Unripe Rubus occidentalis, Ellagic Acid, and Urolithin A Attenuate Inflammatory Responses in IL-1β-Stimulated A549 Cells and PMA-Stimulated Differentiated HL-60 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Unripe Rubus occidentalis Extract, Ellagic Acid, and Urolithin A
2.2. Cell Culture
2.3. A549 Cell Experiment
2.3.1. MTT Assay
2.3.2. ELISA
2.3.3. Gelatin Zymography
2.3.4. Western Blot Assay
2.4. dHL-60 Cell Experiment
2.4.1. NETosis Assay
2.4.2. ROS Assay
2.5. Statistical Analysis
3. Results
3.1. A549 Cell Experiment
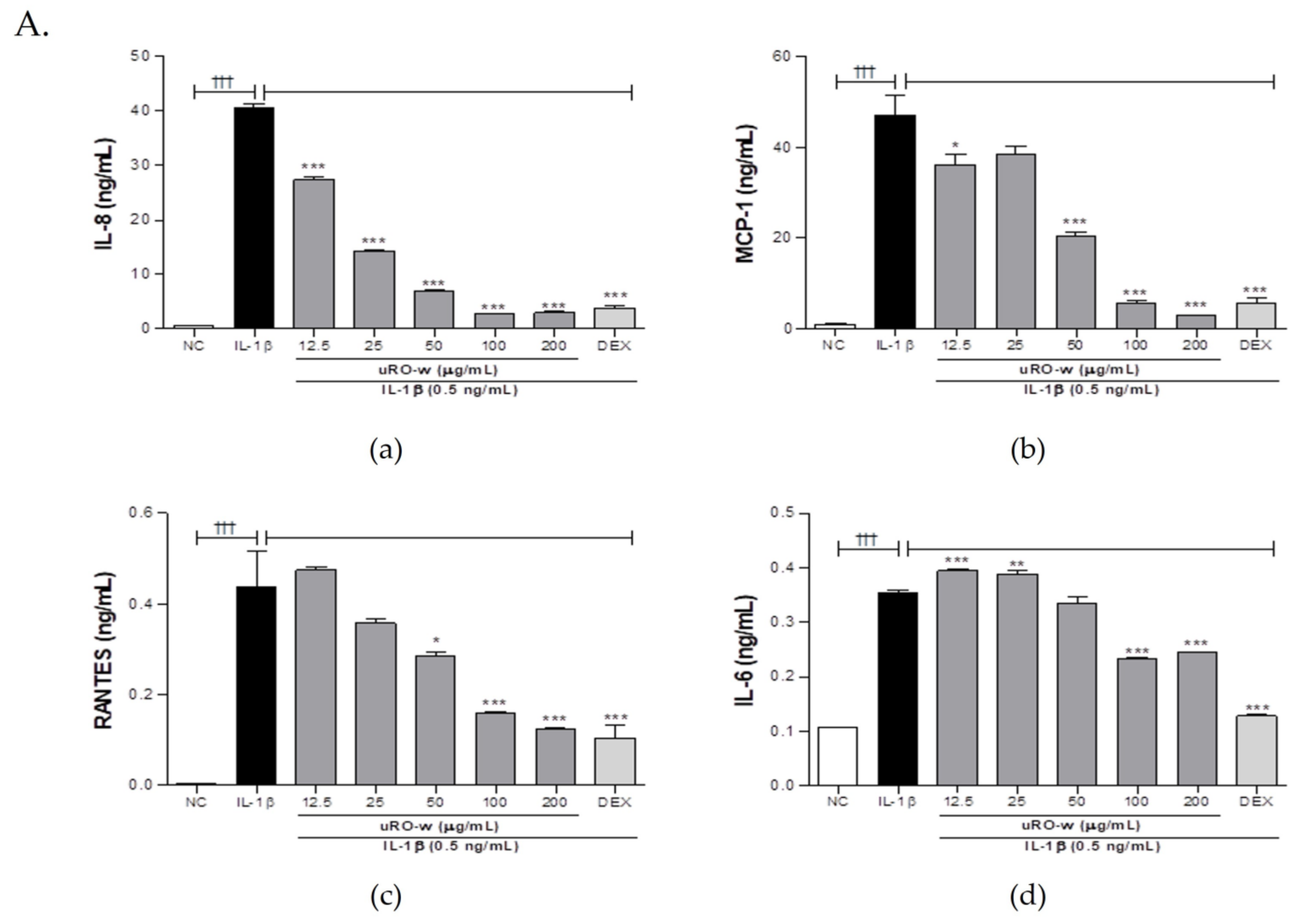
3.1.1. Effect of uRO-w, EA, and UA on Pro-Inflammatory Cytokines and Chemokines
3.1.2. Comparison of IL-8 Lowering Effect of Water or EtOH Extracts of Unripe or Ripe R. occidentalis
3.1.3. Effect of uRO-w, EA, and UA on MMP-9
3.1.4. Effect of uRO-w, EA, and UA on MAPK/AKT/NF-κB Signaling Pathway
3.2. dHL-60 Cell Experiment
3.2.1. Effect of uRO-w, EA, and UA on NETosis
3.2.2. Effect of uRO-w, EA, and UA on ROS Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, Y.; Huang, S.; Kang, J.; Lin, J.; Lai, K.; Sun, Y.; Xiao, W.; Yang, L.; Yao, W.; Cai, S.; et al. Management of airway mucus hypersecretion in chronic airway inflammatory disease: Chinese expert consensus (English edition). Int. J. Chronic Obstr. Pulm. Dis. Engl. Ed. 2018, 13, 399–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postma, D.S.; Kerstjens, H.A. Characteristics of airway hyperresponsiveness in asthma and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1998, 158, S187–S192. [Google Scholar] [CrossRef]
- Cukic, V.; Lovre, V.; Dragisic, D.; Ustamujic, A. Asthma and chronic obstructive pulmonary disease (COPD)—Differences and similarities. Mater. Sociomed. 2012, 24, 100–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GBD. Diseases and Injuries Collaborators. The Lancet. 2019. Available online: https://www.thelancet.com/gbd/summaries (accessed on 18 July 2022).
- Sze, E.; Bhalla, A.; Nair, P. Mechanisms and therapeutic strategies for non-T2 asthma. Allergy 2020, 75, 311–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.C.; Shi, L.S.; Ye, Y.L. Advanced molecular knowledge of therapeutic drugs and natural products focusing on inflammatory cytokines in asthma. Cells 2019, 8, 685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, O.K.; Lee, J.W.; Xu, X.; Harmalkar, D.S.; Song, J.G.; Park, J.W.; Hwang, D.; Min, J.H.; Kim, J.H.; Han, H.K.; et al. DK-1108 exerts anti-inflammatory activity against phorbol 12-myristate 13-acetate-induced inflammation and protective effect against OVA-induced allergic asthma. Biomed. Pharmacother. 2020, 132, 110950. [Google Scholar] [CrossRef] [PubMed]
- Message, S.D.; Johnston, S.L. The immunology of virus infection in asthma. Eur. Respir. J. 2001, 18, 1013–1025. [Google Scholar] [CrossRef]
- Grzela, K.; Litwiniuk, M.; Zagorska, W.; Grzela, T. Airway remodeling in chronic obstructive pulmonary disease and asthma: The role of matrix metalloproteinase-9. Arch. Immunol. Ther. Exp. 2016, 64, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Henderson, I.; Caiazzo, E.; McSharry, C.; Guzik, T.J.; Maffia, P. Why do some asthma patients respond poorly to glucocorticoid therapy? Pharmacol. Res. 2020, 160, 105189. [Google Scholar] [CrossRef]
- Patel, R.; Naqvi, S.A.; Griffiths, C.; Bloom, C.I. Systemic adverse effects from inhaled corticosteroid use in asthma: A systematic review. BMJ Open Respir. Res. 2020, 7, e000756. [Google Scholar] [CrossRef]
- Erdoğan, T.; Karakaya, G.; Kalyoncu, A.F. The frequency and risk factors for oropharyngeal candidiasis in adult asthma patients using inhaled corticosteroids. Turk. Thorac. J. 2019, 20, 136–139. [Google Scholar] [CrossRef]
- Pap, N.; Fidelis, M.; Azevedo, L.; do Carmo, M.A.V.; Wang, D.; Mocan, A.; Pereira, E.P.R.; Xavier-Santos, D.; Sant’Ana, A.S.; Yang, B.; et al. Berry polyphenols and human health: Evidence of antioxidant, anti-inflammatory, microbiota modulation, and cell-protecting effects. Curr. Opin. Food Sci. 2021, 42, 167–186. [Google Scholar] [CrossRef]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer efficacy of polyphenols and their combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [Green Version]
- Golovinskaia, O.; Wang, C.K. Review of functional and pharmacological activities of berries. Molecules 2021, 26, 3904. [Google Scholar] [CrossRef]
- Oh, Y.S.; Shin, S.Y.; Kim, S.; Lee, K.H.; Shin, J.C.; Park, K.M. Comparison of antiaging, anti-melanogenesis effects, and active components of Raspberry (Rubus occidentalis L.) extracts according to maturity. J. Food Biochem. 2020, 44, e13464. [Google Scholar] [CrossRef]
- Choi, H.R.; Lee, S.J.; Lee, J.H.; Kwon, J.W.; Lee, H.K.; Jeong, J.T.; Lee, T. Cholesterol-lowering effects of unripe black raspberry water extract. J. Korean Soc. Food Sci. Nutr. 2013, 42, 1899–1907. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Choi, H.R.; Kwon, J.W.; Kim, Y.S.; Ryu, T.H. Antioxidative and anti-inflammatory effects of unripe black raspberry (Rubus occidentalis) extract and ellagic acid via modulation of NF-κB signaling in lipopolysaccharide-stimulated RAW 264.7 cells. Korean J. Food Cook. Sci. 2022, 38, 321–331. [Google Scholar] [CrossRef]
- Zhou, E.; Fu, Y.; Wei, Z.; Yang, Z. Inhibition of allergic airway inflammation through the blockage of NF-κB activation by ellagic acid in an ovalbumin-induced mouse asthma model. Food Funct. 2014, 5, 2106–2112. [Google Scholar] [CrossRef]
- D’Amico, D.; Andreux, P.A.; Valdés, P.; Singh, A.; Rinsch, C.; Auwerx, J. Impact of the natural compound urolithin A on health, disease, and aging. Trends Mol. Med. 2021, 27, 687–699. [Google Scholar] [CrossRef]
- Lappalainen, U.; Whitsett, J.A.; Wert, S.E.; Tichelaar, J.W.; Bry, K. Interleukin-1β causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am. J. Respir. Cell Mol. Biol. 2005, 32, 311–318. [Google Scholar] [CrossRef]
- Aleman, M.M.; Kesic, M.J.; Mills, K.H.; Peden, D.B.; Hernandez, M.L. The IL-1 axis is associated with airway inflammation after O3 exposure in allergic asthmatic patients. J. Allergy Clin. Immunol. 2015, 136, 1099–1101.e2. [Google Scholar] [CrossRef] [Green Version]
- Liao, Z.; Xiao, H.T.; Zhang, Y.; Tong, R.S.; Zhang, L.J.; Bian, Y.; He, X. IL-1β: A key modulator in asthmatic airway smooth muscle hyper-reactivity. Expert Rev. Respir. Med. 2015, 9, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Kuo, C.T.; Lin, C.C.; Hsieh, H.L.; Yang, C.M. IL-1β induces expression of matrix metalloproteinase-9 and cell migration via a c-Src-dependent, growth factor receptor transactivation in A549 cells. Br. J. Pharmacol. 2010, 160, 1595–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liou, C.J.; Huang, W.C. Casticin inhibits interleukin-1β-induced ICAM-1 and MUC5AC expression by blocking NF-κB, PI3K-Akt, and MAPK signaling in human lung epithelial cells. Oncotarget 2017, 8, 101175–101188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, S.C.M.; Fenwick, P.S.; Barnes, P.J.; Lin, H.S.; Donnelly, L.E. Isorhapontigenin, a bioavailable dietary polyphenol, suppresses airway epithelial cell inflammation through a corticosteroid-independent mechanism. Br. J. Pharmacol. 2017, 174, 2043–2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ham, J.; Kim, J.; Ko, Y.G.; Kim, H.Y. The dynamic contribution of neutrophils in the chronic respiratory diseases. Allergy Asthma Immunol. Res. 2022, 14, 361–378. [Google Scholar] [CrossRef]
- Cesta, M.C.; Zippoli, M.; Marsiglia, C.; Gavioli, E.M.; Mantelli, F.; Allegretti, M.; Balk, R.A. The role of interleukin-8 in lung inflammation and injury: Implications for the management of COVID-19 and hyperinflammatory acute respiratory distress syndrome. Front. Pharmacol. 2021, 12, 808797. [Google Scholar] [CrossRef]
- Kalchiem-Dekel, O.; Yao, X.; Levine, S.J. Meeting the challenge of identifying new treatments for type 2-low neutrophilic asthma. Chest 2020, 157, 26–33. [Google Scholar] [CrossRef] [Green Version]
- de Boer, W.I.; Sont, J.K.; van Schadewijk, A.; Stolk, J.; van Krieken, J.H.; Hiemstra, P.S. Monocyte chemoattractant protein 1, interleukin 8, and chronic airways inflammation in COPD. J. Pathol. 2000, 190, 619–626. [Google Scholar] [CrossRef]
- Elias, J.A. Airway remodeling in asthma. Unanswered questions. Am. J. Respir. Crit. Care Med. 2000, 161, S168–S171. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, G.J.; Fattahi, F.; Rozeveld, D.; Jonker, M.R.; Kliphuis, N.M.; van den Berge, M.; Hylkema, M.N.; ten Hacken, N.H.; van Oosterhout, A.J.; Heijink, I.H. Glucocorticoids induce the production of the chemoattractant CCL20 in airway epithelium. Eur. Respir. J. 2014, 44, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.C.; Kuo, C.T.; Cheng, C.Y.; Wu, C.Y.; Lee, C.W.; Hsieh, H.L.; Lee, I.T.; Yang, C.M. IL-1β promotes A549 cell migration via MAPKs/AP-1- and NF-kappaB-dependent matrix metalloproteinase-9 expression. Cell. Signal. 2009, 21, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- Gilowska, I.; Kasper, Ł.; Bogacz, K.; Szczegielniak, J.; Szymasek, T.; Kasper, M.; Czerwinski, M.; Sładek, K.; Majorczyk, E. Impact of matrix metalloproteinase 9 on COPD development in polish patients: Genetic polymorphism, protein level, and their relationship with lung function. BioMed Res. Int. 2018, 2018, 6417415. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauhan, P.S.; Singh, D.K.; Dash, D.; Singh, R. Intranasal curcumin regulates chronic asthma in mice by modulating NF-ĸB activation and MAPK signaling. Phytomedicine 2018, 51, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Kwon, O.K.; Shin, I.S.; Mali, J.R.; Harmalkar, D.S.; Lim, Y.; Lee, G.; Lu, Q.; Oh, S.R.; Ahn, K.S.; et al. Novel benzofuran derivative DK-1014 attenuates lung inflammation via blocking of MAPK/AP-1 and AKT/mTOR signaling in vitro and in vivo. Sci. Rep. 2019, 9, 862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.H.; Shih, C.H.; Jiang, C.P.; Wen, H.C.; Cheng, W.H.; Chen, B.C. Mammalian target of rapamycin and p70S6K mediate thrombin-induced nuclear factor-κB activation and IL-8/CXCL8 release in human lung epithelial cells. Eur. J. Pharmacol. 2020, 868, 172879. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.U.; Eom, J.E.; Song, H.J.; Jung, S.Y.; Nguyen, T.V.; Lim, K.M.; Chai, O.H.; Kim, H.J.; Kim, G.D.; Shin, H.S.; et al. Camellia sinensis L. alleviates pulmonary inflammation induced by porcine pancreas elastase and cigarette smoke extract. Antioxidants 2022, 11, 1683. [Google Scholar] [CrossRef]
- Yamasaki, A.; Okazaki, R.; Harada, T. Neutrophils and asthma. Diagnostics 2022, 12, 1175. [Google Scholar] [CrossRef]
- Keir, H.R.; Chalmers, J.D. Neutrophil extracellular traps in chronic lung disease: Implications for pathogenesis and therapy. Eur. Respir. Rev. 2022, 31, 210241. [Google Scholar] [CrossRef]
- Jo, A.; Kim, D.W. Neutrophil extracellular traps in airway diseases: Pathological roles and therapeutic implications. Int. J. Mol. Sci. 2023, 24, 5034. [Google Scholar] [CrossRef]
- Porto, B.N.; Stein, R.T. Neutrophil extracellular traps in pulmonary diseases: Too much of a good thing? Front. Immunol. 2016, 7, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pruchniak, M.P.; Demkow, U. Potent NETosis inducers do not show synergistic effects in vitro. Cent. Eur. J. Immunol. 2019, 44, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Hoppenbrouwers, T.; Autar, A.S.A.; Sultan, A.R.; Abraham, T.E.; van Cappellen, W.A.; Houtsmuller, A.B.; van Wamel, W.J.B.; van Beusekom, H.M.M.; van Neck, J.W.; de Maat, M.P.M. In vitro induction of NETosis: Comprehensive live imaging comparison and systematic review. PLoS ONE 2017, 12, e0176472. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Gene Name | IgG | Company | CAT Number | Conc. |
|---|---|---|---|---|
| Primary antibody | ||||
| AKT | R | Cell Signaling Technology (Danvers, MA, USA) | 9272 | 1:1000 |
| Phospho-AKT | R | Cell Signaling Technology | 9271 | 1:1000 |
| p38 | R | Cell Signaling Technology | 8690 | 1:1000 |
| Phospho-p38 | R | Cell Signaling Technology | 4511 | 1:1000 |
| ERK | R | Cell Signaling Technology | 4695 | 1:1000 |
| Phospho-ERK | R | Cell Signaling Technology | 4370 | 1:2000 |
| JNK | R | Cell Signaling Technology | 9252 | 1:1000 |
| Phospho-JNK | R | Cell Signaling Technology | 4668 | 1:1000 |
| NF-κB | R | Cell Signaling Technology | 8242 | 1:1000 |
| Phospho-NF-κB | R | Cell Signaling Technology | 3033 | 1:1000 |
| GAPDH | M | Santa Cruz Biotechnology (Dallas, TX, USA) | sc-365062 | 1:1000 |
| Secondary antibody | ||||
| Mouse | Santa Cruz Biotechnology | Sc-516102 | 1:3000 | |
| Rabbit | Santa Cruz Biotechnology | Sc-2357 | 1:3000 | |
| IL-1β (0.5 ng/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-1β | uRO-w (μg/mL) | EA (μg/mL) | UA (μg/mL) | |||||||||
| 12.5 | 25 | 50 | 100 | 200 | 1.25 | 2.5 | 5 | 1.25 | 2.5 | 5 | ||
| IL-8 (%) | 100 | 67.39 | 35.15 | 17.23 | 6.73 | 7.35 | 47.32 | 37.36 | 25.49 | 72.76 | 65.30 | 73.29 |
| MCP-1 (%) | 77.06 | 81.93 | 43.42 | 12.34 | 6.45 | 42.19 | 37.92 | 27.68 | 73.93 | 84.83 | 71.70 | |
| RANTES (%) | 108.54 | 81.81 | 65.41 | 36.60 | 28.25 | 93.70 | 70.01 | 51.86 | 89.04 | 59.53 | 43.32 | |
| IL-6 (%) | 111.69 | 109.99 | 95.06 | 65.73 | 69.46 | 67.22 | 42.65 | 20.90 | 87.73 | 49.36 | 33.66 | |
| PMA (100 nM) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PMA | uRO-w (μg/mL) | EA (μg/mL) | UA (μg/mL) | |||||||||
| 12.5 | 25 | 50 | 100 | 200 | 1.25 | 2.5 | 5 | 1.25 | 2.5 | 5 | ||
| NET (%) | 100 | 67.45 | 67.93 | 56.28 | 52.74 | 53.06 | 63.16 | 60.19 | 63.59 | 66.3 | 67.4 | 66.8 |
| PMA (100 nM) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PMA | uRO-w (μg/mL) | EA (μg/mL) | UA (μg/mL) | |||||||||
| 12.5 | 25 | 50 | 100 | 200 | 1.25 | 2.5 | 5 | 1.25 | 2.5 | 5 | ||
| ROS (%) | 100 | 79.83 | 80.70 | 80.44 | 73.80 | 75.90 | 77.03 | 75.72 | 74.85 | 81.31 | 81.40 | 82.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Kim, J.; Song, Y.; Kim, S.; Kong, H. Unripe Rubus occidentalis, Ellagic Acid, and Urolithin A Attenuate Inflammatory Responses in IL-1β-Stimulated A549 Cells and PMA-Stimulated Differentiated HL-60 Cells. Nutrients 2023, 15, 3364. https://doi.org/10.3390/nu15153364
Kim S, Kim J, Song Y, Kim S, Kong H. Unripe Rubus occidentalis, Ellagic Acid, and Urolithin A Attenuate Inflammatory Responses in IL-1β-Stimulated A549 Cells and PMA-Stimulated Differentiated HL-60 Cells. Nutrients. 2023; 15(15):3364. https://doi.org/10.3390/nu15153364
Chicago/Turabian StyleKim, Soojin, Jiyeon Kim, Youngcheon Song, Sangbum Kim, and Hyunseok Kong. 2023. "Unripe Rubus occidentalis, Ellagic Acid, and Urolithin A Attenuate Inflammatory Responses in IL-1β-Stimulated A549 Cells and PMA-Stimulated Differentiated HL-60 Cells" Nutrients 15, no. 15: 3364. https://doi.org/10.3390/nu15153364
APA StyleKim, S., Kim, J., Song, Y., Kim, S., & Kong, H. (2023). Unripe Rubus occidentalis, Ellagic Acid, and Urolithin A Attenuate Inflammatory Responses in IL-1β-Stimulated A549 Cells and PMA-Stimulated Differentiated HL-60 Cells. Nutrients, 15(15), 3364. https://doi.org/10.3390/nu15153364






