M.; Spagnuolo, C.; Russo, G.L.; Skalicka-Woźniak, K.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Nrf2 Targeting by Sulforaphane: A Potential Therapy for Cancer Treatment. Crit. Rev. Food Sci. Nutr. 2018, 58, 1391–1405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Bergan, R.; Shannon, J.; Slatore, C.G.; Bobe, G.; Takata, Y. The Role of Cruciferous Vegetables and Isothiocyanates for Lung Cancer Prevention: Current Status, Challenges, and Future Research Directions. Mol. Nutr. Food Res. 2018, 62, e1700936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, Y.; Jiang, L. Sulforaphane Attenuates Angiotensin II-Induced Human Umbilical Vein Endothelial Cell Injury by Modulating ROS-Mediated Mitochondrial Signaling. Hum. Exp. Toxicol. 2020, 39, 734–747. [Google Scholar] [CrossRef] [PubMed]
- Palliyaguru, D.L.; Yuan, J.-M.; Kensler, T.W.; Fahey, J.W. Isothiocyanates: Translating the Power of Plants to People. Mol. Nutr. Food Res. 2018, 62, e1700965. [Google Scholar] [CrossRef]
- Kamal, M.M.; Akter, S.; Lin, C.-N.; Nazzal, S. Sulforaphane as an Anticancer Molecule: Mechanisms of Action, Synergistic Effects, Enhancement of Drug Safety, and Delivery Systems. Arch. Pharm. Res. 2020, 43, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, S.M.; Royce, S.G.; Licciardi, P.V.; Karagiannis, T.C. Dietary Sulforaphane in Cancer Chemoprevention: The Role of Epigenetic Regulation and HDAC Inhibition. Antioxid. Redox Signal. 2015, 22, 1382–1424. [Google Scholar] [CrossRef]
- Wu, G.; Yan, Y.; Zhou, Y.; Duan, Y.; Zeng, S.; Wang, X.; Lin, W.; Ou, C.; Zhou, J.; Xu, Z. Sulforaphane: Expected to Become a Novel Antitumor Compound. Oncol. Res. 2020, 28, 439–446. [Google Scholar] [CrossRef]
- Balachandar, R.; Pullakhandam, R.; Kulkarni, B.; Sachdev, H.S. Relative Efficacy of Vitamin D2 and Vitamin D3 in Improving Vitamin D Status: Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3328. [Google Scholar] [CrossRef]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The Role of Vitamin D in Reducing Cancer Risk and Progression. Nat. Rev. Cancer 2014, 14, 342. [Google Scholar] [CrossRef]
- Ben-Eltriki, M.; Deb, S.; Guns, E.S.T. Calcitriol in Combination Therapy for Prostate Cancer: Pharmacokinetic and Pharmacodynamic Interactions. J. Cancer 2016, 7, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.B.; Nyame, Y.; Martin, I.K.; Catalona, W.J.; Hollowell, C.M.P.; Nadler, R.B.; Kozlowski, J.M.; Perry, K.T.; Kajdacsy-Balla, A.; Kittles, R. Vitamin D Deficiency Predicts Prostate Biopsy Outcomes. Clin. Cancer Res. 2014, 20, 2289–2299. [Google Scholar] [CrossRef]
- Xie, D.-D.; Chen, Y.-H.; Xu, S.; Zhang, C.; Wang, D.-M.; Wang, H.; Chen, L.; Zhang, Z.-H.; Xia, M.-Z.; Xu, D.-X.; et al. Low Vitamin D Status Is Associated with Inflammation in Patients with Prostate Cancer. Oncotarget 2017, 8, 22076–22085. [Google Scholar] [CrossRef]
- Inglis, J.E.; Fernandez, I.D.; van Wijngaarden, E.; Culakova, E.; Reschke, J.E.; Kleckner, A.S.; Lin, P.-J.; Mustian, K.M.; Peppone, L.J. Effects of High-Dose Vitamin D Supplementation on Phase Angle and Physical Function in Patients with Prostate Cancer on ADT. Nutr. Cancer 2021, 73, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Trump, D.L.; Aragon-Ching, J.B. Vitamin D in Prostate Cancer. Asian J. Androl. 2018, 20, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Frederick, A.-L.M.; Jin, Y.; Guo, C.; Xiao, H.; Wood, R.J.; Liu, Z. The Prevention of a High Dose of Vitamin D or Its Combination with Sulforaphane on Intestinal Inflammation and Tumorigenesis in Apc1638N Mice Fed a High-Fat Diet. Mol. Nutr. Food Res. 2019, 63, 1800824. [Google Scholar] [CrossRef]
- Bal-Price, A.; Coecke, S. Guidance on Good Cell Culture Practice (GCCP). In Cell Culture Techniques; Aschner, M., Suñol, C., Bal-Price, A., Eds.; Neuromethods; Humana Press: Totowa, NJ, USA, 2011; pp. 1–25. ISBN 978-1-61779-077-5. [Google Scholar]
- Di Nunzio, M.; Valli, V.; Tomás-Cobos, L.; Tomás-Chisbert, T.; Murgui-Bosch, L.; Danesi, F.; Bordoni, A. Is Cytotoxicity a Determinant of the Different in Vitro and in Vivo Effects of Bioactives? BMC Complement. Altern. Med. 2017, 17, 453. [Google Scholar] [CrossRef] [PubMed]
- Gasper, A.V.; Al-janobi, A.; Smith, J.A.; Bacon, J.R.; Fortun, P.; Atherton, C.; Taylor, M.A.; Hawkey, C.J.; Barrett, D.A.; Mithen, R.F. Glutathione S-Transferase M1 Polymorphism and Metabolism of Sulforaphane from Standard and High-Glucosinolate Broccoli. Am. J. Clin. Nutr. 2005, 82, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M.G.; Trump, D.L.; Muindi, J.R.; Black, J.D.; Bernardi, R.J.; Creaven, P.J.; Schwartz, J.; Brattain, M.G.; Hutson, A.; French, R.; et al. A Phase I Pharmacokinetic and Pharmacodynamic Study of Intravenous Calcitriol in Combination with Oral Gefitinib in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2007, 13, 1216–1223. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Machado, A.R.T.; Tuttis, K.; Santos, P.W.D.S.; Aissa, A.F.; Antunes, L.M.G. Diallyl Disulfide Induces Chemosensitization to Sorafenib, Autophagy, and Cell Cycle Arrest and Inhibits Invasion in Hepatocellular Carcinoma. Pharmaceutics 2022, 14, 2582. [Google Scholar] [CrossRef]
- Tusskorn, O.; Khunluck, T.; Prawan, A.; Senggunprai, L.; Kukongviriyapan, V. Mitochondrial Division Inhibitor-1 Potentiates Cisplatin-Induced Apoptosis via the Mitochondrial Death Pathway in Cholangiocarcinoma Cells. Biomed. Pharmacother. Biomedecine Pharmacother. 2019, 111, 109–118. [Google Scholar] [CrossRef]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.-C.; Sasaki, Y.F. Single Cell Gel/Comet Assay: Guidelines for in Vitro and in Vivo Genetic Toxicology Testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Santos, A.; von Mering, C.; Jensen, L.J.; Bork, P.; Kuhn, M. STITCH 5: Augmenting Protein-Chemical Interaction Networks with Tissue and Affinity Data. Nucleic Acids Res. 2016, 44, D380–D384. [Google Scholar] [CrossRef] [PubMed]
- Foucquier, J.; Guedj, M. Analysis of Drug Combinations: Current Methodological Landscape. Pharmacol. Res. Perspect. 2015, 3, e00149. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Raimondi, M.; Marzagalli, M.; Di Domizio, A.; Limonta, P. Natural Compounds in Prostate Cancer Prevention and Treatment: Mechanisms of Action and Molecular Targets. Cells 2020, 9, 460. [Google Scholar] [CrossRef]
- Rutz, J.; Thaler, S.; Maxeiner, S.; Chun, F.K.-H.; Blaheta, R.A. Sulforaphane Reduces Prostate Cancer Cell Growth and Proliferation In Vitro by Modulating the Cdk-Cyclin Axis and Expression of the CD44 Variants 4, 5, and 7. Int. J. Mol. Sci. 2020, 21, 8724. [Google Scholar] [CrossRef]
- Zhuang, S.-H.; Schwartz, G.G.; Cameron, D.; Burnstein, K.L. Vitamin D Receptor Content and Transcriptional Activity Do Not Fully Predict Antiproliferative Effects of Vitamin D in Human Prostate Cancer Cell Lines. Mol. Cell. Endocrinol. 1997, 126, 83–90. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, G.; Tian, H.; Hu, Y.; Wu, S.; Geng, Y.; Lin, K.; Wu, W. Sulforaphane Metabolites Cause Apoptosis via Microtubule Disruption in Cancer. Endocr. Relat. Cancer 2018, 25, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, P.W.D.S.; Machado, A.R.T.; De Grandis, R.A.; Ribeiro, D.L.; Tuttis, K.; Morselli, M.; Aissa, A.F.; Pellegrini, M.; Antunes, L.M.G. Transcriptome and DNA Methylation Changes Modulated by Sulforaphane Induce Cell Cycle Arrest, Apoptosis, DNA Damage, and Suppression of Proliferation in Human Liver Cancer Cells. Food Chem. Toxicol. 2020, 136, 111047. [Google Scholar] [CrossRef]
- Fraser, M.; Zhao, H.; Luoto, K.R.; Lundin, C.; Coackley, C.; Chan, N.; Joshua, A.M.; Bismar, T.A.; Evans, A.; Helleday, T.; et al. PTEN Deletion in Prostate Cancer Cells Does Not Associate with Loss of RAD51 Function: Implications for Radiotherapy and Chemotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 1015–1027. [Google Scholar] [CrossRef]
- Aggarwal, M.; Saxena, R.; Asif, N.; Sinclair, E.; Tan, J.; Cruz, I.; Berry, D.; Kallakury, B.; Pham, Q.; Wang, T.T.Y.; et al. P53 Mutant-Type in Human Prostate Cancer Cells Determines the Sensitivity to Phenethyl Isothiocyanate Induced Growth Inhibition. J. Exp. Clin. Cancer Res. CR 2019, 38, 307. [Google Scholar] [CrossRef] [PubMed]
- Gano, C.A.; Fatima, S.; Failes, T.W.; Arndt, G.M.; Sajinovic, M.; Mahns, D.; Saedisomeolia, A.; Coorssen, J.R.; Bucci, J.; de Souza, P.; et al. Anti-Cancer Potential of Synergistic Phytochemical Combinations Is Influenced by the Genetic Profile of Prostate Cancer Cell Lines. Front. Nutr. 2023, 10, 1119274. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Hu, J. The Role of JNK in Prostate Cancer Progression and Therapeutic Strategies. Biomed. Pharmacother. 2020, 121, 109679. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-D.; Li, G.; Hu, H.; Jiang, C.; Kang, K.-S.; Lee, Y.-S.; Kim, S.-H.; Lu, J. Involvement of C-Jun N-Terminal Kinase in G2/M Arrest and Caspase-Mediated Apoptosis Induced by Sulforaphane in DU145 Prostate Cancer Cells. Nutr. Cancer 2005, 52, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-M.; Shin, E.-A. Exploring Vitamin D Metabolism and Function in Cancer. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in Cancer Therapy: The Bright Side of the Moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.S.; Seo, Y.R. Understanding of ROS-Inducing Strategy in Anticancer Therapy. Oxid. Med. Cell. Longev. 2019, 2019, 5381692. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shao, R.; Wang, N.; Zhou, N.; Du, K.; Shi, J.; Wang, Y.; Zhao, Z.; Ye, X.; Zhang, X.; et al. Sulforaphane Activates a Lysosome-Dependent Transcriptional Program to Mitigate Oxidative Stress. Autophagy 2021, 17, 872–887. [Google Scholar] [CrossRef]
- Jung, S.; Jeong, H.; Yu, S.-W. Autophagy as a Decisive Process for Cell Death. Exp. Mol. Med. 2020, 52, 921–930. [Google Scholar] [CrossRef]
- Herman-Antosiewicz, A.; Johnson, D.E.; Singh, S.V. Sulforaphane Causes Autophagy to Inhibit Release of Cytochrome C and Apoptosis in Human Prostate Cancer Cells. Cancer Res. 2006, 66, 5828–5835. [Google Scholar] [CrossRef]
- Naumann, P.; Fortunato, F.; Zentgraf, H.; Büchler, M.W.; Herr, I.; Werner, J. Autophagy and Cell Death Signaling Following Dietary Sulforaphane Act Independently of Each Other and Require Oxidative Stress in Pancreatic Cancer. Int. J. Oncol. 2011, 39, 101–109. [Google Scholar] [CrossRef]
- Jo, G.H.; Kim, G.-Y.; Kim, W.-J.; Park, K.Y.; Choi, Y.H. Sulforaphane Induces Apoptosis in T24 Human Urinary Bladder Cancer Cells through a Reactive Oxygen Species-Mediated Mitochondrial Pathway: The Involvement of Endoplasmic Reticulum Stress and the Nrf2 Signaling Pathway. Int. J. Oncol. 2014, 45, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Ferreira de Oliveira, J.M.P.; Remédios, C.; Oliveira, H.; Pinto, P.; Pinho, F.; Pinho, S.; Costa, M.; Santos, C. Sulforaphane Induces DNA Damage and Mitotic Abnormalities in Human Osteosarcoma MG-63 Cells: Correlation with Cell Cycle Arrest and Apoptosis. Nutr. Cancer 2014, 66, 325–334. [Google Scholar] [CrossRef]
- Lewinska, A.; Adamczyk-Grochala, J.; Deregowska, A.; Wnuk, M. Sulforaphane-Induced Cell Cycle Arrest and Senescence Are Accompanied by DNA Hypomethylation and Changes in MicroRNA Profile in Breast Cancer Cells. Theranostics 2017, 7, 3461–3477. [Google Scholar] [CrossRef]
- Hossain, S. Effect of Histone Deacetylase Inhibitors on Vitamin D Dependent Gene Expression in Human Colorectal and Breast Cancers. FASEB J. 2017, 31, 644.7. [Google Scholar]
- Lee, B.H.; Yang, Y.; Liu, Z. Synergistic Mediation of Sulforaphane and Vitamin D on the Wnt-Signaling Pathway. J. Nat. Sci. 2015, 1, 36. [Google Scholar]
- Bulusu, K.C.; Guha, R.; Mason, D.J.; Lewis, R.P.I.; Muratov, E.; Kalantar Motamedi, Y.; Cokol, M.; Bender, A. Modelling of Compound Combination Effects and Applications to Efficacy and Toxicity: State-of-the-Art, Challenges and Perspectives. Drug Discov. Today 2016, 21, 225–238. [Google Scholar] [CrossRef] [PubMed]




| Antibodies | Host | Supplier | CAT Number |
|---|---|---|---|
| Anti-β-Actin | Rabbit | Cell Signaling Technology, (Danvers, MA, USA) | 8457S |
| Anti-Bax | Rabbit | Cell Signaling Technology | 2772S |
| Anti-Bcl-2 | Rabbit | Cell Signaling Technology | 2876S |
| Anti-Caspase-3 | Rabbit | ABclonal (Woburn, MA, USA) | A2156 |
| Anti-Caspase-8 | Rabbit | ABclonal | A0215 |
| Anti-Nrf2 | Rabbit | ABclonal | a1244 |
| Anti-phospho-JNK1/2 | Rabbit | ABclonal | ap0473 |
| HRP Goat Anti-Rabbit IgG | Goat | ABclonal | AS014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuttis, K.; Machado, A.R.T.; Santos, P.W.d.S.; Antunes, L.M.G. Sulforaphane Combined with Vitamin D Induces Cytotoxicity Mediated by Oxidative Stress, DNA Damage, Autophagy, and JNK/MAPK Pathway Modulation in Human Prostate Tumor Cells. Nutrients 2023, 15, 2742. https://doi.org/10.3390/nu15122742
Tuttis K, Machado ART, Santos PWdS, Antunes LMG. Sulforaphane Combined with Vitamin D Induces Cytotoxicity Mediated by Oxidative Stress, DNA Damage, Autophagy, and JNK/MAPK Pathway Modulation in Human Prostate Tumor Cells. Nutrients. 2023; 15(12):2742. https://doi.org/10.3390/nu15122742
Chicago/Turabian StyleTuttis, Katiuska, Ana Rita Thomazela Machado, Patrick Wellington da Silva Santos, and Lusânia Maria Greggi Antunes. 2023. "Sulforaphane Combined with Vitamin D Induces Cytotoxicity Mediated by Oxidative Stress, DNA Damage, Autophagy, and JNK/MAPK Pathway Modulation in Human Prostate Tumor Cells" Nutrients 15, no. 12: 2742. https://doi.org/10.3390/nu15122742
APA StyleTuttis, K., Machado, A. R. T., Santos, P. W. d. S., & Antunes, L. M. G. (2023). Sulforaphane Combined with Vitamin D Induces Cytotoxicity Mediated by Oxidative Stress, DNA Damage, Autophagy, and JNK/MAPK Pathway Modulation in Human Prostate Tumor Cells. Nutrients, 15(12), 2742. https://doi.org/10.3390/nu15122742







