The Joint Effects of Bisphenols and Iodine Exposure on Thyroid during Pregnancy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Urine Collection and BPs Laboratory Measurements
2.3. Assessment of Iodine Nutrition Status
2.4. Thyroid Gland Function Assessment
2.5. EDI and Risk Assessment
2.6. Covariates
2.7. Statistical Analysis
3. Results
3.1. Characteristic of Study Populatio
3.2. Exposure Levels of Urinary BPA and Its Alternatives in Study Participants
3.3. Risk Assessment of Exposure to BPA and Its Alternatives
3.4. Overall Effects of BPs on Thyroid Health
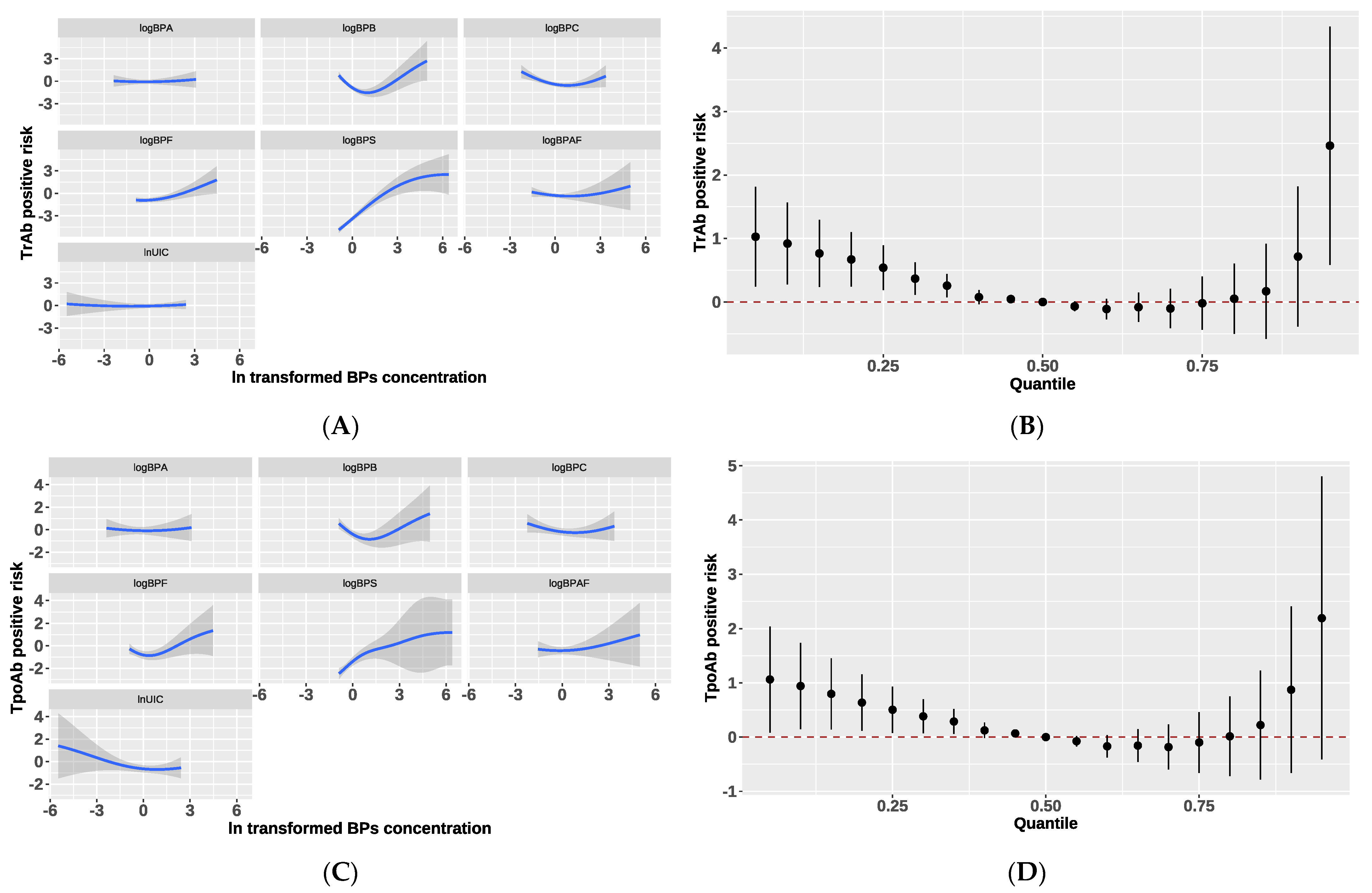
3.5. Association between Each BPs and Thyroid Health
3.6. Interaction Effect between BPs and Iodine on Thyroid Antibodies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.L.; Wu, Y.; Widelka, M. Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity—A Review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef] [PubMed]
- Mustieles, V.; D’Cruz, S.C.; Couderq, S.; Rodríguez-Carrillo, A.; Fini, J.B.; Hofer, T.; Steffensen, I.L.; Dirven, H.; Barouki, R.; Olea, N.; et al. Bisphenol A and its analogues: A comprehensive review to identify and prioritize effect biomarkers for human biomonitoring. Environ. Int. 2020, 144, 105811. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhuang, T.; Shi, W.; Liang, Y.; Liao, C.; Song, M.; Jiang, G. Serum concentration of bisphenol analogues in pregnant women in China. Sci. Total Environ. 2020, 707, 136100. [Google Scholar] [CrossRef]
- Kang, S.; Shin, B.H.; Kwon, J.A.; Lee, C.W.; Park, E.K.; Park, E.Y.; Kim, B. Urinary bisphenol A and its analogues and haemato-biochemical alterations of pregnant women in Korea. Environ. Res. 2020, 182, 109104. [Google Scholar] [CrossRef]
- Bornehag, C.G.; Engdahl, E.; Unenge Hallerbäck, M.; Wikström, S.; Lindh, C.; Rüegg, J.; Tanner, E.; Gennings, C. Prenatal exposure to bisphenols and cognitive function in children at 7 years of age in the Swedish SELMA study. Environ. Int. 2021, 150, 106433. [Google Scholar] [CrossRef] [PubMed]
- Varghese, B.; Jala, A.; Das, P.; Borkar, R.M.; Adela, R. Estimation of parabens and bisphenols in maternal products and urinary concentrations in Indian pregnant women: Daily intake and health risk assessment. Environ. Sci. Pollut. Res. Int. 2022, 29, 21642–21655. [Google Scholar] [CrossRef]
- Eng, L.; Lam, L. Thyroid Function During the Fetal and Neonatal Periods. NeoReviews 2020, 21, e30–e36. [Google Scholar] [CrossRef]
- Kitamura, S.; Suzuki, T.; Sanoh, S.; Kohta, R.; Jinno, N.; Sugihara, K.; Yoshihara, S.; Fujimoto, N.; Watanabe, H.; Ohta, S. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol. Sci. Off. J. Soc. Toxicol. 2005, 84, 249–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevrier, J.; Gunier, R.B.; Bradman, A.; Holland, N.T.; Calafat, A.M.; Eskenazi, B.; Harley, K.G. Maternal urinary bisphenol a during pregnancy and maternal and neonatal thyroid function in the CHAMACOS study. Environ. Health Perspect. 2013, 121, 138–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Tang, N.; Nakayama, S.F.; Fan, P.; Liu, Z.; Zhang, J.; Ouyang, F. Maternal urinary bisphenol A concentration and thyroid hormone levels of Chinese mothers and newborns by maternal body mass index. Environ. Sci. Pollut. Res. Int. 2020, 27, 10939–10949. [Google Scholar] [CrossRef]
- Chen, X.; Wu, C.; Wang, Z.; Wu, C.; Guo, Y.; Zhu, X.; Hu, Y.P.; Shi, Z.; Song, Q.; Cui, X.; et al. Iodine nutrition status and thyroid autoimmunity during pregnancy: A cross-sectional study of 4635 pregnant women. Nutr. J. 2022, 21, 7. [Google Scholar] [CrossRef]
- Nicolucci, C.; Rossi, S.; Menale, C.; del Giudice, E.M.; Perrone, L.; Gallo, P.; Mita, D.G.; Diano, N. A high selective and sensitive liquid chromatography-tandem mass spectrometry method for quantization of BPA urinary levels in children. Anal. Bioanal. Chem. 2013, 405, 9139–9148. [Google Scholar] [CrossRef]
- World Health Organization; United Nations International Children’s Emergency Fund. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers, 2nd ed.; World Health Organization: Genevan, Switzerland, 2001. [Google Scholar]
- Li, Y.; Xu, T.; Mo, Q.; Fu, W.; Yao, C. Thyrotropin receptor antibody: A novel risk indicator for pregnancy loss. Clin. Biochem. 2019, 64, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.P.; Yang, P.; Liu, C.; Chen, P.P.; Deng, Y.L.; Miao, Y.; Luo, Q.; Zhang, M.; Lu, W.Q.; Zeng, Q. Urinary bisphenol A and its alternatives among pregnant women: Predictors and risk assessment. Sci. Total Environ. 2021, 784, 147184. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Nielsen, J.K.; Mørck, T.A.; Hansen, P.W.; Jensen, J.F.; Nielsen, O.; Andersson, A.M.; Knudsen, L.E. Urinary excretion of phthalate metabolites, phenols and parabens in rural and urban Danish mother-child pairs. Int. J. Hyg. Environ. Health 2013, 216, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mustieles, V.; Williams, P.L.; Wylie, B.J.; Souter, I.; Calafat, A.M.; Demokritou, M.; Lee, A.; Vagios, S.; Hauser, R.; et al. Parental preconception exposure to phenol and phthalate mixtures and the risk of preterm birth. Environ. Int. 2021, 151, 106440. [Google Scholar] [CrossRef]
- Bobb, J.F.; Valeri, L.; Claus Henn, B.; Christiani, D.C.; Wright, R.O.; Mazumdar, M.; Godleski, J.J.; Coull, B.A. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2015, 16, 493–508. [Google Scholar] [CrossRef] [Green Version]
- Bobb, J.F.; Claus Henn, B.; Valeri, L.; Coull, B.A. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ. Health Glob. Access Sci. Source 2018, 17, 67. [Google Scholar] [CrossRef] [Green Version]
- Kolatorova, L.; Vitku, J.; Hampl, R.; Adamcova, K.; Skodova, T.; Simkova, M.; Parizek, A.; Starka, L.; Duskova, M. Exposure to bisphenols and parabens during pregnancy and relations to steroid changes. Environ. Res. 2018, 163, 115–122. [Google Scholar] [CrossRef]
- Ao, J.; Huo, X.; Zhang, J.; Mao, Y.; Li, G.; Ye, J.; Shi, Y.; Jin, F.; Bao, S.; Zhang, J. Environmental exposure to bisphenol analogues and unexplained recurrent miscarriage: A case-control study. Environ. Res. 2022, 204, 112293. [Google Scholar] [CrossRef]
- van den Dries, M.A.; Keil, A.P.; Tiemeier, H.; Pronk, A.; Spaan, S.; Santos, S.; Asimakopoulos, A.G.; Kannan, K.; Gaillard, R.; Guxens, M.; et al. Prenatal Exposure to Nonpersistent Chemical Mixtures and Fetal Growth: A Population-Based Study. Environ. Health Perspect. 2021, 129, 117008. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, C.; Zhao, H.; Zhou, Y.; Cao, G.; Yang, Z.; Hong, Y.; Xu, S.; Xia, W.; Cai, Z. Exposure Assessment of Bisphenols in Chinese Women during Pregnancy: A Longitudinal Study. Environ. Sci. Technol. 2019, 53, 7812–7820. [Google Scholar] [CrossRef] [PubMed]
- Jedynak, P.; Rolland, M.; Pin, I.; Thomsen, C.; Sakhi, A.K.; Sabaredzovic, A.; Philippat, C.; Slama, R. Pregnancy Exposure to Phenols and Anthropometric Measures in Gestation and at Birth. Epidemiol. Camb. Mass. 2022, 33, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Miao, M.; Xu, J.; Chen, Y.; Liang, H.; Yang, L.; Liu, X.; Wen, S.; Tu, X.; Yuan, W. Gestational exposure to bisphenol analogues and kisspeptin levels in pregnant women and their children: A pregnancy-birth cohort study. Sci. Total Environ. 2022, 848, 157720. [Google Scholar] [CrossRef]
- Sanchis, Y.; Coscollà, C.; Corpas-Burgos, F.; Vento, M.; Gormaz, M.; Yusà, V. Biomonitoring of bisphenols A, F, S and parabens in urine of breastfeeding mothers: Exposure and risk assessment. Environ. Res. 2020, 185, 109481. [Google Scholar] [CrossRef]
- Lucarini, F.; Krasniqi, T.; Bailat Rosset, G.; Roth, N.; Hopf, N.B.; Broillet, M.C.; Staedler, D. Exposure to New Emerging Bisphenols among Young Children in Switzerland. Int. J. Environ. Res. Public Health 2020, 17, 4793. [Google Scholar] [CrossRef]
- Milczarek-Banach, J.; Rachoń, D.; Bednarczuk, T.; Myśliwiec-Czajka, K.; Wasik, A.; Miśkiewicz, P. Exposure to Bisphenol A Analogs and the Thyroid Function and Volume in Women of Reproductive Age-Cross-Sectional Study. Front. Endocrinol. 2020, 11, 587252. [Google Scholar] [CrossRef]
- Lambré, C.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; Mengelers, M.; et al. Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. Eur. Food Saf. Auth. 2023, 21, e06857. [Google Scholar] [CrossRef]
- Huang, H.; Liang, J.; Tang, P.; Yu, C.; Fan, H.; Liao, Q.; Long, J.; Pan, D.; Zeng, X.; Liu, S.; et al. Associations of bisphenol exposure with thyroid hormones in pregnant women: A prospective birth cohort study in China. Environ. Sci. Pollut. Res. Int. 2022, 29, 87170–87183. [Google Scholar] [CrossRef]
- Moleti, M.; Di Mauro, M.; Sturniolo, G.; Russo, M.; Vermiglio, F. Hyperthyroidism in the pregnant woman: Maternal and fetal aspects. J. Clin. Transl. Endocrinol. 2019, 16, 100190. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, Z.; Liu, Q.; Chen, L. Adverse effects of bisphenol B exposure on the thyroid and nervous system in early life stages of zebrafish. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2021, 250, 109167. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Zhao, F.; Zhang, X.; Liu, W.; Jiang, G.; Wang, H.; Ru, S. Transgenerational thyroid endocrine disruption induced by bisphenol S affects the early development of zebrafish offspring. Environ. Pollut. Barking Essex 2018, 243 (Pt B), 800–808. [Google Scholar] [CrossRef]
- Jang, Y.; Choi, Y.J.; Lim, Y.H.; Lee, K.S.; Kim, B.N.; Shin, C.H.; Lee, Y.A.; Kim, J.I.; Hong, Y.C. Associations between Thyroid Hormone Levels and Urinary Concentrations of Bisphenol A, F, and S in 6-Year-old Children in Korea. J. Prev. Med. Public Health Yebang Uihakhoe Chi 2021, 54, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Aker, A.M.; Ferguson, K.K.; Rosario, Z.Y.; Mukherjee, B.; Alshawabkeh, A.N.; Calafat, A.M.; Cordero, J.F.; Meeker, J.D. A repeated measures study of phenol, paraben and Triclocarban urinary biomarkers and circulating maternal hormones during gestation in the Puerto Rico PROTECT cohort. Environ. Health Glob. Access Sci. Source 2019, 18, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonavane, M.; Gassman, N.R. Bisphenol A co-exposure effects: A key factor in understanding BPA’s complex mechanism and health outcomes. Crit. Rev. Toxicol. 2019, 49, 371–386. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, G.; Xing, Y.; Wang, C.; Yuan, X.; Li, B. Evaluation of single and combined toxicity of bisphenol A and its analogues using a highly-sensitive micro-biosensor. J. Hazard. Mater. 2020, 381, 120908. [Google Scholar] [CrossRef]
- Huang, Y.F.; Wang, P.W.; Huang, L.W.; Lin, M.H.; Yang, W.; Chen, H.C.; Yu, K.P.; Chen, M.L. Interactive effects of nonylphenol and bisphenol A exposure with oxidative stress on fetal reproductive indices. Environ. Res. 2018, 167, 567–574. [Google Scholar] [CrossRef]
- Sarzo, B.; Abumallouh, R.; Marín, N.; Llop, S.; Beneito, A.; Lopez-Flores, I.; Ferrero, N.; Sakhi, A.K.; Ballester, F.; Lopez-Espinosa, M.J. Association between phenols and thyroid hormones: The role of iodothyronine deiodinase genes. Environ. Pollut. Barking Essex 2022, 311, 119926. [Google Scholar] [CrossRef]
- Chen, P.; Wang, R.; Chen, G.; An, B.; Liu, M.; Wang, Q.; Tao, Y. Thyroid endocrine disruption and hepatotoxicity induced by bisphenol AF: Integrated zebrafish embryotoxicity test and deep learning. Sci. Total Environ. 2022, 822, 153639. [Google Scholar] [CrossRef]
- Wu, X.; Yang, X.; Geng, X.; Ji, X.; Zhang, X.; Yue, H.; Li, G.; Sang, N. Bisphenol A Analogs Induce Cellular Dysfunction in Human Trophoblast Cells in a Thyroid Hormone Receptor-Dependent Manner: In Silico and In Vitro Analyses. Environ. Sci. Technol. 2022, 56, 8384–8394. [Google Scholar] [CrossRef]

| BPs | Percent Detection | Mean (95% CI) | Selected Percentiles | Geometric Mean (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| 10% | 25% | 50% | 75% | 90% | ||||
| Unadjusted (ng/mL) | ||||||||
| BPA | 90.36 | 2.35 (1.88, 2.82) | 0.63 | 0.81 | 1.30 | 2.08 | 3.65 | 1.44 (1.34, 1.53) |
| BPB | 77.29 | 0.32 (0.26, 0.37) | 0.02 | 0.04 | 0.10 | 0.36 | 0.85 | 0.11 (0.10, 0.13) |
| BPC | 86.00 | 2.08 (1.86, 2.29) | 0.34 | 0.62 | 1.24 | 2.42 | 4.86 | 1.27 (1.18, 1.39) |
| BPF | 79.01 | 0.77 (0.62, 0.92) | 0.03 | 0.05 | 0.13 | 0.49 | 2.23 | 0.19 (0.16, 0.21) |
| BPS | 84.76 | 0.31 (0.24, 0.39) | 0.04 | 0.06 | 0.11 | 0.26 | 0.65 | 0.14 (0.13, 0.15) |
| BPAF | 79.94 | 0.28 (0.27, 0.29) | 0.20 | 0.22 | 0.26 | 0.33 | 0.40 | 0.27 (0.26, 0.28) |
| ΣBPs | - | 5.29 (4.75, 5.83) | 1.15 | 1.94 | 3.56 | 6.37 | 10.78 | 3.41 (3.15, 3.68) |
| Creatinine-adjusted (μg/g creatinine) | ||||||||
| BPA | - | 8.20 (3.52, 12.88) | 0.99 | 1.52 | 2.64 | 5.19 | 10.42 | 3.03 (2.78, 3.30) |
| BPB | - | 0.73 (0.50, 0.97) | 0.04 | 0.07 | 0.22 | 0.79 | 1.69 | 0.24 (0.21, 0.27) |
| BPC | - | 6.52 (2.75, 10.28) | 0.74 | 1.24 | 2.67 | 5.06 | 10.08 | 2.66 (2.43, 2.92) |
| BPF | - | 1.57 (1.18, 1.97) | 0.06 | 0.12 | 0.25 | 1.01 | 4.64 | 0.36 (0.31, 0.42) |
| BPS | - | 0.76 (0.48, 1.04) | 0.06 | 0.11 | 0.24 | 0.55 | 1.32 | 0.26 (0.24, 0.29) |
| BPAF | - | 1.52 (0.91, 2.12) | 0.22 | 0.37 | 0.61 | 1.25 | 2.72 | 0.72 (0.65, 0.78) |
| ΣBPs | - | 16.72 (8.28, 25.17) | 2.53 | 4.14 | 7.88 | 14.37 | 25.17 | 7.55 (6.94, 8.21) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, W.; Sun, Z.; Wang, Z.; Qu, M.; Shi, Z.; Song, Q.; Shen, L.; Mai, S.; Wang, Y.; Hong, X.; et al. The Joint Effects of Bisphenols and Iodine Exposure on Thyroid during Pregnancy. Nutrients 2023, 15, 3422. https://doi.org/10.3390/nu15153422
Lu W, Sun Z, Wang Z, Qu M, Shi Z, Song Q, Shen L, Mai S, Wang Y, Hong X, et al. The Joint Effects of Bisphenols and Iodine Exposure on Thyroid during Pregnancy. Nutrients. 2023; 15(15):3422. https://doi.org/10.3390/nu15153422
Chicago/Turabian StyleLu, Wei, Zhuo Sun, Zhengyuan Wang, Mengying Qu, Zehuan Shi, Qi Song, Liping Shen, Shupeng Mai, Yuan Wang, Xinyu Hong, and et al. 2023. "The Joint Effects of Bisphenols and Iodine Exposure on Thyroid during Pregnancy" Nutrients 15, no. 15: 3422. https://doi.org/10.3390/nu15153422
APA StyleLu, W., Sun, Z., Wang, Z., Qu, M., Shi, Z., Song, Q., Shen, L., Mai, S., Wang, Y., Hong, X., & Zang, J. (2023). The Joint Effects of Bisphenols and Iodine Exposure on Thyroid during Pregnancy. Nutrients, 15(15), 3422. https://doi.org/10.3390/nu15153422






