Bibliometric and Visual Analysis of Global Research on Taurine, Creatine, Carnosine, and Anserine with Metabolic Syndrome: From 1992 to 2022
Abstract
1. Introduction
2. Methods
2.1. Data Collection
2.2. Data Analysis
3. Results
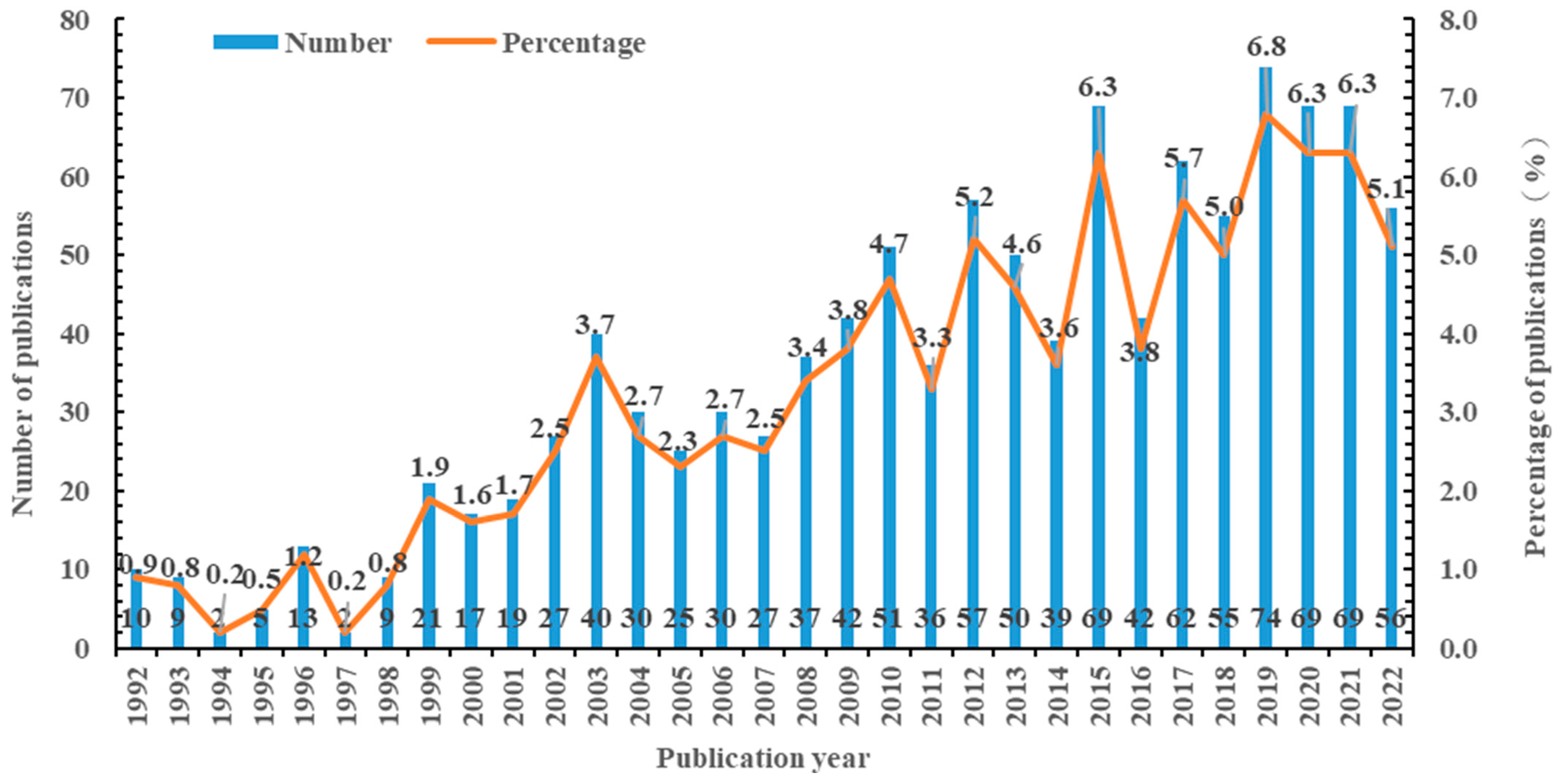
3.1. Annual Publications
3.2. Distribution of Journals and Representative Literature
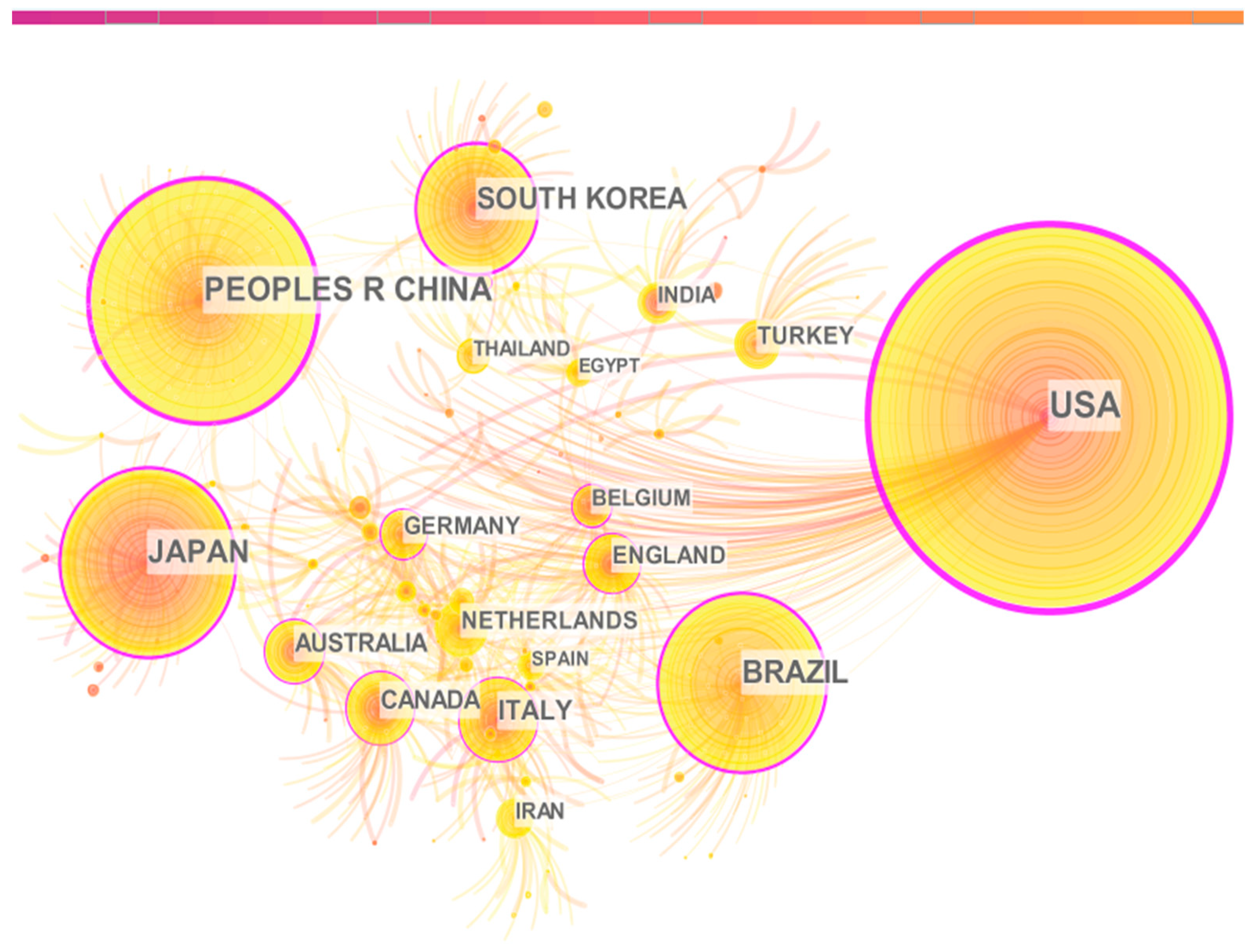
3.3. Collaboration Analysis
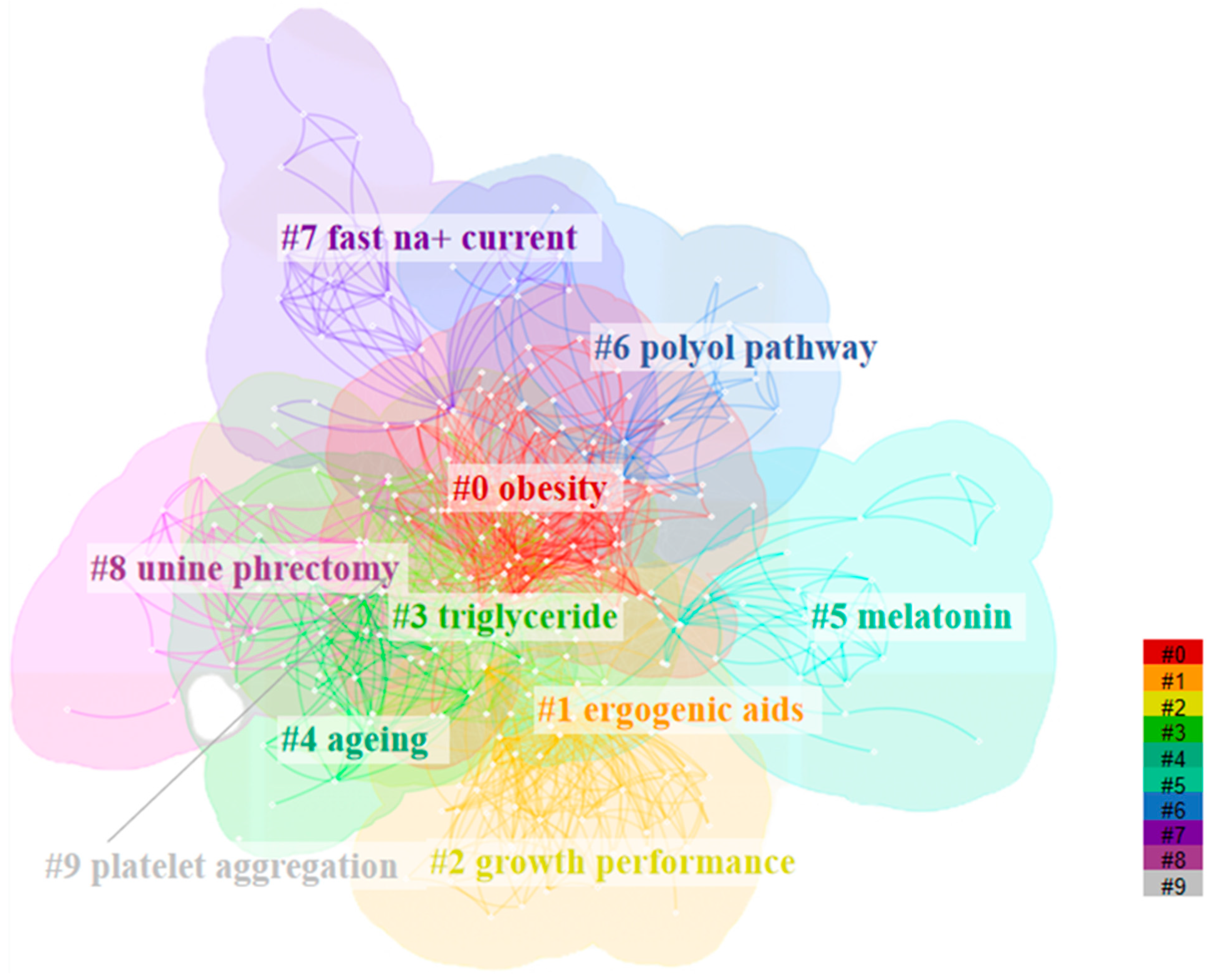
3.4. Keyword Cluster Analysis
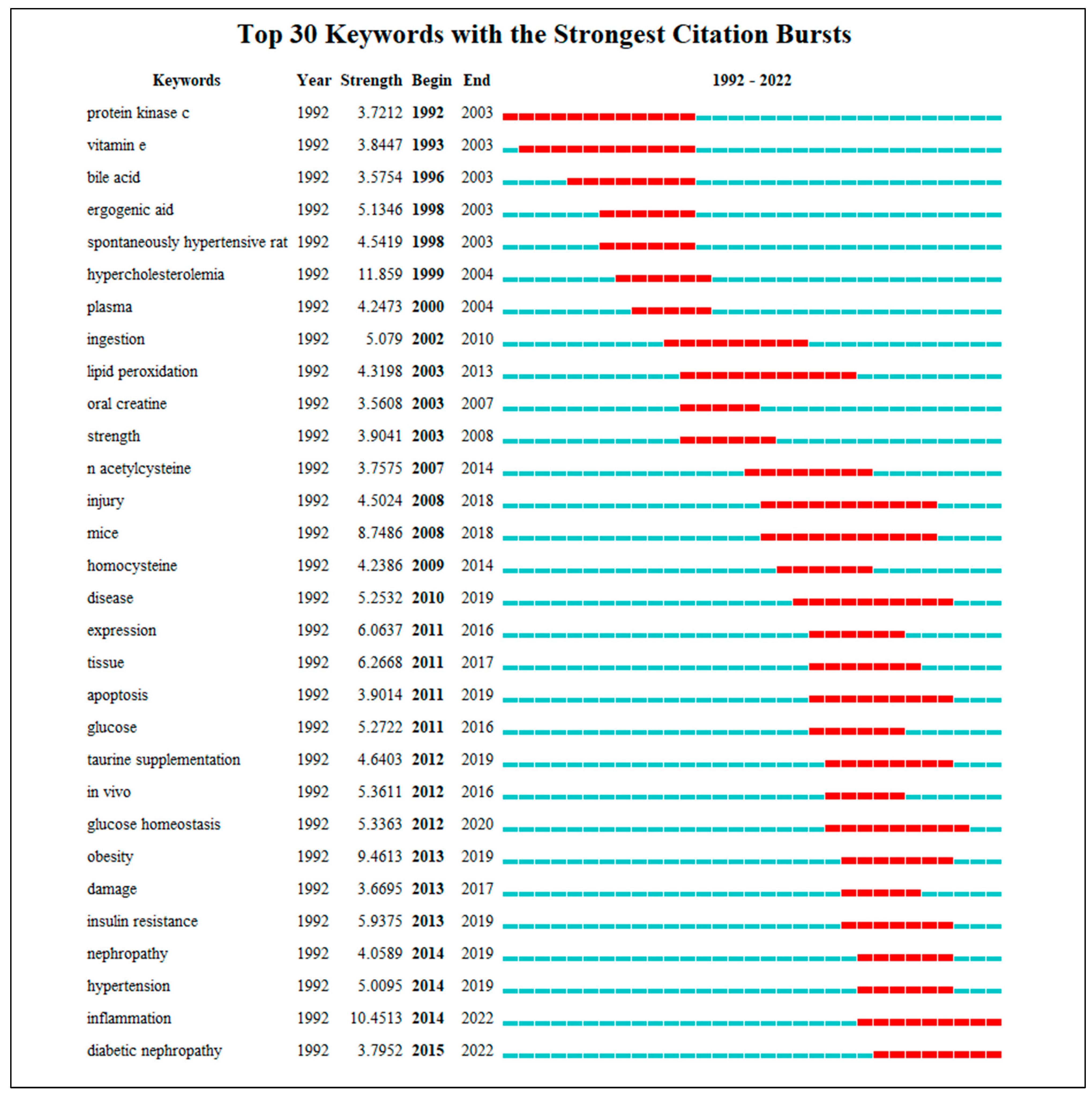
3.5. Burst Keyword Analysis
4. Discussion
4.1. General Summary
4.2. Research Hotspots
4.3. Research Frontiers
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Hirode, G.; Wong, R.J. Trends in the Prevalence of Metabolic Syndrome in the United States, 2011-2016. JAMA 2020, 323, 2526–2528. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Rockstrom, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A. Food in the Anthropocene: The EAT-Lancet Commission on Healthy Diets from Sustainable Food Systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services and US Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th ed.; US Department of Health and Human Services and US Department of Agriculture: Washington, DC, USA, 2015. [Google Scholar]
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Recommendations and Public Health and Policy Implications; World Cancer Research Fund/American Institute for Cancer Research: London, UK, 2018. [Google Scholar]
- International Agency for Research on Cancer, World Health Organization. IARC Monographs Evaluate Consumption of Red Meat and Processed Meat; International Agency for Research on Cancer, World Health Organization: Lyon, France, 2015. [Google Scholar]
- Aldini, G.; de Courten, B.; Regazzoni, L.; Gilardoni, E.; Ferrario, G.; Baron, G.; Altomare, A.; D’Amato, A.; Vistoli, G.; Carini, M. Understanding the Antioxidant and Carbonyl Sequestering Activity of Carnosine: Direct and Indirect Mechanisms. Free. Radic. Res. 2021, 55, 321–330. [Google Scholar] [CrossRef]
- Lescinsky, H.; Afshin, A.; Ashbaugh, C.; Bisignano, C.; Brauer, M.; Ferrara, G.; Hay, S.I.; He, J.; Iannucci, V.; Marczak, L.B. Health Effects Associated with Consumption of Unprocessed Red Meat: A Burden of Proof Study. Nat. Med. 2022, 28, 2075–2082. [Google Scholar] [CrossRef]
- Wu, G. Important Roles of Dietary Taurine, Creatine, Carnosine, Anserine and 4-Hydroxyproline in Human Nutrition and Health. Amino Acids 2020, 52, 329–360. [Google Scholar] [CrossRef]
- Ito, T.; Schaffer, S.; Azuma, J. The Effect of Taurine on Chronic Heart Failure: Actions of Taurine Against Catecholamine and Angiotensin II. Amino Acids 2014, 46, 111–119. [Google Scholar] [CrossRef]
- Ra, S.G.; Choi, Y.; Akazawa, N.; Kawanaka, K.; Ohmori, H.; Maeda, S. Effects of Taurine Supplementation on Vascular Endothelial Function at Rest and After Resistance Exercise. Adv. Exp. Med. Biol. 2019, 1155, 407–414. [Google Scholar] [CrossRef]
- Shimada, K.; Jong, C.J.; Takahashi, K.; Schaffer, S.W. Role of ROS Production and Turnover in the Antioxidant Activity of Taurine. Adv. Exp. Med. Biol. 2015, 803, 581–596. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and Pathophysiology of Carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef] [PubMed]
- Song, B.C.; Joo, N.S.; Aldini, G.; Yeum, K.J. Biological Functions of Histidine-Dipeptides and Metabolic Syndrome. Nutr. Res. Pract. 2014, 8, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.N.; Agharkar, A.S.; Gonzales, E.B. A Review of Creatine Supplementation in Age-Related Diseases: More than a Supplement for Athletes. F1000Res 2014, 3, 222. [Google Scholar] [CrossRef]
- Wyss, M.; Schulze, A. Health Implications of Creatine: Can Oral Creatine Supplementation Protect Against Neurological and Atherosclerotic Disease? Neuroscience 2002, 112, 243–260. [Google Scholar] [CrossRef]
- Hou, Y.; He, W.; Hu, S.; Wu, G. Composition of Polyamines and Amino Acids in Plant-Source Foods for Human Consumption. Amino Acids 2019, 51, 1153–1165. [Google Scholar] [CrossRef]
- Wu, G. Functional Amino Acids in Nutrition and Health. Amino Acids 2013, 45, 407–411. [Google Scholar] [CrossRef]
- Wu, G.; Cross, H.R.; Gehring, K.B.; Savell, J.W.; Arnold, A.N.; McNeill, S.H. Composition of Free and Peptide-Bound Amino Acids in Beef Chuck, Loin, and Round Cuts. J. Anim. Sci. 2016, 94, 2603–2613. [Google Scholar] [CrossRef] [PubMed]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to Conduct a Bibliometric Analysis: An Overview and Guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Chen, C. Searching for Intellectual Turning Points: Progressive Knowledge Domain Visualization. Proc. Natl. Acad. Sci. USA 2004, 101 (Suppl. S1), 5303–5310. [Google Scholar] [CrossRef]
- Chen, Y. The Methodology Function of Cite Space Mapping Knowledge Domains. Stud. Sci. Sci. 2015, 33, 242–253. [Google Scholar] [CrossRef]
- De Courten, B.; Jakubova, M.; de Courten, M.P.; Kukurova, I.J.; Vallova, S.; Krumpolec, P.; Valkovic, L.; Kurdiova, T.; Garzon, D.; Barbaresi, S. Effects of Carnosine Supplementation on Glucose Metabolism: Pilot Clinical Trial. Obesity 2016, 24, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.H. The Role of Taurine in Diabetes and the Development of Diabetic Complications. Diabetes Metab. Res. Rev. 2001, 17, 330–346. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S. Role of Taurine in the Pathogenesis of Obesity. Mol. Nutr. Food Res. 2015, 59, 1353–1363. [Google Scholar] [CrossRef]
- Ito, T.; Schaffer, S.W.; Azuma, J. The Potential Usefulness of Taurine on Diabetes Mellitus and its Complications. Amino Acids 2012, 42, 1529–1539. [Google Scholar] [CrossRef]
- Murakami, S. The Physiological and Pathophysiological Roles of Taurine in Adipose Tissue in Relation to Obesity. Life Sci. 2017, 186, 80–86. [Google Scholar] [CrossRef]
- Schaffer, S.W.; Azuma, J.; Mozaffari, M. Role of Antioxidant Activity of Taurine in Diabetes. Can. J. Physiol. Pharmacol. 2009, 87, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, E.M.; Latorraca, M.Q.; Araujo, E.; Beltra, M.; Oliveras, M.J.; Navarro, M.; Berna, G.; Bedoya, F.J.; Velloso, L.A.; Soria, B. Taurine Supplementation Modulates Glucose Homeostasis and Islet Function. J. Nutr. Biochem. 2009, 20, 503–511. [Google Scholar] [CrossRef]
- Albrecht, T.; Schilperoort, M.; Zhang, S.; Braun, J.D.; Qiu, J.; Rodriguez, A.; Pastene, D.O.; Kramer, B.K.; Koppel, H.; Baelde, H. Carnosine Attenuates the Development of Both Type 2 Diabetes and Diabetic Nephropathy in BTBR Ob/Ob Mice. Sci. Rep. 2017, 7, 44492. [Google Scholar] [CrossRef] [PubMed]
- Aldini, G.; Orioli, M.; Rossoni, G.; Savi, F.; Braidotti, P.; Vistoli, G.; Yeum, K.J.; Negrisoli, G.; Carini, M. The Carbonyl Scavenger Carnosine Ameliorates Dyslipidaemia and Renal Function in Zucker Obese Rats. J. Cell Mol. Med. 2011, 15, 1339–1354. [Google Scholar] [CrossRef] [PubMed]
- Hultman, K.; Alexanderson, C.; Manneras, L.; Sandberg, M.; Holmang, A.; Jansson, T. Maternal Taurine Supplementation in the Late Pregnant Rat Stimulates Postnatal Growth and Induces Obesity and Insulin Resistance in Adult Offspring. J. Physiol. 2007, 579, 823–833. [Google Scholar] [CrossRef]
- Nardelli, T.R.; Ribeiro, R.A.; Balbo, S.L.; Vanzela, E.C.; Carneiro, E.M.; Boschero, A.C.; Bonfleur, M.L. Taurine Prevents Fat Deposition and Ameliorates Plasma Lipid Profile in Monosodium Glutamate-Obese Rats. Amino Acids 2011, 41, 901–908. [Google Scholar] [CrossRef]
- Tsuboyama-Kasaoka, N.; Shozawa, C.; Sano, K.; Kamei, Y.; Kasaoka, S.; Hosokawa, Y.; Ezaki, O. Taurine (2-Aminoethanesulfonic Acid) Deficiency Creates a Vicious Circle Promoting Obesity. Endocrinology 2006, 147, 3276–3284. [Google Scholar] [CrossRef]
- Bonfleur, M.L.; Borck, P.C.; Ribeiro, R.A.; Caetano, L.C.; Soares, G.M.; Carneiro, E.M.; Balbo, S.L. Improvement in the Expression of Hepatic Genes Involved in Fatty Acid Metabolism in Obese Rats Supplemented with Taurine. Life Sci. 2015, 135, 15–21. [Google Scholar] [CrossRef]
- Kim, K.S.; Jang, M.J.; Fang, S.; Yoon, S.G.; Kim, I.Y.; Seong, J.K.; Yang, H.I.; Hahm, D.H. Anti-Obesity Effect of Taurine through Inhibition of Adipogenesis in White Fat Tissue but Not in Brown Fat Tissue in a High-Fat Diet-Induced Obese Mouse Model. Amino Acids 2019, 51, 245–254. [Google Scholar] [CrossRef]
- Camargo, R.L.; Batista, T.M.; Ribeiro, R.A.; Branco, R.; Da Silva, P.; Izumi, C.; Araujo, T.R.; Greene, L.J.; Boschero, A.C.; Carneiro, E.M. Taurine Supplementation Preserves Hypothalamic Leptin Action in Normal and Protein-Restricted Mice Fed on a High-Fat Diet. Amino Acids 2015, 47, 2419–2435. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Figueiredo, H.; Rebuffat, S.A.; Vieira, E.; Gomis, R. Taurine Treatment Modulates Circadian Rhythms in Mice Fed a High Fat Diet. Sci. Rep. 2016, 6, 36801. [Google Scholar] [CrossRef] [PubMed]
- Rosa, F.T.; Freitas, E.C.; Deminice, R.; Jordao, A.A.; Marchini, J.S. Oxidative Stress and Inflammation in Obesity After Taurine Supplementation: A Double-Blind, Placebo-Controlled Study. Eur. J. Nutr. 2014, 53, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Izumi, I.; Kagamimori, S.; Sokejima, S.; Yamagami, T.; Liu, Z.; Qi, B. Role of Taurine Supplementation to Prevent Exercise-Induced Oxidative Stress in Healthy Young Men. Amino Acids 2004, 26, 203–207. [Google Scholar] [CrossRef]
- Baye, E.; Ukropec, J.; de Courten, M.P.J.; Vallova, S.; Krumpolec, P.; Kurdiova, T.; Aldini, G.; Ukropcova, B.; de Courten, B. Effect of Carnosine Supplementation on the Plasma Lipidome in Overweight and Obese Adults: A Pilot Randomised Controlled Trial. Sci. Rep. 2020, 10, 17458. [Google Scholar] [CrossRef]
- Borck, P.C.; Vettorazzi, J.F.; Souto Branco, R.C.; Batista, T.M.; Santos-Silva, J.C.; Nakanishi, V.Y.; Boschero, A.C.; Ribeiro, R.A.; Carneiro, E.M. Taurine Supplementation Induces Long-Term Beneficial Effects on Glucose Homeostasis in ob/ob Mice. Amino Acids 2018, 50, 765–774. [Google Scholar] [CrossRef]
- Guan, L.; Miao, P. The Effects of Taurine Supplementation on Obesity, Blood Pressure and Lipid Profile: A Meta-Analysis of Randomized Controlled Trials. Eur. J. Pharmacol. 2020, 885, 173533. [Google Scholar] [CrossRef]
- Zhang, M.; Bi, L.F.; Fang, J.H.; Su, X.L.; Da, G.L.; Kuwamori, T.; Kagamimori, S. Beneficial Effects of Taurine on Serum Lipids in Overweight or Obese Non-Diabetic Subjects. Amino Acids 2004, 26, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.G.; Kim, M.J.; Moon, B.Y.; Cheong, S.H. Antioxidant and Laxative Effects of Taurine-Xylose, a Synthetic Taurine-Carbohydrate Derivative, in Loperamide-Induced Constipation in Sprague-Dawley Rats. J. Exerc. Nutr. Biochem. 2019, 23, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Takenaga, T.; Imada, K.; Otomo, S. Hypoglycemic Effect of Taurine in Golden Syrian Hamsters. Adv. Exp. Med. Biol. 2000, 187–192. [Google Scholar] [CrossRef]
- Mizushima, S.; Nara, Y.; Sawamura, M.; Yamori, Y. Effects of Oral Taurine Supplementation on Lipids and Sympathetic Nerve Tone. Adv. Exp. Med. Biol. 1996, 403, 615–622. [Google Scholar] [CrossRef]
- Kim, K.S.; Oh, D.H.; Kim, J.Y.; Lee, B.G.; You, J.S.; Chang, K.J.; Chung, H.; Yoo, M.C.; Yang, H.; Kang, J. Taurine Ameliorates Hyperglycemia and Dyslipidemia by Reducing Insulin Resistance and Leptin Level in Otsuka Long-Evans Tokushima Fatty (OLETF) Rats with Long-Term Diabetes. Exp. Mol. Med. 2012, 44, 665–673. [Google Scholar] [CrossRef]
- Mozaffari, M.S.; Patel, C.; Abdelsayed, R.; Schaffer, S.W. Accelerated NaCl-induced Hypertension in Taurine-Deficient Rat: Role of Renal Function. Kidney Int. 2006, 70, 329–337. [Google Scholar] [CrossRef]
- Rahman, M.M.; Park, H.M.; Kim, S.J.; Go, H.K.; Kim, G.B.; Hong, C.U.; Lee, Y.U.; Kim, S.Z.; Kim, J.S.; Kang, H.S. Taurine Prevents Hypertension and Increases Exercise Capacity in Rats with Fructose-Induced Hypertension. Am. J. Hypertens. 2011, 24, 574–581. [Google Scholar] [CrossRef]
- Lv, Q.F.; Dong, G.L.; Cao, S.; Wu, G.F.; Feng, Y.; Mei, L.; Lin, S.M.; Yang, Q.H.; Yang, J.C.; Hu, J.M. Effects of Taurine on Blood Index of Hypothalamic Pituitary Adrenal (HPA) Axis of Stress-Induced Hypertensive Rat. Adv. Exp. Med. Biol. 2015, 803, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.J.; Liu, L.S.; Ding, Y.; Huang, Z.D.; He, B.X.; Sun, S.F.; Zhao, G.S.; Zhang, H.X.; Miki, T.; Mizushima, S. Ethnic and Environmental Differences in Various Markers of Dietary Intake and Blood Pressure Among Chinese Han and Three Other Minority Peoples of China: Results from the WHO Cardiovascular Diseases and Alimentary Comparison (CARDIAC) Study. Hypertens. Res. 2001, 24, 315–322. [Google Scholar] [CrossRef]
- Yamori, Y.; Liu, L.; Ikeda, K.; Miura, A.; Mizushima, S.; Miki, T.; Nara, Y. Distribution of Twenty-Four Hour Urinary Taurine Excretion and Association with Ischemic Heart Disease Mortality in 24 Populations of 16 Countries: Results from the WHO-CARDIAC Study. Hypertens. Res. 2001, 24, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Camargo, R.L.; Batista, T.M.; Ribeiro, R.A.; Velloso, L.A.; Boschero, A.C.; Carneiro, E.M. Effects of Taurine Supplementation upon Food Intake and Central Insulin Signaling in Malnourished Mice Fed on a High-Fat Diet. Adv. Exp. Med. Biol. 2013, 776, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Mancini, D.S.M.; Nakata, M.; Baldini, C.; de Oliveira-Sales, E.B.; Boim, M.A.; Martimbianco, A.; Maquigussa, E. Creatine Supplementation in Type 2 Diabetic Patients: A Systematic Review of Randomized Clinical Trials. Curr. Diabetes Rev. 2022, 18, e120721194709. [Google Scholar] [CrossRef]
- Matthews, J.J.; Dolan, E.; Swinton, P.A.; Santos, L.; Artioli, G.G.; Turner, M.D.; Elliott-Sale, K.J.; Sale, C. Effect of Carnosine or beta-Alanine Supplementation on Markers of Glycemic Control and Insulin Resistance in Humans and Animals: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 2216–2231. [Google Scholar] [CrossRef] [PubMed]
- Nandhini, A.; Thirunavukkarasu, V.; Anuradha, C.V. Stimulation of Glucose Utilization and Inhibition of Protein Glycation and AGE Products by Taurine. Acta Physiol. Scand. 2004, 181, 297–303. [Google Scholar] [CrossRef]
- Harada, N.; Ninomiya, C.; Osako, Y.; Morishima, M.; Mawatari, K.; Takahashi, A.; Nakaya, Y. Taurine Alters Respiratory Gas Exchange and Nutrient Metabolism in Type 2 Diabetic Rats. Obes. Res. 2004, 12, 1077–1084. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, H.Y.; Kim, S.Y.; Lee, I.K.; Song, D.K. Enhancing Effect of Taurine on Glucose Response in UCP2-overexpressing Beta Cells. Diabetes Res. Clin. Pract. 2004, 66 (Suppl. 1), S69–S74. [Google Scholar] [CrossRef]
- Wu, N.; Lu, Y.; He, B.; Zhang, Y.; Lin, J.; Zhao, S.; Zhang, W.; Li, Y.; Han, P. Taurine Prevents Free Fatty Acid-Induced Hepatic Insulin Resistance in Association with Inhibiting JNK1 Activation and Improving Insulin Signaling in Vivo. Diabetes Res. Clin. Pract. 2010, 90, 288–296. [Google Scholar] [CrossRef]
- Everaert, I.; Van der Stede, T.; Stautemas, J.; Hanssens, M.; van Aanhold, C.; Baelde, H.; Vanhaecke, L.; Derave, W. Oral Anserine Supplementation Does Not Attenuate Type-2 Diabetes or Diabetic Nephropathy in BTBR ob/ob Mice. Amino Acids 2021, 53, 1269–1277. [Google Scholar] [CrossRef]
- Kubomura, D.; Matahira, Y.; Nagai, K.; Niijima, A. Effect of Anserine Ingestion on Hyperglycemia and the Autonomic Nerves in Rats and Humans. Nutr. Neurosci. 2010, 13, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Eliot, K.A.; Knehans, A.W.; Bemben, D.A.; Witten, M.S.; Carter, J.; Bemben, M.G. The Effects of Creatine and Whey Protein Supplementation on Body Composition in Men Aged 48 to 72 Years During Resistance Training. J. Nutr. Health Aging 2008, 12, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Ferreira, M.; Wilson, M.; Grindstaff, P.; Plisk, S.; Reinardy, J.; Cantler, E.; Almada, A.L. Effects of Creatine Supplementation on Body Composition, Strength, and Sprint Performance. Med. Sci. Sports Exerc. 1998, 30, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Pakulak, A.; Candow, D.G.; Totosy, D.Z.J.; Forbes, S.C.; Basta, D. Effects of Creatine and Caffeine Supplementation During Resistance Training on Body Composition, Strength, Endurance, Rating of Perceived Exertion and Fatigue in Trained Young Adults. J. Diet. Suppl. 2022, 19, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition Position Stand: Safety and Efficacy of Creatine Supplementation in Exercise, Sport, and Medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef]
- Candow, D.G.; Vogt, E.; Johannsmeyer, S.; Forbes, S.C.; Farthing, J.P. Strategic Creatine Supplementation and Resistance Training in Healthy Older Adults. Appl. Physiol. Nutr. Metab. 2015, 40, 689–694. [Google Scholar] [CrossRef]
- Gualano, B.; Macedo, A.R.; Alves, C.R.; Roschel, H.; Benatti, F.B.; Takayama, L.; de Sa, P.A.; Lima, F.R.; Pereira, R.M. Creatine Supplementation and Resistance Training in Vulnerable Older Women: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Exp. Gerontol. 2014, 53, 7–15. [Google Scholar] [CrossRef]
- Lobo, D.M.; Tritto, A.C.; Da, S.L.; de Oliveira, P.B.; Benatti, F.B.; Roschel, H.; Niess, B.; Gualano, B.; Pereira, R.M. Effects of Long-Term Low-Dose Dietary Creatine Supplementation in Older Women. Exp. Gerontol. 2015, 70, 97–104. [Google Scholar] [CrossRef]
- Rogers, M.E.; Bohlken, R.M.; Beets, M.W.; Hammer, S.B.; Ziegenfuss, T.N.; Sarabon, N. Effects of Creatine, Ginseng, and Astragalus Supplementation on Strength, Body Composition, Mood, and Blood Lipids During Strength-Training in Older Adults. J. Sports Sci. Med. 2006, 5, 60–69. [Google Scholar]
- Han, H.L.; Zhang, J.F.; Yan, E.F.; Shen, M.M.; Wu, J.M.; Gan, Z.D.; Wei, C.H.; Zhang, L.L.; Wang, T. Effects of Taurine on Growth Performance, Antioxidant Capacity, and Lipid Metabolism in Broiler Chickens. Poultry Sci. 2020, 99, 5707–5717. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Hosseini, S.A.; Eskandari, S.; Amirahmadi, M. Effect of Dietary Taurine and Methionine Supplementation on Growth Performance, Body Composition, Taurine Retention and Lipid Status of Persian Sturgeon, Acipenser Persicus (Borodin, 1897), Fed with Plant-Based Diet. Aquac. Nutr. 2018, 24, 324–331. [Google Scholar] [CrossRef]
- Hung, P.N.; Khaoian, P.; Fukada, H.; Suzuki, N.; Masumoto, T. Feeding Fermented Soybean Meal Diet Supplemented with Taurine to Yellowtail Seriola Quinqueradiata Affects Growth Performance and Lipid Digestion. Aquac. Res. 2015, 46, 1101–1110. [Google Scholar] [CrossRef]
- Shi, Y.; Zhong, L.; Zhong, H.; Zhang, J.; Liu, X.; Peng, M.; Fu, G.; Hu, Y. Taurine Supplements in High-Carbohydrate Diets Increase Growth Performance of Monopterus Albus by Improving Carbohydrate and Lipid Metabolism, Reducing Liver Damage, and Regulating Intestinal Microbiota. Aquaculture 2022, 554, 73815. [Google Scholar] [CrossRef]
- Sturman, J.A.; Gaull, G.E. Taurine in the Brain and Liver of the Developing Human and Monkey. J. Neurochem. 1975, 25, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and Molecular Cell Biology of Diabetic Complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Bkaily, G.; Jazzar, A.; Normand, A.; Simon, Y.; Al-Khoury, J.; Jacques, D. Taurine and Cardiac Disease: State of the Art and Perspectives. Can. J. Physiol. Pharm. 2020, 98, 67–73. [Google Scholar] [CrossRef]
- Mozaffari, M.S.; Schaffer, S.W. Chronic Taurine Treatment Ameliorates Reduction in Saline-Induced Diuresis and Natriuresis. Kidney Int. 2002, 61, 1750–1759. [Google Scholar] [CrossRef]
- Karkabounas, S.; Papadopoulos, N.; Anastasiadou, C.; Gubili, C.; Peschos, D.; Daskalou, T.; Fikioris, N.; Simos, Y.V.; Kontargiris, E.; Gianakopoulos, X. Effects of alpha-Lipoic Acid, Carnosine, and Thiamine Supplementation in Obese Patients with Type 2 Diabetes Mellitus: A Randomized, Double-Blind Study. J. Med. Food 2018, 21, 1197–1203. [Google Scholar] [CrossRef]
- Rosca, A.E.; Vladareanu, A.M.; Mirica, R.; Anghel-Timaru, C.M.; Mititelu, A.; Popescu, B.O.; Caruntu, C.; Voiculescu, S.E.; Gologan, S.; Onisai, M. Taurine and its Derivatives: Analysis of the Inhibitory Effect on Platelet Function and their Antithrombotic Potential. J. Clin. Med. 2022, 11, 666. [Google Scholar] [CrossRef]
- Bellentani, S.; Pecorari, M.; Cordoma, P.; Marchegiano, P.; Manenti, F.; Bosisio, E.; De Fabiani, E.; Galli, G. Taurine Increases Bile Acid Pool Size and Reduces Bile Saturation Index in the Hamster. J. Lipid Res. 1987, 28, 1021–1027. [Google Scholar] [CrossRef]
- Kulanthaivel, P.; Cool, D.R.; Ramamoorthy, S.; Mahesh, V.B.; Leibach, F.H.; Ganapathy, V. Transport of Taurine and its Regulation by Protein Kinase C in the JAR Human Placental Choriocarcinoma Cell Line. Biochem. J. 1991, 277 Pt 1, 53–58. [Google Scholar] [CrossRef]
- Nandhini, A.; Balakrishnan, S.D.; Anuradha, C.V. Taurine Improves Lipid Profile in Rats Fed a High Fructose-Diet. Nutr. Res. 2002, 22, 343–354. [Google Scholar] [CrossRef]
- Nandhini, A.; Thirunavukkarasu, V.; Anuradha, C.V. Taurine Modifies Insulin Signaling Enzymes in the Fructose-Fed Insulin Resistant-Rats. Diabetes Metab. 2005, 31, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Haber, C.A.; Lam, T.; Yu, Z.W.; Gupta, N.; Goh, T.; Bogdanovic, E.; Giacca, A.; Fantus, I.G. N-Acetylcysteine and Taurine Prevent Hyperglycemia-Induced Insulin Resistance in Vivo: Possible Role of Oxidative Stress. Am. J. Physiol. Endoc. Metab. 2003, 285, E744–E753. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, H.; Tsujino, T.; Watari, Y.; Emoto, N.; Yokoyama, M. Taurine Prevents the Decrease in Expression and Secretion of Extracellular Superoxide Dismutase Induced by Homocysteine—Amelioration of Homocysteine-Induced Endoplasmic Reticulum Stress by Taurine. Circulation 2001, 104, 1165–1170. [Google Scholar] [CrossRef]
- Ito, T.; Yoshikawa, N.; Ito, H.; Schaffer, S.W. Impact of Taurine Depletion on Glucose Control and Insulin Secretion in Mice. J. Pharmacol. Sci. 2015, 129, 59–64. [Google Scholar] [CrossRef]
- Zhang, X.; Tu, S.; Wang, Y.; Xu, B.; Wan, F. Mechanism of Taurine-Induced Apoptosis in Human Colon Cancer Cells. Acta Biochim. Biophys. Sin. 2014, 46, 261–272. [Google Scholar] [CrossRef]
- Han, X.; Chesney, R.W. The Role of Taurine in Renal Disorders. Amino Acids 2012, 43, 2249–2263. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Eraqi, M.M.; Alfaiz, F.A. Therapeutic Role of Taurine as Antioxidant in Reducing Hypertension Risks in Rats. Heliyon 2020, 6, e03209. [Google Scholar] [CrossRef] [PubMed]
- Qaradakhi, T.; Gadanec, L.K.; McSweeney, K.R.; Abraham, J.R.; Apostolopoulos, V.; Zulli, A. The Anti-Inflammatory Effect of Taurine on Cardiovascular Disease. Nutrients 2020, 12, 2847. [Google Scholar] [CrossRef]
- Ma, J.; Yang, Z.; Jia, S.; Yang, R. A Systematic Review of Preclinical Studies on the Taurine Role During Diabetic Nephropathy: Focused On Anti-Oxidative, Anti-Inflammation, and Anti-Apoptotic Effects. Toxicol. Mech. Method. 2022, 32, 420–430. [Google Scholar] [CrossRef]
- Peters, V.; Yard, B.; Schmitt, C.P. Carnosine and Diabetic Nephropathy. Curr. Med. Chem. 2020, 27, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Key, T.J.; Appleby, P.N.; Bradbury, K.E.; Sweeting, M.; Wood, A.; Johansson, I.; Kuhn, T.; Steur, M.; Weiderpass, E.; Wennberg, M. Consumption of Meat, Fish, Dairy Products, and Eggs and Risk of Ischemic Heart Disease a Prospective Study of 7198 Incident Cases Among 409 885 Participants in the Pan-European EPIC Cohort. Circulation 2019, 139, 2835–2845. [Google Scholar] [CrossRef] [PubMed]
- Knuppel, A.; Papier, K.; Fensom, G.K.; Appleby, P.N.; Schmidt, J.A.; Tong, T.; Travis, R.C.; Key, T.J.; Perez-Cornago, A. Meat Intake and Cancer Risk: Prospective Analyses in UK Biobank. Int. J. Epidemiol. 2020, 49, 1540–1552. [Google Scholar] [CrossRef]
- Lajous, M.; Tondeur, L.; Fagherazzi, G.; de Lauzon-Guillain, B.; Boutron-Ruaualt, M.C.; Clavel-Chapelon, F. Processed and Unprocessed Red Meat Consumption and Incident Type 2 Diabetes Among French Women. Diabetes Care 2012, 35, 128–130. [Google Scholar] [CrossRef] [PubMed]
| No. | Keywords | Frequency a | Keywords | Centrality b |
|---|---|---|---|---|
| 1 | Taurine | 365 | Cholesterol | 0.09 |
| 2 | Oxidative stress | 167 | Blood pressure | 0.08 |
| 3 | Metabolism | 158 | Insulin | 0.07 |
| 4 | Rat | 156 | Kidney | 0.07 |
| 5 | Supplementation | 156 | Expression | 0.06 |
| 6 | Skeletal Muscle | 92 | Damage | 0.06 |
| 7 | Glucose | 91 | Hyperglycemia | 0.06 |
| 8 | Exercise | 90 | Supplementation | 0.05 |
| 9 | Carnosine | 88 | Exercise | 0.05 |
| 10 | Expression | 81 | Diabetes | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Guo, F.; Ran, J.; Wu, H.; Li, Y.; Wang, M.; Wang, X. Bibliometric and Visual Analysis of Global Research on Taurine, Creatine, Carnosine, and Anserine with Metabolic Syndrome: From 1992 to 2022. Nutrients 2023, 15, 3374. https://doi.org/10.3390/nu15153374
Sun J, Guo F, Ran J, Wu H, Li Y, Wang M, Wang X. Bibliometric and Visual Analysis of Global Research on Taurine, Creatine, Carnosine, and Anserine with Metabolic Syndrome: From 1992 to 2022. Nutrients. 2023; 15(15):3374. https://doi.org/10.3390/nu15153374
Chicago/Turabian StyleSun, Jiaru, Fang Guo, Jinjun Ran, Haisheng Wu, Yang Li, Mingxu Wang, and Xiaoqin Wang. 2023. "Bibliometric and Visual Analysis of Global Research on Taurine, Creatine, Carnosine, and Anserine with Metabolic Syndrome: From 1992 to 2022" Nutrients 15, no. 15: 3374. https://doi.org/10.3390/nu15153374
APA StyleSun, J., Guo, F., Ran, J., Wu, H., Li, Y., Wang, M., & Wang, X. (2023). Bibliometric and Visual Analysis of Global Research on Taurine, Creatine, Carnosine, and Anserine with Metabolic Syndrome: From 1992 to 2022. Nutrients, 15(15), 3374. https://doi.org/10.3390/nu15153374







