The Usefulness of Resistant Maltodextrin and Chitosan Oligosaccharide in Management of Gut Leakage and Microbiota in Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tested Chemicals
2.2. In Vitro Study
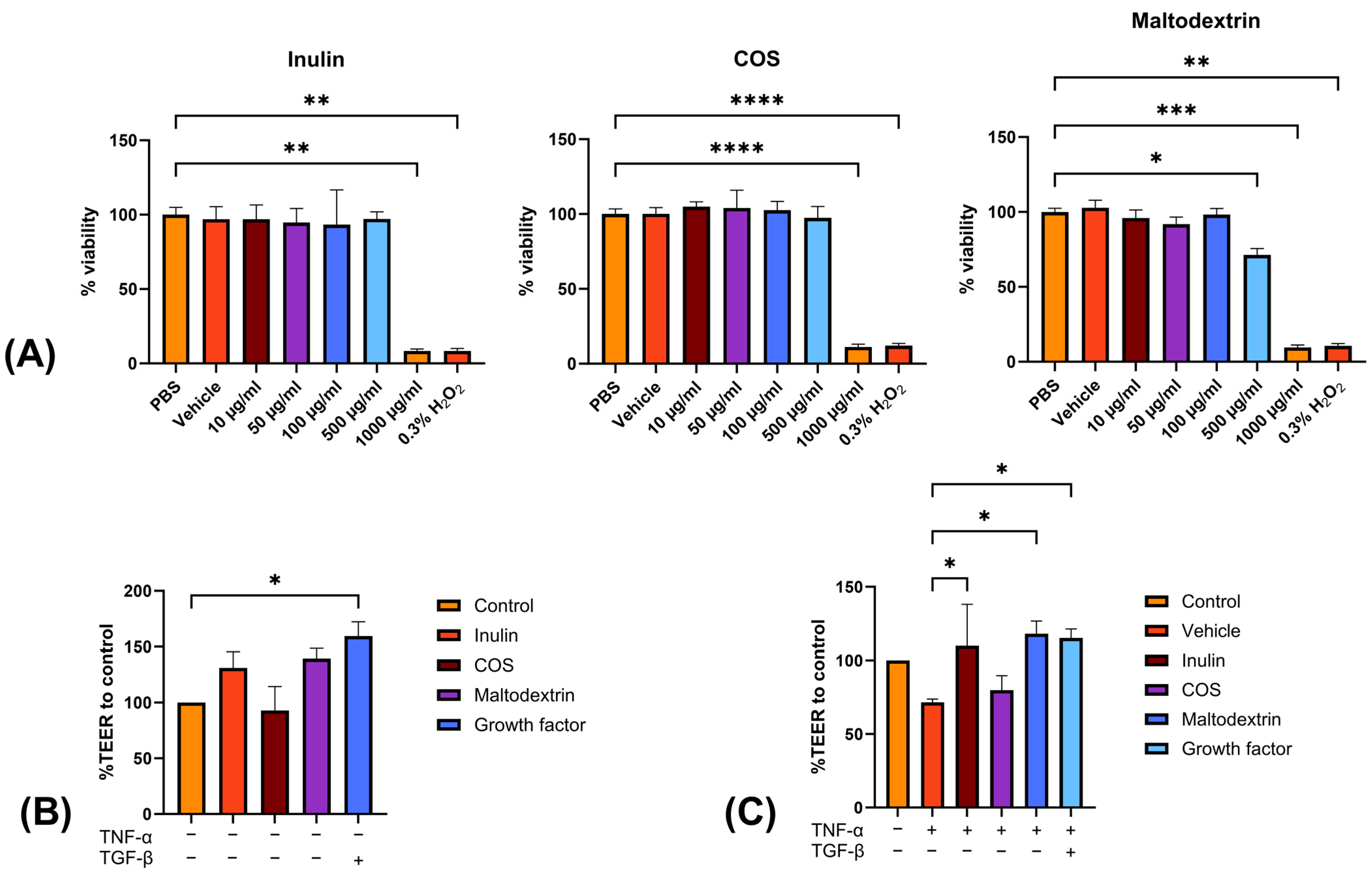
2.2.1. MTT Survivability Test
2.2.2. Transepithelial Electrical Resistance (TEER) Assay
2.3. Animal Study
2.3.1. Animal Preparation, CKD Induction, and Sample Collection
2.3.2. Gene Expression of Tight Junction Protein
2.3.3. Serum Creatinine, Calcium, Phosphate, and PTH Profiling
2.3.4. Histopathological Evaluation
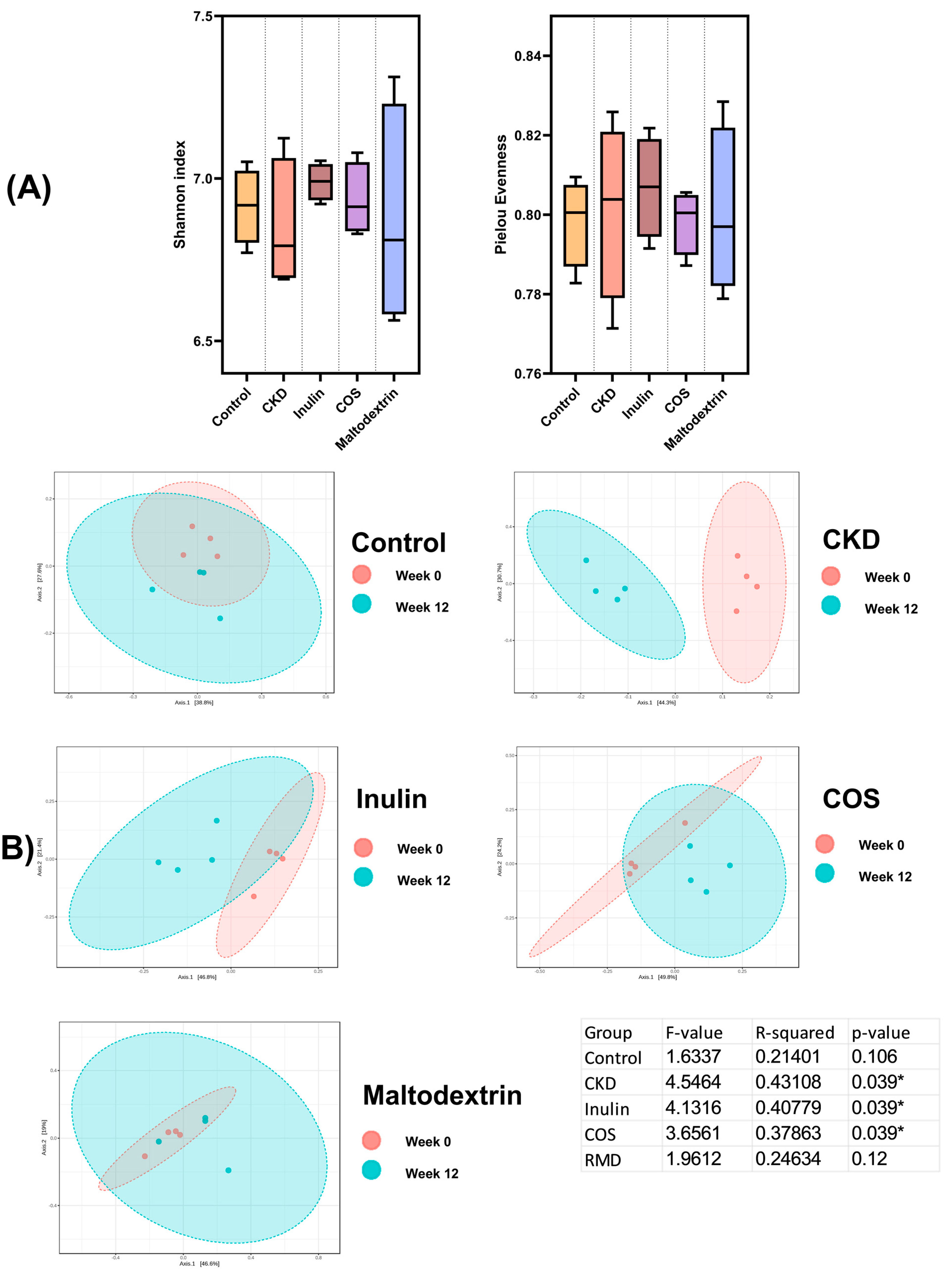
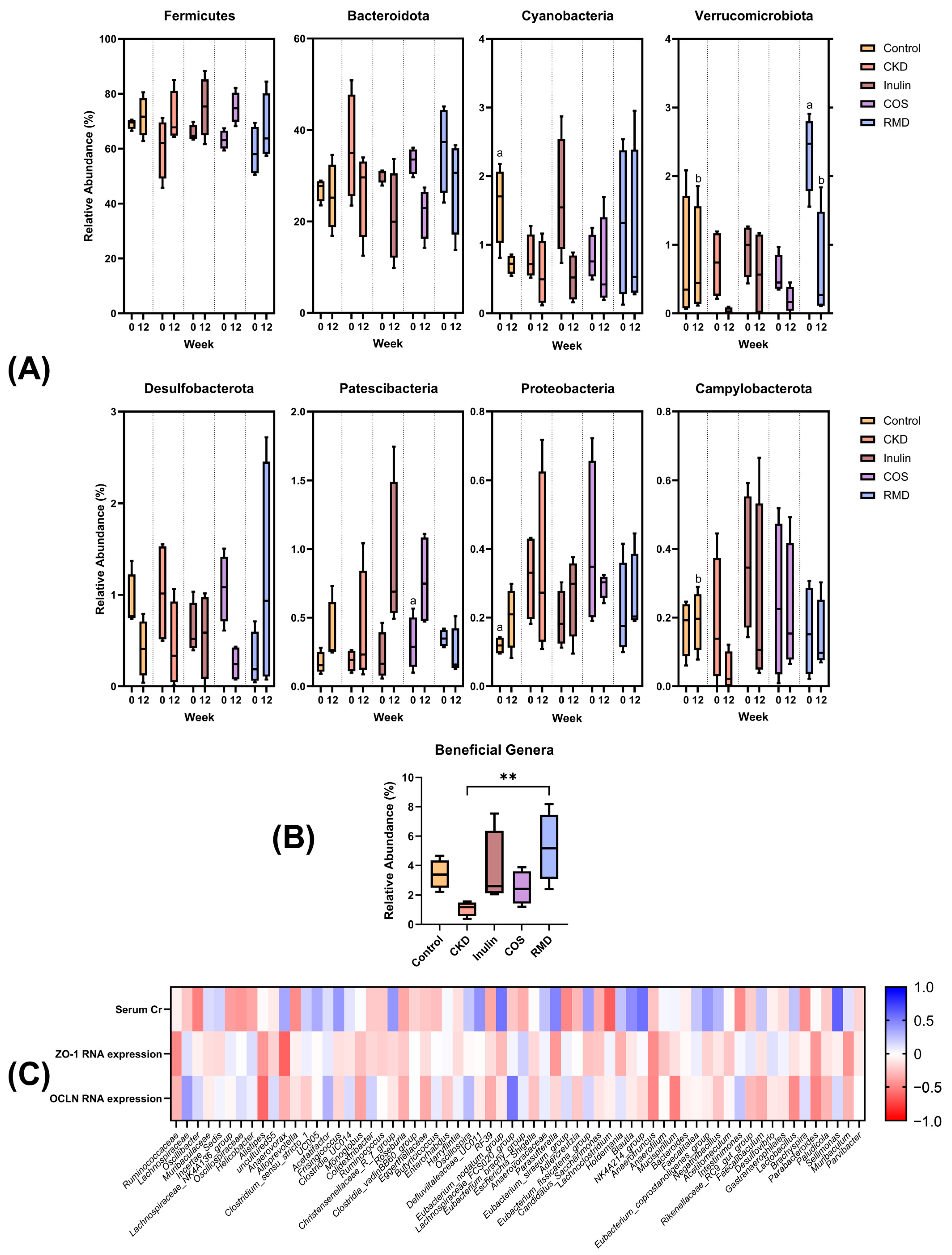
2.3.5. Intestinal Microbiota Analysis
2.4. Statistical Analysis
2.5. Ethical Consideration
3. Results
3.1. In Vitro Study
3.2. Animal Studies
3.3. Gut Microbiome Study
3.3.1. Fecal Microbiota Diversity
3.3.2. Relative Abundances and Correlation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Voroneanu, L.; Burlacu, A.; Brinza, C.; Covic, A.; Balan, G.G.; Nistor, I.; Popa, C.; Hogas, S.; Covic, A. Gut Microbiota in Chronic Kidney Disease: From Composition to Modulation towards Better Outcomes—A Systematic Review. J. Clin. Med. 2023, 12, 1948. [Google Scholar] [CrossRef] [PubMed]
- Rysz, J.; Franczyk, B.; Ławiński, J.; Olszewski, R.; Ciałkowska-Rysz, A.; Gluba-Brzózka, A. The Impact of CKD on Uremic Toxins and Gut Microbiota. Toxins 2021, 13, 252. [Google Scholar] [CrossRef] [PubMed]
- Lydia, A.; Indra, T.A.; Rizka, A.; Abdullah, M. The effects of synbiotics on indoxyl sulphate level, constipation, and quality of life associated with constipation in chronic haemodialysis patients: A randomized controlled trial. BMC Nephrol. 2022, 23, 259. [Google Scholar] [CrossRef]
- Ebrahim, Z.; Proost, S.; Tito, R.Y.; Raes, J.; Glorieux, G.; Moosa, M.R.; Blaauw, R. The Effect of ß-Glucan Prebiotic on Kidney Function, Uremic Toxins and Gut Microbiome in Stage 3 to 5 Chronic Kidney Disease (CKD) Predialysis Participants: A Randomized Controlled Trial. Nutrients 2022, 14, 805. [Google Scholar] [CrossRef]
- Shamloo, M.; Mollard, R.; Wang, H.; Kingra, K.; Tangri, N.; MacKay, D. A randomized double-blind cross-over trial to study the effects of resistant starch prebiotic in chronic kidney disease (ReSPECKD). Trials 2022, 23, 72. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, C.; Krishnasamy, R.; Stanton, T.; Savill, E.; Snelson, M.; Mihala, G.; Kelly, J.T.; Morrison, M.; Johnson, D.W.; Campbell, K.L. Synbiotics Easing Renal Failure by Improving Gut Microbiology II (SYNERGY II): A Feasibility Randomized Controlled Trial. Nutrients 2021, 13, 4481. [Google Scholar] [CrossRef]
- Salvatore, S.; Battigaglia, M.S.; Murone, E.; Dozio, E.; Pensabene, L.; Agosti, M. Dietary Fibers in Healthy Children and in Pediatric Gastrointestinal Disorders: A Practical Guide. Nutrients 2023, 15, 2208. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Levi, Y.; Moraschini, V.; Messora, M.R.; Furlaneto, F.A.C. Effects of Prebiotic Therapy on Gastrointestinal Microbiome of Individuals with Different Inflammatory Conditions: A Systematic Review of Randomized Controlled Trials. Probiotics Antimicrob. Proteins 2023, 1–23. [Google Scholar] [CrossRef]
- Beker, B.M.; Colombo, I.; Gonzalez-Torres, H.; Musso, C.G. Decreasing microbiota-derived uremic toxins to improve CKD outcomes. Clin. Kidney J. 2022, 15, 2214–2219. [Google Scholar] [CrossRef]
- Tain, Y.-L.; Hsu, C.-N. Role of the Gut Microbiota in Children with Kidney Disease. Children 2023, 10, 269. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Tang, D.; Dong, R.; Feng, Q. Chitosan Oligosaccharide Ameliorates Metabolic Syndrome Induced by Overnutrition via Altering Intestinal Microbiota. Front. Nutr. 2021, 8, 743492. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Wang, S.; Lv, Z.; Zhao, W.; Li, S. Low molecular weight chitosan oligosaccharides (LMW-COSs) prevent obesity-related metabolic abnormalities in association with the modification of gut microbiota in high-fat diet (HFD)-fed mice. Food Funct. 2020, 11, 9947–9959. [Google Scholar] [CrossRef] [PubMed]
- Cheon, S.; Kim, G.; Bae, J.-H.; Lee, D.H.; Seong, H.; Kim, D.H.; Han, J.-S.; Lim, S.-Y.; Han, N.S. Comparative analysis of prebiotic effects of four oligosaccharides using in vitro gut model: Digestibility, microbiome, and metabolome changes. FEMS Microbiol. Ecol. 2023, 99, fiad002. [Google Scholar] [CrossRef] [PubMed]
- Pellicer-Valero, Ó.J.; Massaro, G.A.; Casanova, A.G.; Paniagua-Sancho, M.; Fuentes-Calvo, I.; Harvat, M.; Martín-Guerrero, J.D.; Martínez-Salgado, C.; López-Hernández, F.J. Neural Network-Based Calculator for Rat Glomerular Filtration Rate. Biomedicines 2022, 10, 610. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Scott, K.P.; Martin, J.C.; Duncan, S.H.; Flint, H.J. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol. Ecol. 2013, 87, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Quigley, E.M. Prebiotics and Probiotics in Digestive Health. Clin. Gastroenterol. Hepatol. 2018, 17, 333–344. [Google Scholar] [CrossRef]
- Jerez-Morales, A.; Merino, J.S.; Díaz-Castillo, S.T.; Smith, C.T.; Fuentealba, J.; Bernasconi, H.; Echeverría, G.; García-Cancino, A. The Administration of the Synbiotic Lactobacillus bulgaricus 6c3 Strain, Inulin and Fructooligosaccharide Decreases the Concentrations of Indoxyl Sulfate and Kidney Damage in a Rat Model. Toxins 2021, 13, 192. [Google Scholar] [CrossRef]
- Lai, S.; Mazzaferro, S.; Muscaritoli, M.; Mastroluca, D.; Testorio, M.; Perrotta, A.; Esposito, Y.; Carta, M.; Campagna, L.; Di Grado, M.; et al. Prebiotic Therapy with Inulin Associated with Low Protein Diet in Chronic Kidney Disease Patients: Evaluation of Nutritional, Cardiovascular and Psychocognitive Parameters. Toxins 2020, 12, 381. [Google Scholar] [CrossRef]
- Lai, S.; Molfino, A.; Testorio, M.; Perrotta, A.M.; Currado, A.; Pintus, G.; Pietrucci, D.; Unida, V.; La Rocca, D.; Biocca, S.; et al. Effect of Low-Protein Diet and Inulin on Microbiota and Clinical Parameters in Patients with Chronic Kidney Disease. Nutrients 2019, 11, 3006. [Google Scholar] [CrossRef] [Green Version]
- Melekoglu, E.; Cetinkaya, M.A.; Kepekci-Tekkeli, S.E.; Kul, O.; Samur, G. Effects of prebiotic oligofructose-enriched inulin on gut-derived uremic toxins and disease progression in rats with adenine-induced chronic kidney disease. PLoS ONE 2021, 16, e0258145. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.; Pichyangkura, R.; Soodvilai, S.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide as potential therapy of inflammatory bowel disease: Therapeutic efficacy and possible mechanisms of action. Pharmacol. Res. 2012, 66, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef] [PubMed]
- Milkova, V. Electrosteric stabilization of oil/water emulsions by adsorption of chitosan oligosaccharides—An electrokinetic study. Carbohydr. Polym. 2021, 265, 118072. [Google Scholar] [CrossRef]
- Yu, D.; Feng, J.; You, H.; Zhou, S.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. The Microstructure, Antibacterial and Antitumor Activities of Chitosan Oligosaccharides and Derivatives. Mar. Drugs 2022, 20, 69. [Google Scholar] [CrossRef]
- Saito, H.; Sakakibara, Y.; Sakata, A.; Kurashige, R.; Murakami, D.; Kageshima, H.; Saito, A.; Miyazaki, Y. Antibacterial activity of lysozyme-chitosan oligosaccharide conjugates (LYZOX) against Pseudomonas aeruginosa, Acinetobacter baumannii and Methicillin-resistant Staphylococcus aureus. PLoS ONE 2019, 14, e0217504. [Google Scholar] [CrossRef]
- Lee, H.-W.; Park, Y.-S.; Jung, J.-S.; Shin, W.-S. Chitosan oligosaccharides, dp 2–8, have prebiotic effect on the Bifidobacterium bifidium and Lactobacillus sp. Anaerobe 2002, 8, 319–324. [Google Scholar] [CrossRef]
- Guan, Z.; Feng, Q. Chitosan and Chitooligosaccharide: The Promising Non-Plant-Derived Prebiotics with Multiple Biological Activities. Int. J. Mol. Sci. 2022, 23, 6761. [Google Scholar] [CrossRef]
- Tao, W.; Wang, G.; Wei, J. The Role of Chitosan Oligosaccharide in Metabolic Syndrome: A Review of Possible Mechanisms. Mar. Drugs 2021, 19, 501. [Google Scholar] [CrossRef]
- Pan, H.; Fu, C.; Huang, L.; Jiang, Y.; Deng, X.; Guo, J.; Su, Z. Anti-Obesity Effect of Chitosan Oligosaccharide Capsules (COSCs) in Obese Rats by Ameliorating Leptin Resistance and Adipogenesis. Mar. Drugs 2018, 16, 198. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.-H.; Chen, R.-Y.; Chiang, M.-T. Effects and Mechanisms of Chitosan and ChitosanOligosaccharide on Hepatic Lipogenesis and Lipid Peroxidation, Adipose Lipolysis, and Intestinal Lipid Absorption in Rats with High-Fat Diet-Induced Obesity. Int. J. Mol. Sci. 2021, 22, 1139. [Google Scholar] [CrossRef]
- Sepasi, T.; Ghadiri, T.; Ebrahimi-Kalan, A.; Bani, F.; Talebi, M.; Rahbarghazi, R.; Khodakarimi, S.; Beyrampour-Basmenj, H.; Seidi, K.; Abbaspour-Ravasjani, S.; et al. CDX-modified chitosan nanoparticles remarkably reduce therapeutic dose of fingolimod in the EAE model of mice. Int. J. Pharm. 2023, 636, 122815. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Mathew, A.P.; Vasukutty, A.; Uthaman, S.; Joo, S.Y.; Bae, E.H.; Ma, S.K.; Park, I.-K.; Kim, S.W. Glycol chitosan-based tacrolimus-loaded nanomicelle therapy ameliorates lupus nephritis. J. Nanobiotechnology 2021, 19, 109. [Google Scholar] [CrossRef] [PubMed]
- Bai, K.; Hong, B.; He, J.; Huang, W. Antioxidant Capacity and Hepatoprotective Role of Chitosan-Stabilized Selenium Nanoparticles in Concanavalin A-Induced Liver Injury in Mice. Nutrients 2020, 12, 857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khater, S.I.; Mohamed, A.A.-R.; Arisha, A.H.; Ebraheim, L.L.; El-Mandrawy, S.A.; Nassan, M.A.; Mohammed, A.T.; Abdo, S.A. Stabilized-chitosan selenium nanoparticles efficiently reduce renal tissue injury and regulate the expression pattern of aldose reductase in the diabetic-nephropathy rat model. Life Sci. 2021, 279, 119674. [Google Scholar] [CrossRef]
- Chen, X.; Dai, W.; Li, H.; Yan, Z.; Liu, Z.; He, L. Targeted drug delivery strategy: A bridge to the therapy of diabetic kidney disease. Drug Deliv. 2022, 30, 2160518. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Wang, L.; Liu, X.; Wu, Y. Chitooligosaccharide guanidine inhibits high glucose-induced activation of DAG/PKC pathway by regulating expression of GLUT2 in type 2 diabetic nephropathy rats. J. Funct. Foods 2018, 41, 41–47. [Google Scholar] [CrossRef]
- Wu, J.; Xu, Y.; Geng, Z.; Zhou, J.; Xiong, Q.; Xu, Z.; Li, H.; Han, Y. Chitosan oligosaccharide alleviates renal fibrosis through reducing oxidative stress damage and regulating TGF-β1/Smads pathway. Sci. Rep. 2022, 12, 19160. [Google Scholar] [CrossRef]
- Pathomthongtaweechai, N.; Soodvilai, S.; Pichyangkura, R.; Muanprasat, C. Novel Potential Application of Chitosan Oligosaccharide for Attenuation of Renal Cyst Growth in the Treatment of Polycystic Kidney Disease. Molecules 2020, 25, 5589. [Google Scholar] [CrossRef]
- Fastinger, N.D.; Karr-Lilienthal, L.K.; Spears, J.K.; Swanson, K.S.; Zinn, K.E.; Nava, G.M.; Ohkuma, K.; Kanahori, S.; Gordon, D.T.; Fahey, G.C., Jr. A Novel Resistant Maltodextrin Alters Gastrointestinal Tolerance Factors, Fecal Characteristics, and Fecal Microbiota in Healthy Adult Humans. J. Am. Coll. Nutr. 2008, 27, 356–366. [Google Scholar] [CrossRef]
- Burns, A.M.; Solch, R.J.; Dennis-Wall, J.C.; Ukhanova, M.; Nieves, C., Jr.; Mai, V.; Christman, M.C.; Gordon, D.T.; Langkamp-Henken, B. In healthy adults, resistant maltodextrin produces a greater change in fecal bifidobacteria counts and increases stool wet weight: A double-blind, randomized, controlled crossover study. Nutr. Res. 2018, 60, 33–42. [Google Scholar] [CrossRef]
- Baer, D.J.; Stote, K.S.; Henderson, T.; Paul, D.R.; Okuma, K.; Tagami, H.; Kanahori, S.; Gordon, D.T.; Rumpler, W.V.; Ukhanova, M.; et al. The Metabolizable Energy of Dietary Resistant Maltodextrin Is Variable and Alters Fecal Microbiota Composition in Adult Men. J. Nutr. 2014, 144, 1023–1029. [Google Scholar] [CrossRef] [Green Version]
- Nishimoto, Y.; Mizuguchi, Y.; Mori, Y.; Ito, M.; Miyazato, S.; Kishimoto, Y.; Yamada, T.; Fukuda, S. Resistant Maltodextrin Intake Reduces Virulent Metabolites in the Gut Environment: A Randomized Control Study in a Japanese Cohort. Front. Microbiol. 2022, 13, 644146. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, G.; Zhang, Q.; Liu, Z.; Jiang, X.; Xin, Y. Function of Akkermansia muciniphila in type 2 diabetes and related diseases. Front. Microbiol. 2023, 14, 1172400. [Google Scholar] [CrossRef]
- Memon, H.; Abdulla, F.; Reljic, T.; Alnuaimi, S.; Serdarevic, F.; Asimi, Z.V.; Kumar, A.; Semiz, S. Effects of combined treatment of probiotics and metformin in management of type 2 diabetes: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2023, 202, 110806. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Xiong, R.; Li, Y.; Chen, J.; Yan, R. Gut microbiome, metabolome, host immunity associated with inflammatory bowel disease and intervention of fecal microbiota transplantation. J. Autoimmun. 2023, 103062, in press. [Google Scholar] [CrossRef] [PubMed]
- Martín-Del-Campo, F.; Avesani, C.M.; Stenvinkel, P.; Lindholm, B.; Cueto-Manzano, A.M.; Cortés-Sanabria, L. Gut microbiota disturbances and protein-energy wasting in chronic kidney disease: A narrative review. J. Nephrol. 2023, 36, 873–883. [Google Scholar] [CrossRef]
- Andrianova, N.V.; Popkov, V.A.; Klimenko, N.S.; Tyakht, A.V.; Baydakova, G.V.; Frolova, O.Y.; Zorova, L.D.; Pevzner, I.B.; Zorov, D.B.; Plotnikov, E.Y. Microbiome-Metabolome Signature of Acute Kidney Injury. Metabolites 2020, 10, 142. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wang, Q.; Chen, P.; Zhou, C.; Zhang, X.; Chen, L. Ursodeoxycholic Acid Treatment Restores Gut Microbiota and Alleviates Liver Inflammation in Non-Alcoholic Steatohepatitic Mouse Model. Front. Pharmacol. 2021, 12, 788558. [Google Scholar] [CrossRef]
- Li, D.-P.; Cui, M.; Tan, F.; Liu, X.-Y.; Yao, P. High Red Meat Intake Exacerbates Dextran Sulfate-Induced Colitis by Altering Gut Microbiota in Mice. Front. Nutr. 2021, 8, 646819. [Google Scholar] [CrossRef]
- Liu, W.-C.; Pan, Z.-Y.; Zhao, Y.; Guo, Y.; Qiu, S.-J.; Balasubramanian, B.; Jha, R. Effects of Heat Stress on Production Performance, Redox Status, Intestinal Morphology and Barrier-Related Gene Expression, Cecal Microbiome, and Metabolome in Indigenous Broiler Chickens. Front. Physiol. 2022, 13, 761. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Sun, H.; Zhang, L.; Geng, W.; Wang, L.; Zou, C.; Wu, Y.; Ji, C.; Liu, X.; Lu, Z. Microbiome-Metabolomics Analysis Reveals the Protection Mechanism of α-Ketoacid on Adenine-Induced Chronic Kidney Disease in Rats. Front. Pharmacol. 2021, 12, 657827. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xu, X.; Chao, L.; Chen, K.; Shao, A.; Sun, D.; Hong, Y.; Hu, R.; Jiang, P.; Zhang, N.; et al. Alteration of the Gut Microbiome in Chronic Kidney Disease Patients and Its Association With Serum Free Immunoglobulin Light Chains. Front. Immunol. 2021, 12, 609700. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anegkamol, W.; Kamkang, P.; Hunthai, S.; Kaewwongse, M.; Taweevisit, M.; Chuaypen, N.; Rattanachaisit, P.; Dissayabutra, T. The Usefulness of Resistant Maltodextrin and Chitosan Oligosaccharide in Management of Gut Leakage and Microbiota in Chronic Kidney Disease. Nutrients 2023, 15, 3363. https://doi.org/10.3390/nu15153363
Anegkamol W, Kamkang P, Hunthai S, Kaewwongse M, Taweevisit M, Chuaypen N, Rattanachaisit P, Dissayabutra T. The Usefulness of Resistant Maltodextrin and Chitosan Oligosaccharide in Management of Gut Leakage and Microbiota in Chronic Kidney Disease. Nutrients. 2023; 15(15):3363. https://doi.org/10.3390/nu15153363
Chicago/Turabian StyleAnegkamol, Weerapat, Panumas Kamkang, Sittiphong Hunthai, Maroot Kaewwongse, Mana Taweevisit, Natthaya Chuaypen, Pakkapon Rattanachaisit, and Thasinas Dissayabutra. 2023. "The Usefulness of Resistant Maltodextrin and Chitosan Oligosaccharide in Management of Gut Leakage and Microbiota in Chronic Kidney Disease" Nutrients 15, no. 15: 3363. https://doi.org/10.3390/nu15153363
APA StyleAnegkamol, W., Kamkang, P., Hunthai, S., Kaewwongse, M., Taweevisit, M., Chuaypen, N., Rattanachaisit, P., & Dissayabutra, T. (2023). The Usefulness of Resistant Maltodextrin and Chitosan Oligosaccharide in Management of Gut Leakage and Microbiota in Chronic Kidney Disease. Nutrients, 15(15), 3363. https://doi.org/10.3390/nu15153363





