Amino Acid Catabolism: An Overlooked Area of Metabolism
Abstract
1. Introduction
2. Enterohepatic Axis of Amino Acid Catabolism
2.1. Protein Hydrolysis and Amino Acid Absorption in the Gut
2.2. Amino Acid Catabolism in the Intestine
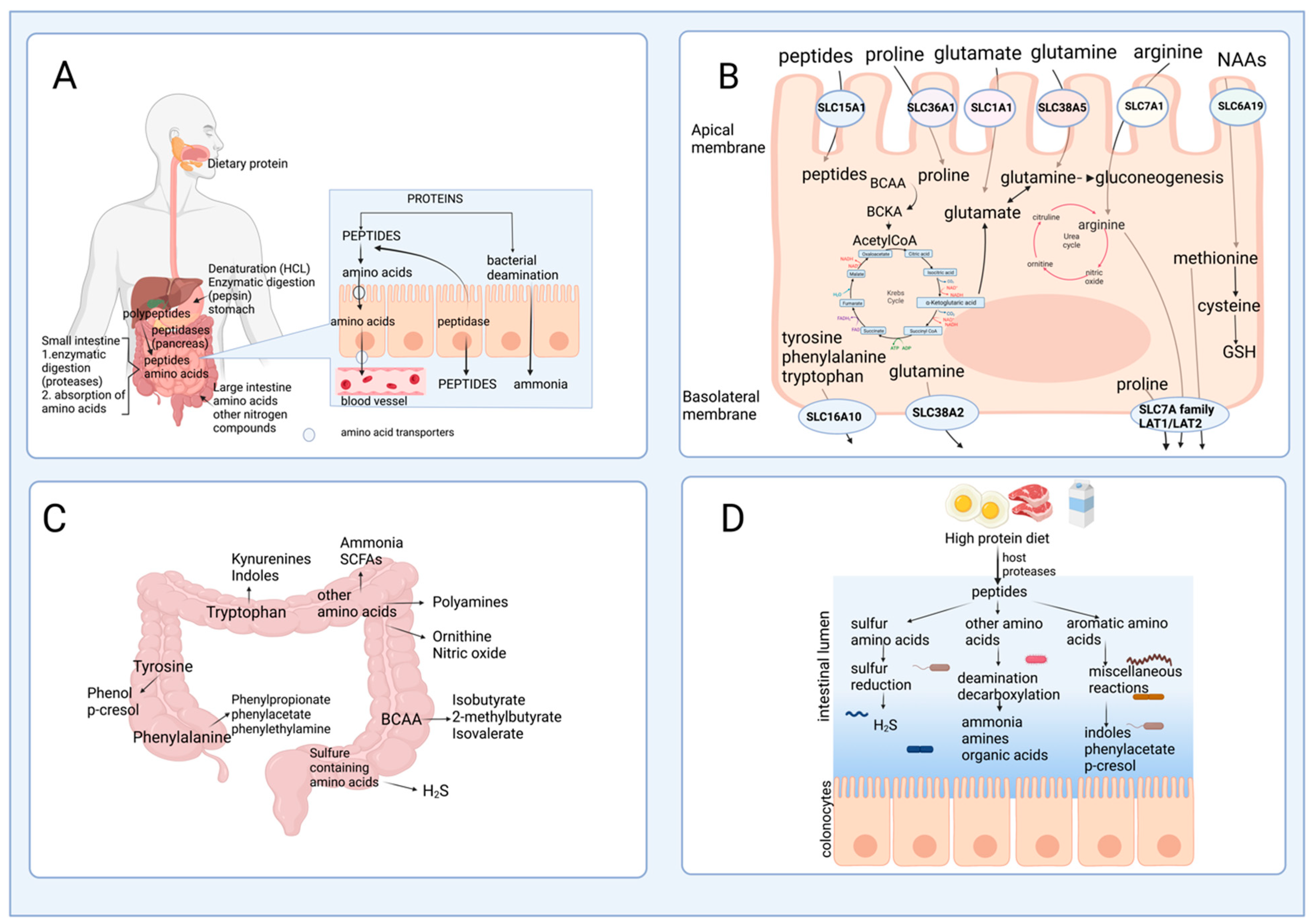
2.3. The Role of Gut Microbiota in Amino Acid Catabolism and Generation of End-Products
3. Mechanisms of Regulation of Amino Acid Oxidation
3.1. Regulation by the Gut Microbiota
3.2. Transcriptional Regulation of Amino Acid Degrading Enzymes
3.3. Regulation of Amino Acid Catabolizing Enzymes by miRNAs
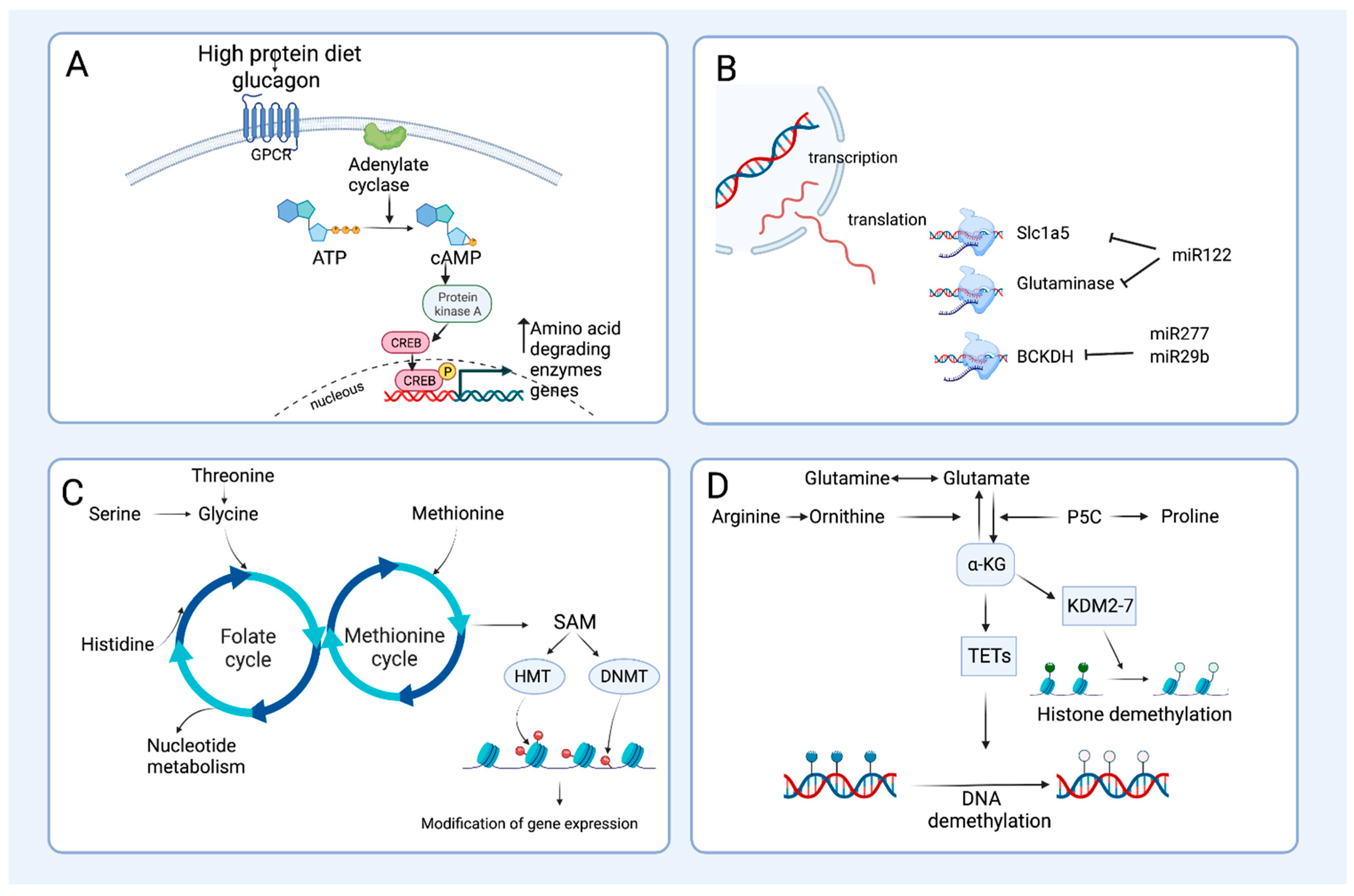
3.4. Amino Acid Catabolism and Epigenetics, Tissue Plasticity and Cellular Reprogramming
4. Amino Acid Catabolism in Health and Disease
4.1. Amino Acid Catabolism and Immunity
4.1.1. Tryptophan Catabolism
4.1.2. Arginine Catabolism
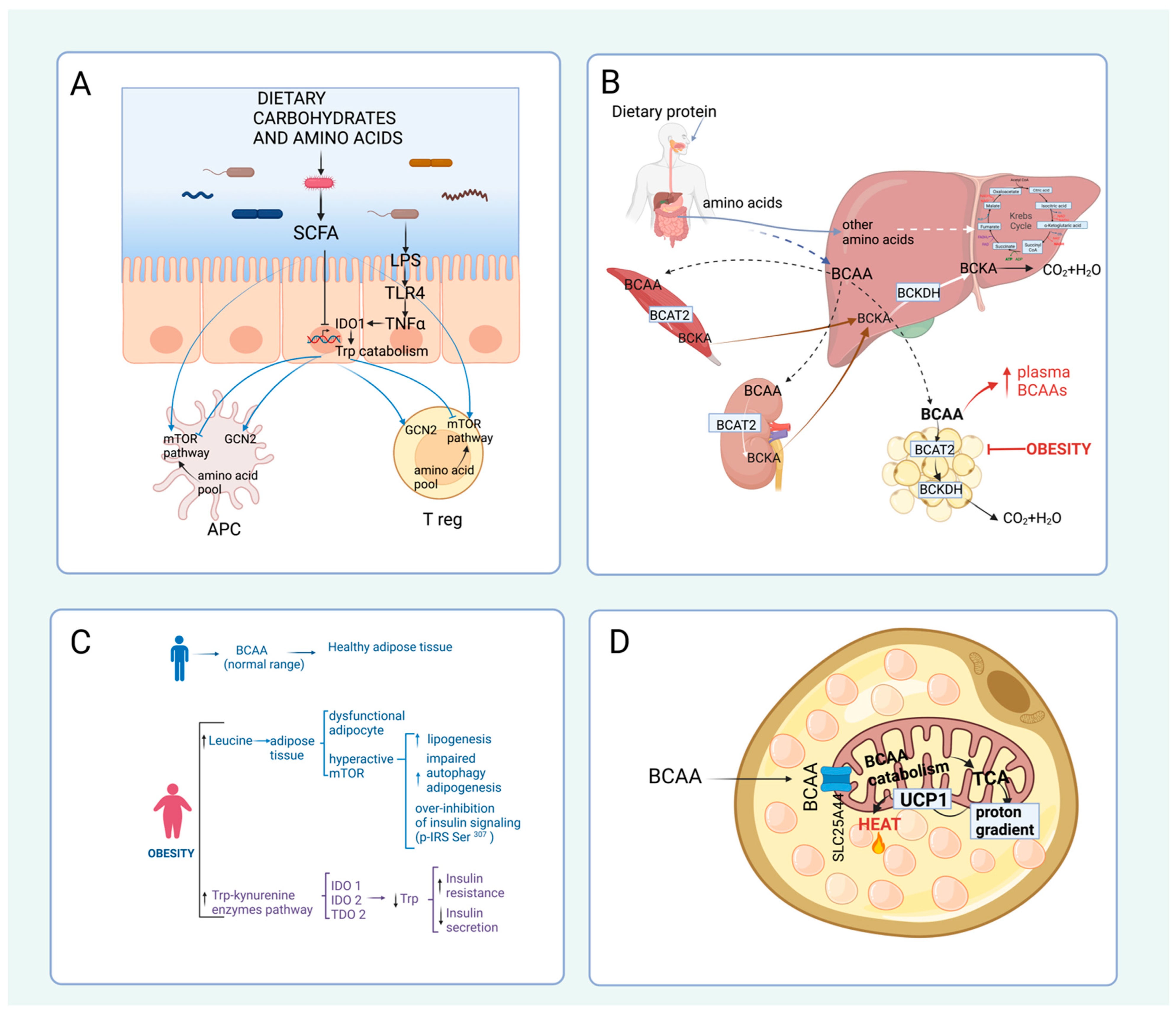
4.2. Amino Acid Catabolism in Obesity and Diabetes
4.3. Amino Acid Catabolism and Thermogenesis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chou, C.J.; Affolter, M.; Kussmann, M. A nutrigenomics view of protein intake: Macronutrient, bioactive peptides, and protein turnover. Prog. Mol. Biol. Transl. Sci. 2012, 108, 51–74. [Google Scholar] [CrossRef]
- Harper, A.E.; Miller, R.H.; Block, K.P. Branched-chain amino acid metabolism. Annu. Rev. Nutr. 1984, 4, 409–454. [Google Scholar] [CrossRef] [PubMed]
- Bender, D.A. The metabolism of “surplus” amino acids. Br. J. Nutr. 2012, 108 (Suppl. S2), S113–S121. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, A.; Kleiner, M. Dietary protein and the intestinal microbiota: An understudied relationship. iScience 2022, 25, 105313. [Google Scholar] [CrossRef] [PubMed]
- Tome, D. Amino acid metabolism and signalling pathways: Potential targets in the control of infection and immunity. Nutr. Diabetes 2021, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Broer, S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 2008, 88, 249–286. [Google Scholar] [CrossRef]
- Loveday, S.M. Protein digestion and absorption: The influence of food processing. Nutr. Res. Rev. 2022, 16, 1–16. [Google Scholar] [CrossRef]
- Bertrand, J.; Goichon, A.; Dechelotte, P.; Coeffier, M. Regulation of intestinal protein metabolism by amino acids. Amino Acids 2013, 45, 443–450. [Google Scholar] [CrossRef]
- Sleisenger, M.H.; Kim, Y.S. Protein digestion and absorption. N. Engl. J. Med. 1979, 300, 659–663. [Google Scholar] [CrossRef]
- Davila, A.M.; Blachier, F.; Gotteland, M.; Andriamihaja, M.; Benetti, P.H.; Sanz, Y.; Tome, D. Intestinal luminal nitrogen metabolism: Role of the gut microbiota and consequences for the host. Pharmacol. Res. 2013, 68, 95–107. [Google Scholar] [CrossRef]
- Chen, C.; Yin, Y.; Tu, Q.; Yang, H. Glucose and amino acid in enterocyte: Absorption, metabolism and maturation. Front. Biosci. 2018, 23, 1721–1739. [Google Scholar] [CrossRef]
- Torres, N.; Lopez, G.; De Santiago, S.; Hutson, S.M.; Tovar, A.R. Dietary protein level regulates expression of the mitochondrial branched-chain aminotransferase in rats. J. Nutr. 1998, 128, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Ma, X. Dietary Amino Acids and the Gut-Microbiome-Immune Axis: Physiological Metabolism and Therapeutic Prospects. Compr. Rev. Food Sci. Food Saf. 2019, 18, 221–242. [Google Scholar] [CrossRef]
- Pink, D.B.; Gatrell, S.K.; Elango, R.; Turchinsky, J.; Kiess, A.S.; Blemings, K.P.; Dixon, W.T.; Ball, R.O. Lysine alpha-ketoglutarate reductase, but not saccharopine dehydrogenase, is subject to substrate inhibition in pig liver. Nutr. Res. 2011, 31, 544–554. [Google Scholar] [CrossRef]
- Mitchell, A.D.; Benevenga, N.J. The role of transamination in methionine oxidation in the rat. J. Nutr. 1978, 108, 67–78. [Google Scholar] [CrossRef]
- Cooper, A.J.; Meister, A. Comparative studies of glutamine transaminases from rat tissues. Comp. Biochem. Physiol. B Biochem. 1981, 69, 137–145. [Google Scholar] [CrossRef]
- Schaart, M.W.; Schierbeek, H.; van der Schoor, S.R.; Stoll, B.; Burrin, D.G.; Reeds, P.J.; van Goudoever, J.B. Threonine utilization is high in the intestine of piglets. J. Nutr. 2005, 135, 765–770. [Google Scholar] [CrossRef]
- Hoerr, R.A.; Matthews, D.E.; Bier, D.M.; Young, V.R. Effects of protein restriction and acute refeeding on leucine and lysine kinetics in young men. Am. J. Physiol. 1993, 264, E567–E575. [Google Scholar] [CrossRef]
- Wu, G. Synthesis of citrulline and arginine from proline in enterocytes of postnatal pigs. Am. J. Physiol. 1997, 272, G1382–G1390. [Google Scholar] [CrossRef]
- Bush, J.A.; Wu, G.; Suryawan, A.; Nguyen, H.V.; Davis, T.A. Somatotropin-induced amino acid conservation in pigs involves differential regulation of liver and gut urea cycle enzyme activity. J. Nutr. 2002, 132, 59–67. [Google Scholar] [CrossRef][Green Version]
- Morris, S.M., Jr. Regulation of enzymes of the urea cycle and arginine metabolism. Annu. Rev. Nutr. 2002, 22, 87–105. [Google Scholar] [CrossRef]
- Wu, G.; Morris, S.M., Jr. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336 Pt 1, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.C.; Chung, T.Y.; Juo, Y.A.; Hsieh, M.H. Glutamine rapidly induces the expression of key transcription factor genes involved in nitrogen and stress responses in rice roots. BMC Genom. 2015, 16, 731. [Google Scholar] [CrossRef] [PubMed]
- Mouille, B.; Robert, V.; Blachier, F. Adaptative increase of ornithine production and decrease of ammonia metabolism in rat colonocytes after hyperproteic diet ingestion. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G344–G351. [Google Scholar] [CrossRef] [PubMed]
- Piper, D.W.; Fenton, B.H. pH stability and activity curves of pepsin with special reference to their clinical importance. Gut 1965, 6, 506–508. [Google Scholar] [CrossRef]
- Sanchez-Tapia, M.; Moreno-Vicencio, D.; Ordaz-Nava, G.; Guevara-Cruz, M.; Granados-Portillo, O.; Vargas-Castillo, A.; Torres, N.; Tovar, A.R. Antibiotic Treatment Reduces the Health Benefits of Soy Protein. Mol. Nutr. Food Res. 2020, 64, e2000532. [Google Scholar] [CrossRef]
- Dai, Z.L.; Li, X.L.; Xi, P.B.; Zhang, J.; Wu, G.; Zhu, W.Y. Metabolism of select amino acids in bacteria from the pig small intestine. Amino Acids 2012, 42, 1597–1608. [Google Scholar] [CrossRef]
- Dai, Z.L.; Zhang, J.; Wu, G.; Zhu, W.Y. Utilization of amino acids by bacteria from the pig small intestine. Amino Acids 2010, 39, 1201–1215. [Google Scholar] [CrossRef]
- Fan, P.; Liu, P.; Song, P.; Chen, X.; Ma, X. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci. Rep. 2017, 7, 43412. [Google Scholar] [CrossRef]
- Windey, K.; De Preter, V.; Verbeke, K. Relevance of protein fermentation to gut health. Mol. Nutr. Food Res. 2012, 56, 184–196. [Google Scholar] [CrossRef]
- Torrallardona, D.; Harris, C.I.; Coates, M.E.; Fuller, M.F. Microbial amino acid synthesis and utilization in rats: Incorporation of 15N from 15NH4Cl into lysine in the tissues of germ-free and conventional rats. Br. J. Nutr. 1996, 76, 689–700. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Allison, C.; Gibson, S.A.; Cummings, J.H. Contribution of the microflora to proteolysis in the human large intestine. J. Appl. Bacteriol. 1988, 64, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Blachier, F.; Mariotti, F.; Huneau, J.F.; Tome, D. Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids 2007, 33, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Gibson, S.A.; Beatty, E.; Cummings, J.H. Estimation of short-chain fatty acid production from protein by human intestinal bacteria based on branched-chain fatty acid measurements. FEMS Microbiol. Lett. 1992, 101, 81–88. [Google Scholar]
- Desvergne, B.; Michalik, L.; Wahli, W. Transcriptional regulation of metabolism. Physiol. Rev. 2006, 86, 465–514. [Google Scholar] [CrossRef]
- Libao-Mercado, A.J.; Zhu, C.L.; Cant, J.P.; Lapierre, H.; Thibault, J.N.; Seve, B.; Fuller, M.F.; de Lange, C.F. Dietary and endogenous amino acids are the main contributors to microbial protein in the upper gut of normally nourished pigs. J. Nutr. 2009, 139, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Larraufie, P.; Jarry, A.; Beguet-Crespel, F.; Marinelli, L.; Ledue, F.; Reimann, F.; Blottiere, H.M.; Lapaque, N. Butyrate Produced by Commensal Bacteria Down-Regulates Indolamine 2,3-Dioxygenase 1 (IDO-1) Expression via a Dual Mechanism in Human Intestinal Epithelial Cells. Front. Immunol. 2018, 9, 2838. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Liu, H.; Brown, M.A.; Qiao, S. Dietary Protein and Gut Microbiota Composition and Function. Curr. Protein Pept. Sci. 2019, 20, 145–154. [Google Scholar] [CrossRef]
- Zhao, M.; Qu, H. Human liver rate-limiting enzymes influence metabolic flux via branch points and inhibitors. BMC Genom. 2009, 10 (Suppl. S3), S31. [Google Scholar] [CrossRef][Green Version]
- Hoffmann, D.; Dvorakova, T.; Schramme, F.; Stroobant, V.; Van den Eynde, B.J. Tryptophan 2,3-Dioxygenase Expression Identified in Murine Decidual Stromal Cells Is Not Essential for Feto-Maternal Tolerance. Front. Immunol. 2020, 11, 601759. [Google Scholar] [CrossRef]
- Labow, B.I.; Souba, W.W.; Abcouwer, S.F. Mechanisms governing the expression of the enzymes of glutamine metabolism—Glutaminase and glutamine synthetase. J. Nutr. 2001, 131, 2467S–2474S; discussion 2486S-2487S. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Fujioka, M.; Su, Y.; Kanamoto, R.; Pitot, H.C. Nutritional regulation and tissue-specific expression of the serine dehydratase gene in rat. J. Biol. Chem. 1991, 266, 20412–20417. [Google Scholar] [CrossRef] [PubMed]
- Serralde-Zuniga, A.E.; Guevara-Cruz, M.; Tovar, A.R.; Herrera-Hernandez, M.F.; Noriega, L.G.; Granados, O.; Torres, N. Omental adipose tissue gene expression, gene variants, branched-chain amino acids, and their relationship with metabolic syndrome and insulin resistance in humans. Genes Nutr. 2014, 9, 431. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Torres, N.; Martinez, L.; Aleman, G.; Bourges, H.; Tovar, A.R. Histidase expression is regulated by dietary protein at the pretranslational level in rat liver. J. Nutr. 1998, 128, 818–824. [Google Scholar] [CrossRef][Green Version]
- Torres, N.; Tovar, A.R.; Harper, A.E. Metabolism of Valine in rat skeletal muscle mitochondria. J. Nutr. Biochem. 1993, 4, 681–689. [Google Scholar] [CrossRef]
- Torres, N.; Vargas, C.; Hernandez-Pando, R.; Orozco, H.; Hutson, S.M.; Tovar, A.R. Ontogeny and subcellular localization of rat liver mitochondrial branched chain amino-acid aminotransferase. Eur. J. Biochem. 2001, 268, 6132–6139. [Google Scholar] [CrossRef]
- Watford, M.; Vincent, N.; Zhan, Z.; Fannelli, J.; Kowalski, T.; Kovacevic, Z. Transcriptional control of rat hepatic glutaminase expression by dietary protein level and starvation. J. Nutr. 1994, 124, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Fagerberg, L.; Hallstrom, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Yu, Y.; Fuscoe, J.C.; Zhao, C.; Guo, C.; Jia, M.; Qing, T.; Bannon, D.I.; Lancashire, L.; Bao, W.; Du, T.; et al. A rat RNA-Seq transcriptomic BodyMap across 11 organs and 4 developmental stages. Nat. Commun. 2014, 5, 3230. [Google Scholar] [CrossRef] [PubMed]
- Yue, F.; Cheng, Y.; Breschi, A.; Vierstra, J.; Wu, W.; Ryba, T.; Sandstrom, R.; Ma, Z.; Davis, C.; Pope, B.D.; et al. A comparative encyclopedia of DNA elements in the mouse genome. Nature 2014, 515, 355–364. [Google Scholar] [CrossRef]
- Tovar, A.R.; Ascencio, C.; Torres, N. Soy protein, casein, and zein regulate histidase gene expression by modulating serum glucagon. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E1016–E1022. [Google Scholar] [CrossRef]
- Kanamoto, R.; Su, Y.; Pitot, H.C. Effects of glucose, insulin, and cAMP on transcription of the serine dehydratase gene in rat liver. Arch. Biochem. Biophys. 1991, 288, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T.; Ewart, H.S.; Squires, S.A. Hormonal control of hepatic glutaminase. Adv. Enzyme Regul. 1995, 35, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Korenfeld, N.; Finkel, M.; Buchshtab, N.; Bar-Shimon, M.; Charni-Natan, M.; Goldstein, I. Fasting Hormones Synergistically Induce Amino Acid Catabolism Genes to Promote Gluconeogenesis. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 1021–1036. [Google Scholar] [CrossRef] [PubMed]
- Schutz, G.; Schmid, W.; Jantzen, M.; Danesch, U.; Gloss, B.; Strahle, U.; Becker, P.; Boshart, M. Molecular basis for the hormonal regulation of the tyrosine aminotransferase and tryptophan oxygenase genes. Ann. N. Y. Acad. Sci. 1986, 478, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Danesch, U.; Gloss, B.; Schmid, W.; Schutz, G.; Schule, R.; Renkawitz, R. Glucocorticoid induction of the rat tryptophan oxygenase gene is mediated by two widely separated glucocorticoid-responsive elements. EMBO J. 1987, 6, 625–630. [Google Scholar] [CrossRef]
- Su, Y.; Kanamoto, R.; Ogawa, H.; Pitot, H.C. Regulatory elements for the tissue-specific expression of the rat serine dehydratase-encoding gene. Gene 1992, 120, 301–306. [Google Scholar] [CrossRef]
- Oyadomari, S.; Matsuno, F.; Chowdhury, S.; Kimura, T.; Iwase, K.; Araki, E.; Shichiri, M.; Mori, M.; Takiguchi, M. The gene for hepatocyte nuclear factor (HNF)-4alpha is activated by glucocorticoids and glucagon, and repressed by insulin in rat liver. FEBS Lett. 2000, 478, 141–146. [Google Scholar] [CrossRef]
- Contreras, A.V.; Rangel-Escareno, C.; Torres, N.; Aleman-Escondrillas, G.; Ortiz, V.; Noriega, L.G.; Torre-Villalvazo, I.; Granados, O.; Velazquez-Villegas, L.A.; Tobon-Cornejo, S.; et al. PPARalpha via HNF4alpha regulates the expression of genes encoding hepatic amino acid catabolizing enzymes to maintain metabolic homeostasis. Genes Nutr. 2015, 10, 452. [Google Scholar] [CrossRef]
- Haas, M.J.; Pitot, H.C. Glucocorticoids stimulate CREB binding to a cyclic-AMP response element in the rat serine dehydratase gene. Arch. Biochem. Biophys. 1999, 362, 317–324. [Google Scholar] [CrossRef]
- Aleman, G.; Ortiz, V.; Langley, E.; Tovar, A.R.; Torres, N. Regulation by glucagon of the rat histidase gene promoter in cultured rat hepatocytes and human hepatoblastoma cells. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E172–E179. [Google Scholar] [CrossRef]
- Velazquez-Villegas, L.A.; Charabati, T.; Contreras, A.V.; Aleman, G.; Torres, N.; Tovar, A.R. PPARalpha Downregulates Hepatic Glutaminase Expression in Mice Fed Diets with Different Protein:Carbohydrate Ratios. J. Nutr. 2016, 146, 1634–1640. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tobon-Cornejo, S.; Vargas-Castillo, A.; Leyva-Martinez, A.; Ortiz, V.; Noriega, L.G.; Velazquez-Villegas, L.A.; Aleman, G.; Furosawa-Carballeda, J.; Torres, N.; Tovar, A.R. PPARalpha/RXRalpha downregulates amino acid catabolism in the liver via interaction with HNF4alpha promoting its proteasomal degradation. Metabolism 2021, 116, 154705. [Google Scholar] [CrossRef]
- Jantzen, H.M.; Strahle, U.; Gloss, B.; Stewart, F.; Schmid, W.; Boshart, M.; Miksicek, R.; Schutz, G. Cooperativity of glucocorticoid response elements located far upstream of the tyrosine aminotransferase gene. Cell 1987, 49, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Nitsch, D.; Schutz, G. The distal enhancer implicated in the developmental regulation of the tyrosine aminotransferase gene is bound by liver-specific and ubiquitous factors. Mol. Cell Biol. 1993, 13, 4494–4504. [Google Scholar] [CrossRef]
- Tovar, A.R.; Becerril, E.; Hernandez-Pando, R.; Lopez, G.; Suryawan, A.; Desantiago, S.; Hutson, S.M.; Torres, N. Localization and expression of BCAT during pregnancy and lactation in the rat mammary gland. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E480–E488. [Google Scholar] [CrossRef]
- DeSantiago, S.; Torres, N.; Suryawan, A.; Tovar, A.R.; Hutson, S.M. Regulation of branched-chain amino acid metabolism in the lactating rat. J. Nutr. 1998, 128, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Christowitz, D.; Mattheyse, F.J.; Balinsky, J.B. Dietary and hormonal regulation of urea cycle enzymes in rat liver. Enzyme 1981, 26, 113–121. [Google Scholar] [CrossRef]
- Valencia-Sanchez, M.A.; Liu, J.; Hannon, G.J.; Parker, R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes. Dev. 2006, 20, 515–524. [Google Scholar] [CrossRef]
- Stark, A.; Brennecke, J.; Russell, R.B.; Cohen, S.M. Identification of Drosophila MicroRNA targets. PLoS Biol. 2003, 1, E60. [Google Scholar] [CrossRef]
- Esslinger, S.M.; Schwalb, B.; Helfer, S.; Michalik, K.M.; Witte, H.; Maier, K.C.; Martin, D.; Michalke, B.; Tresch, A.; Cramer, P.; et al. Drosophila miR-277 controls branched-chain amino acid catabolism and affects lifespan. RNA Biol. 2013, 10, 1042–1056. [Google Scholar] [CrossRef] [PubMed]
- Mersey, B.D.; Jin, P.; Danner, D.J. Human microRNA (miR29b) expression controls the amount of branched chain alpha-ketoacid dehydrogenase complex in a cell. Hum. Mol. Genet. 2005, 14, 3371–3377. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, D.; Cassel, T.; Teng, K.Y.; Aljuhani, M.; Chowdhary, V.K.; Hu, P.; Zhang, X.; Fan, T.W.; Ghoshal, K. Regulation of hepatic glutamine metabolism by miR-122. Mol. Metab. 2020, 34, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Still, E.R.; Yuneva, M.O. Hopefully devoted to Q: Targeting glutamine addiction in cancer. Br. J. Cancer 2017, 116, 1375–1381. [Google Scholar] [CrossRef]
- Song, P.; Yang, F.; Jin, H.; Wang, X. The regulation of protein translation and its implications for cancer. Signal Transduct. Target. Ther. 2021, 6, 68. [Google Scholar] [CrossRef]
- Wu, Y.; Cheng, Z.; Bai, Y.; Ma, X. Epigenetic Mechanisms of Maternal Dietary Protein and Amino Acids Affecting Growth and Development of Offspring. Curr. Protein Pept. Sci. 2019, 20, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Clare, C.E.; Brassington, A.H.; Kwong, W.Y.; Sinclair, K.D. One-Carbon Metabolism: Linking Nutritional Biochemistry to Epigenetic Programming of Long-Term Development. Annu. Rev. Anim. Biosci. 2019, 7, 263–287. [Google Scholar] [CrossRef]
- Zdzisinska, B.; Zurek, A.; Kandefer-Szerszen, M. Alpha-Ketoglutarate as a Molecule with Pleiotropic Activity: Well-Known and Novel Possibilities of Therapeutic Use. Arch. Immunol. Ther. Exp. 2017, 65, 21–36. [Google Scholar] [CrossRef]
- Cheng, Z.; Cheung, P.; Kuo, A.J.; Yukl, E.T.; Wilmot, C.M.; Gozani, O.; Patel, D.J. A molecular threading mechanism underlies Jumonji lysine demethylase KDM2A regulation of methylated H3K36. Genes. Dev. 2014, 28, 1758–1771. [Google Scholar] [CrossRef]
- Ito, S.; D’Alessio, A.C.; Taranova, O.V.; Hong, K.; Sowers, L.C.; Zhang, Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 2010, 466, 1129–1133. [Google Scholar] [CrossRef]
- Tahiliani, M.; Koh, K.P.; Shen, Y.; Pastor, W.A.; Bandukwala, H.; Brudno, Y.; Agarwal, S.; Iyer, L.M.; Liu, D.R.; Aravind, L.; et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 2009, 324, 930–935. [Google Scholar] [CrossRef]
- Chalecka, M.; Kazberuk, A.; Palka, J.; Surazynski, A. P5C as an Interface of Proline Interconvertible Amino Acids and Its Role in Regulation of Cell Survival and Apoptosis. Int. J. Mol. Sci. 2021, 22, 11763. [Google Scholar] [CrossRef]
- Cyr, A.R.; Domann, F.E. The redox basis of epigenetic modifications: From mechanisms to functional consequences. Antioxid. Redox Signal 2011, 15, 551–589. [Google Scholar] [CrossRef]
- Phang, J.M.; Liu, W.; Hancock, C. Bridging epigenetics and metabolism: Role of non-essential amino acids. Epigenetics 2013, 8, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Kilberg, M.S.; Terada, N.; Shan, J. Influence of Amino Acid Metabolism on Embryonic Stem Cell Function and Differentiation. Adv. Nutr. 2016, 7, 780S–789S. [Google Scholar] [CrossRef]
- Vanweert, F.; Schrauwen, P.; Phielix, E. Role of branched-chain amino acid metabolism in the pathogenesis of obesity and type 2 diabetes-related metabolic disturbances BCAA metabolism in type 2 diabetes. Nutr. Diabetes 2022, 12, 35. [Google Scholar] [CrossRef]
- Newsholme, P. Cellular and metabolic mechanisms of nutrient actions in immune function. Eur. J. Clin. Nutr. 2021, 75, 1328–1331. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.; Pearce, E.L. Amino Assets: How Amino Acids Support Immunity. Cell Metab. 2020, 32, 154–175. [Google Scholar] [CrossRef] [PubMed]
- Kedia-Mehta, N.; Finlay, D.K. Competition for nutrients and its role in controlling immune responses. Nat. Commun. 2019, 10, 2123. [Google Scholar] [CrossRef] [PubMed]
- Loftus, R.M.; Finlay, D.K. Immunometabolism: Cellular Metabolism Turns Immune Regulator. J. Biol. Chem. 2016, 291, 1–10. [Google Scholar] [CrossRef]
- Calder, P.C. Branched-chain amino acids and immunity. J. Nutr. 2006, 136, 288S–293S. [Google Scholar] [CrossRef]
- McGaha, T.L.; Huang, L.; Lemos, H.; Metz, R.; Mautino, M.; Prendergast, G.C.; Mellor, A.L. Amino acid catabolism: A pivotal regulator of innate and adaptive immunity. Immunol. Rev. 2012, 249, 135–157. [Google Scholar] [CrossRef] [PubMed]
- Forouhar, F.; Anderson, J.L.; Mowat, C.G.; Vorobiev, S.M.; Hussain, A.; Abashidze, M.; Bruckmann, C.; Thackray, S.J.; Seetharaman, J.; Tucker, T.; et al. Molecular insights into substrate recognition and catalysis by tryptophan 2,3-dioxygenase. Proc. Natl. Acad. Sci. USA 2007, 104, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Rafice, S.A.; Chauhan, N.; Efimov, I.; Basran, J.; Raven, E.L. Oxidation of L-tryptophan in biology: A comparison between tryptophan 2,3-dioxygenase and indoleamine 2,3-dioxygenase. Biochem. Soc. Trans. 2009, 37, 408–412. [Google Scholar] [CrossRef]
- Kanai, M.; Funakoshi, H.; Takahashi, H.; Hayakawa, T.; Mizuno, S.; Matsumoto, K.; Nakamura, T. Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice. Mol. Brain 2009, 2, 8. [Google Scholar] [CrossRef]
- Herr, N.; Bode, C.; Duerschmied, D. The Effects of Serotonin in Immune Cells. Front. Cardiovasc. Med. 2017, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Pilotte, L.; Larrieu, P.; Stroobant, V.; Colau, D.; Dolusic, E.; Frederick, R.; De Plaen, E.; Uyttenhove, C.; Wouters, J.; Masereel, B.; et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc. Natl. Acad. Sci. USA 2012, 109, 2497–2502. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef]
- Currier, A.R.; Ziegler, M.H.; Riley, M.M.; Babcock, T.A.; Telbis, V.P.; Carlin, J.M. Tumor necrosis factor-alpha and lipopolysaccharide enhance interferon-induced antichlamydial indoleamine dioxygenase activity independently. J. Interferon Cytokine Res. 2000, 20, 369–376. [Google Scholar] [CrossRef]
- Taylor, M.W.; Feng, G.S. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991, 5, 2516–2522. [Google Scholar] [CrossRef]
- Mezrich, J.D.; Fechner, J.H.; Zhang, X.; Johnson, B.P.; Burlingham, W.J.; Bradfield, C.A. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 2010, 185, 3190–3198. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ryu, H.; Ferrante, R.J.; Morris, S.M., Jr.; Ratan, R.R. Translational control of inducible nitric oxide synthase expression by arginine can explain the arginine paradox. Proc. Natl. Acad. Sci. USA 2003, 100, 4843–4848. [Google Scholar] [CrossRef] [PubMed]
- Rath, M.; Muller, I.; Kropf, P.; Closs, E.I.; Munder, M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front. Immunol. 2014, 5, 532. [Google Scholar] [CrossRef] [PubMed]
- Stout, R.D.; Suttles, J. Functional plasticity of macrophages: Reversible adaptation to changing microenvironments. J. Leukoc. Biol. 2004, 76, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C.; Rollinghoff, M.; Diefenbach, A. The role of nitric oxide in innate immunity. Immunol. Rev. 2000, 173, 17–26. [Google Scholar] [CrossRef]
- Kepka-Lenhart, D.; Mistry, S.K.; Wu, G.; Morris, S.M., Jr. Arginase I: A limiting factor for nitric oxide and polyamine synthesis by activated macrophages? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R2237–R2242. [Google Scholar] [CrossRef]
- Bronte, V.; Zanovello, P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 2005, 5, 641–654. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Grohmann, U.; Bronte, V. Control of immune response by amino acid metabolism. Immunol. Rev. 2010, 236, 243–264. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef]
- Guevara-Cruz, M.; Vargas-Morales, J.M.; Mendez-Garcia, A.L.; Lopez-Barradas, A.M.; Granados-Portillo, O.; Ordaz-Nava, G.; Rocha-Viggiano, A.K.; Gutierrez-Leyte, C.A.; Medina-Cerda, E.; Rosado, J.L.; et al. Amino acid profiles of young adults differ by sex, body mass index and insulin resistance. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 393–401. [Google Scholar] [CrossRef]
- Medina-Vera, I.; Sanchez-Tapia, M.; Noriega-Lopez, L.; Granados-Portillo, O.; Guevara-Cruz, M.; Flores-Lopez, A.; Avila-Nava, A.; Fernandez, M.L.; Tovar, A.R.; Torres, N. A dietary intervention with functional foods reduces metabolic endotoxaemia and attenuates biochemical abnormalities by modifying faecal microbiota in people with type 2 diabetes. Diabetes Metab. 2019, 45, 122–131. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Neinast, M.; Murashige, D.; Arany, Z. Branched Chain Amino Acids. Annu. Rev. Physiol. 2019, 81, 139–164. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Alcalde, I.; Tenorio-Guzman, M.R.; Tovar, A.R.; Salinas-Rubio, D.; Torre-Villalvazo, I.; Torres, N.; Noriega, L.G. Metabolic Fate of Branched-Chain Amino Acids During Adipogenesis, in Adipocytes From Obese Mice and C2C12 Myotubes. J. Cell Biochem. 2017, 118, 808–818. [Google Scholar] [CrossRef]
- Lackey, D.E.; Lynch, C.J.; Olson, K.C.; Mostaedi, R.; Ali, M.; Smith, W.H.; Karpe, F.; Humphreys, S.; Bedinger, D.H.; Dunn, T.N.; et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1175–E1187. [Google Scholar] [CrossRef]
- Salinas-Rubio, D.; Tovar, A.R.; Torre-Villalvazo, I.; Granados-Portillo, O.; Torres, N.; Pedraza-Chaverri, J.; Noriega, L.G. Interaction between leucine and palmitate catabolism in 3T3-L1 adipocytes and primary adipocytes from control and obese rats. J. Nutr. Biochem. 2018, 59, 29–36. [Google Scholar] [CrossRef] [PubMed]
- She, P.; Van Horn, C.; Reid, T.; Hutson, S.M.; Cooney, R.N.; Lynch, C.J. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1552–E1563. [Google Scholar] [CrossRef] [PubMed]
- Zoncu, R.; Bar-Peled, L.; Efeyan, A.; Wang, S.; Sancak, Y.; Sabatini, D.M. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 2011, 334, 678–683. [Google Scholar] [CrossRef]
- Zoncu, R.; Efeyan, A.; Sabatini, D.M. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Green, C.R.; Wallace, M.; Divakaruni, A.S.; Phillips, S.A.; Murphy, A.N.; Ciaraldi, T.P.; Metallo, C.M. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat. Chem. Biol. 2016, 12, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Halama, A.; Horsch, M.; Kastenmuller, G.; Moller, G.; Kumar, P.; Prehn, C.; Laumen, H.; Hauner, H.; Hrabe de Angelis, M.; Beckers, J.; et al. Metabolic switch during adipogenesis: From branched chain amino acid catabolism to lipid synthesis. Arch. Biochem. Biophys. 2016, 589, 93–107. [Google Scholar] [CrossRef]
- Hong, S.; Zhou, W.; Fang, B.; Lu, W.; Loro, E.; Damle, M.; Ding, G.; Jager, J.; Zhang, S.; Zhang, Y.; et al. Dissociation of muscle insulin sensitivity from exercise endurance in mice by HDAC3 depletion. Nat. Med. 2017, 23, 223–234. [Google Scholar] [CrossRef]
- Lerin, C.; Goldfine, A.B.; Boes, T.; Liu, M.; Kasif, S.; Dreyfuss, J.M.; De Sousa-Coelho, A.L.; Daher, G.; Manoli, I.; Sysol, J.R.; et al. Defects in muscle branched-chain amino acid oxidation contribute to impaired lipid metabolism. Mol. Metab. 2016, 5, 926–936. [Google Scholar] [CrossRef]
- Muoio, D.M. Metabolic inflexibility: When mitochondrial indecision leads to metabolic gridlock. Cell 2014, 159, 1253–1262. [Google Scholar] [CrossRef]
- Chandel, N.S. Amino Acid Metabolism. Cold Spring Harb. Perspect. Biol. 2021, 13, a040584. [Google Scholar] [CrossRef]
- Oxenkrug, G.F. Genetic and hormonal regulation of tryptophan kynurenine metabolism: Implications for vascular cognitive impairment, major depressive disorder, and aging. Ann. N. Y. Acad. Sci. 2007, 1122, 35–49. [Google Scholar] [CrossRef]
- Schwarcz, R. The kynurenine pathway of tryptophan degradation as a drug target. Curr. Opin. Pharmacol. 2004, 4, 12–17. [Google Scholar] [CrossRef]
- Chen, T.; Zheng, X.; Ma, X.; Bao, Y.; Ni, Y.; Hu, C.; Rajani, C.; Huang, F.; Zhao, A.; Jia, W.; et al. Tryptophan Predicts the Risk for Future Type 2 Diabetes. PLoS ONE 2016, 11, e0162192. [Google Scholar] [CrossRef] [PubMed]
- Breum, L.; Rasmussen, M.H.; Hilsted, J.; Fernstrom, J.D. Twenty-four-hour plasma tryptophan concentrations and ratios are below normal in obese subjects and are not normalized by substantial weight reduction. Am. J. Clin. Nutr. 2003, 77, 1112–1118. [Google Scholar] [CrossRef]
- Muzik, O.; Burghardt, P.; Yi, Z.; Kumar, A.; Seyoum, B. Successful metformin treatment of insulin resistance is associated with down-regulation of the kynurenine pathway. Biochem. Biophys. Res. Commun. 2017, 488, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Oxenkrug, G.; van der Hart, M.; Summergrad, P. Elevated anthranilic acid plasma concentrations in type 1 but not type 2 diabetes mellitus. Integr. Mol. Med. 2015, 2, 365–368. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kotake, Y.; Ueda, T.; Mori, T.; Igaki, S.; Hattori, M. Abnormal tryptophan metabolism and experimental diabetes by xanthurenic acid (XA). Acta Vitaminol. Enzymol. 1975, 29, 236–239. [Google Scholar] [PubMed]
- Lin, H.V.; Efanov, A.M.; Fang, X.; Beavers, L.S.; Wang, X.; Wang, J.; Gonzalez Valcarcel, I.C.; Ma, T. GPR142 Controls Tryptophan-Induced Insulin and Incretin Hormone Secretion to Improve Glucose Metabolism. PLoS ONE 2016, 11, e0157298. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef]
- Nicholls, D.G. The hunt for the molecular mechanism of brown fat thermogenesis. Biochimie 2017, 134, 9–18. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Q.A.; Liu, Y.; Jiang, L. Energy metabolism in brown adipose tissue. FEBS J. 2021, 288, 3647–3662. [Google Scholar] [CrossRef]
- Yoneshiro, T.; Wang, Q.; Tajima, K.; Matsushita, M.; Maki, H.; Igarashi, K.; Dai, Z.; White, P.J.; McGarrah, R.W.; Ilkayeva, O.R.; et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 2019, 572, 614–619. [Google Scholar] [CrossRef]

| Amino Acid (1-Letter) | AADE | NameAADE | Tissue Expression Humans [48] | Tissue Expression Rat [49] | Tissue Expression Mouse [50] |
|---|---|---|---|---|---|
| Alanine (A) | Glutamic pyruvate transaminase | Gpt | Liver, Kidney, fat, colon, duodenum small intestine, stomach | Liver, lung muscle, thymus, uterus | Liver, duodenum, large intestine, subcutaneous fat pad, stomach |
| Arginine (R) | Arginase 1 | Arg1 | Liver, skyn, bone marrow | Liver, lung, testes, uterus | Liver, lung, ovary |
| Asparagine (N) | Asparaginase | Aspg | Liver, kidney, heart, ovary, colon, sky, stomach, lung | Liver, kidney, lung. Adrenal, testes | Liver, kidney, mammary gland, colon, large intestine |
| Aspartic acid (D) | glutamic-oxaloacetic transaminase 1 | Got1 | Heart, liver, kidney, brain, duodenum, colon, small intestine | Heart, muscle, brain, liver, lung | Heart, liver, kidney, cerebellum, cortex |
| Cysteine (C) | Cysteine dioxygenase 1 | Cdo1 | Liver, fat, placenta, testis, brain, lung | Liver, thymus, adrenal, lung | Liver, genital fat, mammary gland, subcutaneous fat pad |
| Glutamic acid (E) | Glutamate dehydrogenase 1 | Glud1 | Liver, kidney, prostate, brain, small intestine, stomach, adrenal (16) | Liver, kidney, brain, uterus, muscle, heart | Liver, kidney, small intestine, adrenal |
| Glutamine (Q) | Glutaminase | Gls | Liver, kidney, small intestine, brain, duodenum, lung | Kidney, brain, adrenal, lung, muscle, testes, thymus, liver | CNS E18, Cortex, frontal lobe, cerebelum, thymus |
| Glycine (G) | Serine hydroximethyl transferase 1 | Shmt1 | Kidney, liver, fat, duodenum, small intestine, esophagus, thyroid | Kidney, liver, adrenal, testes, thymus | Liver, kidney, placenta, testis |
| Histidine (H) | Histidine ammonia lyase | Hal | Liver, skin, bone marrow, appendix, spleen. | Liver, lung, testes, uterus. | Liver, genital fat pad, placenta |
| Lysine (K) | Glutaryl-CoA dehydrogenase | Gcdh | Liver, kidney, ovary, fat, heart | Kidney, liver, adrenal, lung, muscle | Liver, kidney, adrenal, heart |
| Methionine (M) | Methionine adenosyl transferase | Mat1a | Liver, pancreas, ovary, skin, testis | Liver, lung, uterus, adrenal | Liver |
| Phenylalanine (F) | Phenylalanine hidroxylase | Pah | Liver, kidney, gall bladder | Liver, kidney, lung, testes, uterus | Kidney, liver |
| Proline (P) | Proline dehydrogenase | Prodh | Small intestine, skin, lung, duodenum, brain, kidney | Liver, kidney, heart, thymus, brain | Kidney, liver, large intestine, small intestine, genital, duodenum |
| Serine (S) | Serine dehydratase | Sds | Liver, stomach, brain | Liver, kidney, testes | Liver, heart |
| Threonine (T) | Serine dehydratase like | sdsl | Kidney, liver, thyroid, adrenal, colon duodenum | Testes, liver, kidney, brain, uterus | Adrenal, duodenum, ovary, colon |
| Tryptophan (W) | Tryptophan 2,3-dioxygenase | Tdo2 | Liver, appendix, urinary bladder, small intestine | Liver, lung, uterus | Liver, placenta |
| Aminocarboxymuconate semialdehyde decarboxylase | Acmsd | Kidney, liver, gall bladder | Kidney, liver | Kidney, liver | |
| Tyrosine (Y) | Tyrosine aminotransferase | Tat | Liver | Liver, kidney, colon, large intestine | Liver, lung, uterus, adrenal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, N.; Tobón-Cornejo, S.; Velazquez-Villegas, L.A.; Noriega, L.G.; Alemán-Escondrillas, G.; Tovar, A.R. Amino Acid Catabolism: An Overlooked Area of Metabolism. Nutrients 2023, 15, 3378. https://doi.org/10.3390/nu15153378
Torres N, Tobón-Cornejo S, Velazquez-Villegas LA, Noriega LG, Alemán-Escondrillas G, Tovar AR. Amino Acid Catabolism: An Overlooked Area of Metabolism. Nutrients. 2023; 15(15):3378. https://doi.org/10.3390/nu15153378
Chicago/Turabian StyleTorres, Nimbe, Sandra Tobón-Cornejo, Laura A. Velazquez-Villegas, Lilia G. Noriega, Gabriela Alemán-Escondrillas, and Armando R. Tovar. 2023. "Amino Acid Catabolism: An Overlooked Area of Metabolism" Nutrients 15, no. 15: 3378. https://doi.org/10.3390/nu15153378
APA StyleTorres, N., Tobón-Cornejo, S., Velazquez-Villegas, L. A., Noriega, L. G., Alemán-Escondrillas, G., & Tovar, A. R. (2023). Amino Acid Catabolism: An Overlooked Area of Metabolism. Nutrients, 15(15), 3378. https://doi.org/10.3390/nu15153378







