Dietary Fat Modulation of Gut Microbiota and Impact on Regulatory Pathways Controlling Food Intake
Abstract
:1. Introduction
2. Microbiota-Gut Brain Axis in Controlling Food Intake
2.1. Short-Chain Fatty Acids and Nutrient Sensing Receptors
2.2. High-Fat Diet, Gut Bacteria, Intestinal Permeability, and Inflammation
2.3. Endocannabinoids, Gut Microbiota, and High-Fat Diets
3. Microbiota and Satiation Signals
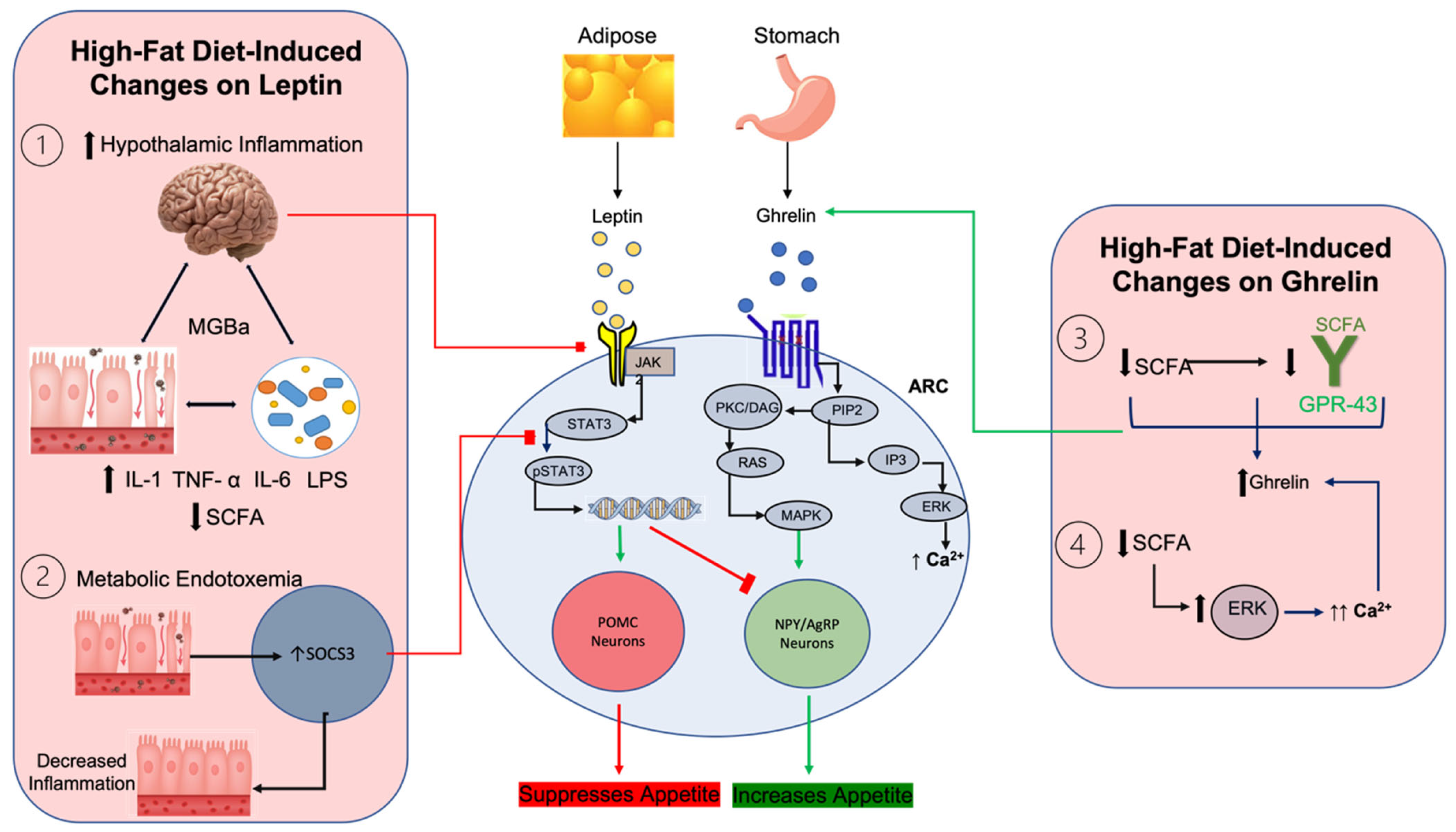
3.1. Gut Microbiota and Its Effects on Leptin and Ghrelin
3.2. Enteroendocrine Signaling, GLP-1, PYY, and Gut Microbiota
3.3. CCK, Gut Microbiota, and Obesity
3.4. Gut Microbiota and Its Effects on Orexigenic Neuropeptides
3.5. Gut Microbiota and Effects on POMC and CART
4. Dietary Interventions and Satiation Signals
4.1. Saturated Fats
4.2. Omega-3 Polyunsaturated Fatty Acids
4.3. Mediterranean Diet
5. Microbiota Intervention and Satiation Signals
5.1. Probiotics
5.2. Prebiotics
5.3. Synbiotics
5.4. Weight Loss Surgery
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Endalifer, M.L.; Diress, G. Epidemiology, Predisposing Factors, Biomarkers, and Prevention Mechanism of Obesity: A Systematic Review. J. Obes. 2020, 2020, 6134362. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Ataey, A.; Jafarvand, E.; Adham, D.; Moradi-Asl, E. The Relationship Between Obesity, Overweight, and the Human Development Index in World Health Organization Eastern Mediterranean Region Countries. J. Prev. Med. Public. Health 2020, 53, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Golden, A. Obesity’s Impact. Nurs. Clin. N. Am. 2021, 56, xiii–xiv. [Google Scholar] [CrossRef]
- Malone, J.I.; Hansen, B.C. Does obesity cause type 2 diabetes mellitus (T2DM)? Or is it the opposite? Pediatr. Diabetes 2019, 20, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Cuevas-Sierra, A.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019, 10 (Suppl. S1), S17–S30. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef] [Green Version]
- Asadi, A.; Shadab Mehr, N.; Mohamadi, M.H.; Shokri, F.; Heidary, M.; Sadeghifard, N.; Khoshnood, S. Obesity and gut-microbiota-brain axis: A narrative review. J. Clin. Lab. Anal. 2022, 36, e24420. [Google Scholar] [CrossRef]
- Hamamah, S.; Hajnal, A.; Covasa, M. Impact of Nutrition, Microbiota Transplant and Weight Loss Surgery on Dopaminergic Alterations in Parkinson’s Disease and Obesity. Int. J. Mol. Sci. 2022, 23, 7503. [Google Scholar] [CrossRef]
- Barber, T.M.; Valsamakis, G.; Mastorakos, G.; Hanson, P.; Kyrou, I.; Randeva, H.S.; Weickert, M.O. Dietary Influences on the Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2021, 22, 3502. [Google Scholar] [CrossRef]
- Mann, P.E.; Huynh, K.; Widmer, G. Maternal high fat diet and its consequence on the gut microbiome: A rat model. Gut Microbes 2018, 9, 143–154. [Google Scholar] [CrossRef]
- Indiani, C.; Rizzardi, K.F.; Castelo, P.M.; Ferraz, L.F.C.; Darrieux, M.; Parisotto, T.M. Childhood Obesity and Firmicutes/Bacteroidetes Ratio in the Gut Microbiota: A Systematic Review. Child. Obes. 2018, 14, 501–509. [Google Scholar] [CrossRef]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef]
- Chianese, R.; Coccurello, R.; Viggiano, A.; Scafuro, M.; Fiore, M.; Coppola, G.; Operto, F.F.; Fasano, S.; Laye, S.; Pierantoni, R.; et al. Impact of Dietary Fats on Brain Functions. Curr. Neuropharmacol. 2018, 16, 1059–1085. [Google Scholar] [CrossRef]
- Jais, A.; Paeger, L.; Sotelo-Hitschfeld, T.; Bremser, S.; Prinzensteiner, M.; Klemm, P.; Mykytiuk, V.; Widdershooven, P.J.M.; Vesting, A.J.; Grzelka, K.; et al. PNOC(ARC) Neurons Promote Hyperphagia and Obesity upon High-Fat-Diet Feeding. Neuron 2020, 106, 1009–1025.e10. [Google Scholar] [CrossRef]
- Schéle, E.; Grahnemo, L.; Anesten, F.; Hallén, A.; Bäckhed, F.; Jansson, J.O. The gut microbiota reduces leptin sensitivity and the expression of the obesity-suppressing neuropeptides proglucagon (Gcg) and brain-derived neurotrophic factor (Bdnf) in the central nervous system. Endocrinology 2013, 154, 3643–3651. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.; Fan, C.; Fan, X.; Lu, Y.; Wang, Y.; Wang, R.; Tang, T.; Qi, K. Effects of gut microbiota on leptin expression and body weight are lessened by high-fat diet in mice. Br. J. Nutr. 2020, 124, 396–406. [Google Scholar] [CrossRef] [Green Version]
- Gu, M.; Liu, C.; Yang, T.; Zhan, M.; Cai, Z.; Chen, Y.; Chen, Q.; Wang, Z. High-Fat Diet Induced Gut Microbiota Alterations Associating with Ghrelin/Jak2/Stat3 up-Regulation to Promote Benign Prostatic Hyperplasia Development. Front. Cell Dev. Biol. 2021, 9, 615928. [Google Scholar] [CrossRef]
- Martín, M.; Rodríguez, A.; Gómez-Ambrosi, J.; Ramírez, B.; Becerril, S.; Catalán, V.; López, M.; Diéguez, C.; Frühbeck, G.; Burrell, M.A. Caloric Restriction Prevents Metabolic Dysfunction and the Changes in Hypothalamic Neuropeptides Associated with Obesity Independently of Dietary Fat Content in Rats. Nutrients 2021, 13, 2128. [Google Scholar] [CrossRef]
- Curone, G.; Biscarini, F.; Cotozzolo, E.; Menchetti, L.; Dal Bosco, A.; Riva, F.; Cremonesi, P.; Agradi, S.; Mattioli, S.; Castiglioni, B.; et al. Could Dietary Supplementation with Different Sources of N-3 Polyunsaturated Fatty Acids Modify the Rabbit Gut Microbiota? Antibiotics 2022, 11, 227. [Google Scholar] [CrossRef]
- D’Angelo, S.; Motti, M.L.; Meccariello, R. ω-3 and ω-6 Polyunsaturated Fatty Acids, Obesity and Cancer. Nutrients 2020, 12, 2751. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front. Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sergeev, I.N.; Aljutaily, T.; Walton, G.; Huarte, E. Effects of Synbiotic Supplement on Human Gut Microbiota, Body Composition and Weight Loss in Obesity. Nutrients 2020, 12, 222. [Google Scholar] [CrossRef] [Green Version]
- Furet, J.P.; Kong, L.C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.L.; Mariat, D.; Corthier, G.; Doré, J.; Henegar, C.; et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: Links with metabolic and low-grade inflammation markers. Diabetes 2010, 59, 3049–3057. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Osadchiy, V.; Mayer, E.A. Brain-gut-microbiome interactions in obesity and food addiction. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 655–672. [Google Scholar] [CrossRef] [PubMed]
- Clemmensen, C.; Müller, T.D.; Woods, S.C.; Berthoud, H.R.; Seeley, R.J.; Tschöp, M.H. Gut-Brain Cross-Talk in Metabolic Control. Cell 2017, 168, 758–774. [Google Scholar] [CrossRef] [Green Version]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar]
- Perez-Burgos, A.; Wang, B.; Mao, Y.K.; Mistry, B.; McVey Neufeld, K.A.; Bienenstock, J.; Kunze, W. Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G211–G220. [Google Scholar] [CrossRef] [Green Version]
- Tanida, M.; Yamano, T.; Maeda, K.; Okumura, N.; Fukushima, Y.; Nagai, K. Effects of intraduodenal injection of Lactobacillus johnsonii La1 on renal sympathetic nerve activity and blood pressure in urethane-anesthetized rats. Neurosci. Lett. 2005, 389, 109–114. [Google Scholar] [CrossRef]
- Loper, H.; Leinen, M.; Bassoff, L.; Sample, J.; Romero-Ortega, M.; Gustafson, K.J.; Taylor, D.M.; Schiefer, M.A. Both high fat and high carbohydrate diets impair vagus nerve signaling of satiety. Sci. Rep. 2021, 11, 10394. [Google Scholar] [CrossRef]
- Nefti, W.; Chaumontet, C.; Fromentin, G.; Tomé, D.; Darcel, N. A high-fat diet attenuates the central response to within-meal satiation signals and modifies the receptor expression of vagal afferents in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1681–R1686. [Google Scholar] [CrossRef]
- Sen, T.; Cawthon, C.R.; Ihde, B.T.; Hajnal, A.; DiLorenzo, P.M.; de La Serre, C.B.; Czaja, K. Diet-driven microbiota dysbiosis is associated with vagal remodeling and obesity. Physiol. Behav. 2017, 173, 305–317. [Google Scholar] [CrossRef] [Green Version]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, M.T.; Cresci, G.A.M. The Immunomodulatory Functions of Butyrate. J. Inflamm. Res. 2021, 14, 6025–6041. [Google Scholar] [CrossRef]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.; Ding, Y.; Wang, L.; Xiao, Y. Gut microbiome improves postoperative cognitive function by decreasing permeability of the blood-brain barrier in aged mice. Brain Res. Bull. 2020, 164, 249–256. [Google Scholar] [CrossRef]
- Li, G.; Lin, J.; Zhang, C.; Gao, H.; Lu, H.; Gao, X.; Zhu, R.; Li, Z.; Li, M.; Liu, Z. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes 2021, 13, 1968257. [Google Scholar] [CrossRef]
- Park, M.J.; Sohrabji, F. The histone deacetylase inhibitor, sodium butyrate, exhibits neuroprotective effects for ischemic stroke in middle-aged female rats. J. Neuroinflamm. 2016, 13, 300. [Google Scholar] [CrossRef] [Green Version]
- May, A.A.; Liu, M.; Woods, S.C.; Begg, D.P. CCK increases the transport of insulin into the brain. Physiol. Behav. 2016, 165, 392–397. [Google Scholar] [CrossRef] [Green Version]
- Banks, W.A.; Farr, S.A.; Salameh, T.S.; Niehoff, M.L.; Rhea, E.M.; Morley, J.E.; Hanson, A.J.; Hansen, K.M.; Craft, S. Triglycerides cross the blood-brain barrier and induce central leptin and insulin receptor resistance. Int. J. Obes. 2018, 42, 391–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, A.A.; Spencer, S.J. Obesity and neuroinflammation: A pathway to cognitive impairment. Brain Behav. Immun. 2014, 42, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef] [PubMed]
- Duca, F.A.; Swartz, T.D.; Sakar, Y.; Covasa, M. Increased oral detection, but decreased intestinal signaling for fats in mice lacking gut microbiota. PLoS ONE 2012, 7, e39748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ang, Z.; Ding, J.L. GPR41 and GPR43 in Obesity and Inflammation-Protective or Causative? Front. Immunol. 2016, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Fan, C.; Li, P.; Lu, Y.; Chang, X.; Qi, K. Short Chain Fatty Acids Prevent High-fat-diet-induced Obesity in Mice by Regulating G Protein-coupled Receptors and Gut Microbiota. Sci. Rep. 2016, 6, 37589. [Google Scholar] [CrossRef] [Green Version]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Page, A.J. Altered Vagal Signaling and Its Pathophysiological Roles in Functional Dyspepsia. Front. Neurosci. 2022, 16, 858612. [Google Scholar] [CrossRef]
- Nighot, M.; Rawat, M.; Al-Sadi, R.; Castillo, E.F.; Nighot, P.; Ma, T.Y. Lipopolysaccharide-Induced Increase in Intestinal Permeability Is Mediated by TAK-1 Activation of IKK and MLCK/MYLK Gene. Am. J. Pathol. 2019, 189, 797–812. [Google Scholar] [CrossRef]
- Cawthon, C.R.; de La Serre, C.B. Gut bacteria interaction with vagal afferents. Brain Res. 2018, 1693 Pt B, 134–139. [Google Scholar] [CrossRef]
- Cawthon, C.R.; Kirkland, R.A.; Pandya, S.; Brinson, N.A.; de La Serre, C.B. Non-neuronal crosstalk promotes an inflammatory response in nodose ganglia cultures after exposure to byproducts from gram positive, high-fat-diet-associated gut bacteria. Physiol. Behav. 2020, 226, 113124. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Park, S.J.; Beyak, M.J. Inducible nitric oxide synthase-derived nitric oxide reduces vagal satiety signalling in obese mice. J. Physiol. 2019, 597, 1487–1502. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cheng, M.; Wang, L.; Zhang, L.; Xu, D.; Cao, P.; Wang, F.; Herzog, H.; Song, S.; Zhan, C. A Vagal-NTS Neural Pathway that Stimulates Feeding. Curr. Biol. 2020, 30, 3986–3998.e5. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Yu, Y.; Zides, C.G.; Beyak, M.J. Mechanisms of reduced leptin-mediated satiety signaling during obesity. Int. J. Obes. 2022, 46, 1212–1221. [Google Scholar] [CrossRef]
- Ayush, E.A.; Iwasaki, Y.; Iwamoto, S.; Nakabayashi, H.; Kakei, M.; Yada, T. Glucagon directly interacts with vagal afferent nodose ganglion neurons to induce Ca2+ signaling via glucagon receptors. Biochem. Biophys. Res. Commun. 2015, 456, 727–732. [Google Scholar] [CrossRef]
- Lichtman, A.H.; Blankman, J.L.; Cravatt, B.F. Endocannabinoid overload. Mol. Pharmacol. 2010, 78, 993–995. [Google Scholar] [CrossRef] [Green Version]
- Forte, N.; Fernández-Rilo, A.C.; Palomba, L.; Di Marzo, V.; Cristino, L. Obesity Affects the Microbiota-Gut-Brain Axis and the Regulation Thereof by Endocannabinoids and Related Mediators. Int. J. Mol. Sci. 2020, 21, 1554. [Google Scholar] [CrossRef] [Green Version]
- Forte, N.; Boccella, S.; Tunisi, L.; Fernández-Rilo, A.C.; Imperatore, R.; Iannotti, F.A.; De Risi, M.; Iannotta, M.; Piscitelli, F.; Capasso, R.; et al. Orexin-A and endocannabinoids are involved in obesity-associated alteration of hippocampal neurogenesis, plasticity, and episodic memory in mice. Nat. Commun. 2021, 12, 6137. [Google Scholar] [CrossRef]
- Davidson, T.L.; Jones, S.; Roy, M.; Stevenson, R.J. The Cognitive Control of Eating and Body Weight: It’s More Than What You “Think”. Front. Psychol. 2019, 10, 62. [Google Scholar] [CrossRef] [Green Version]
- Christie, S.; O’Rielly, R.; Li, H.; Wittert, G.A.; Page, A.J. High fat diet induced obesity alters endocannabinoid and ghrelin mediated regulation of components of the endocannabinoid system in nodose ganglia. Peptides 2020, 131, 170371. [Google Scholar] [CrossRef]
- Christie, S.; O’Rielly, R.; Li, H.; Nunez-Salces, M.; Wittert, G.A.; Page, A.J. Modulatory effect of methanandamide on gastric vagal afferent satiety signals depends on nutritional status. J. Physiol. 2020, 598, 2169–2182. [Google Scholar] [CrossRef]
- Senin, L.L.; Al-Massadi, O.; Folgueira, C.; Castelao, C.; Pardo, M.; Barja-Fernandez, S.; Roca-Rivada, A.; Amil, M.; Crujeiras, A.B.; Garcia-Caballero, T.; et al. The gastric CB1 receptor modulates ghrelin production through the mTOR pathway to regulate food intake. PLoS ONE 2013, 8, e80339. [Google Scholar] [CrossRef]
- Liu, J.; Batkai, S.; Pacher, P.; Harvey-White, J.; Wagner, J.A.; Cravatt, B.F.; Gao, B.; Kunos, G. Lipopolysaccharide induces anandamide synthesis in macrophages via CD14/MAPK/phosphoinositide 3-kinase/NF-kappaB independently of platelet-activating factor. J. Biol. Chem. 2003, 278, 45034–45039. [Google Scholar] [CrossRef] [Green Version]
- Moosavi Sohroforouzani, A.; Shakerian, S.; Ghanbarzadeh, M.; Alaei, H. Treadmill exercise improves LPS-induced memory impairments via endocannabinoid receptors and cyclooxygenase enzymes. Behav. Brain Res. 2020, 380, 112440. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Varela, L.; Horvath, T.L. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep. 2012, 13, 1079–1086. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Yang, X.; Yu, S.; Zheng, R. The Leptin Resistance. Adv. Exp. Med. Biol. 2018, 1090, 145–163. [Google Scholar]
- Shin, A.C.; MohanKumar, S.M.J.; Balasubramanian, P.; Sirivelu, M.P.; Linning, K.; Woolcock, A.; James, M.; MohanKumar, P.S. Responsiveness of hypothalamo-pituitary-adrenal axis to leptin is impaired in diet-induced obese rats. Nutr. Diabetes 2019, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Du, T.; Li, C.; Yang, G. STAT3 phosphorylation in central leptin resistance. Nutr. Metab. 2021, 18, 39. [Google Scholar] [CrossRef]
- Palanivel, R.; Fullerton, M.D.; Galic, S.; Honeyman, J.; Hewitt, K.A.; Jorgensen, S.B.; Steinberg, G.R. Reduced Socs3 expression in adipose tissue protects female mice against obesity-induced insulin resistance. Diabetologia 2012, 55, 3083–3093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, H.; Hanada, R.; Hanada, T.; Aki, D.; Mashima, R.; Nishinakamura, H.; Torisu, T.; Chien, K.R.; Yasukawa, H.; Yoshimura, A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat. Med. 2004, 10, 739–743. [Google Scholar] [CrossRef] [PubMed]
- González, F.; Considine, R.V.; Abdelhadi, O.A.; Acton, A.J. Saturated Fat Ingestion Promotes Lipopolysaccharide-Mediated Inflammation and Insulin Resistance in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2019, 104, 934–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, K.; Ushiki, T.; Ishiguro, H.; Tamura, S.; Araki, M.; Suwabe, T.; Katagiri, T.; Watanabe, M.; Fujimoto, Y.; Ohashi, R.; et al. Altered microbiota by a high-fat diet accelerates lethal myeloid hematopoiesis associated with systemic SOCS3 deficiency. iScience 2021, 24, 103117. [Google Scholar] [CrossRef]
- Mirpuri, J.; Sotnikov, I.; Myers, L.; Denning, T.L.; Yarovinsky, F.; Parkos, C.A.; Denning, P.W.; Louis, N.A. Lactobacillus rhamnosus (LGG) regulates IL-10 signaling in the developing murine colon through upregulation of the IL-10R2 receptor subunit. PLoS ONE 2012, 7, e51955. [Google Scholar] [CrossRef]
- Thaler, J.P.; Yi, C.X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Valdearcos, M.; Robblee, M.M.; Benjamin, D.I.; Nomura, D.K.; Xu, A.W.; Koliwad, S.K. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014, 9, 2124–2138. [Google Scholar] [CrossRef] [Green Version]
- Mossad, O.; Batut, B.; Yilmaz, B.; Dokalis, N.; Mezö, C.; Nent, E.; Nabavi, L.S.; Mayer, M.; Maron, F.J.M.; Buescher, J.M.; et al. Gut microbiota drives age-related oxidative stress and mitochondrial damage in microglia via the metabolite N(6)-carboxymethyllysine. Nat. Neurosci. 2022, 25, 295–305. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the, C.N.S. Nat Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Heiss, C.N.; Mannerås-Holm, L.; Lee, Y.S.; Serrano-Lobo, J.; Håkansson Gladh, A.; Seeley, R.J.; Drucker, D.J.; Bäckhed, F.; Olofsson, L.E. The gut microbiota regulates hypothalamic inflammation and leptin sensitivity in Western diet-fed mice via a GLP-1R-dependent mechanism. Cell Rep. 2021, 35, 109163. [Google Scholar] [CrossRef]
- Grasset, E.; Puel, A.; Charpentier, J.; Klopp, P.; Christensen, J.E.; Lelouvier, B.; Servant, F.; Blasco-Baque, V.; Tercé, F.; Burcelin, R. Gut microbiota dysbiosis of type 2 diabetic mice impairs the intestinal daily rhythms of GLP-1 sensitivity. Acta Diabetol. 2022, 59, 243–258. [Google Scholar] [CrossRef]
- Berkseth, K.E.; Guyenet, S.J.; Melhorn, S.J.; Lee, D.; Thaler, J.P.; Schur, E.A.; Schwartz, M.W. Hypothalamic gliosis associated with high-fat diet feeding is reversible in mice: A combined immunohistochemical and magnetic resonance imaging study. Endocrinology 2014, 155, 2858–2867. [Google Scholar] [CrossRef] [Green Version]
- Tschöp, M.; Smiley, D.L.; Heiman, M.L. Ghrelin induces adiposity in rodents. Nature 2000, 407, 908–913. [Google Scholar] [CrossRef]
- Rahat-Rozenbloom, S.; Fernandes, J.; Cheng, J.; Wolever, T.M.S. Acute increases in serum colonic short-chain fatty acids elicited by inulin do not increase GLP-1 or PYY responses but may reduce ghrelin in lean and overweight humans. Eur. J. Clin. Nutr. 2017, 71, 953–958. [Google Scholar] [CrossRef]
- Stevenson, J.L.; Paton, C.M.; Cooper, J.A. Hunger and satiety responses to high-fat meals after a high-polyunsaturated fat diet: A randomized trial. Nutrition 2017, 41, 14–23. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Golubeva, A.V.; Zhdanov, A.V.; Wallace, S.; Arboleya, S.; Papkovsky, D.B.; Aidy, S.E.; Ross, P.; Roy, B.L.; Stanton, C.; et al. Short-chain fatty acids and microbiota metabolites attenuate ghrelin receptor signaling. FASEB J. 2019, 33, 13546–13559. [Google Scholar] [CrossRef] [Green Version]
- Engelstoft, M.S.; Park, W.M.; Sakata, I.; Kristensen, L.V.; Husted, A.S.; Osborne-Lawrence, S.; Piper, P.K.; Walker, A.K.; Pedersen, M.H.; Nøhr, M.K.; et al. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol. Metab. 2013, 2, 376–392. [Google Scholar] [CrossRef]
- Woźniak, D.; Cichy, W.; Przysławski, J.; Drzymała-Czyż, S. The role of microbiota and enteroendocrine cells in maintaining homeostasis in the human digestive tract. Adv. Med. Sci. 2021, 66, 284–292. [Google Scholar] [CrossRef]
- Posovszky, C.; Wabitsch, M. Regulation of appetite, satiation, and body weight by enteroendocrine cells. Part 1: Characteristics of enteroendocrine cells and their capability of weight regulation. Horm. Res. Paediatr. 2015, 83, 1–10. [Google Scholar] [CrossRef]
- Lu, V.B.; Gribble, F.M.; Reimann, F. Free Fatty Acid Receptors in Enteroendocrine Cells. Endocrinology 2018, 159, 2826–2835. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria with Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Farzi, A.; Ip, C.K.; Reed, F.; Enriquez, R.; Zenz, G.; Durdevic, M.; Zhang, L.; Holzer, P.; Herzog, H. Lack of peptide YY signaling in mice disturbs gut microbiome composition in response to high-fat diet. FASEB J. 2021, 35, e21435. [Google Scholar] [CrossRef] [PubMed]
- Mazzawi, T.; Hausken, T.; El-Salhy, M. Changes in colonic enteroendocrine cells of patients with irritable bowel syndrome following fecal microbiota transplantation. Scand. J. Gastroenterol. 2022, 57, 792–796. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, D. Is GLP-1 a hormone: Whether and When? J. Diabetes Investig. 2016, 7 (Suppl. S1), 50–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drucker, D.J. GLP-1 physiology informs the pharmacotherapy of obesity. Mol. Metab. 2022, 57, 101351. [Google Scholar] [CrossRef]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.H.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K.; et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021, 6, 563–573. [Google Scholar] [CrossRef]
- Joffe, Y.T.; van der Merwe, L.; Evans, J.; Collins, M.; Lambert, E.V.; September, A.V.; Goedecke, J.H. Interleukin-6 gene polymorphisms, dietary fat intake, obesity and serum lipid concentrations in black and white South African women. Nutrients 2014, 6, 2436–2465. [Google Scholar] [CrossRef] [Green Version]
- Ren, M.; Zhang, H.; Qi, J.; Hu, A.; Jiang, Q.; Hou, Y.; Feng, Q.; Ojo, O.; Wang, X. An Almond-Based Low Carbohydrate Diet Improves Depression and Glycometabolism in Patients with Type 2 Diabetes through Modulating Gut Microbiota and GLP-1: A Randomized Controlled Trial. Nutrients 2020, 12, 3036. [Google Scholar] [CrossRef]
- Cawthon, C.R.; de La Serre, C.B. The critical role of CCK in the regulation of food intake and diet-induced obesity. Peptides 2021, 138, 170492. [Google Scholar] [CrossRef]
- Covasa, M.; Grahn, J.; Ritter, R.C. Reduced hindbrain and enteric neuronal response to intestinal oleate in rats maintained on high-fat diet. Auton. Neurosci. 2000, 84, 8–18. [Google Scholar] [CrossRef]
- Kim, J.S.; Kirkland, R.A.; Lee, S.H.; Cawthon, C.R.; Rzepka, K.W.; Minaya, D.M.; de Lartigue, G.; Czaja, K.; de La Serre, C.B. Gut microbiota composition modulates inflammation and structure of the vagal afferent pathway. Physiol. Behav. 2020, 225, 113082. [Google Scholar] [CrossRef]
- Muzio, L.; Viotti, A.; Martino, G. Microglia in Neuroinflammation and Neurodegeneration: From Understanding to Therapy. Front. Neurosci. 2021, 15, 742065. [Google Scholar] [CrossRef]
- Wang, L.; Jacobs, J.P.; Lagishetty, V.; Yuan, P.Q.; Wu, S.V.; Million, M.; Reeve, J.R.; Pisegna, J.R., Jr.; Taché, Y. High-protein diet improves sensitivity to cholecystokinin and shifts the cecal microbiome without altering brain inflammation in diet-induced obesity in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R473–R486. [Google Scholar] [CrossRef] [Green Version]
- Chelakkot, C.; Choi, Y.; Kim, D.K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.S.; Jee, Y.K.; Gho, Y.S.; et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef] [Green Version]
- Alhabeeb, H.; AlFaiz, A.; Kutbi, E.; AlShahrani, D.; Alsuhail, A.; AlRajhi, S.; Alotaibi, N.; Alotaibi, K.; AlAmri, S.; Alghamdi, S.; et al. Gut Hormones in Health and Obesity: The Upcoming Role of Short Chain Fatty Acids. Nutrients 2021, 13, 481. [Google Scholar] [CrossRef]
- Lal, S.; Kirkup, A.J.; Brunsden, A.M.; Thompson, D.G.; Grundy, D. Vagal afferent responses to fatty acids of different chain length in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G907–G915. [Google Scholar] [CrossRef]
- Klingbeil, E.A.; Cawthon, C.; Kirkland, R.; de La Serre, C.B. Potato-Resistant Starch Supplementation Improves Microbiota Dysbiosis, Inflammation, and Gut-Brain Signaling in High Fat-Fed Rats. Nutrients 2019, 11, 2710. [Google Scholar] [CrossRef] [Green Version]
- Ahn, W.; Latremouille, J.; Harris, R.B.S. Leptin receptor-expressing cells in the ventromedial nucleus of the hypothalamus contribute to enhanced CCK-induced satiety following central leptin injection. Am. J. Physiol. Endocrinol. Metab. 2022, 323, E267–E280. [Google Scholar] [CrossRef]
- Smith, M.A.; Choudhury, A.I.; Glegola, J.A.; Viskaitis, P.; Irvine, E.E.; de Campos Silva, P.C.C.; Khadayate, S.; Zeilhofer, H.U.; Withers, D.J. Extrahypothalamic GABAergic nociceptin-expressing neurons regulate AgRP neuron activity to control feeding behavior. J. Clin. Investig. 2020, 130, 126–142. [Google Scholar] [CrossRef] [Green Version]
- Krashes, M.J.; Koda, S.; Ye, C.; Rogan, S.C.; Adams, A.C.; Cusher, D.S.; Maratos-Flier, E.; Roth, B.L.; Lowell, B.B. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Investig. 2011, 121, 1424–1428. [Google Scholar] [CrossRef] [Green Version]
- Jikomes, N.; Ramesh, R.N.; Mandelblat-Cerf, Y.; Andermann, M.L. Preemptive Stimulation of AgRP Neurons in Fed Mice Enables Conditioned Food Seeking under Threat. Curr. Biol. 2016, 26, 2500–2507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglass, J.D.; Dorfman, M.D.; Fasnacht, R.; Shaffer, L.D.; Thaler, J.P. Astrocyte IKKβ/NF-κB signaling is required for diet-induced obesity and hypothalamic inflammation. Mol. Metab. 2017, 6, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Rauf, N.; Nabi, G.; Yi, S.; Yu-Dong, Z.; Fu, J. Mechanistic insight into high-fat diet-induced metabolic inflammation in the arcuate nucleus of the hypothalamus. Biomed. Pharmacother. 2021, 142, 112012. [Google Scholar] [CrossRef]
- Olofsson, L.E.; Unger, E.K.; Cheung, C.C.; Xu, A.W. Modulation of AgRP-neuronal function by SOCS3 as an initiating event in diet-induced hypothalamic leptin resistance. Proc. Natl. Acad. Sci. USA 2013, 110, E697–E706. [Google Scholar] [CrossRef] [PubMed]
- Hamamah, S.; Covasa, M. Gut Microbiota Restores Central Neuropeptide Deficits in Germ-Free Mice. Int. J. Mol. Sci. 2022, 23, 11756. [Google Scholar] [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [Green Version]
- Breton, J.; Tirelle, P.; Hasanat, S.; Pernot, A.; L’Huillier, C.; do Rego, J.C.; Déchelotte, P.; Coëffier, M.; Bindels, L.B.; Ribet, D. Gut microbiota alteration in a mouse model of Anorexia Nervosa. Clin. Nutr. 2021, 40, 181–189. [Google Scholar] [CrossRef]
- Wang, T.; Yan, H.; Lu, Y.; Li, X.; Wang, X.; Shan, Y.; Yi, Y.; Liu, B.; Zhou, Y.; Lü, X. Anti-obesity effect of Lactobacillus rhamnosus LS-8 and Lactobacillus crustorum MN047 on high-fat and high-fructose diet mice base on inflammatory response alleviation and gut microbiota regulation. Eur. J. Nutr. 2020, 59, 2709–2728. [Google Scholar] [CrossRef]
- Hao, L.; Sheng, Z.; Potian, J.; Deak, A.; Rohowsky-Kochan, C.; Routh, V.H. Lipopolysaccharide (LPS) and tumor necrosis factor alpha (TNFα) blunt the response of Neuropeptide Y/Agouti-related peptide (NPY/AgRP) glucose inhibited (GI) neurons to decreased glucose. Brain Res. 2016, 1648 Pt A, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Hill, J.W. Gene Expression and the Control of Food Intake by Hypothalamic POMC/CART Neurons. Open Neuroendocrinol. J. 2010, 3, 21–27. [Google Scholar]
- Wang, Z.W.; Zhou, Y.T.; Kakuma, T.; Lee, Y.; Higa, M.; Kalra, S.P.; Dube, M.G.; Kalra, P.S.; Unger, R.H. Comparing the hypothalamic and extrahypothalamic actions of endogenous hyperleptinemia. Proc. Natl. Acad. Sci. USA 1999, 96, 10373–10378. [Google Scholar] [CrossRef]
- Srisai, D.; Gillum, M.P.; Panaro, B.L.; Zhang, X.M.; Kotchabhakdi, N.; Shulman, G.I.; Ellacott, K.L.; Cone, R.D. Characterization of the hyperphagic response to dietary fat in the MC4R knockout mouse. Endocrinology 2011, 152, 890–902. [Google Scholar] [CrossRef] [Green Version]
- Tam, J.; Szanda, G.; Drori, A.; Liu, Z.; Cinar, R.; Kashiwaya, Y.; Reitman, M.L.; Kunos, G. Peripheral cannabinoid-1 receptor blockade restores hypothalamic leptin signaling. Mol. Metab. 2017, 6, 1113–1125. [Google Scholar] [CrossRef]
- Morello, G.; Imperatore, R.; Palomba, L.; Finelli, C.; Labruna, G.; Pasanisi, F.; Sacchetti, L.; Buono, L.; Piscitelli, F.; Orlando, P.; et al. Orexin-A represses satiety-inducing POMC neurons and contributes to obesity via stimulation of endocannabinoid signaling. Proc. Natl. Acad. Sci. USA 2016, 113, 4759–4764. [Google Scholar] [CrossRef]
- Kreutzer, C.; Peters, S.; Schulte, D.M.; Fangmann, D.; Türk, K.; Wolff, S.; van Eimeren, T.; Ahrens, M.; Beckmann, J.; Schafmayer, C.; et al. Hypothalamic Inflammation in Human Obesity Is Mediated by Environmental and Genetic Factors. Diabetes 2017, 66, 2407–2415. [Google Scholar] [CrossRef] [Green Version]
- Xu, A.A.; Kennedy, L.K.; Hoffman, K.; White, D.L.; Kanwal, F.; El-Serag, H.B.; Petrosino, J.F.; Jiao, L. Dietary Fatty Acid Intake and the Colonic Gut Microbiota in Humans. Nutrients 2022, 14, 2722. [Google Scholar] [CrossRef]
- Bailén, M.; Bressa, C.; Martínez-López, S.; González-Soltero, R.; Montalvo Lominchar, M.G.; San Juan, C.; Larrosa, M. Microbiota Features Associated with a High-Fat/Low-Fiber Diet in Healthy Adults. Front. Nutr. 2020, 7, 583608. [Google Scholar] [CrossRef]
- De Wit, N.; Derrien, M.; Bosch-Vermeulen, H.; Oosterink, E.; Keshtkar, S.; Duval, C.; de Vogel-van den Bosch, J.; Kleerebezem, M.; Müller, M.; van der Meer, R. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G589–G599. [Google Scholar] [CrossRef] [Green Version]
- Ju, T.; Bourrie, B.C.T.; Forgie, A.J.; Pepin, D.M.; Tollenaar, S.; Sergi, C.M.; Willing, B.P. The Gut Commensal Escherichia coli Aggravates High-Fat-Diet-Induced Obesity and Insulin Resistance in Mice. Appl. Environ. Microbiol. 2023, 89, e0162822. [Google Scholar] [CrossRef]
- van der Heijden, R.A.; Sheedfar, F.; Morrison, M.C.; Hommelberg, P.P.; Kor, D.; Kloosterhuis, N.J.; Gruben, N.; Youssef, S.A.; de Bruin, A.; Hofker, M.H.; et al. High-fat diet induced obesity primes inflammation in adipose tissue prior to liver in C57BL/6j mice. Aging 2015, 7, 256–268. [Google Scholar] [CrossRef] [Green Version]
- De La Serre, C.B.; de Lartigue, G.; Raybould, H.E. Chronic exposure to low dose bacterial lipopolysaccharide inhibits leptin signaling in vagal afferent neurons. Physiol. Behav. 2015, 139, 188–194. [Google Scholar] [PubMed] [Green Version]
- Nguyen, A.T.; Mandard, S.; Dray, C.; Deckert, V.; Valet, P.; Besnard, P.; Drucker, D.J.; Lagrost, L.; Grober, J. Lipopolysaccharides-mediated increase in glucose-stimulated insulin secretion: Involvement of the GLP-1 pathway. Diabetes 2014, 63, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, G.D.; Lira, F.S.; Rosa, J.C.; Oliveira, J.L.; Losinskas-Hachul, A.C.; Souza, G.I.; das Graças, T.d.C.M.; Santos, R.V.; de Mello, M.T.; Tufik, S.; et al. Intake of trans fatty acids during gestation and lactation leads to hypothalamic inflammation via TLR4/NFκBp65 signaling in adult offspring. J. Nutr. Biochem. 2012, 23, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Ben Fradj, S.; Nédélec, E.; Salvi, J.; Fouesnard, M.; Huillet, M.; Pallot, G.; Cansell, C.; Sanchez, C.; Philippe, C.; Gigot, V.; et al. Evidence for Constitutive Microbiota-Dependent Short-Term Control of Food Intake in Mice: Is There a Link with Inflammation, Oxidative Stress, Endotoxemia, and GLP-1? Antioxid. Redox Signal 2022, 37, 349–369. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, X.; Zhu, Y.; Huang, Y.; Zhang, Q.; Lin, S.; Fang, C.; Li, L.; Lv, Y.; Mei, W.; et al. Effect of Plant-Derived n-3 Polyunsaturated Fatty Acids on Blood Lipids and Gut Microbiota: A Double-Blind Randomized Controlled Trial. Front. Nutr. 2022, 9, 830960. [Google Scholar] [PubMed]
- Watson, H.; Mitra, S.; Croden, F.C.; Taylor, M.; Wood, H.M.; Perry, S.L.; Spencer, J.A.; Quirke, P.; Toogood, G.J.; Lawton, C.L.; et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 2018, 67, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar]
- Cheng, L.; Hu, T.; Shi, H.; Chen, X.; Wang, H.; Zheng, K.; Huang, X.F.; Yu, Y. DHA reduces hypothalamic inflammation and improves central leptin signaling in mice. Life Sci. 2020, 257, 118036. [Google Scholar] [CrossRef]
- Zheng, X.; Niu, S. Leptin-induced basal Akt phosphorylation and its implication in exercise-mediated improvement of insulin sensitivity. Biochem. Biophys. Res. Commun. 2018, 496, 37–43. [Google Scholar] [CrossRef]
- Cintra, D.E.; Ropelle, E.R.; Moraes, J.C.; Pauli, J.R.; Morari, J.; Souza, C.T.; Grimaldi, R.; Stahl, M.; Carvalheira, J.B.; Saad, M.J.; et al. Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. PLoS ONE 2012, 7, e30571. [Google Scholar] [CrossRef]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet; a Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosato, V.; Temple, N.J.; La Vecchia, C.; Castellan, G.; Tavani, A.; Guercio, V. Mediterranean diet and cardiovascular disease: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2019, 58, 173–191. [Google Scholar] [CrossRef] [PubMed]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet: A Review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 742065. [Google Scholar] [CrossRef] [Green Version]
- Dahl, W.J.; Rivero Mendoza, D.; Lambert, J.M. Diet, nutrients and the microbiome. Prog. Mol. Biol. Transl. Sci. 2020, 171, 237–263. [Google Scholar]
- Newman, T.M.; Shively, C.A.; Register, T.C.; Appt, S.E.; Yadav, H.; Colwell, R.R.; Fanelli, B.; Dadlani, M.; Graubics, K.; Nguyen, U.T.; et al. Diet, obesity, and the gut microbiome as determinants modulating metabolic outcomes in a non-human primate model. Microbiome 2021, 9, 100. [Google Scholar] [CrossRef]
- Song, W.; Song, C.; Li, L.; Wang, T.; Hu, J.; Zhu, L.; Yue, T. Lactobacillus alleviated obesity induced by high-fat diet in mice. J. Food Sci. 2021, 86, 5439–5451. [Google Scholar] [CrossRef]
- Lee, Y.K.; Park, J.E.; Lee, M.; Hardwick, J.P. Hepatic lipid homeostasis by peroxisome proliferator-activated receptor gamma 2. Liver Res. 2018, 2, 209–215. [Google Scholar] [CrossRef]
- Jiang, H.; Gan, T.; Zhang, J.; Ma, Q.; Liang, Y.; Zhao, Y. The Structures and Bioactivities of Fatty Acid Synthase Inhibitors. Curr. Med. Chem. 2019, 26, 7081–7101. [Google Scholar] [CrossRef]
- Meslier, V.; Laiola, M.; Roager, H.M.; De Filippis, F.; Roume, H.; Quinquis, B.; Giacco, R.; Mennella, I.; Ferracane, R.; Pons, N.; et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 2020, 69, 1258–1268. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Liang, R.; Zhang, W.; Tian, K.; Li, J.; Chen, X.; Yu, T.; Chen, Q. Faecalibacterium prausnitzii-derived microbial anti-inflammatory molecule regulates intestinal integrity in diabetes mellitus mice via modulating tight junction protein expression. J. Diabetes 2020, 12, 224–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maioli, T.U.; Borras-Nogues, E.; Torres, L.; Barbosa, S.C.; Martins, V.D.; Langella, P.; Azevedo, V.A.; Chatel, J.M. Possible Benefits of Faecalibacterium prausnitzii for Obesity-Associated Gut Disorders. Front. Pharmacol. 2021, 12, 740636. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, M.; Wang, Y.; Dorfman, R.G.; Liu, H.; Yu, T.; Chen, X.; Tang, D.; Xu, L.; Yin, Y.; et al. Faecalibacterium prausnitzii Produces Butyrate to Maintain Th17/Treg Balance and to Ameliorate Colorectal Colitis by Inhibiting Histone Deacetylase 1. Inflamm. Bowel Dis. 2018, 24, 1926–1940. [Google Scholar] [CrossRef] [Green Version]
- Rusch, C.; Beke, M.; Tucciarone, L.; Nieves, C.; Ukhanova, M., Jr.; Tagliamonte, M.S.; Mai, V.; Suh, J.H.; Wang, Y.; Chiu, S.; et al. Mediterranean Diet Adherence in People with Parkinson’s Disease Reduces Constipation Symptoms and Changes Fecal Microbiota after a 5-Week Single-Arm Pilot Study. Front. Neurol. 2021, 12, 794640. [Google Scholar]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia intestinalis: A Beneficial Gut Organism from the Discoveries in Genus and Species. Front. Cell. Infect. Microbiol. 2021, 11, 757718. [Google Scholar] [CrossRef]
- Wu, X.; Pan, S.; Luo, W.; Shen, Z.; Meng, X.; Xiao, M.; Tan, B.; Nie, K.; Tong, T.; Wang, X. Roseburia intestinalis-derived flagellin ameliorates colitis by targeting miR-223-3p-mediated activation of NLRP3 inflammasome and pyroptosis. Mol. Med. Rep. 2020, 22, 2695–2704. [Google Scholar] [CrossRef]
- Gan, R.; Liu, H.; Wu, S.; Huang, R.; Tang, Z.; Zhang, N.; Hu, L. Curcumin Alleviates Arsenic Trioxide-Induced Inflammation and Pyroptosis via the NF-κB/NLRP3 Signaling Pathway in the Hypothalamus of Ducks. Biol. Trace Elem. Res. 2023, 201, 2503–2511. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Díaz-López, A.; Ruiz-Canela, M.; Basora, J.; Fitó, M.; Corella, D.; Serra-Majem, L.; Wärnberg, J.; Romaguera, D.; Estruch, R.; et al. Effect of a Lifestyle Intervention Program with Energy-Restricted Mediterranean Diet and Exercise on Weight Loss and Cardiovascular Risk Factors: One-Year Results of the PREDIMED-Plus Trial. Diabetes Care 2019, 42, 777–788. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Pérez, A.; Sánchez-Jiménez, F.; Vilariño-García, T.; Sánchez-Margalet, V. Role of Leptin in Inflammation and Vice Versa. Int. J. Mol. Sci. 2020, 21, 5887. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Zafar, H.; Saier, M.H., Jr. Gut Bacteroides species in health and disease. Gut Microbes 2021, 13, 1848158. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, J.; Yi, J.; Liu, Y.; Yu, Z.; Chen, S.; Liu, X. Increased mucin-degrading bacteria by high protein diet leads to thinner mucus layer and aggravates experimental colitis. J. Gastroenterol. Hepatol. 2021, 36, 2864–2874. [Google Scholar] [PubMed]
- Pellizoni, F.P.; Leite, A.Z.; Rodrigues, N.C.; Ubaiz, M.J.; Gonzaga, M.I.; Takaoka, N.N.C.; Mariano, V.S.; Omori, W.P.; Pinheiro, D.G.; Matheucci Junior, E.; et al. Detection of Dysbiosis and Increased Intestinal Permeability in Brazilian Patients with Relapsing-Remitting Multiple Sclerosis. Int. J. Environ. Res. Public. Health 2021, 18, 4621. [Google Scholar] [CrossRef]
- Ding, N.; Zhang, X.; Zhang, X.D.; Jing, J.; Liu, S.S.; Mu, Y.P.; Peng, L.L.; Yan, Y.J.; Xiao, G.M.; Bi, X.Y.; et al. Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes. Gut 2020, 69, 1608–1619. [Google Scholar] [CrossRef] [Green Version]
- Mima, A.; Hiraoka-Yamomoto, J.; Li, Q.; Kitada, M.; Li, C.; Geraldes, P.; Matsumoto, M.; Mizutani, K.; Park, K.; Cahill, C.; et al. Protective effects of GLP-1 on glomerular endothelium and its inhibition by PKCβ activation in diabetes. Diabetes 2012, 61, 2967–2979. [Google Scholar] [CrossRef] [Green Version]
- Ceriello, A.; Esposito, K.; Testa, R.; Bonfigli, A.R.; Marra, M.; Giugliano, D. The possible protective role of glucagon-like peptide 1 on endothelium during the meal and evidence for an “endothelial resistance” to glucagon-like peptide 1 in diabetes. Diabetes Care 2011, 34, 697–702. [Google Scholar] [CrossRef] [Green Version]
- Di Mauro, A.; Tuccinardi, D.; Watanabe, M.; Del Toro, R.; Monte, L.; Giorgino, R.; Rampa, L.; Rossini, G.; Kyanvash, S.; Soare, A.; et al. The Mediterranean diet increases glucagon-like peptide 1 and oxyntomodulin compared with a vegetarian diet in patients with type 2 diabetes: A randomized controlled cross-over trial. Diabetes Metab. Res. Rev. 2021, 37, e3406. [Google Scholar] [CrossRef]
- Papamichou, D.; Panagiotakos, D.B.; Holmes, E.; Koutsakis, P.; Katsoulotos, H.; Loo, R.L.; Itsiopoulos, C. The rationale and design of a Mediterranean diet accompanied by time restricted feeding to optimise the management of type 2 diabetes: The MedDietFast randomised controlled trial. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 220–230. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Li, H.; Liu, X. The potential of proteins, hydrolysates and peptides as growth factors for Lactobacillus and Bifidobacterium: Current research and future perspectives. Food Funct. 2020, 11, 1946–1957. [Google Scholar] [CrossRef]
- Zhang, X.F.; Guan, X.X.; Tang, Y.J.; Sun, J.F.; Wang, X.K.; Wang, W.D.; Fan, J.M. Clinical effects and gut microbiota changes of using probiotics, prebiotics or synbiotics in inflammatory bowel disease: A systematic review and meta-analysis. Eur. J. Nutr. 2021, 60, 2855–2875. [Google Scholar] [CrossRef]
- Xue, L.; He, J.; Gao, N.; Lu, X.; Li, M.; Wu, X.; Liu, Z.; Jin, Y.; Liu, J.; Xu, J.; et al. Probiotics may delay the progression of nonalcoholic fatty liver disease by restoring the gut microbiota structure and improving intestinal endotoxemia. Sci. Rep. 2017, 7, 45176. [Google Scholar] [PubMed] [Green Version]
- Nagpal, R.; Shively, C.A.; Appt, S.A.; Register, T.C.; Michalson, K.T.; Vitolins, M.Z.; Yadav, H. Gut Microbiome Composition in Non-human Primates Consuming a Western or Mediterranean Diet. Front. Nutr. 2018, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Paliwoda, A.; Błasiak, J. Anti-proliferative, pro-apoptotic and anti-oxidative activity of Lactobacillus and Bifidobacterium strains: A review of mechanisms and therapeutic perspectives. Crit. Rev. Food Sci. Nutr. 2019, 59, 3456–3467. [Google Scholar] [CrossRef]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Narmaki, E.; Borazjani, M.; Ataie-Jafari, A.; Hariri, N.; Doost, A.H.; Qorbani, M.; Saidpour, A. The combined effects of probiotics and restricted calorie diet on the anthropometric indices, eating behavior, and hormone levels of obese women with food addiction: A randomized clinical trial. Nutr. Neurosci. 2022, 25, 963–975. [Google Scholar] [CrossRef]
- Nam, Y.; Yoon, S.; Baek, J.; Kim, J.H.; Park, M.; Hwang, K.; Kim, W. Heat-Killed Lactiplantibacillus plantarum LRCC5314 Mitigates the Effects of Stress-Related Type 2 Diabetes in Mice via Gut Microbiome Modulation. J. Microbiol. Biotechnol. 2022, 32, 324–332. [Google Scholar]
- Lee, H.; An, J.; Kim, J.; Choi, D.; Song, Y.; Lee, C.K.; Kong, H.; Kim, S.B.; Kim, K. A Novel Bacterium, Butyricimonas virosa, Preventing HFD-Induced Diabetes and Metabolic Disorders in Mice via GLP-1 Receptor. Front. Microbiol. 2022, 13, 858192. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, H.; Gao, F.; Qian, Z.; Mao, W.; Yin, Y.; Tan, J.; Chen, D. Antidiabetic effects of selenium-enriched Bifidobacterium longum DD98 in type 2 diabetes model of mice. Food Funct. 2020, 11, 6528–6541. [Google Scholar]
- Nicolucci, A.C.; Hume, M.P.; Martínez, I.; Mayengbam, S.; Walter, J.; Reimer, R.A. Prebiotics Reduce Body Fat and Alter Intestinal Microbiota in Children Who Are Overweight or With Obesity. Gastroenterology 2017, 153, 711–722. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Yu, H.; Xiao, X.; Hu, L.; Xin, F.; Yu, X. Inulin-type fructan improves diabetic phenotype and gut microbiota profiles in rats. PeerJ 2018, 6, e4446. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Zapata, R.C.; Pezeshki, A.; Reidelberger, R.D.; Chelikani, P.K. Inulin fiber dose-dependently modulates energy balance, glucose tolerance, gut microbiota, hormones and diet preference in high-fat-fed male rats. J. Nutr. Biochem. 2018, 59, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Parnell, J.A.; Reimer, R.A. Prebiotic fibres dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR:LA-cp rats. Br. J. Nutr. 2012, 107, 601–613. [Google Scholar] [CrossRef] [Green Version]
- Rabiei, S.; Hedayati, M.; Rashidkhani, B.; Saadat, N.; Shakerhossini, R. The Effects of Synbiotic Supplementation on Body Mass Index, Metabolic and Inflammatory Biomarkers, and Appetite in Patients with Metabolic Syndrome: A Triple-Blind Randomized Controlled Trial. J. Diet. Suppl. 2019, 16, 294–306. [Google Scholar] [CrossRef]
- Hosseinifard, E.S.; Bavafa-Valenlia, K.; Saghafi-Asl, M.; Morshedi, M. Antioxidative and Metabolic Effects of Lactobacillus plantarum, Inulin, and Their Synbiotic on the Hypothalamus and Serum of Healthy Rats. Nutr. Metab. Insights. 2020, 13, 1178638820925092. [Google Scholar] [CrossRef]
- Choi, B.R.; Kwon, E.Y.; Kim, H.J.; Choi, M.S. Role of Synbiotics Containing d-Allulose in the Alteration of Body Fat and Hepatic Lipids in Diet-Induced Obese Mice. Nutrients 2018, 10, 1797. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Haase, N.; Haange, S.B.; Sucher, R.; Münzker, J.; Jäger, E.; Schischke, K.; Seyfried, F.; von Bergen, M.; Hankir, M.K.; et al. Roux-en-Y gastric bypass contributes to weight loss-independent improvement in hypothalamic inflammation and leptin sensitivity through gut-microglia-neuron-crosstalk. Mol. Metab. 2021, 48, 101214. [Google Scholar] [CrossRef]
- Dischinger, U.; Corteville, C.; Otto, C.; Fassnacht, M.; Seyfried, F.; Hankir, M.K. GLP-1 and PYY(3-36) reduce high-fat food preference additively after Roux-en-Y gastric bypass in diet-induced obese rats. Surg. Obes. Relat. Dis. 2019, 15, 1483–1492. [Google Scholar] [CrossRef]
- McCarty, T.R.; Jirapinyo, P.; Thompson, C.C. Effect of Sleeve Gastrectomy on Ghrelin, GLP-1, PYY, and GIP Gut Hormones: A Systematic Review and Meta-analysis. Ann Surg. 2020, 272, 72–80. [Google Scholar] [CrossRef]
- Chaudhari, S.N.; Luo, J.N.; Harris, D.A.; Aliakbarian, H.; Yao, L.; Paik, D.; Subramaniam, R.; Adhikari, A.A.; Vernon, A.H.; Kiliç, A.; et al. A microbial metabolite remodels the gut-liver axis following bariatric surgery. Cell Host Microbe 2021, 29, 408–424.e7. [Google Scholar] [CrossRef]
- Valent, D.; Arroyo, L.; Fàbrega, E.; Font, I.F.M.; Rodríguez-Palmero, M.; Moreno-Muñoz, J.A.; Tibau, J.; Bassols, A. Effects of a high-fat-diet supplemented with probiotics and ω3-fatty acids on appetite regulatory neuropeptides and neurotransmitters in a pig model. Benef. Microbes 2020, 11, 347–359. [Google Scholar] [CrossRef]
- Gioacchini, G.; Ciani, E.; Pessina, A.; Cecchini, C.; Silvi, S.; Rodiles, A.; Merrifield, D.L.; Olivotto, I.; Carnevali, O. Effects of Lactogen 13, a New Probiotic Preparation, on Gut Microbiota and Endocrine Signals Controlling Growth and Appetite of Oreochromis niloticus Juveniles. Microb. Ecol. 2018, 76, 1063–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burmester, V.; Nicholls, D.; Buckle, A.; Stanojevic, B.; Crous-Bou, M. Review of eating disorders and oxytocin receptor polymorphisms. J. Eat. Disord. 2021, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Sailike, J.; Sun, X.; Abuduwaili, N.; Tuoliuhan, H.; Yusufu, M.; Nabi, X.H. Fourteen composite probiotics alleviate type 2 diabetes through modulating gut microbiota and modifying M1/M2 phenotype macrophage in db/db mice. Pharmacol. Res. 2020, 161, 105150. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, M.; Sundralingam, U.; Palanisamy, U.D. Polyphenols as Prebiotics in the Management of High-Fat Diet-Induced Obesity: A Systematic Review of Animal Studies. Foods 2021, 10, 299. [Google Scholar] [CrossRef]
- Gurpilhares, D.B.; Cinelli, L.P.; Simas, N.K.; Pessoa, A.; Sette, L.D., Jr. Marine prebiotics: Polysaccharides and oligosaccharides obtained by using microbial enzymes. Food Chem. 2019, 280, 175–186. [Google Scholar] [CrossRef]
- Meyer, R.K.; Bime, M.A.; Duca, F.A. Small intestinal metabolomics analysis reveals differentially regulated metabolite profiles in obese rats and with prebiotic supplementation. Metabolomics 2022, 18, 60. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.; Wadie, W.; Abdallah, D.M.; Lentzen, G.; Khayyal, M.T. Novel effects of ectoine, a bacteria-derived natural tetrahydropyrimidine, in experimental colitis. Phytomedicine 2013, 20, 585–591. [Google Scholar] [CrossRef]
- Brial, F.; Chilloux, J.; Nielsen, T.; Vieira-Silva, S.; Falony, G.; Andrikopoulos, P.; Olanipekun, M.; Hoyles, L.; Djouadi, F.; Neves, A.L.; et al. Human and preclinical studies of the host-gut microbiome co-metabolite hippurate as a marker and mediator of metabolic health. Gut 2021, 70, 2105–2114. [Google Scholar] [CrossRef]
- Da Silva Borges, D.; Fernandes, R.; Thives Mello, A.; da Silva Fontoura, E.; Soares Dos Santos, A.R.; Santos de Moraes Trindade, E.B. Prebiotics may reduce serum concentrations of C-reactive protein and ghrelin in overweight and obese adults: A systematic review and meta-analysis. Nutr. Rev. 2020, 78, 235–248. [Google Scholar]
- Delbès, A.S.; Castel, J.; Denis, R.G.P.; Morel, C.; Quiñones, M.; Everard, A.; Cani, P.D.; Massiera, F.; Luquet, S.H. Prebiotics Supplementation Impact on the Reinforcing and Motivational Aspect of Feeding. Front. Endocrinol. 2018, 9, 273. [Google Scholar] [CrossRef]
- Van der Beek, C.M.; Canfora, E.E.; Kip, A.M.; Gorissen, S.H.M.; Olde Damink, S.W.M.; van Eijk, H.M.; Holst, J.J.; Blaak, E.E.; Dejong, C.H.C.; Lenaerts, K. The prebiotic inulin improves substrate metabolism and promotes short-chain fatty acid production in overweight to obese men. Metabolism 2018, 87, 25–35. [Google Scholar] [CrossRef]
- Chiou, W.C.; Chang, B.H.; Tien, H.H.; Cai, Y.L.; Fan, Y.C.; Chen, W.J.; Chu, H.F.; Chen, Y.H.; Huang, C. Synbiotic Intervention with an Adlay-Based Prebiotic and Probiotics Improved Diet-Induced Metabolic Disturbance in Mice by Modulation of the Gut Microbiota. Nutrients 2021, 13, 3161. [Google Scholar] [CrossRef]
- Ke, X.; Walker, A.; Haange, S.B.; Lagkouvardos, I.; Liu, Y.; Schmitt-Kopplin, P.; von Bergen, M.; Jehmlich, N.; He, X.; Clavel, T.; et al. Synbiotic-driven improvement of metabolic disturbances is associated with changes in the gut microbiome in diet-induced obese mice. Mol. Metab. 2019, 22, 96–109. [Google Scholar] [CrossRef]
- Mischke, M.; Arora, T.; Tims, S.; Engels, E.; Sommer, N.; van Limpt, K.; Baars, A.; Oozeer, R.; Oosting, A.; Bäckhed, F.; et al. Specific synbiotics in early life protect against diet-induced obesity in adult mice. Diabetes Obes. Metab. 2018, 20, 1408–1418. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, B.M.; Kvach, E.; Eckel, R.H. Treatment of Obesity: Weight Loss and Bariatric Surgery. Circ. Res. 2016, 118, 1844–1855. [Google Scholar] [CrossRef]
- Rossell, J.; Brindefalk, B.; Baena-Fustegueras, J.A.; Peinado-Onsurbe, J.; Udekwu, K.I. Diet change affects intestinal microbiota restoration and improves vertical sleeve gastrectomy outcome in diet-induced obese rats. Eur. J. Nutr. 2020, 59, 3555–3564. [Google Scholar] [CrossRef] [Green Version]
- Martin, O.A.; Grant-Beurmann, S.; Orellana, E.R.; Hajnal, A.; Fraser, C.M. Changes in the Gut Microbiota Following Bariatric Surgery Are Associated with Increased Alcohol Intake in a Female Rat Model. Alcohol. Alcohol. 2021, 56, 605–613. [Google Scholar] [CrossRef]
- Paganelli, F.L.; Luyer, M.; Hazelbag, C.M.; Uh, H.W.; Rogers, M.R.C.; Adriaans, D.; Berbers, R.M.; Hendrickx, A.P.A.; Viveen, M.C.; Groot, J.A.; et al. Roux-Y Gastric Bypass and Sleeve Gastrectomy directly change gut microbiota composition independent of surgery type. Sci. Rep. 2019, 9, 10979. [Google Scholar] [CrossRef] [Green Version]
- Koutoukidis, D.A.; Jebb, S.A.; Zimmerman, M.; Otunla, A.; Henry, J.A.; Ferrey, A.; Schofield, E.; Kinton, J.; Aveyard, P.; Marchesi, J.R. The association of weight loss with changes in the gut microbiota diversity, composition, and intestinal permeability: A systematic review and meta-analysis. Gut Microbes 2022, 14, 2020068. [Google Scholar] [CrossRef]
- Shao, Y.; Ding, R.; Xu, B.; Hua, R.; Shen, Q.; He, K.; Yao, Q. Alterations of Gut Microbiota after Roux-en-Y Gastric Bypass and Sleeve Gastrectomy in Sprague-Dawley Rats. Obes. Surg. 2017, 27, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alcoholado, L.; Gutiérrez-Repiso, C.; Gómez-Pérez, A.M.; García-Fuentes, E.; Tinahones, F.J.; Moreno-Indias, I. Gut microbiota adaptation after weight loss by Roux-en-Y gastric bypass or sleeve gastrectomy bariatric surgeries. Surg. Obes. Relat. Dis. 2019, 15, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Hankir, M.K.; Seyfried, F.; Miras, A.D.; Cowley, M.A. Brain Feeding Circuits after Roux-en-Y Gastric Bypass. Trends Endocrinol. Metab. 2018, 29, 218–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Repiso, C.; Garrido-Sánchez, L.; Alcaide-Torres, J.; Cornejo-Pareja, I.; Ocaña-Wilhelmi, L.; García-Fuentes, E.; Moreno-Indias, I.; Tinahones, F.J. Predictive Role of Gut Microbiota in Weight Loss Achievement after Bariatric Surgery. J. Am. Coll. Surg. 2022, 234, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Jahansouz, C.; Staley, C.; Kizy, S.; Xu, H.; Hertzel, A.V.; Coryell, J.; Singroy, S.; Hamilton, M.; DuRand, M.; Bernlohr, D.A.; et al. Antibiotic-induced Disruption of Intestinal Microbiota Contributes to Failure of Vertical Sleeve Gastrectomy. Ann. Surg. 2019, 269, 1092–1100. [Google Scholar] [CrossRef]
- Fries, C.M.; Haange, S.B.; Rolle-Kampczyk, U.; Till, A.; Lammert, M.; Grasser, L.; Medawar, E.; Dietrich, A.; Horstmann, A.; von Bergen, M.; et al. Metabolic Profile and Metabolite Analyses in Extreme Weight Responders to Gastric Bypass Surgery. Metabolites 2022, 12, 417. [Google Scholar] [CrossRef]
- Castellanos-Jankiewicz, A.; Guzmán-Quevedo, O.; Fénelon, V.S.; Zizzari, P.; Quarta, C.; Bellocchio, L.; Tailleux, A.; Charton, J.; Fernandois, D.; Henricsson, M.; et al. Hypothalamic bile acid-TGR5 signaling protects from obesity. Cell Metab. 2021, 33, 1483–1492.e10. [Google Scholar] [CrossRef]
- Dischinger, U.; Kötzner, L.; Kovatcheva-Datchary, P.; Kleinschmidt, H.; Haas, C.; Perez, J.; Presek, C.; Koschker, A.C.; Miras, A.D.; Hankir, M.K.; et al. Hypothalamic integrity is necessary for sustained weight loss after bariatric surgery: A prospective, cross-sectional study. Metabolism 2023, 138, 155341. [Google Scholar] [CrossRef]
- Huang, H.H.; Lin, T.L.; Lee, W.J.; Chen, S.C.; Lai, W.F.; Lu, C.C.; Lai, H.C.; Chen, C.Y. Impact of Metabolic Surgery on Gut Microbiota and Sera Metabolomic Patterns among Patients with Diabetes. Int. J. Mol. Sci. 2022, 23, 7797. [Google Scholar] [CrossRef]
- Su, D.W.; Wei, W.W.; Yao, R.R.; Yang, C.J.; Tian, H. Research on sleeve gastrectomy for the treatment of rats with type 2 diabetes mellitus and the regulation of ghrelin and intestinal lesions. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10653–10662. [Google Scholar]
- Chaudhari, S.N.; Harris, D.A.; Aliakbarian, H.; Luo, J.N.; Henke, M.T.; Subramaniam, R.; Vernon, A.H.; Tavakkoli, A.; Sheu, E.G.; Devlin, A.S. Bariatric surgery reveals a gut-restricted TGR5 agonist with anti-diabetic effects. Nat. Chem. Biol. 2021, 17, 20–29. [Google Scholar] [CrossRef]
- Calikoglu, F.; Barbaros, U.; Uzum, A.K.; Tutuncu, Y.; Satman, I. The Metabolic Effects of Pre-probiotic Supplementation after Roux-en-Y Gastric Bypass (RYGB) Surgery: A Prospective, Randomized Controlled Study. Obes. Surg. 2021, 31, 215–223. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [Green Version]
- Park, J.C.; Im, S.H. Of men in mice: The development and application of a humanized gnotobiotic mouse model for microbiome therapeutics. Exp. Mol. Med. 2020, 52, 1383–1396. [Google Scholar] [CrossRef]
- Alard, J.; Cudennec, B.; Boutillier, D.; Peucelle, V.; Descat, A.; Decoin, R.; Kuylle, S.; Jablaoui, A.; Rhimi, M.; Wolowczuk, I.; et al. Multiple Selection Criteria for Probiotic Strains with High Potential for Obesity Management. Nutrients 2021, 13, 713. [Google Scholar] [CrossRef]
- Dudík, B.; Kiňová Sepová, H.; Greifová, G.; Bilka, F.; Bílková, A. Next generation probiotics: An overview of the most promising candidates. Epidemiol. Mikrobiol. Imunol. 2022, 71, 48–56. [Google Scholar]
- Remely, M.; Hippe, B.; Zanner, J.; Aumueller, E.; Brath, H.; Haslberger, A.G. Gut Microbiota of Obese, Type 2 Diabetic Individuals is Enriched in Faecalibacterium prausnitzii, Akkermansia muciniphila and Peptostreptococcus anaerobius after Weight Loss. Endocr. Metab. Immune Disord. Drug Targets 2016, 16, 99–106. [Google Scholar] [CrossRef]
- Legrand, R.; Lucas, N.; Dominique, M.; Azhar, S.; Deroissart, C.; Le Solliec, M.A.; Rondeaux, J.; Nobis, S.; Guérin, C.; Léon, F.; et al. Commensal Hafnia alvei strain reduces food intake and fat mass in obese mice-a new potential probiotic for appetite and body weight management. Int. J. Obes. 2020, 44, 1041–1051. [Google Scholar] [CrossRef] [Green Version]
- Déchelotte, P.; Breton, J.; Trotin-Picolo, C.; Grube, B.; Erlenbeck, C.; Bothe, G.; Fetissov, S.O.; Lambert, G. The Probiotic Strain H. alvei HA4597® Improves Weight Loss in Overweight Subjects under Moderate Hypocaloric Diet: A Proof-of-Concept, Multicenter Randomized, Double-Blind Placebo-Controlled Study. Nutrients 2021, 13, 1902. [Google Scholar] [CrossRef]

| Therapeutic Intervention | Study Period | Species Involved/Outcome Measured | Results/Implications | Subject Type | Reference |
|---|---|---|---|---|---|
| Mediterranean Diet | 1 year | Metabolic Parameters |
| Human | [158] |
| 8 weeks | ↑ Faecalibacterium ↓ Ruminococcus |
| Human | [150] | |
| 210 days | Hunger/Satiety |
| Human | [167] | |
| 24 weeks | Metabolic Parameters/Satiety Peptides |
| Human | [168] | |
| Probiotics | 12 weeks | Satiety Peptides/Weight/Eating Behavior |
| Human | [176] |
| 12 weeks | ↑ Lactobacillus, Alistipes, Akkermansia ↓ Ruminococcus, Dorea, Clostridium |
| Mice | [177] | |
| 6 weeks | Metabolic Parameters/Effect on HFD-induced obesity |
| Mice | [178] | |
| Metabolic Parameters/Satiety Peptides |
| Mice | [179] | ||
| Prebiotics—Inulin/Oligofructose | 16 weeks | Metabolic Parameters ↑ Bifidobacterium ↓ Bacteroides |
| Human | [180] |
| 12 weeks | Satiety Peptides ↑ Lachnospiraceae ↓ Desulfovibrio |
| Mice | [181] | |
| 21 days | ↑ Bacteroidetes, Bifidobacterium ↓ Clostridium |
| Rats | [182] | |
| 10 weeks | ↑ Lactobacillus, Bifidobacterium |
| Rats | [183] | |
| Synbiotics | 12 weeks | Metabolic Parameters/Satiety Peptides |
| Human | [184] |
| 8 weeks | Metabolic and Oxidative Parameters |
| Rats | [185] | |
| 12 weeks | Metabolic and Inflammatory Parameters |
| Mice | [186] | |
| Roux-en-Y Gastric Bypass (RYGB) | 12 weeks | Satiety/Inflammatory Markers |
| Rats | [187] |
| 4 to 6 weeks | Satiety Peptides |
| Rats | [188] | |
| Vertical Sleeve Gastrectomy (VSG) | Meta Analysis | Satiety Peptides |
| Human | [189] |
| 6 weeks | Microbiota Metabolites/Satiety Peptides |
| Mice | [190] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamamah, S.; Amin, A.; Al-Kassir, A.L.; Chuang, J.; Covasa, M. Dietary Fat Modulation of Gut Microbiota and Impact on Regulatory Pathways Controlling Food Intake. Nutrients 2023, 15, 3365. https://doi.org/10.3390/nu15153365
Hamamah S, Amin A, Al-Kassir AL, Chuang J, Covasa M. Dietary Fat Modulation of Gut Microbiota and Impact on Regulatory Pathways Controlling Food Intake. Nutrients. 2023; 15(15):3365. https://doi.org/10.3390/nu15153365
Chicago/Turabian StyleHamamah, Sevag, Arman Amin, Abdul Latif Al-Kassir, Judith Chuang, and Mihai Covasa. 2023. "Dietary Fat Modulation of Gut Microbiota and Impact on Regulatory Pathways Controlling Food Intake" Nutrients 15, no. 15: 3365. https://doi.org/10.3390/nu15153365
APA StyleHamamah, S., Amin, A., Al-Kassir, A. L., Chuang, J., & Covasa, M. (2023). Dietary Fat Modulation of Gut Microbiota and Impact on Regulatory Pathways Controlling Food Intake. Nutrients, 15(15), 3365. https://doi.org/10.3390/nu15153365





