Gait Analysis, Metabolic Parameters and Adherence to the Mediterranean Diet in Patients with Type 2 Diabetes Mellitus Compared with Healthy Controls: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adherence to MedDiet
2.2. Anthropometric Data
2.3. Diabetes Distress Scale
2.4. Biochemical Data
2.5. Gait Analysis
2.6. Statistical Analysis
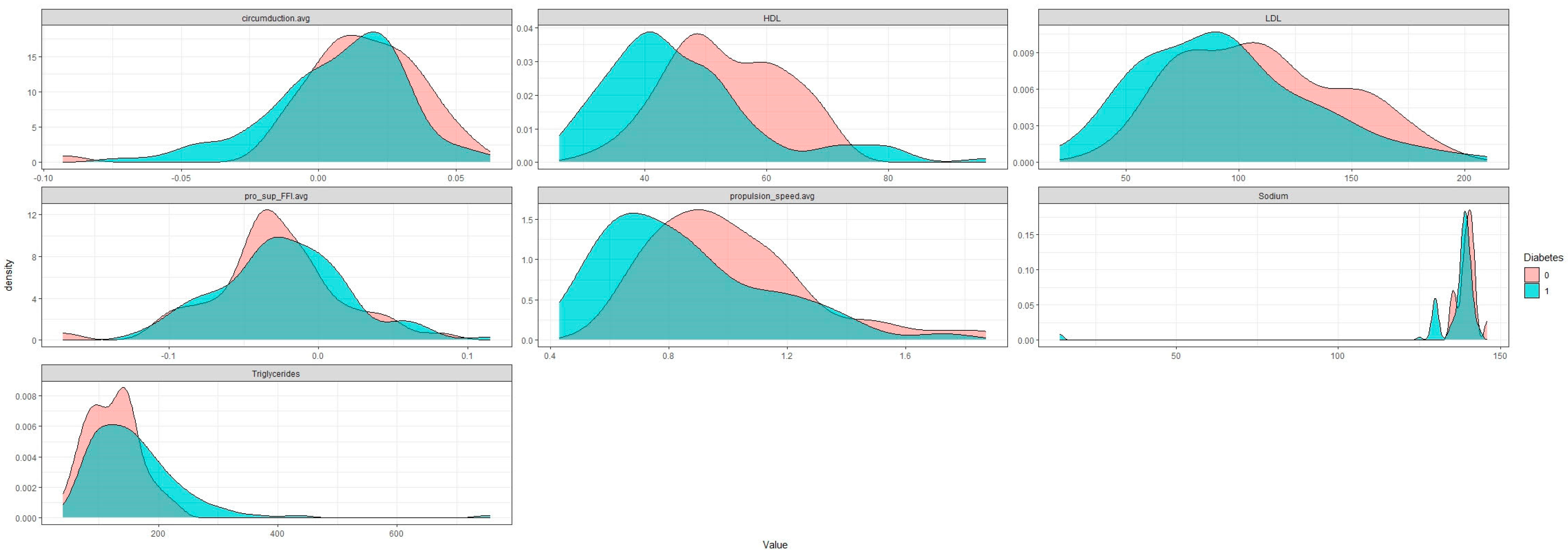
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variables | T2DM | HC | p-Value |
|---|---|---|---|
| stride_length.avg | 1.04 (±0.13) | 0.94 (±0.15) | 0.000 |
| walking_speed.avg | 2.96 (±0.52) | 2.64 (0.54) | 0.001 |
| angle_attack.avg | 0.3 (±0.07) | 0.26 (±0.09) | 0.001 |
| oscillation_speed.avg | 1.33 (±0.17) | 1.23 (±0.18) | 0.002 |
| time_digitigrade_phase.avg | 279.09 (±37.21) | 259.98 (±41.77) | 0.007 |
| pro_sup_TO.avg | meion 0.03 (±0.07) | 0 (±0.07) | 0.009 |
| clearance.avg | 11.97 (±6.3) | 9.48 (±5.98) | 0.020 |
| time_plantigrade_phase.avg | 367.81 (±102.8) | 338.81 (121.86) | 0.151 |
| pro_sup_FFI.avg | MEION 0.03 (0.04) | MEION 0.02 (±0.04) | 0.393 |
| pro_sup_HO.avg | 0.03 (±0.05) | 0.03 (±0.07) | 0.501 |
| cadence.avg | 47.31 (±4.9) | 46.94 (±4.96) | 0.667 |
| flight_time.avg | 490.57 (±46.512 | 489.27 (±55.46) | 0.887 |
| gaitline_TO.avg | 3 (0) | 2.99 (0.02) | <0.001 |
| propulsion_speed.avg | 0.81 (0.26) | 0.94 (0.26) | <0.001 |
| foot_progression_angle.avg | 0.19 (0.11) | 0.12 (0.13) | 0.006 |
| time_taligrade_phase.avg | 148.93 (108.95) | 117.35 (60.34) | 0.016 |
| circumduction.avg | 0.01 (0.02) | 0.02 (0.02) | 0.039 |
| gaitline_TS.avg | 2.74 (0.2) | 2.67 (0.21) | 0.339 |
| pro_sup_HS.avg | MEION 0.12 (0.06) | MEION 0.12 0.06) | 0.404 |
| gaitline_HS.avg | 2.18 (0.44) | 2.05 (0.47) | 0.482 |
| Confusions | 75 (13.34) | 78 (10.38) | 0.825 |
| gaitline_HO.avg | 2.77 (0.33) | 2.82 (0.25) | 0.939 |
References
- American Diabetes Association. American Diabetes Association Standards of Medical Care in Diabetes—2012. Diabetes Care 2011, 35 (Suppl. S1), S11–S63. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Peng, S.; Zhang, H.; Sun, H.; Hu, J. Gait Parameters and Peripheral Neuropathy in Patients with Diabetes: A Meta-Analysis. Front. Endocrinol. 2022, 13, 891356. [Google Scholar] [CrossRef] [PubMed]
- Hicks, C.W.; Selvin, E. Epidemiology of Peripheral Neuropathy and Lower Extremity Disease in Diabetes. Curr. Diabetes Rep. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Paschou, S.A.; Siasos, G.; Katsiki, N.; Tentolouris, N.; Tousoulis, D. The Role of microRNAs in the Development of Type 2 Diabetes Complications. Curr. Pharm. Des. 2020, 26, 5969–5979. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.; Armstrong, D.; Krisner, R.; Attinger, C.; Lavery, L.; Lipsky, B.; Mills, J.; Steinberg, J. Diagnosis and Management of Diabetic Foot Complications. ADA Clin. Compend. 2018, 2018, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Pirker, W.; Katzenschlager, R. Gait disorders in adults and the elderly: A clinical guide. Wien. Klin. Wochenschr. 2016, 129, 81–95. [Google Scholar] [CrossRef] [Green Version]
- Hulleck, A.A.; Mohan, D.M.; Abdallah, N.; El Rich, M.; Khalaf, K. Present and future of gait assessment in clinical practice: Towards the application of novel trends and technologies. Front. Med Technol. 2022, 4, 901331. [Google Scholar] [CrossRef]
- Brach, J.S.; Talkowski, J.B.; Strotmeyer, E.S.; Newman, A.B. Diabetes Mellitus and Gait Dysfunction: Possible Explanatory Factors. Phys. Ther. 2008, 88, 1365–1374. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Deng, F.; Rui, S.; Ma, Y.; Wang, M.; Deng, B.; Wang, H.; Du, C.; Chen, B.; Yang, X.; et al. The Evaluation of Gait and Balance for Patients with Early Diabetic Peripheral Neuropathy: A Cross-Sectional Study. Risk Manag. Healthc. Policy 2022, 15, 543–552. [Google Scholar] [CrossRef]
- Kanji, J.N.; Anglin, R.E.S.; Hunt, D.L.; Panju, A. Does This Patient With Diabetes Have Large-Fiber Peripheral Neuropathy? JAMA 2010, 303, 1526–1532. [Google Scholar] [CrossRef]
- Ko, S.-U.; Stenholm, S.; Chia, C.W.; Simonsick, E.M.; Ferrucci, L. Gait pattern alterations in older adults associated with type 2 diabetes in the absence of peripheral neuropathy—Results from the Baltimore Longitudinal Study of Aging. Gait Posture 2011, 34, 548–552. [Google Scholar] [CrossRef] [Green Version]
- Brown, S.; Boulton, A.; Bowling, F.; Reeves, N. Benefits, Challenges, and Potential Utility of a Gait Database for Diabetes Patients. J. Diabetes Sci. Technol. 2016, 10, 1065–1072. [Google Scholar] [CrossRef] [Green Version]
- Brach, J.S.; Studenski, S.; Perera, S.; VanSwearingen, J.M.; Newman, A.B. Stance time and step width variability have unique contributing impairments in older persons. Gait Posture 2008, 27, 431–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volpato, S.; Bianchi, L.; Lauretani, F.; Lauretani, F.; Bandinelli, S.; Guralnik, J.M.; Zuliani, G.; Ferrucci, L. Role of Muscle Mass and Muscle Quality in the Association Between Diabetes and Gait Speed. Diabetes Care 2012, 35, 1672–1679. [Google Scholar] [CrossRef] [Green Version]
- Raspovic, A. Gait characteristics of people with diabetes-related peripheral neuropathy, with and without a history of ulceration. Gait Posture 2013, 38, 723–728. [Google Scholar] [CrossRef]
- Dananberg, H.J. Gait style as an etiology to chronic postural pain. Part II. Postural compensatory process. J. Am. Podiatr. Med Assoc. 1993, 83, 615–624. [Google Scholar] [CrossRef] [PubMed]
- McClure, R.; Villani, A. Greater adherence to a Mediterranean Diet is associated with better gait speed in older adults with type 2 diabetes mellitus. Clin. Nutr. ESPEN 2019, 32, 33–39. [Google Scholar] [CrossRef]
- Didangelos, T.; Karlafti, E.; Kotzakioulafi, E.; Kontoninas, Z.; Margaritidis, C.; Giannoulaki, P.; Kantartzis, K. Efficacy and Safety of the Combination of Superoxide Dismutase, Alpha Lipoic Acid, Vitamin B12, and Carnitine for 12 Months in Patients with Diabetic Neuropathy. Nutrients 2020, 12, 3254. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary patterns: A Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef]
- Kintzoglanakis, K.; Vonta, P.; Copanitsanou, P. Diabetes-Related Distress and Associated Characteristics in Patients with Type 2 Diabetes in an Urban Primary Care Setting in Greece. Chronic Stress 2020, 4, 2470547020961538. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- “Podosmart”. Available online: http://www.podosmart.tech/en/smart-insoles (accessed on 15 July 2023).
- Efthymiou, D.; Zekakos, D.X.; Papatriantafyllou, E.; Ziagkas, E.; Petrelis, A.N.; Vassilopoulou, E. Gait Alterations in the Prediction of Metabolic Syndrome in Patients with Schizophrenia: A Pilot Study With PODOSmart® Insoles. Front. Psychiatry 2022, 13, 756600. [Google Scholar] [CrossRef]
- Leys, C.; Ley, C.; Klein, O.; Bernard, P.; Licata, L. Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. J. Exp. Soc. Psychol. 2013, 49, 764–766. [Google Scholar] [CrossRef] [Green Version]
- IBM. Forest Is a Commonly, both Classification and Regression Problems. Available online: https://www.ibm.com/topics/random-forest#:~:text=Random (accessed on 15 July 2023).
- Henderson, A.D.; Johnson, A.W.; Ridge, S.T.; Egbert, J.S.; Curtis, K.P.; Berry, L.J.; Bruening, D.A. Diabetic Gait Is Not Just Slow Gait: Gait Compensations in Diabetic Neuropathy. J. Diabetes Res. 2019, 2019, 4512501. [Google Scholar] [CrossRef] [Green Version]
- Yavuzer, G.; Yetkin, I.; Toruner, F.B.; Koca, N.; Bolukbasi, N. Gait deviations of patients with diabetes mellitus: Looking beyond peripheral neuropathy. Eur. Medicophys. 2006, 42, 127–133. [Google Scholar]
- Allet, L.; Armand, S.; Golay, A.; Monnin, D.; de Bie, R.A.; de Bruin, E.D. Gait characteristics of diabetic patients: A systematic review. Diabetes/Metab. Res. Rev. 2008, 24, 173–191. [Google Scholar] [CrossRef]
- Alam, U.; Riley, D.R.; Jugdey, R.S.; Azmi, S.; Rajbhandari, S.; D’août, K.; Malik, R.A. Diabetic Neuropathy and Gait: A Review. Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2017, 8, 1253–1264. [Google Scholar] [CrossRef] [Green Version]
- Loukovitis, A.; Ziagkas, E.; Zekakos, D.X.; Petrelis, A.; Grouios, G. Test-Retest Reliability of PODOSmart® Gait Analysis Insoles. Sensors 2021, 21, 7532. [Google Scholar] [CrossRef]
- Tian, T.; Wang, C.; Xu, Y.; Bai, Y.; Wang, J.; Long, Z.; Wang, X.; Zhou, L. A Wearable Gait Analysis System Used in Type 2 Diabetes Mellitus Patients: A Case–Control Study. Diabetes Metab. Syndr. Obesity Targets Ther. 2021, 14, 1799–1808. [Google Scholar] [CrossRef]
- Almurdhi, M.M.; Brown, S.J.; Bowling, F.L.; Boulton, A.J.M.; Jeziorska, M.; Malik, R.A.; Reeves, N.D. Altered walking strategy and increased unsteadiness in participants with impaired glucose tolerance and Type 2 diabetes relates to small-fibre neuropathy but not vitamin D deficiency. Diabet. Med. 2017, 34, 839–845. [Google Scholar] [CrossRef]
- Khalaf, K.; Al-Angari, H.M.; Khandoker, A.H.; Lee, S.; Almahmeed, W.; Al Safar, H.S.; Jelinek, H.F. Gait alterations in the UAE population with and without diabetic complications using both traditional and entropy measures. Gait Posture 2017, 58, 72–77. [Google Scholar] [CrossRef]
- Nadeau, D.A. Physiologic and Weight-Focused Treatment Strategies for Managing Type 2 Diabetes Mellitus: The Metformin, Glucagon-Like Peptide-1 Receptor Agonist, and Insulin (MGI) Approach. Postgrad. Med. 2013, 125, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Boye, K.S.; Shinde, S.; Kennedy-Martin, T.; Robinson, S.; Thieu, V.T. Weight Change and the Association with Adherence and Persistence to Diabetes Therapy: A Narrative Review. Patient Prefer. Adherence 2022, 16, 23–39. [Google Scholar] [CrossRef]
- Ross, S.A.; Dzida, G.; Vora, J.; Khunti, K.; Kaiser, M.; Ligthelm, R.J. Impact of weight gain on outcomes in type 2 diabetes. Curr. Med. Res. Opin. 2011, 27, 1431–1438. [Google Scholar] [CrossRef]
- Aldhahi, M.I. Effect of Gait Alteration on Fatigability during Walking in Adult Women with High Body Fat Composition. Medicina 2022, 59, 85. [Google Scholar] [CrossRef]
- Itani, L.; Kreidieh, D.; El Masri, D.; Tannir, H.; El Ghoch, M. The Impact of Sarcopenic Obesity on Health-Related Quality of Life of Treatment-Seeking Patients with Obesity. Curr. Diabetes Rev. 2020, 16, 635–640. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, B.; Huang, G.; Zhang, G.; Ding, Z.; Li, Z.; Sinclair, J.; Fan, Y. Sarcopenia: Body Composition and Gait Analysis. Front. Aging Neurosci. 2022, 14, 909551. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Maiorino, M.I.; Bellastella, G.; Chiodini, P.; Panagiotakos, D.; Giugliano, D. A journey into a Mediterranean diet and type 2 diabetes: A systematic review with meta-analyses. BMJ Open 2015, 5, e008222. [Google Scholar] [CrossRef] [Green Version]
- Buchanan, A.; Villani, A. Association of Adherence to a Mediterranean Diet with Excess Body Mass, Muscle Strength and Physical Performance in Overweight or Obese Adults with or without Type 2 Diabetes: Two Cross-Sectional Studies. Healthcare 2021, 9, 1255. [Google Scholar] [CrossRef] [PubMed]

| Variables | T2DM Group | HC Group | p-Value |
|---|---|---|---|
| Gender, n | 78F/21M | 34F/16M | 0.78 |
| Age, years (mean ± SD) | 66.4 ± 11.5 | 62.1 ± 7.9 | 0.657 |
| Height, m (mean ± SD) | 1.7 ± 0.1 | 1.7 ± 0.1 | 0.564 |
| Weight, kg (median, MAD) | 85.0 (13.3) | 75.5 (15.6) | <0.001 |
| BMI, kg/m2 (median, MAD) | 30.5 (4.4) | 27.5 (6.0) | <0.001 |
| WC, cm (mean ± SD) | 103 ± 17 | 93 ± 19 | <0.001 |
| SBP, mmHg (median, MAD) | 131.0 (13.3) | 120 (5.9) | <0.001 |
| Glucose, mg/dL (median, MAD) | 130.0 (25.2) | 88.0 (9.6) | <0.001 |
| HBA1c, % (median, MAD) | 6.5 (0.7) | 4.6 (0.3) | <0.001 |
| Total Cholesterol, mg/dL (mean ± SD) | 176 ± 4 | 196 ± 35 | 0.002 |
| HDL-C, mg/dL (median, MAD) | 43 (10) | 51 (10) | <0.001 |
| LDL-C, mg/dL (median, MAD) | 90 (40) | 107 (44) | 0.007 |
| Triglycerides, mg/dL (median, MAD) | 148 (68) | 126 (44) | 0.005 |
| Sodium, mEq/L (median, MAD) | 139.0 (1.5) | 140.0 (1.5) | <0.001 |
| WBC, 109/L (median, MAD) | 7.56 (2.00) | 6.70 (1.36) | <0.001 |
| CRP, mg/dL (median, MAD) | 0.20 (0.03) | 0.22 (0.01) | <0.001 |
| Uric Acid, mg/dL (median, MAD) | 5.6 (1.7) | 4.3 (0.8) | <0.001 |
| Urea, mg/dL (median, MAD) | 34.5 (9.6) | 31 (5.9) | <0.001 |
| Creatinine, mg/dL (median, MAD) | 0.9 (0.2) | 0.8 (0.2) | 0.002 |
| Food Type | T2DM Mean (±SD) | HC Mean (±SD) | p-Value |
|---|---|---|---|
| Potatoes | 1.66 (±0.79) | 1.86 (±0.53) | 0.790 |
| Whole grains | 1 (±1.48) | 1 (±1.48) | 0.078 |
| Olive oil | 5 (±0) | 5 (±0) | 0.115 |
| Vegetables | 2 (±1.93) | 2 (±2.08) | 0.118 |
| Full Fat Dairies | 4.95 (±0.22) | 4.9 (±0.3) | 0.251 |
| Fruits | 2 (±0) | 2 (±0) | 0.339 |
| Alcohol | 5 (±0) | 5 (±0) | 0.380 |
| White meat | 5 (±0) | 5 (±0) | 0.521 |
| Red meat | 4.61 (±0) | 4.55 (±0) | 0.618 |
| Legumes | 2 (±0) | 2 (±0) | 0.868 |
| Fish/shelfish | 1 (±0) | 1 (±0) | 0.888 |
| MedDiet Score | 34 (±3) | 34 (±1) | 0.718 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efthymiou, D.; Katsiki, N.; Zekakos, D.X.; Vassiliadis, P.; Petrelis, A.; Vassilopoulou, E. Gait Analysis, Metabolic Parameters and Adherence to the Mediterranean Diet in Patients with Type 2 Diabetes Mellitus Compared with Healthy Controls: A Pilot Study. Nutrients 2023, 15, 3421. https://doi.org/10.3390/nu15153421
Efthymiou D, Katsiki N, Zekakos DX, Vassiliadis P, Petrelis A, Vassilopoulou E. Gait Analysis, Metabolic Parameters and Adherence to the Mediterranean Diet in Patients with Type 2 Diabetes Mellitus Compared with Healthy Controls: A Pilot Study. Nutrients. 2023; 15(15):3421. https://doi.org/10.3390/nu15153421
Chicago/Turabian StyleEfthymiou, Dimitris, Niki Katsiki, Dimitrios Xipolias Zekakos, Panagiotis Vassiliadis, Alexandros Petrelis, and Emilia Vassilopoulou. 2023. "Gait Analysis, Metabolic Parameters and Adherence to the Mediterranean Diet in Patients with Type 2 Diabetes Mellitus Compared with Healthy Controls: A Pilot Study" Nutrients 15, no. 15: 3421. https://doi.org/10.3390/nu15153421
APA StyleEfthymiou, D., Katsiki, N., Zekakos, D. X., Vassiliadis, P., Petrelis, A., & Vassilopoulou, E. (2023). Gait Analysis, Metabolic Parameters and Adherence to the Mediterranean Diet in Patients with Type 2 Diabetes Mellitus Compared with Healthy Controls: A Pilot Study. Nutrients, 15(15), 3421. https://doi.org/10.3390/nu15153421






