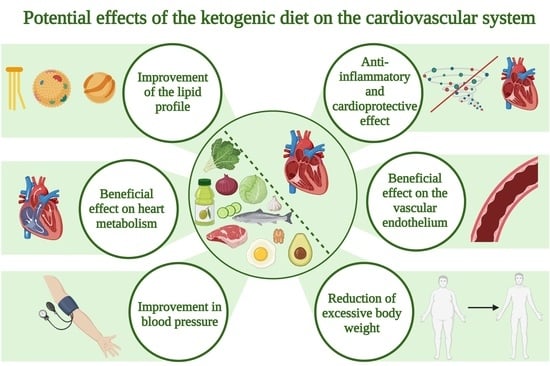
The Ketogenic Diet and Cardiovascular Diseases
Abstract
1. Introduction
2. The Ketogenic Diet and Blood Lipid Profile
2.1. Lipid Profile and Cardiovascular Diseases
2.2. The Effect of the Ketogenic Diet on the Blood Lipid Profile
3. Anti-Inflammatory Potential of the Ketogenic Diet in Cardiovascular Diseases
3.1. Anti-Inflammatory, Cardioprotective Potential of the State of Ketosis (Ketone Bodies)
3.2. Anti-Inflammatory, Cardioprotective Effects of Elimination of Simple Sugars
3.3. Anti-Inflammatory, Cardioprotective Effects of Total Carbohydrate Restriction
3.4. Anti-Inflammatory, Cardioprotective Effects of Omega-3 Fatty Acids
4. Ketone Bodies and Cardiac Energy Metabolism
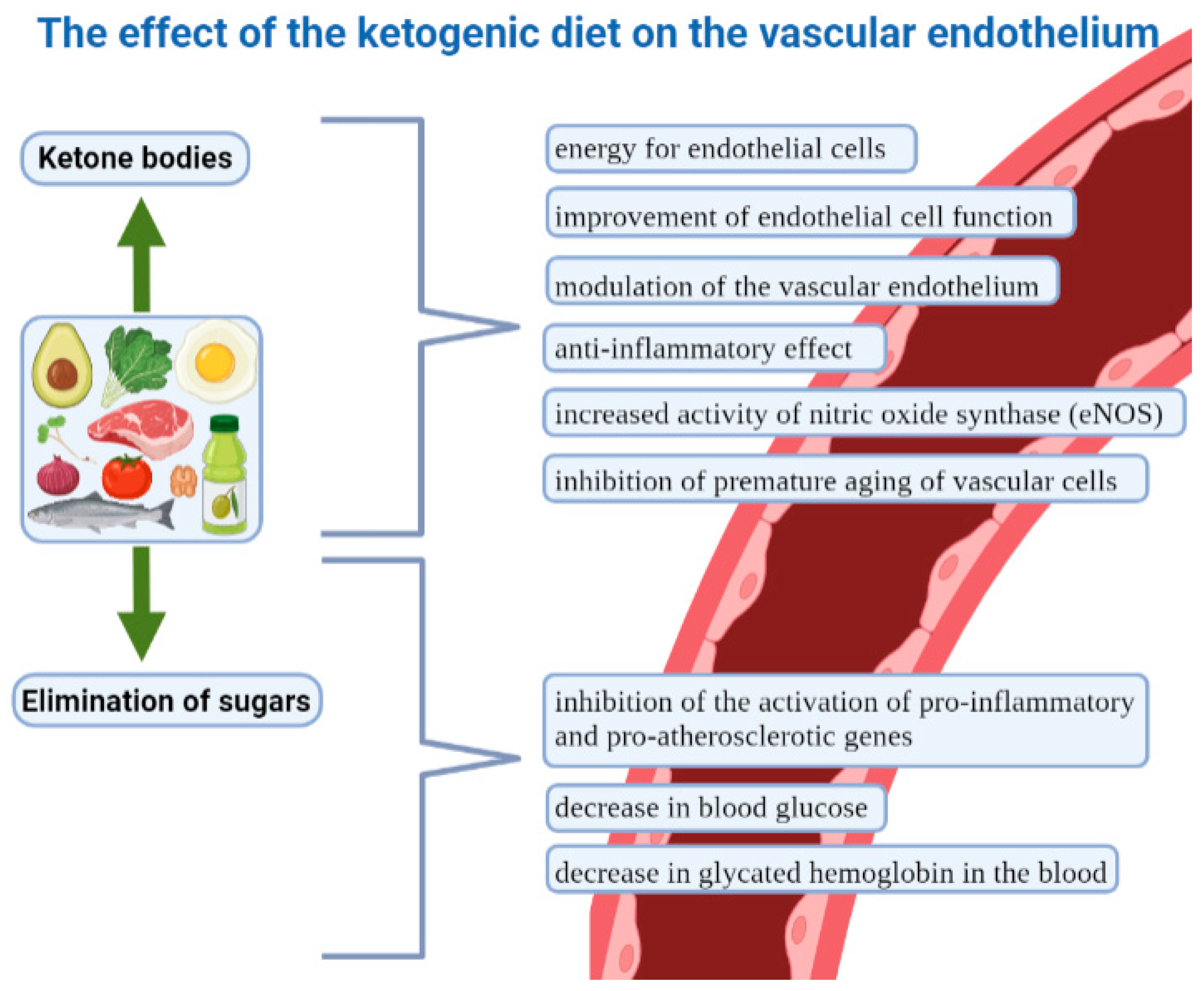
5. The Ketogenic Diet and the Vascular Endothelium
6. The Ketogenic Diet and Blood Pressure
7. The Ketogenic Diet and Weight Loss as a Factor in CVD Prevention and Therapy
8. The Effect of the Ketogenic Diet among Patients with CVD and Healthy People
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 4 March 2023).
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639, Erratum in Circulation 2022, 146, e141. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 4 March 2023).
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef]
- Available online: https://www.cdc.gov/chronicdisease/resources/publications/factsheets/heart-disease-stroke.htm (accessed on 4 March 2023).
- Vancheri, F.; Longo, G.; Vancheri, E.; Henein, M.Y. MentalStress and Cardiovascular Health—Part I. J. Clin. Med. 2022, 11, 3353. [Google Scholar] [CrossRef]
- Cosentino, N.; Campodonico, J.; Milazzo, V.; De Metrio, M.; Brambilla, M.; Camera, M.; Marenzi, G. Vitamin D and Cardiovascular Disease: Current Evidence and Future Perspectives. Nutrients 2021, 13, 3603. [Google Scholar] [CrossRef]
- Muzaffar, R.; Khan, M.A.; Mushtaq, M.H.; Nasir, M.; Khan, A.; Haq, I.U.; Muhammad, J. Hyperhomocysteinemia as an Independent Risk Factor for Coronary Heart Disease. Comparison with Conventional Risk Factors. Braz. J. Biol. 2021, 83, e249104. [Google Scholar] [CrossRef]
- Khan, M.S.; Saeedullah, A.; Andrews, S.C.; Iqbal, K.; Qadir, S.A.; Shahzad, B.; Ahmed, Z.; Shahzad, M. Adolescent Afghan Refugees Display a High Prevalence of Hyperhomocysteinemia and Associated Micronutrients Deficiencies Indicating an Enhanced Risk of Cardiovascular Disease in Later Life. Nutrients 2022, 14, 1751. [Google Scholar] [CrossRef]
- Xiao, K.; Chen, Y.; Xiao, L.; Sun, H.; He, Z.; Huang, G.; Chen, L.; Xv, L.; Peng, L.; Li, J.; et al. The relationship between hyperhomocysteinemia and total coronary artery occlusion: A cross-sectional study from Southwest China. Coron. Artery Dis. 2023, 34, 138–145. [Google Scholar] [CrossRef]
- Tyrovola, D.; Soulaidopoulos, S.; Tsioufis, C.; Lazaros, G. The Role of Nutrition in Cardiovascular Disease: Current Concepts and Trends. Nutrients 2023, 15, 1064. [Google Scholar] [CrossRef]
- Kahleova, H.; Levin, S.; Barnard, N.D. Vegetarian Dietary Patterns and Cardiovascular Disease. Prog. Cardiovasc. Dis. 2018, 61, 54–61. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, 596–646. [Google Scholar]
- Mitrou, P.N.; Kipnis, V.; Thiébaut, A.C.; Reedy, J.; Subar, A.F.; Wirfält, E.; Flood, A.; Mouw, T.; Hollenbeck, A.R.; Leitzmann, M.F.; et al. Mediterranean dietary pattern and prediction of all-cause mortality in a US population: Results from the NIH–AARP diet and health study. Arch. Intern. Med. 2007, 167, 2461–2468. [Google Scholar] [CrossRef]
- Fung, T.T.; Rexrode, K.M.; Mantzoros, C.S.; Manson, J.E.; Willett, W.C.; Hu, F.B. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009, 119, 1093–1100. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Viguiliouk, E.; Nishi, S.K.; Blanco Mejia, S.; Rahelić, D.; Kahleová, H.; Salas-Salvadó, J.; Kendall, C.W.; Sievenpiper, J.L. DASH Dietary Pattern and Cardiometabolic Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients 2019, 11, 338. [Google Scholar] [CrossRef]
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; de Jesus, J.M.; Miller, N.H.; Hubbard, V.S.; Lee, I.M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J. Am. Coll. Cardiol. 2014, 63, 2960–2984. [Google Scholar] [CrossRef]
- Wilson, J.; Lowery, R. The Ketogenic Bible; Victory Belt Publishing Inc.: Las Vegas, NV, USA, 2017; ISBN 13:978-1-628601-04-6. [Google Scholar]
- Gardner, C.D.; Vadiveloo, M.K.; Petersen, K.S.; Anderson, C.A.M.; Springfield, S.; Van Horn, L.; Khera, A.; Lamendola, C.; Mayo, S.M.; Joseph, J.J. American Heart Association Council on Lifestyle and Cardiometabolic Health. Popular Dietary Patterns: Alignment with American Heart Association 2021 Dietary Guidance: A Scientific Statement from the American Heart Association. Circulation 2023, 147, 1715–1730. [Google Scholar] [CrossRef]
- Du, Z.; Qin, Y. Dyslipidemia and Cardiovascular Disease: Current Knowledge, Existing Challenges, and New Opportunities for Management Strategies. J. Clin. Med. 2023, 12, 363. [Google Scholar] [CrossRef]
- Jung, E.; Kong, S.Y.; Ro, Y.S.; Ryu, H.H.; Shin, S.D. Serum Cholesterol Levels and Risk of Cardiovascular Death: A Systematic Review and a Dose-Response Meta-Analysis of Prospective Cohort Studies. Int. J. Environ. Res. Public Health 2022, 19, 8272. [Google Scholar] [CrossRef]
- Dong, J.; Yang, S.; Zhuang, Q.; Sun, J.; Wei, P.; Zhao, X.; Chen, Y.; Chen, X.; Li, M.; Wei, L.; et al. The Associations of Lipid Profiles With Cardiovascular Diseases and Death in a 10-Year Prospective Cohort Study. Front. Cardiovasc. Med. 2021, 8, 745539. [Google Scholar] [CrossRef]
- Yi, S.W.; Yi, J.J.; Ohrr, H. Total cholesterol and all-cause mortality by sex and age: A prospective cohort study among 12.8 million adults. Sci. Rep. 2019, 9, 1596. [Google Scholar] [CrossRef]
- BHF-HEART STATS and WHO-MORTALITY (Adapted). Total Cholesterol Levels vs Mortality Data from 164 Countries, 2005. Available online: https://renegadewellness.files.wordpress.com/2011/02/cholesterol-mortality-chart.pdf (accessed on 23 July 2023).
- Ravnskov, U.; de Lorgeril, M.; Diamond, D.M.; Hama, R.; Hamazaki, T.; Hammarskjöld, B.; Hynes, N.; Kendrick, M.; Langsjoen, P.H.; Mascitelli, L.; et al. LDL-C does not cause cardiovascular disease: A comprehensive review of the current literature. Expert Rev. Clin. Pharmacol. 2018, 11, 959–970. [Google Scholar] [CrossRef]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef]
- Peng, K.M.; Li, X.; Wang, Z.; Li, M.M.; Yang, Y. Association of low-density lipoprotein cholesterol levels with the risk of mortality and cardiovascular events: A meta-analysis of cohort studies with 1,232,694 participants. Medicine 2022, 101, e32003. [Google Scholar] [CrossRef]
- Bhargava, S.; de la Puente-Secades, S.; Schurgers, L.; Jankowski, J. Lipids and lipoproteins in cardiovascular diseases: A classification. Trends Endocrinol. Metab. 2022, 33, 409–423. [Google Scholar] [CrossRef]
- Kim, Y.G.; Jeong, J.H.; Han, K.D.; Roh, S.Y.; Min, K.; Lee, H.S.; Choi, Y.Y.; Shim, J.; Choi, J.I.; Kim, Y.H. Association between low-density lipoprotein cholesterol and sudden cardiac arrest in people with diabetes mellitus. Cardiovasc. Diabetol. 2023, 22, 36. [Google Scholar] [CrossRef]
- Rong, S.; Li, B.; Chen, L.; Sun, Y.; Du, Y.; Liu, B.; Robinson, J.G.; Bao, W. Association of Low-Density Lipoprotein Cholesterol Levels with More than 20-Year Risk of Cardiovascular and All-Cause Mortality in the General Population. J. Am. Heart Assoc. 2022, 11, e023690. [Google Scholar] [CrossRef]
- Feingold, K.R. Utility of Advanced Lipoprotein Testing in Clinical Practice; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., Eds.; Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Nicholls, S.J.; Nelson, A.J. HDL and cardiovascular disease. Pathology 2019, 51, 142–147. [Google Scholar] [CrossRef]
- Trimarco, V.; Izzo, R.; Morisco, C.; Mone, P.; Virginia Manzi, M.; Falco, A.; Pacella, D.; Gallo, P.; Lembo, M.; Santulli, G.; et al. High HDL (High-Density Lipoprotein) Cholesterol Increases Cardiovascular Risk in Hypertensive Patients. Hypertension 2022, 79, 2355–2363. [Google Scholar] [CrossRef]
- Cho, Y.K.; Jung, C.H. HDL-C and Cardiovascular Risk: You Don’t Need to Worry about Extremely High HDL-C Levels. J. Lipid Atheroscler. 2021, 10, 57–61. [Google Scholar] [CrossRef]
- Farnier, M.; Zeller, M.; Masson, D.; Cottin, Y. Triglycerides and risk of atherosclerotic cardiovascular disease: An update. Arch Cardiovasc. Dis. 2021, 114, 132–139. [Google Scholar] [CrossRef]
- Xia, T.L.; Li, Y.M.; Huang, F.Y.; Chai, H.; Huang, B.T.; Li, Q.; Zhao, Z.-G.; Liao, Y.-B.; Zuo, Z.L.; Peng, Y.; et al. The triglyceride paradox in the mortality of coronary artery disease. Lipids Health Dis. 2019, 18, 21. [Google Scholar] [CrossRef]
- Jain, M.; Jain, A.; Yerragondu, N.; Brown, R.D.; Rabinstein, A.; Jahromi, B.S.; Vaidyanathan, L.; Blyth, B.; Stead, L.G. The triglyceride paradox in stroke survivors: A prospective study. Neurosci. J. 2013, 2013, 870608. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Rodriguez Polanco, S.; Bousvarou, M.D.; Papakonstantinou, E.J.; Peña Genao, E.; Guzman, E.; Kostara, C.E. The Triglyceride/High-Density Lipoprotein Cholesterol (TG/HDL-C) Ratio as a Risk Marker for Metabolic Syndrome and Cardiovascular Disease. Diagnostics 2023, 13, 929. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, J.; Zhang, H.; Pan, H.; Li, Z.; Liu, Y.; Miao, X.; Han, Z.; Kang, X.; Li, X.; et al. Longitudinal association of remnant cholesterol with joint arteriosclerosis and atherosclerosis progression beyond LDL cholesterol. BMC Med. 2023, 21, 42. [Google Scholar] [CrossRef]
- Varbo, A.; Freiberg, J.J.; Nordestgaard, B.G. Extreme Nonfasting Remnant Cholesterol vs Extreme LDL Cholesterol as Contributors to Cardiovascular Disease and All-Cause Mortality in 90,000 Individuals from the General Population. Clin. Chem. 2015, 61, 533–543. [Google Scholar] [CrossRef]
- Tamang, H.K.; Timilsina, U.; Singh, K.P.; Shrestha, S.; Raman, R.K.; Panta, P.; Karna, P.; Khadka, L.; Dahal, C. Apo B/Apo A-I Ratio is Statistically A Better Predictor of Cardiovascular Disease (CVD) than Conventional Lipid Profile: A Study from Kathmandu Valley, Nepal. J. Clin. Diagn. Res. 2014, 8, 34–36. [Google Scholar] [CrossRef]
- Li, S.; Lin, G.; Chen, J.; Chen, Z.; Xu, F.; Zhu, F.; Zhang, J.; Yuan, S. The effect of periodic ketogenic diet on newly diagnosed overweight or obese patients with type 2 diabetes. BMC Endocr. Disord. 2022, 22, 34. [Google Scholar] [CrossRef]
- Gardner, C.D.; Landry, M.J.; Perelman, D.; Petlura, C.; Durand, L.R.; Aronica, L.; Crimarco, A.; Cunanan, K.M.; Chang, A.; Dant, C.C.; et al. Effect of a ketogenic diet versus Mediterranean diet on glycated hemoglobin in individuals with prediabetes and type 2 diabetes mellitus: The interventional Keto-Med randomized crossover trial. Am. J. Clin. Nutr. 2022, 116, 640–652, Erratum in Am. J. Clin. Nutr. 2022, 116, 1904. [Google Scholar] [CrossRef]
- Schiavo, L.; Pierro, R.; Asteria, C.; Calabrese, P.; Di Biasio, A.; Coluzzi, I.; Severino, L.; Giovanelli, A.; Pilone, V.; Silecchia, G. Low-Calorie Ketogenic Diet with Continuous Positive Airway Pressure to Alleviate Severe Obstructive Sleep Apnea Syndrome in Patients with Obesity Scheduled for Bariatric/Metabolic Surgery: A Pilot, Prospective, Randomized Multicenter Comparative Study. Obes. Surg. 2022, 32, 634–642. [Google Scholar] [CrossRef]
- Vidić, V.; Ilić, V.; Toskić, L.; Janković, N.; Ugarković, D. Effects of calorie restricted low carbohydrate high fat ketogenic vs. non-ketogenic diet on strength, body-composition, hormonal and lipid profile in trained middle-aged men. Clin. Nutr. 2021, 40, 1495–1502. [Google Scholar] [CrossRef]
- Burén, J.; Ericsson, M.; Damasceno, N.R.T.; Sjödin, A. A Ketogenic Low-Carbohydrate High-Fat Diet Increases LDL Cholesterol in Healthy, Young, Normal-Weight Women: A Randomized Controlled Feeding Trial. Nutrients 2021, 13, 814. [Google Scholar] [CrossRef] [PubMed]
- Ravnskov, U. Is High Cholesterol Deleterious? An Alternative Point of View. Comment on Burén et al. A Ketogenic Low-Carbohydrate High-Fat Diet Increases LDL Cholesterol in Healthy, Young, Normal-Weight Women: A Randomized Controlled Feeding Trial. Nutrients 2021, 13, 814, Nutrients2021, 13, 2119. [Google Scholar] [CrossRef] [PubMed]
- Basciani, S.; Camajani, E.; Contini, S.; Persichetti, A.; Risi, R.; Bertoldi, L.; Strigari, L.; Prossomariti, G.; Watanabe, M.; Mariani, S.; et al. Very-Low-Calorie Ketogenic Diets with Whey, Vegetable, or Animal Protein in Patients with Obesity: A Randomized Pilot Study. J. Clin. Endocrinol. Metab. 2020, 105, dgaa336. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, M.M.; Klonek, G.; Maszczyk, A.; Zajac, A. The Effects of a Low Calorie Ketogenic Diet on Glycaemic Control Variables in Hyperinsulinemic Overweight/Obese Females. Nutrients 2020, 12, 1854. [Google Scholar] [CrossRef]
- Saslow, L.R.; Daubenmier, J.J.; Moskowitz, J.T.; Kim, S.; Murphy, E.J.; Phinney, S.D.; Ploutz-Snyder, R.; Goldman, V.; Cox, R.M.; Mason, A.E.; et al. Twelve-month outcomes of a randomized trial of a moderate-carbohydrate versus very low-carbohydrate diet in overweight adults with type 2 diabetes mellitus or prediabetes. Nutr. Diabetes 2017, 7, 304. [Google Scholar] [CrossRef]
- Paoli, A.; Moro, T.; Bosco, G.; Bianco, A.; Grimaldi, K.A.; Camporesi, E.; Mangar, D. Effects of n-3 polyunsaturated fatty acids (ω-3) supplementation on some cardiovascular risk factors with a ketogenic Mediterranean diet. Mar. Drugs. 2015, 13, 996–1009. [Google Scholar] [CrossRef]
- Partsalaki, I.; Karvela, A.; Spiliotis, B.E. Metabolic impact of a ketogenic diet compared to a hypocaloric diet in obese children and adolescents. J. Pediatr. Endocrinol. Metab. 2012, 25, 697–704. [Google Scholar] [CrossRef]
- Amanollahi, A.; Khazdouz, M.; Malekahmadi, M.; Klement, R.J.; Lee, D.; Khodabakhshi, A. Effect of Ketogenic Diets on Cardio-Metabolic Outcomes in Cancer Patients: A Systematic Review and Meta-Analysis of Controlled Clinical Trials. Nutr. Cancer 2023, 75, 95–111. [Google Scholar] [CrossRef]
- Li, M.; Yuan, J. Effects of very low-carbohydrate ketogenic diet on lipid metabolism in patients with type II diabetes mellitus: A meta-analysis. Nutr. Hosp. 2022, 39, 916–923, English. [Google Scholar] [CrossRef]
- Rafiullah, M.; Musambil, M.; David, S.K. Effect of a very low-carbohydrate ketogenic diet vs recommended diets in patients with type 2 diabetes: A meta-analysis. Nutr. Rev. 2022, 80, 488–502. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, J.; Yang, S.; Gao, M.; Cao, L.; Li, X.; Hong, D.; Tian, S.; Sun, C. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: A systematic review and meta-analysis. Nutr. Diabetes 2020, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- López-Espinoza, M.Á.; Chacón-Moscoso, S.; Sanduvete-Chaves, S.; Ortega-Maureira, M.J.; Barrientos-Bravo, T. Effect of a Ketogenic Diet on the Nutritional Parameters of Obese Patients: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 2946. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; El Ghoch, M.; Colao, A.; Hassapidou, M.; Yumuk, V.; Busetto, L. Obesity Management Task Force (OMTF) of the European Association for the Study of Obesity (EASO). European Guidelines for Obesity Management in Adults with a Very Low-Calorie Ketogenic Diet: A Systematic Review and Meta-Analysis. Obes. Facts. 2021, 14, 222–245. [Google Scholar] [CrossRef] [PubMed]
- Castellana, M.; Conte, E.; Cignarelli, A.; Perrini, S.; Giustina, A.; Giovanella, L.; Giorgino, F.; Trimboli, P. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2020, 21, 5–16. [Google Scholar] [CrossRef]
- Kern, F., Jr. Normal plasma cholesterol in an 88-year-old man who eats 25 eggs a day. Mechanisms of adaptation. N. Engl. J. Med. 1991, 324, 896–899. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Murillo, A.G. Is There a Correlation between Dietary and Blood Cholesterol? Evidence from Epidemiological Data and Clinical Interventions. Nutrients 2022, 14, 2168. [Google Scholar] [CrossRef] [PubMed]
- Ravnskov, U.; Diamond, D.M.; Hama, R.; Hamazaki, T.; Hammarskjöld, B.; Hynes, N.; Kendrick, M.; Langsjoen, P.H.; Malhotra, A.; Mascitelli, L.; et al. Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: A systematic review. BMJ Open 2016, 6, e010401. [Google Scholar] [CrossRef]
- Ravnskov, U.; de Lorgeril, M.; Diamond, D.M.; Hama, R.; Hamazaki, T.; Hammarskjöld, B.; Harcombe, Z.; Kendrick, M.; Langsjoen, P.H.; McCully, K.S.; et al. The LDL paradox: Higher LDL-cholesterol is associated with greater longevity. Ann. Epidemiol. Public Health 2020, 3, 1040–1047. [Google Scholar]
- Sorriento, D.; Iaccarino, G. Inflammation and Cardiovascular Diseases: The Most Recent Findings. Int. J. Mol. Sci. 2019, 20, 3879. [Google Scholar] [CrossRef]
- Fiordelisi, A.; Iaccarino, G.; Morisco, C.; Coscioni, E.; Sorriento, D. NFkappaB is a Key Player in the Crosstalk between Inflammation Cardiovascular Diseases. Int. J. Mol. Sci. 2019, 20, 1599. [Google Scholar] [CrossRef]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 1414. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, V.; Lobasso, A.; Barbieri, L.; Parrella, P.; Ciervo, D.; Liccardo, B.; Bonaduce, D.; Tocchetti, C.G.; De Paulis, A.; Rossi, F.W. Inflammatory, Serological and Vascular Determinants of Cardiovascular Disease in Systemic Lupus Erythematosus Patients. Int. J. Mol. Sci. 2019, 20, 2154. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Loffredo, S.; Borriello, F.; Pecoraro, A.; Rivellese, F.; Genovese, A.; Spadaro, G.; Marone, G. Superantigenic Activation of Human Cardiac Mast Cells. Int. J. Mol. Sci. 2019, 20, 1828. [Google Scholar] [CrossRef] [PubMed]
- Brigant, B.; Metzinger-Le Meuth, V.; Rochette, J.; Metzinger, L. TRIMming down to TRIM37: Relevance to Inflammation, Cardiovascular Disorders, and Cancer in MULIBREY Nanism. Int. J. Mol. Sci. 2018, 20, 67. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef]
- Sklerov, M.; Dayan, E.; Browner, N. Functional neuroimaging of the central autonomic network: Recent developments and clinical implications. Clin. Auton. Res. 2019, 29, 555–566. [Google Scholar] [CrossRef]
- Kraynak, T.E.; Marsland, A.L.; Gianaros, P.J. Neural Mechanisms Linking Emotion with Cardiovascular Disease. Curr. Cardiol. Rep. 2018, 20, 128. [Google Scholar] [CrossRef]
- Muscatell, K.A.; Dedovic, K.; Slavich, G.M.; Jarcho, M.R.; Breen, E.C.; Bower, J.E.; Irwin, M.R.; Eisenberger, N.I. Greater amygdala activity and dorsomedial prefrontal–amygdala coupling are associated with enhanced inflammatory responses to stress. Brain Behav. Immun. 2015, 43, 46–53. [Google Scholar] [CrossRef]
- Tracey, K.J. The inflammatory reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef]
- Shah, S.M.; Meadows, J.L.; Burg, M.M.; Pfau, S.; Soufer, R. Effects of Psychological Stress on Vascular Physiology: Beyond the Current Imaging Signal. Curr. Cardiol. Rep. 2020, 22, 156. [Google Scholar] [CrossRef] [PubMed]
- Tawakol, A.; Ishai, A.; Takx, R.A.P.; Figueroa, A.L.; Ali, A.; Kaiser, Y.; Truong, Q.A.; Solomon, C.J.E.; Calcagno, C.; Mani, V.; et al. Relation between resting amygdalar activity and cardiovascular events: A longitudinal and cohort study. Lancet 2017, 389, 834–845. [Google Scholar] [CrossRef]
- Pondel, N.; Liśkiewicz, D.; Liśkiewicz, A. Dieta ketogeniczna–mechanizm działania i perspektywy zastosowania w terapii: Dane z badań klinicznych. Postępy Biochem. 2020, 66, 270–286. [Google Scholar] [CrossRef]
- Dyńka, D.; Kowalcze, K.; Paziewska, A. The Role of Ketogenic Diet in the Treatment of Neurological Diseases. Nutrients 2022, 14, 5003. [Google Scholar] [CrossRef]
- Hwang, C.Y.; Choe, W.; Yoon, K.-S.; Ha, J.; Kim, S.S.; Yeo, E.-J.; Kang, I. Molecular Mechanisms for Ketone Body Metabolism, Signaling Functions, and Therapeutic Potential in Cancer. Nutrients 2022, 14, 4932. [Google Scholar] [CrossRef] [PubMed]
- Dyńka, D.; Kowalcze, K.; Ambrozkiewicz, F.; Paziewska, A. Effect of the Ketogenic Diet on the Prophylaxis and Treatment of Diabetes Mellitus: A Review of the Meta-Analyses and Clinical Trials. Nutrients 2023, 15, 500. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Yan, X.; Liu, Y.; Huang, L.; Zhu, Y.; He, J.; Gao, R.; Kalady, M.F.; Goel, A.; Qin, H.; et al. Ketogenic diet alleviates colitis by reduction of colonic group 3 innate lymphoid cells through altering gut microbiome. Signal Transduct. Target. Ther. 2021, 6, 154. [Google Scholar] [CrossRef]
- Field, R.; Pourkazemi, F.; Rooney, K. Effects of a Low-Carbohydrate Ketogenic Diet on Reported Pain, Blood Biomarkers and Quality of Life in Patients with Chronic Pain: A Pilot Randomized Clinical Trial. Pain Med. 2022, 23, 326–338. [Google Scholar] [CrossRef]
- Alkhorayef, N.; Almutery, F.T.; Rasheed, Z.; Althwab, S.A.; Aljohani, A.S.M.; Alhawday, Y.A.N.; Salem, T.; Alharbi, A.M.; Wahaq, A.A.A.B.; Alharbi, F.S.; et al. Regulatory effects of ketogenic diet on the inflammatory response in obese Saudi women. J. Taibah Univ. Med. Sci. 2023, 18, 1101–1107. [Google Scholar] [CrossRef]
- Mohammadifard, N.; Haghighatdoost, F.; Rahimlou, M.; Rodrigues, A.P.S.; Gaskarei, M.K.; Okhovat, P.; de Oliveira, C.; Silveira, E.A.; Sarrafzadegan, N. The Effect of Ketogenic Diet on Shared Risk Factors of Cardiovascular Disease and Cancer. Nutrients 2022, 14, 3499. [Google Scholar] [CrossRef]
- Mezzaroma, E.; Toldo, S.; Farkas, D.; Seropian, I.M.; Van Tassell, B.W.; Salloum, F.N.; Kannan, H.R.; Menna, A.C.; Voelkel, N.F.; Abbate, A. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc. Natl. Acad. Sci. USA 2011, 108, 19725–19730. [Google Scholar] [CrossRef]
- Tong, Y.; Wang, Z.; Cai, L.; Lin, L.; Liu, J.; Cheng, J. NLRP3 Inflammasome and Its Central Role in the Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2020, 2020, 4293206. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, L.; Dong, N.; Li, F. NLRP3 inflammasome: The rising star in cardiovascular diseases. Front. Cardiovasc. Med. 2022, 9, 927061. [Google Scholar] [CrossRef] [PubMed]
- Poff, A.; Kesl, S.; Koutnik, A.; Ward, N.; Ari, C.; Deblasi, J.; D’Agostino, D. Characterizing the metabolic effects of exogenous ketone supplementation—An alternative or adjuvant to the ketogenic diet. FASEB J. 2017, 31, 970.7. [Google Scholar] [CrossRef]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Graff, E.C.; Fang, H.; Wanders, D.; Judd, R.L. Anti-inflammatory effects of the hydroxycarboxylic acid receptor 2. Metabolism 2016, 65, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Offermanns, S. Free fatty acid (FFA) and hydroxy carboxylic acid (HCA) receptors. Annu. Rev. Pharm. Toxicol. 2014, 54, 407–434. [Google Scholar] [CrossRef]
- Qi, J.; Gan, L.; Fang, J.; Zhang, J.; Yu, X.; Guo, H.; Cai, D.; Cui, H.; Gou, L.; Deng, J.; et al. Beta-Hydroxybutyrate: A Dual Function Molecular and Immunological Barrier Function Regulator. Front. Immunol. 2022, 13, 805881. [Google Scholar] [CrossRef]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef]
- Bae, H.R.; Kim, D.H.; Park, M.H.; Lee, B.; Kim, M.J.; Lee, E.K.; Chung, K.W.; Kim, S.M.; Im, D.S.; Chung, H.Y. β-Hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation. Oncotarget 2016, 7, 66444–66454. [Google Scholar] [CrossRef]
- Nasser, S.; Vialichka, V.; Biesiekierska, M.; Balcerczyk, A.; Pirola, L. Effects of ketogenic diet and ketone bodies on the cardiovascular system: Concentration matters. World J. Diabetes 2020, 11, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Yurista, S.R.; Chong, C.R.; Badimon, J.J.; Kelly, D.P.; de Boer, R.A.; Westenbrink, B.D. Therapeutic Potential of Ketone Bodies for Patients with Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Westman, E.C.; Feinman, R.D.; Mavropoulos, J.C.; Vernon, M.C.; Volek, J.S.; Wortman, J.A.; Yancy, W.S.; Phinney, S.D. Lowcarbohydrate nutrition and metabolism. Am. J. Clin. Nutr. 2007, 86, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Nan, F.; Liang, H.; Shu, P.; Fan, X.; Song, X.; Hou, Y.; Zhang, D. Excessive intake of sugar: An accomplice of inflammation. Front. Immunol. 2022, 13, 988481. [Google Scholar] [CrossRef]
- Della Corte, K.W.; Perrar, I.; Penczynski, K.J.; Schwingshackl, L.; Herder, C.; Buyken, A.E. Effect of Dietary Sugar Intake on Biomarkers of Subclinical Inflammation: A Systematic Review and Meta-Analysis of Intervention Studies. Nutrients 2018, 10, 606. [Google Scholar] [CrossRef]
- O’Connor, L.; Imamura, F.; Brage, S.; Griffin, S.J.; Wareham, N.J.; Forouhi, N.G. Intakes and sources of dietary sugars and their association with metabolic and inflammatory markers. Clin. Nutr. 2018, 37, 1313–1322. [Google Scholar] [CrossRef]
- McGandy, R.B.; Hegsted, D.M.; Stare, F.J. Dietary fats, carbohydratesand atherosclerotic vascular disease. N. Engl. J. Med. 1967, 277, 186–192. [Google Scholar] [CrossRef]
- Carbone, S.; Billingsley, H.E.; Lavie, C.J. The Effects of Dietary Sugars on Cardiovascular Disease and Cardiovascular Disease-Related Mortality: Finding the Sweet Spot. Mayo Clin. Proc. 2019, 94, 2375–2377. [Google Scholar] [CrossRef]
- Howard, B.V.; Van Horn, L.; Hsia, J.; Manson, J.E.; Stefanick, M.L.; Wassertheil-Smoller, S.; Kuller, L.H.; LaCroix, A.Z.; Langer, L.D.; Lasser, N.L.; et al. Low-fat dietary patternand risk of cardiovascular disease: The Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006, 295, 655–666. [Google Scholar] [CrossRef]
- Billingsley, H.E.; Carbone, S.; Lavie, C.J. Dietary fats and chronicnoncommunicable diseases. Nutrients 2018, 10, 1385. [Google Scholar] [CrossRef]
- Huang, C.; Huang, J.; Tian, Y.; Yang, X.; Gu, D. Sugar sweetened beverages consumption and risk of coronary heart disease: A metaanalysis of prospective studies. Atherosclerosis 2014, 234, 11–16. [Google Scholar] [CrossRef]
- Li, Y.; Hruby, A.; Bernstein, A.M.; Ley, S.H.; Wang, D.D.; Chiuve, S.E.; Sampson, L.; Rexrode, K.M.; Rimm, E.B.; Willett, W.C.; et al. Saturated fats comparedwith unsaturated fats and sources of carbohydrates in relationto risk of coronary heart disease: A prospective cohort study. J. Am. Coll. Cardiol. 2015, 66, 1538–1548. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, Z.; Gregg, E.W.; Flanders, W.D.; Merritt, R.; Hu, F.B. Added sugar intake and cardiovascular diseases mortalityamong US adults. JAMA Intern. Med. 2014, 174, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.health.harvard.edu/heart-health/the-sweet-danger-of-sugar (accessed on 23 July 2023).
- Yang, B.; Glenn, A.J.; Liu, Q.; Madsen, T.; Allison, M.A.; Shikany, J.M.; Manson, J.E.; Chan, K.H.K.; Wu, W.C.; Li, J.; et al. Added Sugar, Sugar-Sweetened Beverages, and Artificially Sweetened Beverages and Risk of Cardiovascular Disease: Findings from the Women’s Health Initiative and a Network Meta-Analysis of Prospective Studies. Nutrients 2022, 14, 4226. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.K.; Tong, T.Y.N.; Watling, C.Z.; Reynolds, A.; Piernas, C.; Schmidt, J.A.; Papier, K.; Carter, J.L.; Key, T.J.; Perez-Cornago, A. Associations between types and sources of dietary carbohydrates and cardiovascular disease risk: A prospective cohort study of UK Biobank participants. BMC Med. 2023, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, B.; Berthon, B.S.; Saedisomeolia, A.; Starkey, M.R.; Collison, A.; Wark, P.A.B.; Wood, L.G. Effects of fruit and vegetable consumption on inflammatory biomarkers and immune cell populations: A systematic literature review and meta-analysis. Am. J. Clin. Nutr. 2018, 108, 136–155. [Google Scholar] [CrossRef]
- Cheng, H.; Zhou, J.; Sun, Y.; Zhan, Q.; Zhang, D. High fructose diet: A risk factor for immune system dysregulation. Hum. Immunol. 2022, 83, 538–546. [Google Scholar] [CrossRef]
- Lubawy, M.; Formanowicz, D. High-Fructose Diet–Induced Hyperuricemia Accompanying Metabolic Syndrome–Mechanisms and Dietary Therapy Proposals. Int. J. Environ. Res. Public Health 2023, 20, 3596. [Google Scholar] [CrossRef]
- Choi, H.K.; Willett, W.; Curhan, G. Fructose-Rich Beverages and the Risk of Gout in Women. JAMA J. Am. Med. Assoc. 2010, 304, 2270–2278. [Google Scholar] [CrossRef]
- Kanbay, M.; Guler, B.; Ertuglu, L.A.; Dagel, T.; Afsar, B.; Incir, S.; Baygul, A.; Covic, A.; Andres-Hernando, A.; Sánchez-Lozada, L.G.; et al. The Speed of Ingestion of a Sugary Beverage Has an Effect on the Acute Metabolic Response to Fructose. Nutrients 2021, 13, 1916. [Google Scholar] [CrossRef]
- Public Health England. Why 5%? An Explanation of SACN’s Recommendations about Sugars and Health. PHE Publications Gateway Number 2015193. 2015. Available online: https://www.gov.uk/government/publications/sacns-sugars-and-health-recommendations-why-5 (accessed on 23 July 2023).
- Rawal, G.; Yadav, S.; Kumar, R.; Singh, A. Glycosylated hemoglobin (HbA1C): A brief overview for clinicians. IP Indian J. Immunol. Respir. Med. 2016, 1, 33–36. [Google Scholar]
- Goto, A.; Noda, M.; Matsushita, Y.; Goto, M.; Kato, M.; Isogawa, A.; Takahashi, Y.; Kurotani, K.; Oba, S.; Nanri, A.; et al. JPHC Study Group. Hemoglobin a1c levels and the risk of cardiovascular disease in people without known diabetes: A population-based cohort study in Japan. Medicine 2015, 94, e785. [Google Scholar] [CrossRef] [PubMed]
- Sinning, C.; Makarova, N.; Völzke, H.; Schnabel, R.B.; Ojeda, F.; Dörr, M.; Felix, S.B.; Koenig, W.; Peters, A.; Rathmann, W.; et al. Association of glycated hemoglobin A1c levels with cardiovascular outcomes in the general population: Results from the BiomarCaRE (Biomarker for Cardiovascular Risk Assessment in Europe) consortium. Cardiovasc. Diabetol. 2021, 20, 223. [Google Scholar] [CrossRef]
- Prasad, K. Does HbA1cc Play a Role in the Development of Cardiovascular Diseases? Curr. Pharm. Des. 2018, 24, 2876–2882. [Google Scholar] [CrossRef]
- Zaki, H.A.; Iftikhar, H.; Bashir, K.; Gad, H.; Fahmy, A.S.; Elmoheen, A. A Comparative Study Evaluating the Effectiveness Between Ketogenic and Low-Carbohydrate Diets on Glycemic and Weight Control in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Cureus 2022, 14, e25528. [Google Scholar] [PubMed]
- Zhou, C.; Wang, M.; Liang, J.; He, G.; Chen, N. Ketogenic Diet Benefits to Weight Loss, Glycemic Control, and Lipid Profiles in Overweight Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trails. Int. J. Environ. Res. Public Health 2022, 19, 10429. [Google Scholar] [CrossRef]
- Choi, Y.J.; Jeon, S.M.; Shin, S. Impact of a Ketogenic Diet on Metabolic Parameters in Patients with Obesity or Overweight and with or without Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 2005. [Google Scholar] [CrossRef]
- Parry-Strong, A.; Wright-McNaughton, M.; Weatherall, M.; Hall, R.M.; Coppell, K.J.; Barthow, C.; Krebs, J.D. Very low carbohydrate (ketogenic) diets in type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2022, 24, 2431–2442. [Google Scholar] [CrossRef]
- Jayedi, A.; Zeraattalab-Motlagh, S.; Jabbarzadeh, B.; Hosseini, Y.; Jibril, A.T.; Shahinfar, H.; Mirrafiei, A.; Hosseini, F.; Bidar, S.S. Dose-dependent effect of carbohydrate restriction for type 2 diabetes management: A systematic review and dose-response meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2022, 116, 40–56. [Google Scholar] [CrossRef]
- Waldman, H.S.; Smith, J.W.; Lamberth, J.; Fountain, B.J.; Bloomer, R.J.; Butawan, M.B.; McAllister, M.J. A 28-Day Carbohydrate-Restricted Diet Improves Markers of Cardiovascular Disease in Professional Firefighters. J. Strength Cond. Res. 2020, 34, 2785–2792. [Google Scholar] [CrossRef]
- Karimi, E.; Yarizadeh, H.; Setayesh, L.; Sajjadi, S.F.; Ghodoosi, N.; Khorraminezhad, L.; Mirzaei, K. High carbohydrate intakes may predict more inflammatory status than high fat intakes in pre-menopause women with overweight or obesity: A cross-sectional study. BMC Res. Notes 2021, 14, 279. [Google Scholar] [CrossRef]
- Tavakoli, A.; Mirzababaei, A.; Sajadi, F.; Mirzaei, K. Circulating inflammatory markers may mediate the relationship between low carbohydrate diet and circadian rhythm in overweight and obese women. BMC Women’s Health 2021, 21, 87. [Google Scholar] [CrossRef]
- Forsythe, C.E.; Phinney, S.D.; Fernandez, M.L.; Quann, E.E.; Wood, R.J.; Bibus, D.M.; Kraemer, W.J.; Feinman, R.D.; Volek, J.S. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids 2008, 43, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.J.; Volek, J.S.; Davis, S.R.; Dell’Ova, C.; Fernandez, M.L. Effects of a carbohydrate-restricted diet on emerging plasma markers for cardiovascular disease. Nutr. Metab. 2006, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Guo, M.; Zhang, P.; Sun, G.; Chen, B. The effects of low-carbohydrate diets on cardiovascular risk factors: A meta-analysis. PLoS ONE 2020, 15, e0225348. [Google Scholar] [CrossRef]
- Simonetto, M.; Infante, M.; Sacco, R.L.; Rundek, T.; Della-Morte, D. A Novel Anti-Inflammatory Role of Omega-3 PUFAs in Prevention and Treatment of Atherosclerosis and Vascular Cognitive Impairment and Dementia. Nutrients 2019, 11, 2279. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-X.; Yu, J.-H.; Sun, J.-H.; Ma, W.-Q.; Wang, J.-J.; Sun, G.-J. Effects of Omega-3 Fatty Acids Supplementation on Serum Lipid Profile and Blood Pressure in Patients with Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Foods 2023, 12, 725. [Google Scholar] [CrossRef]
- Rodriguez, D.; Lavie, C.J.; Elagizi, A.; Milani, R.V. Update on Omega-3 Polyunsaturated Fatty Acids on Cardiovascular Health. Nutrients 2022, 14, 5146. [Google Scholar] [CrossRef]
- Fatahi, S.; Sohouli, M.H.; da Silva Magalhães, E.I.; da Cruz Silveira, V.N.; Zanghelini, F.; Rahmani, P.; Kord-Varkaneh, H.; Sharifi-Zahabi, E.; Shidfar, F. Comparing the effects of docosahexaenoic and eicosapentaenoic acids on cardiovascular risk factors: Pairwise and network meta-analyses of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 11–21. [Google Scholar] [CrossRef]
- Yang, B.; Tseng, P.T.; Hu, X.; Zeng, B.Y.; Chang, J.P.; Liu, Y.; Chu, W.J.; Zhang, S.S.; Zhou, Z.L.; Chu, C.S.; et al. Comparative efficacy of omega-3 polyunsaturated fatty acids on major cardiovascular events: A network meta-analysis of randomized controlled trials. Prog. Lipid Res. 2022, 88, 101196, Erratum in Prog. Lipid Res. 2022, 101206. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Kuno, T.; Morita, S.X.; Slipczuk, L.; Takagi, H.; Briasoulis, A.; Latib, A.; Bangalore, S.; Heffron, S.P. Eicosapentaenoic Acid for Cardiovascular Events Reduction- Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. J. Cardiol. 2022, 80, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, L.; Wang, D.; Yan, N.; Li, C.; Wu, M.; Wang, F.; Mi, B.; Chen, F.; Jia, W.; et al. Omega-3 polyunsaturated fatty acid biomarkers and risk of type 2 diabetes, cardiovascular disease, cancer, and mortality. Clin. Nutr. 2022, 41, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.; Domingo, J.C.; Izaola, O.; Casanueva, F.F.; Bellido, D.; Sajoux, I. Effect of DHA supplementation in a very low-calorie ketogenic diet in the treatment of obesity: A randomized clinical trial. Endocrine 2016, 54, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Perna, S.; Ilyas, Z.; Peroni, G.; Bazire, P.; Sajuox, I.; Maugeri, R.; Nichetti, M.; Gasparri, C. Effect of very low-calorie ketogenic diet in combination with omega-3 on inflammation, satiety hormones, body composition, and metabolic markers. A pilot study in class I obese subjects. Endocrine 2022, 75, 129–136. [Google Scholar] [CrossRef]
- Liu, S.H.; Chen, Y.X.; Tzeng, H.P.; Chiang, M.T. Fish Oil Enriched n-3 Polyunsaturated Fatty Acids Improve Ketogenic Low-Carbohydrate/High-Fat Diet-Caused Dyslipidemia, Excessive Fat Accumulation, and Weight Control in Rats. Nutrients 2022, 14, 1796. [Google Scholar] [CrossRef]
- Stoll, S.; Leimena, C.; Qiu, H. Mitochondria and Heart Disease; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Brown, D.A.; Perry, J.B.; Allen, M.E.; Sabbah, H.N.; Stauffer, B.L.; Shaikh, S.R.; Cleland, J.G.F.; Colucci, W.S.; Butler, J.; Voors, A.A.; et al. Expert consensus document: Mitochondrial function as a therapeutictarget in heart failure. Nat. Rev. Cardiol. 2017, 14, 238–250. Available online: https://www.researchgate.net/publication/327299198_Mitochondria_and_Heart_Disease (accessed on 24 April 2023).
- Murashige, D.; Jang, C.; Neinast, M.; Edwards, J.J.; Cowan, A.; Hyman, M.C.; Rabinowitz, J.D.; Frankel, D.S.; Arany, Z. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 2020, 370, 364–368. [Google Scholar] [CrossRef]
- Abdul Kadir, A.; Clarke, K.; Evans, R.D. Cardiac ketone body metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165739. [Google Scholar] [CrossRef]
- Sack, M.N.; Rader, T.A.; Park, S.; Bastin, J.; McCune, S.A.; Kelly, D.P. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation 1996, 94, 2837–2842. [Google Scholar]
- Carley, A.N.; Taegtmeyer, H.; Lewandowski, E.D. Matrix revisited: Mechanisms linking energy substrate metabolism to the function of the heart. Circ. Res. 2014, 114, 717–729. [Google Scholar]
- Horton, J.L.; Davidson, M.T.; Kurishima, C.; Vega, R.B.; Powers, J.C.; Matsuura, T.R.; Petucci, C.; Lewandowski, E.D.; Crawford, P.A.; Muoio, D.M.; et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight 2019, 4, e124079. [Google Scholar] [CrossRef]
- Bedi, K.C.; Snyder, N.W.; Brandimarto, J.; Aziz, M.; Mesaros, C.; Worth, A.J.; Wang, L.L.; Javaheri, A.; Blair, I.A.; Margulies, K.B.; et al. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation 2016, 133, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Aubert, G.; Martin, O.J.; Horton, J.L.; Lai, L.; Vega, R.B.; Leone, T.C.; Koves, T.; Gardell, S.J.; Krüger, M.; Hoppel, C.L.; et al. The Failing Heart Relies on Ketone Bodies as a Fuel. Circulation 2016, 133, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Lommi, M.D.J. Blood ketone bodies in congestive heart failure. J. Am. Coll. Cardiol. 1996, 28, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Voros, G.; Ector, J.; Garweg, C.; Droogne, W.; Van Cleemput, J.; Peersman, N.; Vermeersch, P.; Janssens, S. Increased cardiac uptake of ketone bodies and free fatty acids in human heart failure and hypertrophic left ventricular remodeling. Circ. Heart Fail. 2018, 11, e004953. [Google Scholar] [CrossRef]
- Gormsen, L.C.; Svart, M.; Thomsen, H.H.; Sondergaard, E.; Vendelbo, M.H.; Chris-tensen, N.; Tolbod, L.P.; Harms, H.J.; Nielsen, R.; Wiggers, H.; et al. Ketone Body Infusion with 3-Hydroxybutyrate Reduces Myocardial Glucose Uptake and Increases Blood Flow in Humans: A Positron Emission Tomography Study. J. Am. Heart Assoc. 2017, 6, e005066. [Google Scholar]
- Svart, M.; Gormsen, L.C.; Hansen, J.; Zeidler, D.; Gejl, M.; Vang, K.; Aanerud, J.; Moeller, N. Regional cerebral effects of ketone body infusion with 3-hydroxybutyrate in humans: Reduced glucose uptake, unchanged oxygen consumption and increased blood flow by positron emission tomography. A randomized, controlled trial. PLoS ONE 2018, 13, e0190556. [Google Scholar]
- Lauritsen, K.M.; Søndergaard, E.; Luong, T.V.; Møller, N.; Gormsen, L.C. Acute Hyperketonemia Does Not Affect Glucose or Palmitate Uptake in Abdominal Organs or Skeletal Muscle. J. Clin. Endocrinol. Metab. 2020, 105, 1785–1790. [Google Scholar] [CrossRef]
- Du, Z.; Shen, A.; Huang, Y.; Su, L.; Lai, W.; Wang, P.; Xie, Z.; Xie, Z.; Zeng, Q.; Ren, H.; et al. 1H-NMR-based metabolic analysis of human serum reveals novel markers of myocardial energy expenditure in heart failure patients. PLoS ONE 2014, 9, e88102. [Google Scholar] [CrossRef]
- Seki, M.; Powers, J.C.; Maruyama, S.; Zuriaga, M.A.; Wu, C.L.; Kurishima, C.; Kim, L.; Johnson, J.; Poidomani, A.; Wang, T.; et al. Acute and chronic increases of circulating FSTL1 normalize energy substrate metabolism in pacing-induced heart failure. Circulation 2018, 11, e004486. [Google Scholar]
- Guo, Y.; Liu, X.; Li, T.; Zhao, J.; Yang, Y.; Yao, Y.; Wang, L.; Yang, B.; Ren, G.; Tan, Y.; et al. Alternate-Day Ketogenic Diet Feeding Protects against Heart Failure through Preservation of Ketogenesis in the Liver. Oxid. Med. Cell. Longev. 2022, 2022, 4253651. [Google Scholar] [CrossRef] [PubMed]
- Obokata, M.; Negishi, K.; Sunaga, H.; Ishida, H.; Ito, K.; Ogawa, T.; Iso, T.; Ando, Y.; Kurabayashi, M. Association between Circulating Ketone Bodies and Worse Outcomes in Hemodialysis Patients. J. Am. Heart Assoc. 2017, 6, e006885. [Google Scholar] [CrossRef] [PubMed]
- Flores-Guerrero, J.L.; Westenbrink, B.D.; Connelly, M.A.; Otvos, J.D.; Groothof, D.; Shalaurova, I.; Garcia, E.; Navis, G.; de Boer, R.A.; Bakker, S.J.L.; et al. Association of beta-hydroxybutyrate with development of heart failure: Sex differences in a Dutch population cohort. Eur. J. Clin. Investig. 2021, 51, e13468. [Google Scholar] [CrossRef]
- Manolis, A.S.; Manolis, T.A.; Manolis, A.A. Ketone Bodies and Cardiovascular Disease: An Alternate Fuel Source to the Rescue. Int. J. Mol. Sci. 2023, 24, 3534. [Google Scholar] [CrossRef]
- Kashiwagi, Y.; Nagoshi, T.; Inoue, Y.; Tanaka, Y.; Takahashi, H.; Oi, Y.; Kimura, H.; Minai, K.; Yoshimura, M. Close linkage between blood total ketone body levels and B-type natriuretic peptide levels in patients with cardiovascular disorders. Sci. Rep. 2021, 11, 6498. [Google Scholar] [CrossRef]
- Marcondes-Braga, F.G.; Batista, G.L.; Gutz, I.G.R.; Saldiva, P.H.N.; Mangini, S.; Issa, V.S.; Ayub-Ferreira, S.M.; Bocchi, E.A.; Pereira, A.C.; Bacal, F. Impact of exhaled breath acetone in the prognosis of patients with heart failure with reduced ejection fraction (HFrEF). One year of clinical follow-up. PLoS ONE 2016, 11, e0168790. [Google Scholar]
- Voorrips, S.N.; Boorsma, E.M.; Beusekamp, J.C.; DE-Boer, R.A.; Connelly, M.A.; Dullaart, R.P.F.; VAN-DER-Meer, P.; VAN-Veldhuisen, D.J.; Voors, A.A.; Damman, K.; et al. Longitudinal Changes in Circulating Ketone Body Levels in Patients with Acute Heart Failure: A Post Hoc Analysis of the EMPA-Response-AHF Trial. J. Card. Fail. 2023, 29, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.; Møller, N.; Gormsen, L.C.; Tolbod, L.P.; Hansson, N.H.; Sorensen, J.; Harms, H.J.; Frøkiær, J.; Eiskjaer, H.; Jespersen, N.R.; et al. Cardiovascular Effects of Treatment With the Ketone Body 3-Hydroxybutyrate in Chronic Heart Failure Patients. Circulation 2019, 139, 2129–2141. [Google Scholar] [CrossRef]
- Kashiwaya, Y.; Sato, K.; Tsuchiya, N.; Thomas, S.; Fell, D.A.; Veech, R.L.; Passonneau, J.V. Control of glucose utilization in working perfused rat heart. J. Biol. Chem. 1994, 269, 25502–25514. [Google Scholar] [CrossRef]
- Sato, K.; Kashiwaya, Y.; Keon, C.A.; Tsuchiya, N.; King, M.T.; Radda, G.K.; Chance, B.; Clarke, K.; Veech, R.L. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 1995, 9, 651–658. [Google Scholar] [CrossRef]
- Schugar, R.C.; Moll, A.R.; André d’Avignon, D.; Weinheimer, C.J.; Kovacs, A.; Crawford, P.A. Cardiomyocyte-specific deficiency of ketone body metabolism promotes accelerated pathological remodeling. Mol. Metab. 2014, 3, 754–769. [Google Scholar] [CrossRef]
- Luong, T.V.; Abild, C.B.; Bangshaab, M.; Gormsen, L.C.; Søndergaard, E. Ketogenic Diet and Cardiac Substrate Metabolism. Nutrients 2022, 14, 1322. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, T.R.; Puchalska, P.; Crawford, P.A.; Kelly, D.P. Ketones and the Heart: Meta-bolic Principles and Therapeutic Implications. Circ Res. 2023, 132, 882–898. [Google Scholar] [CrossRef] [PubMed]
- Likhodii, S.S.; Musa, K.; Mendonca, A.; Dell, C.; Burnham, W.M.; Cunnane, S.C. Dietary fat, ketosis, and seizure resistance in rats on the ketogenic diet. Epilepsia 2000, 41, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Z.; Han, Y.; Xu, J.; Huang, W.; Li, Z. Medium Chain Triglycerides enhances exercise endurance through the increased mitochondrial biogenesis and metabolism. PLoS ONE 2018, 13, e0191182. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, D.A.; Moreira, A.S.; de Oliveira, G.M.; Raggio Luiz, R.; Rosa, G. A Coconut Extra Virgin Oil-Rich Diet Increases Hdl Cholesterol and Decreases Waist Circumference and Body Mass in Coronary Artery Disease Patients. Nutr. Hosp. 2015, 32, 2144–2152. [Google Scholar] [CrossRef] [PubMed]
- Galley, H.F.; Webster, N.R. Physiology of the endothelium. Br. J. Anaesth. 2004, 93, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Fiore, A.; Masiglat, J.; Cavuoti, T.; Romandini, M.; Nappi, P.; Avtaar Singh, S.S.; Couetil, J.-P. Endothelium-Derived Relaxing Factors and Endothelial Function: A Systematic Review. Biomedicines 2022, 10, 2884. [Google Scholar] [CrossRef]
- Weis, E.M.; Puchalska, P.; Nelson, A.B.; Taylor, J.; Moll, I.; Hasan, S.S.; Dewenter, M.; Hagenmüller, M.; Fleming, T.; Poschet, G.; et al. Ketone body oxidation increases cardiac endothelial cell proliferation. EMBO Mol. Med. 2022, 14, e14753. [Google Scholar] [CrossRef]
- Devaraj, S.; Cheung, A.T.; Jialal, I.; Griffen, S.C.; Nguyen, D.; Glaser, N.; Aoki, T. Evidence of increased inflammation and microcirculatory abnormalities in patients with type 1 diabetes and their role inmicrovascular complications. Diabetes 2007, 56, 2790–2796. [Google Scholar] [CrossRef]
- White, N.H. Diabetic ketoacidosis in children. Endocrinol. Metab. Clin. North Am. 2000, 29, 657–682. [Google Scholar] [CrossRef]
- Bialo, S.R.; Agrawal, S.; Boney, C.M.; Quintos, J.B. Rare complications of pediatric diabetic ketoacidosis. World J. Diabetes 2015, 6, 167–174. [Google Scholar] [CrossRef]
- Ma, D.; Wang, A.C.; Parikh, I.; Green, S.J.; Hoffman, J.D.; Chlipala, G.; Murphy, M.P.; Sokola, B.S.; Bauer, B.; Hartz, A.M.S.; et al. Ketogenic diet enhances neurovascular function with altered gut microbiome in young healthy mice. Sci Rep. 2018, 8, 6670. [Google Scholar] [CrossRef] [PubMed]
- Mccarthy, C.G.; Chakraborty, S.; Schreckenberger, Z.; Wenceslau, C.F.; Joe, B. β-hydroxybutyrate (βHOB) increases nitric oxide synthase activity in resistance arteries from dahl salt-sensitive rats. FASEB J. 2019, 33, 829. [Google Scholar] [CrossRef]
- Newman, J.C.; Covarrubias, A.J.; Zhao, M.; Yu, X.; Gut, P.; Ng, C.P.; Huang, Y.; Haldar, S.; Verdin, E. Ketogenic Diet Reduces Midlife Mortality and Improves Memory in Aging Mice. Cell Metab. 2017, 26, 547–557.e8. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.M.; Bedarida, T.; Ding, Y.; Somba, B.K.; Lu, Q.; Wang, Q.; Song, P.; Zou, M.H. β-Hydroxybutyrate Prevents Vascular Senescence through hnRNP A1-Mediated Upregulation of Oct4. Mol Cell. 2018, 71, 1064–1078.e5. [Google Scholar] [CrossRef]
- Meroni, E.; Papini, N.; Criscuoli, F.; Casiraghi, M.C.; Massaccesi, L.; Basilico, N.; Erba, D. Metabolic Responses in Endothelial Cells Following Exposure to Ketone Bodies. Nutrients 2018, 10, 250. [Google Scholar] [CrossRef] [PubMed]
- Pirola, L.; Balcerczyk, A.; Tothill, R.W.; Haviv, I.; Kaspi, A.; Lunke, S.; Ziemann, M.; Karagiannis, T.; Tonna, S.; Kowalczyk, A.; et al. Genome-wide analysis distinguishes hyperglycemia regulated epigenetic signatures of primary vascular cells. Genome Res. 2011, 21, 1601–1615. [Google Scholar] [CrossRef]
- Coppola, G.; Natale, F.; Torino, A.; Capasso, R.; D’Aniello, A.; Pironti, E.; Santoro, E.; Calabrò, R.; Verrotti, A. The impact of the ketogenic diet on arterial morphology and en-dothelial function in children and young adults with epilepsy: A case-control study. Seizure 2014, 23, 260–265. [Google Scholar] [CrossRef]
- Buscemi, S.; Verga, S.; Tranchina, M.R.; Cottone, S.; Cerasola, G. Effects of hypocaloric very-low-carbohydrate diet vs. Mediterranean diet on endothelial function in obese women. Eur. J. Clin. Investig. 2009, 39, 339–347. [Google Scholar] [CrossRef]
- DeFronzo, R.A. The effect of insulin on renal sodium metabolism. A review with clinical implications. Diabetologia 1981, 21, 165–171. [Google Scholar] [CrossRef]
- Brands, M.W. Role of Insulin-Mediated Antinatriuresis in Sodium Homeostasis and Hypertension. Hypertension 2018, 72, 1255–1262. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. Physiology and pathophysiology of potassium homeostasis. Adv. Physiol. Educ. 2016, 40, 480–490. [Google Scholar] [CrossRef]
- Harvey, C.J.D.C.; Schofield, G.M.; Williden, M. The use of nutritional supplements to induce ketosis and reduce symptomsassociated with keto-induction: A narrative review. PeerJ 2018, 6, e4488. [Google Scholar] [CrossRef] [PubMed]
- Zupec-Kania, B.; Zupanc, M.L. Long-term management of the ketogenic diet: Seizure monitoring, nutrition, and supplementation. Epilepsia 2008, 49 (Suppl. S8), 23–26. [Google Scholar] [CrossRef]
- Cordain, L. Nutritional Deficiencies of Ketogenic Diets. 2018. Available online: https://www.researchgate.net/publication/332098774_Nutritional_Deficiencies_of_Ketogenic_Diets?channel=doi&linkId=5c9f99e2a6fdccd46045868c&showFulltext=true (accessed on 23 July 2023). License CC BY-NC-ND 4.0.
- Iqbal, S.; Klammer, N.; Ekmekcioglu, C. The Effect of Electrolytes on Blood Pressure: A Brief Summary of Meta-Analyses. Nutrients 2019, 11, 1362. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Volpe, M.; Savoia, C. Endothelial Dysfunction in Hypertension: Current Concepts and Clinical Implications. Front. Med. 2021, 8, 798958. [Google Scholar] [CrossRef] [PubMed]
- Kostov, K. The Causal Relationship between Endothelin-1 and Hypertension: Focusing on Endothelial Dysfunction, Arterial Stiffness, Vascular Remodeling, and Blood Pressure Regulation. Life 2021, 11, 986. [Google Scholar] [CrossRef]
- Polito, R.; Messina, G.; Valenzano, A.; Scarinci, A.; Villano, I.; Monda, M.; Cibelli, G.; Porro, C.; Pisanelli, D.; Monda, V.; et al. The Role of Very Low Calorie Ketogenic Diet in Sympathetic Activation through Cortisol Secretion in Male Obese Population. J. Clin. Med. 2021, 10, 4230. [Google Scholar] [CrossRef]
- Polito, R.; Valenzano, A.; Monda, V.; Cibelli, G.; Monda, M.; Messina, G.; Villano, I.; Messina, A. Heart Rate Variability and Sympathetic Activity Is Modulated by Very Low-Calorie Ketogenic Diet. Int. J. Environ. Res. Public Health 2022, 19, 2253. [Google Scholar] [CrossRef]
- Barrea, L.; Verde, L.; Camajani, E.; Šojat, A.S.; Marina, L.; Savastano, S.; Colao, A.; Caprio, M.; Muscogiuri, G. Effects of very low-calorie ketogenic diet on hypothalamic–pituitary–adrenal axis and renin–angiotensin–aldosterone system. J. Endocrinol. Investig. 2023, 46, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Belany, P.; Kackley, M.L.; Zhao, S.; Kluwe, B.; Buga, A.; Crabtree, C.D.; Nedungadi, D.; Kline, D.; Brock, G.; Simonetti, O.P.; et al. Effects of Hypocaloric Low-Fat, Ketogenic, and Ketogenic and Ketone Supplement Diets on Aldosterone and Renin. J. Clin. Endocrinol. Metab. 2023, 108, 1727–1739. [Google Scholar] [CrossRef] [PubMed]
- Di Raimondo, D.; Buscemi, S.; Musiari, G.; Rizzo, G.; Pirera, E.; Corleo, D.; Pinto, A.; Tuttolomondo, A. Ketogenic Diet, Physical Activity, and Hypertension—A Narrative Review. Nutrients 2021, 13, 2567. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Tas k Force for the Management of Arterial Hypertension. J. Hypertens. 2018, 36, 2284–2309. [Google Scholar] [CrossRef]
- Di Raimondo, D.; Musiari, G.; Miceli, G.; Arnao, V.; Pinto, A. Preventive and Therapeutic Role of Muscle Contraction against Chronic Diseases. Curr. Pharm. Des. 2016, 22, 4686–4699. [Google Scholar] [CrossRef]
- Barrea, L.; Verde, L.; Santangeli, P.; Lucà, S.; Docimo, A.; Savastano, S.; Colao, A.; Muscogiuri, G. Very low-calorie ketogenic diet (VLCKD): An antihypertensive nutritional approach. J. Transl. Med. 2023, 21, 128. [Google Scholar] [CrossRef]
- Rinaldi, R.; De Nucci, S.; Castellana, F.; Di Chito, M.; Giannuzzi, V.; Shahini, E.; Zupo, R.; Lampignano, L.; Piazzolla, G.; Triggiani, V.; et al. The Effects of Eight Weeks’ Very Low-Calorie Ketogenic Diet (VLCKD) on Liver Health in Subjects Affected by Overweight and Obesity. Nutrients 2023, 15, 825. [Google Scholar] [CrossRef]
- Yancy, W.S., Jr.; Westman, E.C.; McDuffie, J.R.; Grambow, S.C.; Jeffreys, A.S.; Bolton, J.; Chalecki, A.; Oddone, E.Z. A randomized trial of a low-carbohydrate diet vs orlistat plus a low-fat diet for weight loss. Arch. Intern. Med. 2010, 170, 136–145, Erratum in JAMA Intern. Med. 2015, 175, 470. [Google Scholar] [CrossRef]
- Foster, G.D.; Wyatt, H.R.; Hill, J.O.; Makris, A.P.; Rosenbaum, D.L.; Brill, C.; Stein, R.I.; Mohammed, B.S.; Miller, B.; Rader, D.J.; et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: A randomized trial. Ann. Intern. Med. 2010, 153, 147–157. [Google Scholar] [CrossRef]
- Samaha, F.F.; Iqbal, N.; Seshadri, P.; Chicano, K.L.; Daily, D.A.; McGrory, J.; Williams, T.; Williams, M.; Gracely, E.J.; Stern, L. A low-carbohydrate as compared with a low-fat diet in severe obesity. N. Engl. J. Med. 2003, 348, 2074–2081. [Google Scholar] [CrossRef]
- Tzenios, N.; Lewis, E.D.; Crowley, D.C.; Chahine, M.; Evans, M. Examining the Efficacy of a Very-Low-Carbohydrate Ketogenic Diet on Cardiovascular Health in Adults with Mildly Elevated Low-Density Lipoprotein Cholesterol in an Open-Label Pilot Study. Metab. Syndr. Relat. Disord. 2022, 20, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Bueno, N.; De Melo, I.; De Oliveira, S.; Da Rocha Ataide, T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Markovikj, G.; Knights, V.; Kljusurić, J.G. Ketogenic Diet Applied in Weight Reduction of Overweight and Obese Individuals with Progress Prediction by Use of the Modified Wishnofsky Equation. Nutrients 2023, 15, 927. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Laudisio, D.; Pugliese, G.; Salzano, C.; Savastano, S.; Colao, A. The management of very low-calorie ketogenic diet in obesity outpatient clinic: A practical guide. J. Transl. Med. 2019, 17, 356. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; and Stroke Council. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]

| Research Type, Year | Purpose of the Study | Type of Diet | Changes in the Lipid Profile | References |
|---|---|---|---|---|
| RCT, 2022 | Observation of periodic ketogenic diet for effect on overweight or obese patients newly diagnosed as T2DM. | Ketogenic diet (KD) vs. standard diabetes diet (SDD) | KD vs. SDD: -decrease in cholesterol from 4.54 ± 0.69 mmol/L to 4.02 ± 0.43 mmol/L (SDD from 4.56 ± 0.67 mmol/L to 4.23 ± 0.47 mmol/L) -decrease in triglycerides from 1.76 ± 0.59 mmol/L to 1.44 ± 0.26 mmol/L (SDD from 1.81 ± 0.78 mmol/L to 1.66 ± 0.46 mmol/L) -decrease in LDL from 2.75 ± 0.65 mmol/L to 2.34 ± 0.45 mmol/L (SDD from 2.77 ± 0.69 mmol/L to 2.59 ± 0.58 mmol/L) -increase in HDL from 1.08 ± 0.11 mmol/L to 1.21 ± 0.23 mmol/L (SDD from 1.09 ± 0.19 mmol/L to 1.12 ± 0.20 mmol/L) | [43] |
| RCT, 2022 | Comparison of 2 low-carbohydrate diets with 3 key similarities and 3 key differences for their effects on glucose control and cardiometabolic risk factors in individuals with prediabetes and T2DM. | Well-formulated ketogenic diet (WFKD) vs. the Mediterranean-plus diet (Med-Plus) | WFKD vs. Med-Plus: -reduction in triglycerides from 118.8 mg/dL to 99.5 mg/dL (in Med-Plus from 131.1 mg/dL to 121.7 mg/dL) -increase in HDL concentration from 49.1 mg/dL to 54.1 mg/dL (in Med-Plus from 48 mg/dL to 47.9 mg/dL) -increase in LDL concentration from 97.8 mg/dL to 111.3 mg/dL (in Med-Plus from 111.5 mg/dL to 95.3 mg/dL) | [44] |
| RCT, 2022 | Assessment of the clinical advantage of combining two preoperative strategies (continuous positive airway pressure (CPAP) and low-calorie ketogenic diet (LCKD)) compared to CPAP alone, to improve apnea–hypopnea index (AHI) score, hypertension (HTN), dyslipidemia (DLP), insulin resistance (IR) and C-reactive protein (CRP) levels in patients with severe obesity and obstructive sleep apnea syndrome (OSAS) scheduled for bariatric surgery (BS). | Low-calorie ketogenic diet (LCKD) + continuous positive airway pressure (CPAP) vs. only continuous positive airway pressure (CPAP) | LCKD + CPAP vs. CPAP: -reduction in total cholesterol from 200.1 ± 30.1 mg/dL to 180.4 ± 35.2 mg/dL (CPAP from 196.1 ± 32.9 mg/dL to 180.8 ± 33.0 mg/dL) -decrease in LDL from 127.4 ± 26.8 mg/dL to 107.1 ± 37.1 mg/dL (CPAP from 128 ± 30.2 mg/dL to 112.9 ± 34.9 mg/dL) -decrease in triglycerides from 191 ± 41.7 mg/dL to 130 ± 79 mg/dL (CPAP from 151.6 ± 62.5 mg/dL to 129.7 ± 62.2 mg/dL) -insignificant increase in HDL from 48.3 ± 9.41 mg/dL to 48.8 ± 10.4 mg/dL (CPAP from 46.4 ± 10.3 mg/dL to 47.3 ± 9.8 mg/dL) | [45] |
| RCT, 2021 | Investigation and comparison of the effects of two iso-energetic hypo-caloric ketogenic hyper-ketonemic and non-ketogenic low-carbohydrate high-fat high-cholesterol diets on body-composition, muscle strength and hormonal profile in experienced resistance-trained middle-aged men. | Ketogenic diets (KD) vs. non-ketogenic diets (NKD) (in several variants) | No significant differences in lipid profile. -In KD—change in TC from 4.44 ± 0.37 mmol/L to 4.43 ± 0.30 mmol/L (NKD from 4.49 ± 0.31 mmol/L to 4.52 ± 0.30 mol/L) -In KD—change in TG from 0.99 ± 0.25 mmol/L to 0.95 ± 0.26 mmol/L (NKD from 0.90 ± 0.14 mmol/L to 0.85 ± 0.13 mmol/L) -In KD—change in HDL from 1.28 ± 0.13 mmol/L to 1.36 ± 0.12 mmol/L (NKD from 1.28 ± 0.13 mmol/L to 1.36 ± 0.12 mmol/L) -In KD—change in LDL from 2.40 ± 0.21 mmol/L to 2.45 ± 0.25 mmol/L (NKD from 2.57 ± 0.41 mmol/L to 2.59 ± 0.39 mmol/L) | [46] |
| RCT, 2021 | Investigation of the effect of a ketogenic LCHF diet on low-density lipoprotein (LDL) cholesterol (primary outcome), LDL cholesterol subfractions and conventional cardiovascular risk factors in the blood of healthy, young, and normal-weight women. | Ketogenic low-carbohydrate high-fat (LCHF) diet vs. National Food Agency recommended control diet (NFACD) | The LCHF diet: -increases in LDL cholesterol in every woman with a treatment effect of 1.82 mM (p < 0.001) (primary outcome at baseline = 2.1 ± 0.6 mM) -increases in apolipoprotein B-100 (ApoB) (treatment effect (95% Cl) = 0.50 [0.35, 0.65], primary outcome at baseline = 0.70 ± 0.15 g/L) -increases in LDL 1–2 (large, buoyant LDL) (treatment effect (95% Cl) = 31.56 [21.60, 41.51], primary outcome at baseline = 42.1 ± 14.6 mg/dL) -increases in LDL 3–7 (small, dense LDL) (treatment effect (95% Cl) = 4.51 [1.87, 7.16], primary outcome at baseline = 2.7 ± 2.5 mg/dL) | [47] |
| RCT, 2020 | Comparison of the efficacy, safety and effect of 45-day isocaloric very-low-calorie ketogenic diets (VLCKDs) incorporating whey, vegetable or animal protein on the microbiota in patients with obesity and insulin resistance, to test the hypothesis that protein source may modulate the response to VLCKD interventions. | Isocaloric VLCKD regimens (≤800 kcal/day) containing whey (WPG), plant (VPG) or animal protein (APG) | Significant reductions in total cholesterol (TC), LDL and triglycerides (TG) in all VLCKD groups: -TC in WPG from 214.8 ± 31.5 mg/dL to 166.2 ± 43.6 mg/dL, in VPG from 220.9 ± 51.6 mg/dL to 170.7 ± 36.3 mg/dL, in APG from 226.9 ± 32.7 mg/dL to 191.2 ± 34.2 mg/dL -LDL in WPG from 132.8 ± 30.8 mg/dL to 100.8 ± 38.4 mg/dL, in VPG from 136.1 ± 41.3 mg/dL to 97.5 ± 32.3 mg/dL, in APG from 143.9 ± 25.8 mg/dL to 118.5 ± 23.1 mg/dL -TG in WPG from 131.0 ± 44.9 mg/dL to 94.6 ± 32.0 mg/dL, in VPG from 170.1 ± 126.9 mg/dL to 117.6 ± 42.7 mg/dL, in APG from 124.25 ± 58 mg/dL to 82.25 ± 33.32 mg/dL -insignificant changes in HDL: in WPG from 51.7 ± 12.3 mg/dL to 46.1 ± 7.5 mg/dL, in VPG from 51.2 ± 12.8 mg/dL to 49.0 ± 9.5 mg/dL, in APG from 57.9 ± 23.7 mg/dL to 56.2 ± 18.0 mg/dL | [49] |
| RCT, 2020 | Comparison of the influence of a 12-week, well-planned, low-calorie ketogenic diet (LCKD) on hyperglycemic, hyperinsulinemic and lipid profiles in adult, overweight or obese females. | Low-calorie ketogenic diet (LCKD) vs. control group (CG) (typical diet) | Significant reduction in TG and increase in HDL in LCKD compared to CG: -TG in LCKD decreased from 213.45 ± 63.60 mg/dL to 129.13 ± 46.23 mg/dL (in CG from 210.57 ± 36.45 mg/dL to 206.44 ± 50.03 mg/dL) -HDL in LCKD increased from 36.71 ± 4.42 mg/dL to 52.99 ± 7.77 mg/dL (in CG from 44.14 ± 5.07 to 43.01 ± 5.03 mg/dL) | [50] |
| RCT, 2017 | Comparison of the effects of a ketogenic diet vs. a moderate-carbohydrate diet on overweight adults with type 2 diabetes mellitus or pre-diabetes. | Very low-carbohydrate ketogenic diet (VLCKD) vs. moderate-carbohydrate, calorie-restricted, low-fat diet (MCCRD) | -In VLCKD, there was a significant reduction in TG from 102.6 mg/dL (81.8, 123.4) to 86.2 mg/dL (68.6, 103.7) in the 6th month and 92.7 mg/dL (73.6, 111.7) in the 12th month (in MCCRD from 158.9 mg/dL (128.8, 189.1) to 143.2 mg/dL (115.6, 170.9) in the 6th month and 173.4 mg/dL (138.1, 208.7) in the 12th month) -In VLCKD, there was an increase in HDL from 48.4 mg/dL (42.6, 54.2) to 51.9 mg/dL (45.7, 58.2) in the 6th month and 53.3 mg/dL (46.8, 59.8) in the 12th month (in MCCRD from 45.8 mg/dL (40.6, 51.0) to 48.1 mg/dL (42.5, 53.6) in the 6th month and 48.9 mg/dL (43.3, 54.5) in the 12th month) -In VLCKD, there was an increase in LDL from 88.7 mg/dL (76.3, 101.1) to 97.9 mg/dL (85.4, 110.5) in the 6th month and 95.6 mg/dL (82.3, 108.9) in the 12th month (in MCCRD from 98.1 mg/dL (86.4, 109.8) to 88.1 mg/dL (76.0, 100.1) in the 6th month and 96.1 mg/dL (83.7, 108.5) in the 12th month) | [51] |
| RCT, 2015 | Evaluating the effects of ω-3 supplementation during a ketogenic diet in overweight subjects. | Ketogenic diet (KD) vs. ketogenic diet + ω-3 supplementation (KDO3) | In both dietary versions, there was a reduction in TC, LDL, TG and an increase in HDL. -TC in KD decreased from 217.25 ± 15.84 mg/dL to 201.28 ± 6.79 mg/dL (in KDO3 from 222.39 ± 6.10 mg/dL to 204.52 ± 9.78 mg/dL) -LDL in KD decreased from 133.41 ± 15.86 mg/dL to 123.60 ± 7.99 mg/dL (in KDO3 from 136.98 ± 7.06 mg/dL to 127.56 ± 7.19 mg/dL) -TG in KD decreased from 237.81 ± 20.26 mg/dL to 197.27 ± 6.1 mg/dL (in KDO3 from 230.79 ± 25.66 mg/dL to 185.54 ± 9.64 mg/dL) -HDL in KD increased slightly from 36.28 ± 2.23 mg/dL to 39.25 ± 1.37 mg/dL (in KDO3 from 39.55 ± 2.99 to 40.25 ± 2.63 mg/dL) | [52] |
| RCT, 2012 | Comparison of the efficacy and metabolic impact of ketogenic and hypocaloric diets in obese children and adolescents. | Ketogenic diet (KD) vs. hypocaloric diet (HD) | -In KD, there was an increase in TC from 4.4 ± 0.85 mmol/L to 4.63 ± 0.75 mmol/L (in HD from 4.05 ± 0.94 mmol/L to 4.03 ± 0.89 mmol/L) -In KD, there was an increase in HDL from 1.27 ± 0.26 mmol/L to 1.38 ± 0.25 mmol/L (in HD from 1.13 ± 0.20 mmol/L to 1.23 ± 0.23 mmol/L) -In KD, there was an increase in LDL from 2.72 ± 0.69 mmol/L to 2.86 ± 0.65 mmol/L (in HD from 2.6 ± 0.83 mmol/L to 2.55 ± 0.77 mmol/L) -In KD, there was a reduction in TG from 0.83 ± 0.35 mmol/L to 0.81 ± 0.39 mmol/L (in HD from 0.89 ± 0.57 mmol/L to 0.80 ± 0.40 mmol/L) | [53] |
| Type of Research, Year | Purpose of the Study | Diet Type | Blood Pressure Changes | References |
|---|---|---|---|---|
| Prospective pilot clinical trial, 2023 | Evaluate the effect of very low-calorie ketogenic diet (VLCKD) on blood pressure (BP) in women with obesity and hypertension. | Very low-calorie ketogenic diet (VLCKD) | Relative to baseline values, after 45 days, there was: -a reduction in systolic blood pressure by an average of −12.89% (from an average of 140.88 ± 8.99 mmHg to 122.56 ± 10.08 mmHg) -a reduction in diastolic blood pressure by a mean of −10.77% (from a mean of 88.90 ± 6.71 mmHg to 78.94 ± 6.68 mmHg). | [206] |
| Prospective study, 2023 | Evaluate the efficacy and safety of VLCKD on non-alcoholic fatty liver disease (NAFLD) and parameters commonly associated with this condition in overweight and obese subjects who did not take any drugs. | Very low-calorie ketogenic diet (VLCKD) | Relative to the initial values, after 8 weeks, there was: -a reduction in systolic blood pressure from an average of 133.51 ± 12.86 mmHg to 123.27 ± 10.51 mmHg -a reduction in diastolic blood pressure from a mean of 81.73 ± 8.09 mmHg to 75.27 ± 7.84 mmHg. | [207] |
| RCT, 2022 | Assessment of the clinical advantage of combining two preoperative strategies (continuous positive airway pressure (CPAP) and low-calorie ketogenic diet (LCKD)) compared to CPAP alone, to improve apnea–hypopnea index (AHI) score, hypertension (HTN), dyslipidemia (DLP), insulin resistance (IR) and C-reactive protein (CRP) levels in patients with severe obesity and obstructive sleep apnea syndrome (OSAS) scheduled for bariatric surgery (BS). | Low-calorie ketogenic diet (LCKD) + continuous positive airway pressure (CPAP) vs. only continuous positive airway pressure (CPAP) | LCKD + CPAP vs. CPAP: -greater mean reduction in systolic blood pressure from 142.8 ± 13.3 mmHg to 133 ± 11.9 mmHg (in CPAP from 134.2 ± 10.4 mmHg to 130 ± 9.7 mmHg) -increased mean diastolic blood pressure reduction from 85.4 ± 8.38 mmHg to 78.7 ± 6.43 mmHg (on CPAP from 87 ± 11.6 mmHg to 82 ± 9.5 mmHg). | [45] |
| Pilot clinical trial, 2022 | Investigate the efficacy of a very-low-carbohydrate ketogenic diet (VLCKD), known as Nic’s Ketogenic Diet, for 140 days on cardiometabolic markers in healthy adults with mildly elevated low-density lipoprotein cholesterol (LDL-C). | Very-low-carbohydrate ketogenic diet | -Systolic blood pressure decreased by 5.3% from baseline on day 140 of VLCKD. -There was a significant increase in diastolic blood pressure on day 28; however, there was no significant change on days 56, 70, 84, 112 and 140. | [211] |
| RCT, 2020 | Comparison of the efficacy, safety and effect of 45-day isocaloric very-low-calorie ketogenic diets (VLCKDs) incorporating whey, vegetable or animal protein on the microbiota in patients with obesity and insulin resistance, to test the hypothesis that the protein source may modulate the response to VLCKD interventions. | Isocaloric VLCKD regimens (≤800 kcal/day) containing whey (WPG), plant (VPG) or animal protein (APG) | Relative to baseline values, after 45 days, there was: -a reduction in mean systolic pressure values (in WPG from 132 ± 10 mmHg to 124 ± 13 mmHg, in VPG from 131 ± 8 mmHg to 121 ± 10 mmHg, in APG from 129 ± 9 mmHg to 121 ± 16 mmHg) -a reduction in mean diastolic pressure values (on WPG from 78 ± 11 mmHg to 70 ± 9 mmHg, on VPG from 78 ± 10 mmHg to 72 ± 10 mmHg, on APG from 78 ± 10 mmHg to 71 ± 9 mmHg) | [49] |
| Meta-analysis, 2020 | Evaluation of the efficacy and safety of VLCKD in overweight and obese patients. | Very-low-calorie ketogenic diet (VLCKD) | VLCKD was associated with an average reduction in systolic blood pressure of −8 mmHg and diastolic blood pressure of −7 mmHg. | [60] |
| RCT, 2017 | Comparison of the effects of a ketogenic diet vs. a moderate-carbohydrate diet in overweight adults with type 2 diabetes mellitus or pre-diabetes. | Very-low-carbohydrate ketogenic diet (VLCKD) vs. moderate-carbohydrate, calorie-restricted, low-fat diet (MCCRD) | There was a slight reduction in diastolic blood pressure in both groups: -in LCK from an average of 77.1 mmHg (74.0, 80.3) to 77.1 mmHg (74.0, 80.1) in the 6th month and to 75.6 mmHg (72.5, 78.8) in the 12th month -in MCCRD from an average of 81.1 mmHg (78.2, 84.1) to 80.8 mmHg (77.9, 83.7) in the 6th month and 78.4 mmHg (75.5, 81.4) in the 12th month. There were small changes in systolic blood pressure in both groups: -in LCK from an average of 127.1 mmHg (121.9, 132.3) to 130.7 mmHg (125.7, 135.7) in the 6th month and 130.3 mmHg (125.2, 135.4) in the 12th month -in MCCRD from an average of 129.2 mmHg (124.6, 133.7) to 130.4 mmHg (125.6, 135.1) in the 6th month and 127.5 mmHg (122.7, 132.4) in the 12th month. | [51] |
| Systematic review with meta-analysis, 2013 | Investigate whether individuals assigned to a VLCKD (i.e., a diet with no more than 50 g carbohydrates/d) achieve better long-term body weight and cardiovascular risk factor management when compared with individuals assigned to a conventional low-fat diet (LFD, i.e., a restricted-energy diet with less than 30% of energy from fat). | Very-low-carbohydrate ketogenic diet (VLCKD) vs. dieta niskotłuszczowa z deficytem kalorycznym (LFD) | -There was a significant difference in favor of the VLCKD in lowering diastolic blood pressure (WMD—1–43 (95% CI—2–49, 0–37) mmHg) -to a lesser extent, there was a difference in lowering systolic blood pressure (WMD in favor of the VLCKD—1–47 (95% CI—3–44, 0–50) mmHg). | [212] |
| RCT, 2012 | To compare the efficacy and metabolic impact of ketogenic and hypocaloric diets in obese children and adolescents. | Ketogenic diet (KD) vs. hypocaloric diet (HD) | Mean systolic blood pressure decreased in KD from 110 ± 13 mmHg to 108 ± 13 mmHg, while diastolic blood pressure increased from a mean of 66 ± 10 mmHg to 68 ± 8 mmHg. In HD, there was a non-significant reduction in systolic blood pressure from 107 ± 9 mmHg to 106 ± 11 mmHg, and diastolic blood pressure from a mean of 65 ± 10 mmHg to 62 ± 11 mmHg. | [53] |
| RCT, 2010 | Comparison of the effects of a low-carbohydrate ketogenic diet (LCKD) and orlistat therapy in combination with a low-fat diet (O + LFD) as a weight loss therapy on key parameters, i.e., body weight, blood pressure, fasting serum lipids and glycemic parameters. | Low-Carb Ketogenic Diet (LCKD) vs. low-fat diet in combination with orlistat (LFD + O) | Relative to baseline values after 48 weeks, there was a significantly greater reduction in blood pressure in the LCKD group compared to LFD + O: -average systolic blood pressure decreased by −5.94 mmHg (−1.5 mmHg in LFD + O) -average diastolic blood pressure decreased by −4.53 mmHg (in LFD + O by −0.43 mmHg). | [208] |
| RCT, 2010 | To evaluate the effects of 2-year treatment with a low-carbohydrate or low-fat diet, each of which was combined with a comprehensive lifestyle modification program. | Low-carbohydrate diet vs. low-fat diet with a caloric deficit | There was a greater reduction in mean diastolic blood pressure in the low-carbohydrate group: -5.53 mmHg (−6.70 to −4.36) (vs. −3.05 mmHg (−4.29 to −1.81)) in the 3rd month; −5.15 mmHg (−6.49 to −3.82) (vs. −2.50 mmHg (−3.76 to −1.25)) in the 6th month; −3.25 mmHg (−4.74 to −1.76) (vs. −2.19 mmHg (−3.58 to −0.79)) in the 12th month; −3.19 mmHg (−4.66 to −1.73) (vs. −0.50 mmHg (−2.13 to 1.13)) in the 24th month. There was a slightly greater reduction in mean systolic blood pressure in the low-carbohydrate group: -7.74 mmHg (−9.59 to −5.89) (vs. −5.20 mmHg (−7.09 to −3.31)) in the 3rd month; −7.36 mmHg (−9.26 to −5.47) (vs. −6.97 mmHg (−8.89 to −5.05)) in the 6th month; −5. 64 mmHg (−7.62 to −3.67) (vs. −4.06 mmHg (−6.07 to −2.05)) in the 12th month; −2.68 mmHg (−5.08 to −0.27) (vs. −2.59 mmHg (−5.07 to −0.12)) in the 24th month. | [209] |
| RCT, 2003 | Testing the hypothesis that severely obese subjects with a high prevalence of diabetes or metabolic syndrome would achieve greater weight loss, without detrimental effects on risk factors for atherosclerosis, while on a carbohydrate-restricted (low-carbohydrate) diet than on a calorie- and fat-restricted (low-fat) diet. | Low-carbohydrate diet (<30 g/d) vs. calorie- and fat-restricted diet | Relative to baseline values, after 6 months, there was: -a non-significant mean reduction in systolic blood pressure of 2 mmHg and diastolic blood pressure of 1 mmHg in the low-carbohydrate group -a non-significant mean reduction in systolic blood pressure of 2 mmHg and diastolic blood pressure of 2 mmHg in the low-carbohydrate and low-fat groups. | [210] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyńka, D.; Kowalcze, K.; Charuta, A.; Paziewska, A. The Ketogenic Diet and Cardiovascular Diseases. Nutrients 2023, 15, 3368. https://doi.org/10.3390/nu15153368
Dyńka D, Kowalcze K, Charuta A, Paziewska A. The Ketogenic Diet and Cardiovascular Diseases. Nutrients. 2023; 15(15):3368. https://doi.org/10.3390/nu15153368
Chicago/Turabian StyleDyńka, Damian, Katarzyna Kowalcze, Anna Charuta, and Agnieszka Paziewska. 2023. "The Ketogenic Diet and Cardiovascular Diseases" Nutrients 15, no. 15: 3368. https://doi.org/10.3390/nu15153368
APA StyleDyńka, D., Kowalcze, K., Charuta, A., & Paziewska, A. (2023). The Ketogenic Diet and Cardiovascular Diseases. Nutrients, 15(15), 3368. https://doi.org/10.3390/nu15153368