The Association of Vitamin D during Pregnancy and mRNA Expression Levels of Inflammatory Factors with Preterm Birth and Prelabor Rupture of Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Information and Sample Collection
2.2.1. Cohort Study
2.2.2. Case-Control Study
2.3. Measurement of 25(OH)D Concentrations
2.4. Measurement of mRNA Expression Levels of Inflammasome Related Molecules
2.5. Variable Definition
2.6. Statistical Analysis
3. Results
3.1. Cohort Study
3.1.1. Characteristics of Study Population
3.1.2. The Association between Maternal Vitamin D Level and PTB or PROM
3.2. Case-Control Study
3.2.1. Characteristics of Study Population
3.2.2. The Association between Maternal Vitamin D during Delivery and PPROM
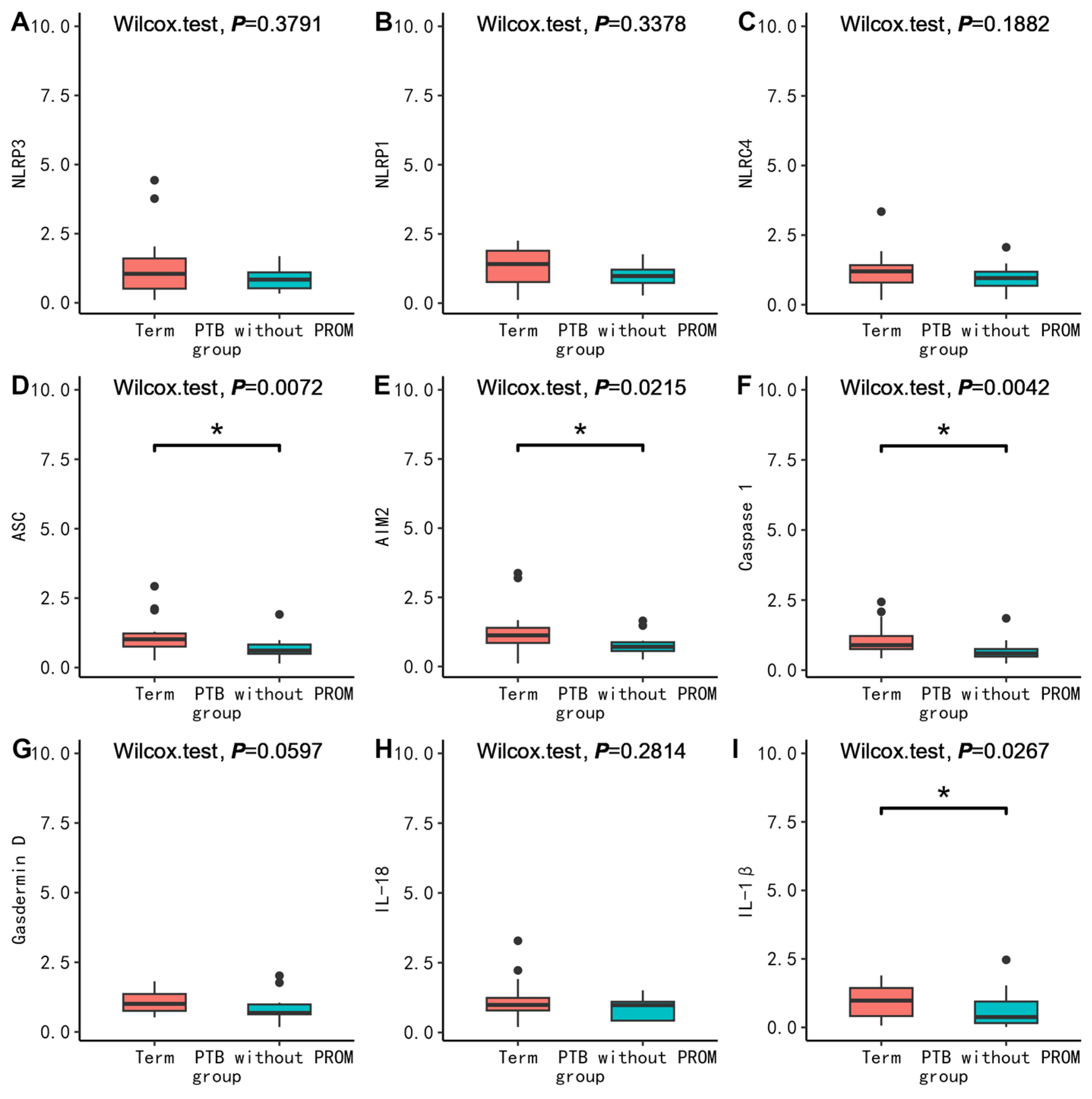
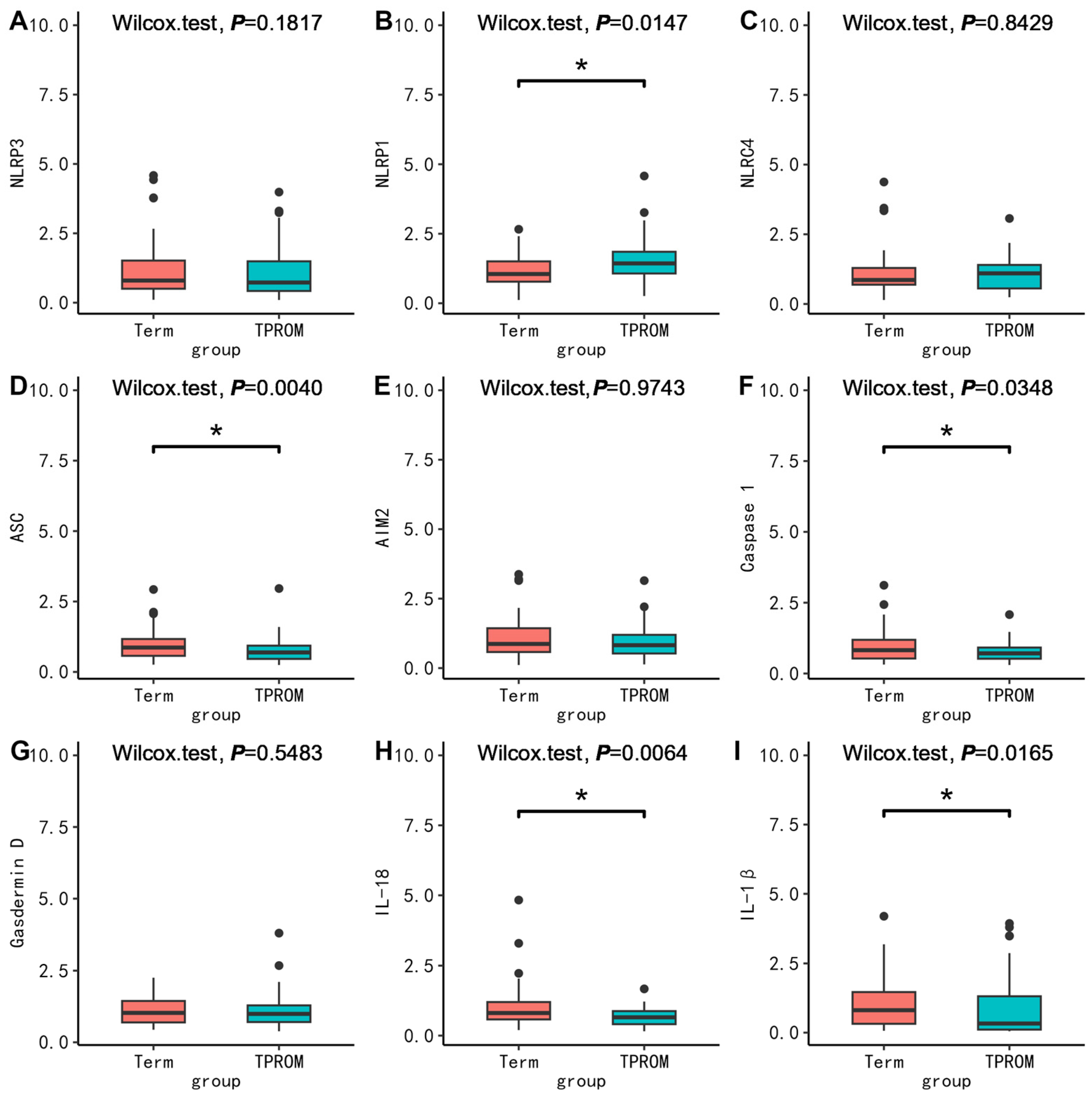
3.2.3. Comparison of mRNA Expression of Inflammatory Factors in PBMC, Placenta, and Fetal Membrane between Cases and Corresponding Controls between Groups
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Preterm Birth. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed on 1 January 2023).
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.-B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, e37–e46. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, J.W.; Xia, H.W.; Zhang, H.X.; Betran, A.P.; Zhang, L.; Hua, X.L.; Feng, L.P.; Chen, D.; Sun, K.; et al. Preterm Birth in China Between 2015 and 2016. Am. J. Public Health 2019, 109, 1597–1604. [Google Scholar] [CrossRef]
- Yang, L.; Xie, G.; Yang, W.; Wang, R.; Zhang, B.; Xu, M.; Sun, L.; Xu, X.; Xiang, W.; Cui, X.; et al. Short-term effects of air pollution exposure on the risk of preterm birth in Xi’an, China. Ann. Med. 2023, 55, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xin, X.; Luo, W.; Mo, M.; Si, S.; Shao, B.; Shen, Y.; Cheng, H.; Yu, Y. Association of vitamin D and gene variants in the vitamin D metabolic pathway with preterm birth. Nutrition 2021, 89, 111349. [Google Scholar] [CrossRef] [PubMed]
- Global, Regional, and National Causes of under-5 Mortality in 2000–19: An Updated Systematic Analysis with Implications for the Sustainable Development Goals. Available online: https://pubmed.ncbi.nlm.nih.gov/34800370/ (accessed on 1 June 2023).
- American College of Obstetricians and Gynecologists. Prelabor Rupture of Membranes: ACOG Practice Bulletin, Number 217. Obstet. Gynecol. 2020, 135, e80–e97. [Google Scholar] [CrossRef]
- Clark, E.A.S.; Varner, M. Impact of preterm PROM and its complications on long-term infant outcomes. Clin. Obstet. Gynecol. 2011, 54, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Tian, Y.; Zheng, H.; Shan, S.; Zhao, X.; Liu, C. Maternal exposure to ambient fine particulate matter and risk of premature rupture of membranes in Wuhan, Central China: A cohort study. Environ. Health Glob. Access Sci. Source 2019, 18, 96. [Google Scholar] [CrossRef]
- Huang, S.; Xia, W.; Sheng, X.; Qiu, L.; Zhang, B.; Chen, T.; Xu, S.; Li, Y. Maternal lead exposure and premature rupture of membranes: A birth cohort study in China. BMJ Open 2018, 8, e021565. [Google Scholar] [CrossRef]
- Liu, J.; Feng, Z.C.; Wu, J. The Incidence Rate of Premature Rupture of Membranes and its Influence on Fetal-Neonatal Health: A report from mainland China. J. Trop. Pediatr. 2010, 56, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xiao, Y.; Zhang, L.; Gao, Q. Maternal early pregnancy vitamin D status in relation to low birth weight and small-for-gestational-age offspring. J. Steroid Biochem. Mol. Biol. 2018, 175, 146–150. [Google Scholar] [CrossRef]
- Haugen, M.; Brantsaeter, A.L.; Trogstad, L.; Alexander, J.; Roth, C.; Magnus, P.; Meltzer, H.M. Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiol. Camb. Mass. 2009, 20, 720–726. [Google Scholar] [CrossRef]
- Lipinska, K.; Malone, K.E.; Moerland, M.; Kluft, C. Applying caspase-1 inhibitors for inflammasome assays in human whole blood. J. Immunol. Methods 2014, 411, 66–69. [Google Scholar] [CrossRef]
- Koga, K.; Izumi, G.; Mor, G.; Fujii, T.; Osuga, Y. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy complications. Am. J. Reprod. Immunol. 2014, 72, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Lindström, T.M.; Bennett, P.R. The role of nuclear factor kappa B in human labour. Reproduction 2005, 130, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Colotta, F.; Jansson, B.; Bonelli, F. Modulation of inflammatory and immune responses by vitamin D. J. Autoimmun. 2017, 85, 78–97. [Google Scholar] [CrossRef] [PubMed]
- Waiden, J.; Heydarian, M.; Oak, P.; Koschlig, M.; Kamgari, N.; Hagemann, M.; Wjst, M.; Hilgendorff, A. Prenatal vitamin D supplementation mitigates inflammation-related alveolar remodeling in neonatal mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 325, L95–L103. [Google Scholar] [CrossRef]
- Thota, C.; Laknaur, A.; Farmer, T.; Ladson, G.; Al-Hendy, A.; Ismail, N. Vitamin D regulates contractile profile in human uterine myometrial cells via NF-κB pathway. Am. J. Obstet. Gynecol. 2014, 210, 347.e1–347.e10. [Google Scholar] [CrossRef]
- Shao, B.; Mo, M.; Xin, X.; Jiang, W.; Wu, J.; Huang, M.; Wang, S.; Muyiduli, X.; Si, S.; Shen, Y.; et al. The interaction between prepregnancy BMI and gestational vitamin D deficiency on the risk of gestational diabetes mellitus subtypes with elevated fasting blood glucose. Clin. Nutr. 2020, 39, 2265–2273. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Zhao, R.; Zhou, L.; Wang, S.; Yin, H.; Yang, X.; Hao, L. Effect of maternal vitamin D status on risk of adverse birth outcomes: A systematic review and dose–response meta-analysis of observational studies. Eur. J. Nutr. 2022, 61, 2881–2907. [Google Scholar] [CrossRef]
- Tahsin, T.; Khanam, R.; Chowdhury, N.H.; Hasan, A.T.; Hosen, M.B.; Rahman, S.; Roy, A.K.; Ahmed, S.; Raqib, R.; Baqui, A.H. Vitamin D deficiency in pregnancy and the risk of preterm birth: A nested case-control study. BMC Pregnancy Childbirth 2023, 23, 322. [Google Scholar] [CrossRef]
- Liu, C.C.; Huang, J.P. Potential benefits of vitamin D supplementation on pregnancy. J. Formos. Med. Assoc. Taiwan Yi Zhi 2023, 122, 557–563. [Google Scholar] [CrossRef]
- Fisher, M.; Marro, L.; Arbuckle, T.E.; Potter, B.K.; Little, J.; Weiler, H.; Morisset, A.-S.; Lanphear, B.; Oulhote, Y.; Braun, J.M.; et al. Association between toxic metals, vitamin D and preterm birth in the Maternal–Infant research on environmental chemicals study. Paediatr. Perinat. Epidemiol. 2023, 37, 447–457. [Google Scholar] [CrossRef]
- Brannon, P.M.; Picciano, M.F. Vitamin D in pregnancy and lactation in humans. Annu. Rev. Nutr. 2011, 31, 89–115. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Van Assche, F.A.; Van Baelen, H.; Heyns, W.; De Moor, P. Influence of the vitamin D-binding protein on the serum concentration of 1,25-dihydroxyvitamin D3. Significance of the free 1,25-dihydroxyvitamin D3 concentration. J. Clin. Investig. 1981, 67, 589–596. [Google Scholar] [CrossRef]
- Vivanti, A.J.; Monier, I.; Salakos, E.; Elie, C.; Tsatsaris, V.; Senat, M.-V.; Jani, J.; Jouannic, J.-M.; Winer, N.; Zeitlin, J.; et al. Vitamin D and pregnancy outcomes: Overall results of the FEPED study. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101883. [Google Scholar] [CrossRef]
- Zhou, J.; Su, L.; Liu, M.; Liu, Y.; Cao, X.; Wang, Z.; Xiao, H. Associations between 25-hydroxyvitamin D levels and pregnancy outcomes: A prospective observational study in southern China. Eur. J. Clin. Nutr. 2014, 68, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, R.P.; Thorp, J.M. Vitamin D in pregnancy: Current concepts. Curr. Opin. Obstet. Gynecol. 2012, 24, 57–64. [Google Scholar] [CrossRef]
- Kook, S.Y.; Park, K.H.; Jang, J.A.; Kim, Y.M.; Park, H.; Jeon, S.J. Vitamin D-binding protein in cervicovaginal fluid as a non-invasive predictor of intra-amniotic infection and impending preterm delivery in women with preterm labor or preterm premature rupture of membranes. PLoS ONE 2018, 13, e0198842. [Google Scholar] [CrossRef] [PubMed]
- Kalagiri, R.R.; Carder, T.; Choudhury, S.; Vora, N.; Ballard, A.R.; Govande, V.; Drever, N.; Beeram, M.R.; Uddin, M.N. Inflammation in Complicated Pregnancy and Its Outcome. Am. J. Perinatol. 2016, 33, 1337–1356. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Andrews, W.W.; Mercer, B.M.; Moawad, A.H.; Meis, P.J.; Iams, J.D.; Das, A.; Caritis, S.N.; Roberts, J.M.; Miodovnik, M.; et al. The preterm prediction study: Granulocyte colony-stimulating factor and spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am. J. Obstet. Gynecol. 2000, 182, 625–630. [Google Scholar] [CrossRef]
- Vogel, I.; Goepfert, A.R.; Thorsen, P.; Skogstrand, K.; Hougaard, D.M.; Curry, A.H.; Cliver, S.; Andrews, W.W. Early second-trimester inflammatory markers and short cervical length and the risk of recurrent preterm birth. J. Reprod. Immunol. 2007, 75, 133–140. [Google Scholar] [CrossRef]
- Shirasuna, K.; Takano, H.; Seno, K.; Ohtsu, A.; Karasawa, T.; Takahashi, M.; Ohkuchi, A.; Suzuki, H.; Matsubara, S.; Iwata, H.; et al. Palmitic acid induces interleukin-1β secretion via NLRP3 inflammasomes and inflammatory responses through ROS production in human placental cells. J. Reprod. Immunol. 2016, 116, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Mulla, M.J.; Myrtolli, K.; Potter, J.; Boeras, C.; Kavathas, P.B.; Sfakianaki, A.K.; Tadesse, S.; Norwitz, E.R.; Guller, S.; Abrahams, V.M. Uric acid induces trophoblast IL-1β production via the inflammasome: Implications for the pathogenesis of preeclampsia. Am. J. Reprod. Immunol. 2011, 65, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Faro, J.; Romero, R.; Schwenkel, G.; Garcia-Flores, V.; Arenas-Hernandez, M.; Leng, Y.; Xu, Y.; Miller, D.; Hassan, S.S.; Gomez-Lopez, N. Intra-amniotic inflammation induces preterm birth by activating the NLRP3 inflammasome. Biol. Reprod. 2019, 100, 1290–1305. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Romero, R.; Garcia-Flores, V.; Leng, Y.; Miller, D.; Hassan, S.S.; Hsu, C.-D.; Panaitescu, B. Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth, and adverse neonatal outcomes†. Biol. Reprod. 2019, 100, 1306–1318. [Google Scholar] [CrossRef]
- Zhu, J.; Ma, C.; Luan, X.; Li, J.; Peng, F.; Huang, L. Inflammasome components and ADAMTS4 in premature rupture of membranes. Mol. Med. Rep. 2021, 23, 101. [Google Scholar] [CrossRef]
- Ida, A.; Tsuji, Y.; Muranaka, J.; Kanazawa, R.; Nakata, Y.; Adachi, S.; Okamura, H.; Koyama, K. IL-18 in pregnancy; the elevation of IL-18 in maternal peripheral blood during labour and complicated pregnancies. J. Reprod. Immunol. 2000, 47, 65–74. [Google Scholar] [CrossRef]
- Kyathanahalli, C.; Snedden, M.; Hirsch, E. Is human labor at term an inflammatory condition? Biol. Reprod. 2022, 108, 23–40. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Z.J.; Myatt, L.; Sun, K. 11β-HSD1 in Human Fetal Membranes as a Potential Therapeutic Target for Preterm Birth. Endocr. Rev. 2018, 39, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Biran, V.; Decobert, F.; Bednarek, N.; Boizeau, P.; Benoist, J.F.; Claustrat, B.; Barré, J.; Colella, M.; Frérot, A.; Garnotel, R.; et al. Melatonin Levels in Preterm and Term Infants and Their Mothers. Int. J. Mol. Sci. 2019, 20, 2077. [Google Scholar] [CrossRef] [PubMed]
- Rashid, C.S.; Preston, J.D.; Tenlep, S.Y.N.; Cook, M.K.; Blalock, E.M.; Zhou, C.; Swanson, H.I.; Pearson, K.J. PCB126 exposure during pregnancy alters maternal and fetal gene expression. Reprod. Toxicol. 2023, 119, 108385. [Google Scholar] [CrossRef] [PubMed]
- Christofi, C.; Lamnis, L.; Stark, A.; Palm, H.; Römer, K.; Vogt, T.; Reichrath, J. Cross-talk of Aryl Hydrocarbon Receptor (AHR)- and Vitamin D Receptor (VDR)-signaling in Human Keratinocytes. Anticancer. Res. 2022, 42, 5049–5067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, S.; Wu, Y.; Chen, D.; Liang, Z. Association between maternal second-trimester stress and adverse pregnancy outcomes according to pre-pregnancy body mass index and gestational weight gain. Front. Psychiatry 2023, 14, 1129014. [Google Scholar] [CrossRef]

| Variable | Term (n = 5274) | TPROM (n = 798) | Preterm Birth without PROM (n = 237) | PPROM (n = 72) | p |
|---|---|---|---|---|---|
| Mean ± SD | |||||
| Age, year | 29.31 ± 4.01 | 29.26 ± 4.04 | 30.08 ± 4.05 | 29.97 ± 4.76 | 0.016 |
| BMI at first visit, kg/m2 | 21.24 ± 2.91 | 21.37 ± 2.93 | 21.95 ± 3.16 | 21.94 ± 3.18 | 0.001 |
| Weight gain, kg | 12.83 ± 3.82 | 12.43 ± 3.83 | 11.43 ± 3.65 | 11.02 ± 3.17 | <0.001 |
| N (%) | |||||
| Education | 0.025 | ||||
| Primary school and below | 783 (14.85) | 93 (11.65) | 30 (12.66) | 11 (15.28) | |
| High school | 485 9.20) | 73 (9.15) | 28 (11.81) | 8 (11.11) | |
| College and above | 3429 (65.02) | 512 (64.16) | 150 (63.29) | 43 (59.72) | |
| Unknown | 577 (10.94) | 120 (15.04) | 29 (12.24) | 10 (13.89) | |
| Parity | 0.011 | ||||
| 0 | 3761 (71.31) | 612 (76.69) | 173 (73.00) | 54 (75.00) | |
| ≥1 | 1513 (28.69) | 186 (23.31) | 64 (27.00) | 18 (25.00) | |
| DM * | 0.195 | ||||
| No | 4435 (84.17) | 657 (82.33) | 196 (83.76) | 55 (76.39) | |
| Yes | 834 (15.83) | 141 (17.67) | 38 (16.24) | 17 (23.61) | |
| HDP † | <0.001 | ||||
| No | 5023 (95.33) | 776 (97.24) | 205 (87.61) | 71 (98.61) | |
| Yes | 246 (4.67) | 22 (2.76) | 29 (12.39) | 1 (1.39) | |
| Delivery mode | <0.001 | ||||
| Vaginal delivery | 2929 (55.54) | 600 (75.19) | 88 (37.13) | 47 (65.28) | |
| Caesarean section | 2345 (44.46) | 198 (24.81) | 149 (62.87) | 25 (34.72) | |
| Variable | Term | TPROM | Preterm Birth without PROM | PPROM | p |
|---|---|---|---|---|---|
| Mean ± SD | |||||
| 25(OH)D, ng/mL | |||||
| T1 | 17.85 ± 8.10 | 18.01 ± 8.28 | 17.97 ± 7.70 | 19.36 ± 9.33 | 0.508 |
| T2 | 28.00 ± 11.08 | 28.24 ± 10.90 | 28.91 ± 10.85 | 28.01 ± 11.17 | 0.859 |
| T3 | 29.11 ± 12.25 | 30.50 ± 11.03 | 25.96 ± 12.15 | 26.16 ± 11.31 | 0.031 |
| Gestational age of Vitamin D detection, week | |||||
| T1 | 11.92 ± 0.92 | 11.96 ± 0.80 | 11.83 ± 0.96 | 12.06 ± 0.75 | 0.197 |
| T2 | 24.12 ± 3.42 | 24.02 ± 3.28 | 23.58 ± 3.58 | 24.23 ± 2.67 | 0.442 |
| T3 | 33.65 ± 3.72 | 34.47 ± 3.33 | 31.86 ± 3.65 | 32.52 ± 3.89 | <0.001 |
| N (%) | |||||
| 25(OH)D, ng/mL | |||||
| T1 | 0.575 | ||||
| ≥20 | 1427 (34.16) | 223 (36.44) | 72 (34.29) | 25 (39.68) | |
| <20 | 2750 (65.84) | 389 (63.56) | 138 (65.71) | 38 (60.32) | |
| T2 | 0.742 | ||||
| ≥20 | 1910 (75.05) | 338 (74.61) | 71 (73.96) | 29 (82.86) | |
| <20 | 635 (24.95) | 115 (25.39) | 25 (26.04) | 6 (17.14) | |
| T3 | 0.037 | ||||
| ≥20 | 1964 (73.89) | 322 (78.73) | 27 (61.36) | 9 (69.23) | |
| <20 | 694 (26.11) | 87 (21.27) | 17 (38.64) | 4 (30.77) | |
| Detection season of Vitamin D | |||||
| T1 | 0.329 | ||||
| Summer and autumn | 2062 (49.37) | 313 (51.14) | 113 (53.81) | 36 (57.14) | |
| Winter and spring | 2115 (50.63) | 299 (48.86) | 97 (46.19) | 27 (42.86) | |
| T2 | 0.326 | ||||
| Summer and autumn | 1329 (52.22) | 247 (54.53) | 47 (48.96) | 14 (40.00) | |
| Winter and spring | 1216 (47.78) | 206 (45.47) | 49 (51.04) | 21 (60.00) | |
| T3 | 0.360 | ||||
| Summer and autumn | 1293 (48.65) | 218 (53.30) | 21 (47.73) | 6 (46.15) | |
| Winter and spring | 1365 (51.35) | 191 (46.70) | 23 (52.27) | 7 (53.85) | |
| 25(OH)D, ng/mL | Term | TPROM | Preterm Birth without PROM | PPROM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | OR (95% CI) | p | N (%) | OR (95% CI) | p | N (%) | OR (95% CI) | p | |
| Model 1 a | ||||||||||
| T1 | 4177 | 612 | 1.00 (0.99–1.01) | 0.6539 | 210 | 1.00 (0.98–1.02) | 0.8344 | 63 | 1.02 (0.99–1.05) | 0.1422 |
| ≥20 | 1427 (81.7) | 223 (12.8) | ref. | - | 72 (4.1) | ref. | - | 25 (1.4) | ref. | - |
| <20 | 2750 (83.0) | 389 (11.7) | 0.91 (0.76–1.08) | 0.2688 | 138 (4.2) | 0.99 (0.74–1.33) | 0.9694 | 38 (1.1) | 0.79 (0.47–1.31) | 0.3594 |
| T2 | 2545 | 453 | 1.00 (0.99–1.01) | 0.6715 | 96 | 1.01 (0.99–1.03) | 0.4281 | 35 | 1.00 (0.97–1.03) | 0.9986 |
| ≥20 | 1910 (81.3) | 338 (14.4) | ref. | - | 71 (3.0) | ref. | - | 29 (1.2) | ref. | - |
| <20 | 635 (81.3) | 115 (14.7) | 1.02 (0.81–1.29) | 0.8437 | 25 (3.2) | 1.06 (0.67–1.69) | 0.8086 | 6 (0.8) | 0.62 (0.26–1.51) | 0.2928 |
| T3 | 2658 | 409 | 1.01 (1.00–1.02) | 0.0307 | 44 | 0.98 (0.95–1.00) | 0.0870 | 13 | 0.98 (0.93–1.03) | 0.3797 |
| ≥20 | 1964 (84.6) | 322 (13.9) | ref. | - | 27 (1.2) | ref. | - | 9 (0.4) | ref. | - |
| <20 | 694 (86.5) | 87 (10.8) | 0.76 (0.59–0.98) | 0.0370 | 17 (2.1) | 1.78 (0.97–3.29) | 0.0648 | 4 (0.5) | 1.26 (0.39–4.10) | 0.7036 |
| Model 2 b | ||||||||||
| T1 | 4177 | 612 | 1.01 (0.99–1.02) | 0.3456 | 210 | 1.00 (0.98–1.02) | 0.9850 | 63 | 1.02 (0.99–1.05) | 0.1395 |
| ≥20 | 1427 (81.7) | 223 (12.8) | ref. | - | 72 (4.1) | ref. | - | 25(1.4) | ref. | - |
| <20 | 2750 (83.0) | 389 (11.7) | 0.87 (0.73–1.05) | 0.1418 | 138 (4.2) | 1.04 (0.77–1.40) | 0.8077 | 38 (1.1) | 0.79 (0.47–1.33) | 0.3786 |
| T2 | 2545 | 453 | 1.00 (0.99–1.01) | 0.6751 | 96 | 1.01 (0.99–1.03) | 0.2398 | 35 | 1.00 (0.97–1.04) | 0.8332 |
| ≥20 | 1910 (81.3) | 338 (14.4) | ref. | - | 71 (3.0) | ref. | - | 29 (1.2) | ref. | - |
| <20 | 635 (81.3) | 115 (14.7) | 1.02 (0.81–1.29) | 0.8353 | 25 (3.2) | 1.03 (0.64–1.65) | 0.9134 | 6 (0.8) | 0.59 (0.24–1.44) | 0.2455 |
| T3 | 2658 | 409 | 1.01 (1.00–1.02) | 0.0310 | 44 | 0.98 (0.95–1.00) | 0.0806 | 13 | 0.98 (0.94–1.03) | 0.4860 |
| ≥20 | 1964 (84.6) | 322 (13.9) | ref. | - | 27 (1.2) | ref. | - | 9 (0.4) | ref. | - |
| <20 | 694 (86.5) | 87 (10.8) | 0.76 (0.59–0.98) | 0.0373 | 17 (2.1) | 1.90 (1.02–3.55) | 0.0446 | 4 (0.5) | 1.07 (0.33–3.51) | 0.9109 |
| Model 3 c | ||||||||||
| T1 | 4177 | 612 | 1.00 (0.99–1.02) | 0.3690 | 210 | 1.00 (0.98–1.02) | 0.7261 | 63 | 1.02 (0.99–1.05) | 0.2149 |
| ≥20 | 1427 (81.7) | 223 (12.8) | ref. | - | 72 (4.1) | ref. | - | 25 (1.4) | ref. | - |
| <20 | 2750 (83.0) | 389 (11.7) | 0.89 (0.74–1.07) | 0.2022 | 138 (4.2) | 1.08 (0.80–1.47) | 0.6148 | 38 (1.1) | 0.85 (0.50–1.44) | 0.5509 |
| T2 | 2545 | 453 | 1.00 (0.99–1.01) | 0.7113 | 96 | 1.02 (1.00–1.04) | 0.0882 | 35 | 1.01 (0.97–1.04) | 0.7179 |
| ≥20 | 1910 (81.3) | 338 (14.4) | ref. | - | 71 (3.0) | ref. | - | 29 (1.2) | ref. | - |
| <20 | 635 (81.3) | 115 (14.7) | 1.05 (0.82–1.34) | 0.7182 | 25 (3.2) | 0.91 (0.55–1.51) | 0.7104 | 6 (0.8) | 0.53 (0.21–1.34) | 0.1791 |
| T3 | 2658 | 409 | 1.00 (0.99–1.01) | 0.5635 | 44 | 0.99 (0.96–1.02) | 0.3634 | 13 | 0.99 (0.94–1.04) | 0.6188 |
| ≥20 | 1964 (84.6) | 322 (13.9) | ref. | - | 27 (1.2) | ref. | - | 9 (0.4) | ref. | - |
| <20 | 694 (86.5) | 87 (10.8) | 0.92 (0.70–1.20) | 0.5318 | 17 (2.1) | 1.50 (0.76–2.93) | 0.2405 | 4 (0.5) | 0.94 (0.26–3.39) | 0.9289 |
| 25(OH)D, ng/mL | Term | TPROM | Preterm Birth without PROM | PPROM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | OR (95% CI) | p | N (%) | OR (95% CI) | p | N (%) | OR (95% CI) | p | |
| Model 1 a | ||||||||||
| Change of 25(OH)D | ||||||||||
| ≥10.8 | 1022 (83.8) | 181 (14.8) | ref. | - | 10 (0.8) | ref. | - | 7 (0.6) | ref. | - |
| <10.8 | 1054 (86.3) | 131 (10.7) | 0.70 (0.55–0.89) | 0.0039 | 30 (2.5) | 2.91 (1.41–5.98) | 0.0037 | 6 (0.5) | 0.83 (0.28–2.48) | 0.7403 |
| Model 2 b | ||||||||||
| Change of 25(OH)D | ||||||||||
| ≥10.8 | 1022 (83.8) | 181 (14.8) | ref. | - | 10 (0.8) | ref. | - | 7 (0.6) | ref. | - |
| <10.8 | 1054 (86.3) | 131 (10.7) | 0.67 (0.52–0.86) | 0.0018 | 30 (2.5) | 2.89 (1.37–6.12) | 0.0054 | 6 (0.5) | 0.87 (0.28–2.69) | 0.8034 |
| Model 3 c | ||||||||||
| Change of 25(OH)D | ||||||||||
| ≥10.8 | 1022 (83.8) | 181 (14.8) | ref. | - | 10 (0.8) | ref. | - | 7 (0.6) | ref. | - |
| <10.8 | 1054 (86.3) | 131 (10.7) | 0.73 (0.56–0.96) | 0.0235 | 30 (2.5) | 2.32 (1.07–5.06) | 0.0337 | 6 (0.5) | 0.71 (0.21–2.39) | 0.5843 |
| Variable | Term (N = 37) † | PPROM (N = 37) | p | Term (N = 15) ‡ | Preterm without PROM (N = 16) | p | Term (N = 55) § | TPROM (N = 58) | p |
|---|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | |||||||
| Gravidity | 0.913 | 0.547 | 0.047 | ||||||
| 1 | 16 (43.2) | 17 (45.9) | 3 (20.0) | 3 (18.8) | 21 (38.2) | 24 (41.4) | |||
| 2 | 11 (29.7) | 9 (24.3) | 10 (66.7) | 8 (50.0) | 21 (38.2) | 11 (19.0) | |||
| ≥3 | 10 (27.0) | 11 (29.7) | 2 (13.3) | 5 (31.2) | 13 (23.6) | 23 (39.7) | |||
| Parity | 0.813 | 1.000 | 0.259 | ||||||
| 0 | 21 (56.8) | 23 (62.2) | 5 (33.3) | 5 (31.2) | 28 (50.9) | 36 (62.1) | |||
| ≥1 | 16 (43.2) | 14 (37.8) | 10 (66.7) | 11 (68.8) | 27 (49.1) | 22 (37.9) | |||
| Delivery mode | 1.000 | 0.716 | 0.001 | ||||||
| Vaginal delivery | 20 (54.1) | 21 (56.8) | 5 (33.3) | 7 (43.8) | 28 (50.9) | 47 (81.0) | |||
| Caesarean delivery | 17 (45.9) | 16 (43.2) | 10 (66.7) | 9 (56.2) | 27 (49.1) | 11 (19.0) | |||
| VD deficiency | 1.000 | 0.101 * | 0.304 | ||||||
| No | 24 (64.9) | 25 (67.6) | 15 (100.0) | 12 (75.0) | 42 (76.4) | 39 (67.2) | |||
| Yes | 13 (35.1) | 12 (32.4) | 0 (0.0) | 4 (25.0) | 13 (23.6) | 19 (32.8) | |||
| Mean ± SD | Mean ± SD | Mean ± SD | |||||||
| Delivery week, week | 40.04 ± 0.95 | 34.91 ± 1.78 | <0.001 | 39.48 ± 0.79 | 34.94 ± 2.05 | <0.001 | 39.84 ± 0.94 | 39.15 ± 1.30 | 0.001 |
| Maternal age, years | 28.70 ± 3.81 | 28.51 ± 3.78 | 0.831 | 29.60 ± 4.05 | 29.75 ± 3.28 | 0.910 | 28.96 ± 3.77 | 29.00 ± 4.75 | 0.964 |
| Pre-pregnancy BMI, kg/m2 | 21.47 ± 3.01 | 23.93 ± 3.44 | 0.089 | 22.55 ± 4.18 | 21.76 ± 4.17 | 0.603 | 22.19 ± 3.41 | 21.66 ± 3.68 | 0.423 |
| 25(OH)D, ng/mL | 27.02 ± 10.46 | 25.79 ± 5.77 | 0.620 | 39.31 ± 14.59 | 28.47 ± 15.20 | 0.052 | 30.66 ± 12.62 | 26.46 ± 14.20 | 0.100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alifu, X.; Si, S.; Qiu, Y.; Cheng, H.; Huang, Y.; Chi, P.; Zhuang, Y.; Zhou, H.; Zhang, L.; Ainiwan, D.; et al. The Association of Vitamin D during Pregnancy and mRNA Expression Levels of Inflammatory Factors with Preterm Birth and Prelabor Rupture of Membranes. Nutrients 2023, 15, 3423. https://doi.org/10.3390/nu15153423
Alifu X, Si S, Qiu Y, Cheng H, Huang Y, Chi P, Zhuang Y, Zhou H, Zhang L, Ainiwan D, et al. The Association of Vitamin D during Pregnancy and mRNA Expression Levels of Inflammatory Factors with Preterm Birth and Prelabor Rupture of Membranes. Nutrients. 2023; 15(15):3423. https://doi.org/10.3390/nu15153423
Chicago/Turabian StyleAlifu, Xialidan, Shuting Si, Yiwen Qiu, Haoyue Cheng, Ye Huang, Peihan Chi, Yan Zhuang, Haibo Zhou, Libi Zhang, Diliyaer Ainiwan, and et al. 2023. "The Association of Vitamin D during Pregnancy and mRNA Expression Levels of Inflammatory Factors with Preterm Birth and Prelabor Rupture of Membranes" Nutrients 15, no. 15: 3423. https://doi.org/10.3390/nu15153423
APA StyleAlifu, X., Si, S., Qiu, Y., Cheng, H., Huang, Y., Chi, P., Zhuang, Y., Zhou, H., Zhang, L., Ainiwan, D., Peng, Z., Liu, H., & Yu, Y. (2023). The Association of Vitamin D during Pregnancy and mRNA Expression Levels of Inflammatory Factors with Preterm Birth and Prelabor Rupture of Membranes. Nutrients, 15(15), 3423. https://doi.org/10.3390/nu15153423








