Diet and Nutritional Status of Polish Girls with Rett Syndrome—A Case-Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Anthropometric Measurements
2.2. Assessment of Nutrition
2.3. Statistical Methods
2.4. Approval of the Bioethical Committee
3. Results
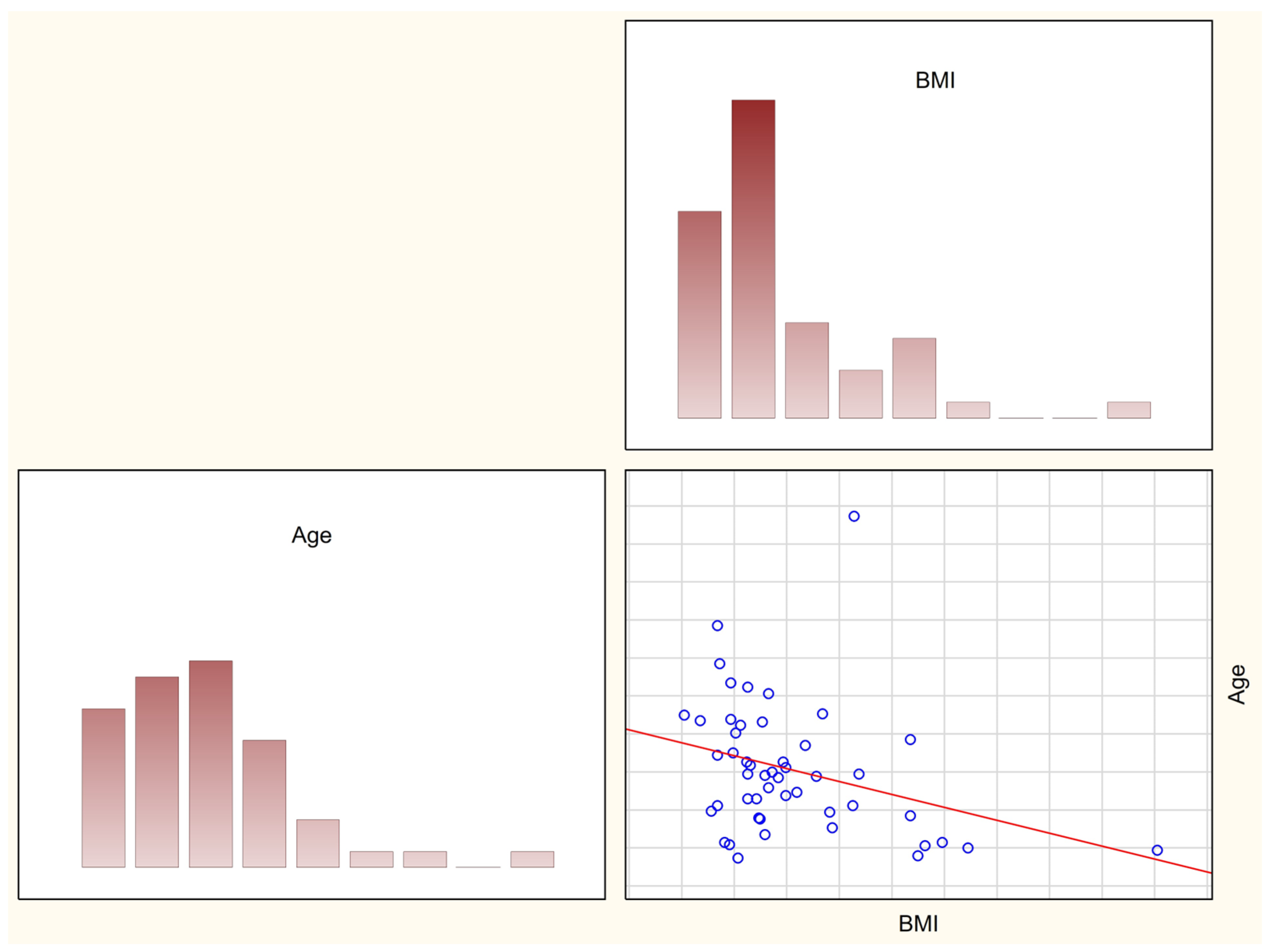
3.1. Assessment of Nutritional Status
3.2. Assessment of Nutrition
3.3. Dietary Intake
4. Discussion
5. Conclusions
- Girls with Rett syndrome have reduced weight, poor growth that deteriorates with age, and are at risk of nutritional deficiencies.
- Various nutritional intervention strategies should be explored to reduce and, if possible, prevent malnutrition and cachexia in girls with Rett syndrome.
- There is a need to systematically review the literature and gather expertise to identify current best practices in relation to nutritional assessment and management.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amir, R.E.; Van den Veyver, I.B.; Schultz, R.; Malicki, D.M.; Tran, C.Q.; Dahle, E.J.; Philippi, A.; Timar, L.; Percy, A.K.; Motil, K.J.; et al. Influence of mutation type and X chromosome inactivation on Rett syndrome phenotypes. Ann. Neurol. 2000, 47, 670–679. [Google Scholar] [CrossRef]
- Schwartzman, F.; Vítolo, M.R.; Schwartzman, J.S.; de Morais, M.B. Eating practices, nutritional status and constipation in patients with Rett syndrome. Arq. Gastroenterol. 2008, 45, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Neul, J.L.; Kaufmann, W.E.; Glaze, D.G.; Christodoulou, J.; Clarke, A.J.; Bahi-Buisson, N.; Leonard, H.; Bailey, M.E.S.; Schanen, N.C.; Zappella, M.; et al. Rett syndrome: Revised diagnostic criteria and nomenclature. Ann. Neurol. 2010, 68, 944–950. [Google Scholar] [CrossRef]
- Jian, L.; Nagarajan, L.; de Klerk, N.; Ravine, D.; Christodoulou, J.; Leonard, H. Seizures in Rett syndrome: An overview from a one-year calendar study. Eur. J. Paediatr. Neurol. 2007, 11, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Ager, S.; Fyfe, S.; Christodoulou, J.; Jacoby, P.; Schmitt, L.; Leonard, H. Predictors of Scoliosis in Rett Syndrome. J. Child Neurol. 2006, 21, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Motil, K.J.; Caeg, E.; Barrish, J.O.; Geerts, S.; Lane, J.B.; Percy, A.K.; Annese, F.; McNair, L.; Skinner, S.A.; Lee, H.-S.; et al. Gastrointestinal and Nutritional Problems Occur Frequently Throughout Life in Girls and Women with Rett Syndrome. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 292–298. [Google Scholar] [CrossRef]
- Oddy, W.H.; Webb, K.G.; Baikie, G.; Thompson, S.M.; Reilly, S.; Fyfe, S.D.; Young, D.; Anderson, A.M.; Leonard, H. Feeding Experiences and Growth Status in a Rett Syndrome Population. J. Pediatr. Gastroenterol. Nutr. 2007, 45, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Bebbington, A.; Anderson, A.; Ravine, D.; Fyfe, S.; Pineda, M.; de Klerk, N.; Ben-Zeev, B.; Yatawara, N.; Percy, A.; Kaufmann, W.E.; et al. Investigating genotype-phenotype relationships in Rett syndrome using an international dataset. Neurology 2008, 70, 868–875. [Google Scholar] [CrossRef]
- Isaacs, J.S.; Murdock, A.; Lane, J.; Percy, A.K. Eating difficulties in girls with Rett syndrome compared with other developmental disabilities. J. Am. Diet Assoc. 2003, 103, 224–230. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health: National Institute of Neurological Disorders and Stroke: Rett Syndrome Fact Sheet. Available online: https://www.ninds.nih.gov/rett-syndrome-fact-sheet (accessed on 3 December 2022).
- Leonard, H.; Ravikumara, M.; Baikie, G.; Naseem, N.; Ellaway, C.; Percy, A.; Abraham, S.; Geerts, S.; Lane, J.; Jones, M.; et al. Assessment and Management of Nutrition and Growth in Rett Syndrome. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Polish Rett Syndrome Association. Available online: https://rettsyndrome.pl (accessed on 6 January 2023).
- Książyk, J.; Kulak, W.; Toporowska-Kowalska, E.; Kmieć, T.; Świąder, A.; Szlagatys-Siodorkiewicz, A.; Romanowska, H.; Żyła, A.; Żelazowska, E.; Popińska, K.; et al. Guidelines on nutritional suport in children with chronic neurological disorders. Neurol. Dziecięca 2011, 20, 79–86. (In Polish) [Google Scholar]
- Kułaga, Z.; Różdżyńska-Świątkowska, A.; Palczewska, I.; Grajda, A.; Gorzkowska, B.; Napieralska, E.; Litwin, M. Percentile charts of height, body mass and body mass index in children and adolescents in Poland—Results of the OLAF study. Stand. Med. Pediatr. 2010, 7, 690–700. (In Polish) [Google Scholar]
- Slaughter, M.H.; Lohman, T.G.; Boileau, R.A.; Horswill, C.A.; Stillman, R.J.; Van Loan, M.D.; Bemben, D.A. Skinfold equations for estimation of body fatness in children and youth. Hum. Biol. 1988, 60, 709–723. [Google Scholar] [PubMed]
- Dziechciarz, P. Assessment of Nutritional Status. In Nutrition and Nutritional Treatment of Children and Adolescents; Szajewska, H., Horvath, A., Eds.; Medycyna Praktyczna: Cracow, Poland, 2017; pp. 7–14. (In Polish) [Google Scholar]
- NIH Publications. National Institutes of Health: Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. The Evidence Reports. 98-4083. 1998. Available online: https://www.nhlbi.nih.gov/files/docs/guidelines/ob_gdlns.pdf (accessed on 20 December 2022).
- Wądołowska, L. Validation of the frequency of consumption questionnaire—FFQ. Repeatability assessment. Bromat. Chem. Toksykol. 2005, 37, 27–33. (In Polish) [Google Scholar]
- Jarosz, M.; Rychlik, E.; Stos, K.; Charzewska, J. Nutrition Standards for the Polish Population and Their Application; NIZP PZH-PIB: Warsaw, Poland, 2020. Available online: https://ncez.pzh.gov.pl/wp-content/uploads/2021/03/normy_zywienia_2020web.pdf (accessed on 12 December 2022). (In Polish)
- Thommessen, M.; Kase, B.; Heiberg, A. Growth and nutrition in 10 girls with Rett syndrome. Acta Paediatr. 1992, 81, 686–690. [Google Scholar] [CrossRef]
- Reilly, S.; Cass, H. Growth and Nutrition in Rett syndrome. Disabil. Rehabil. 2001, 10, 118–128. [Google Scholar]
- Wong, L.C.; Chen, Y.T.; Tsai, S.M.; Lin, Y.J.; Hsu, C.J.; Wang, H.P.; Hu, S.C.; Shen, H.Y.; Tsai, W.C.; Lee, W.T. Dietary intake and growth deficits in Rett syndrome–A cross-section study. Autism Res. 2021, 14, 1512–1521. [Google Scholar] [CrossRef]
- Kennedy, M.; McCombie, L.; Dawes, P.; McConnell, K.N.; Dunningan, M.G. Nutritional support for patients with intellectual disability and nutrition/dysphagia disorders in community care. J. Intellect. Disabil. Res. 1997, 41, 430–436. [Google Scholar] [CrossRef]
- Rice, M.A.; Haas, R.H. The Nutritional Aspects of Rett Syndrome. J. Child Neurol. 1988, 3, S35–S42. [Google Scholar] [CrossRef]
- Bebbington, A.; Percy, A.; Christodoulou, J.; Ravine, D.; Ho, G.; Jacoby, P.; Anderson, A.; Pineda, M.; Ben-Zeev, B.; Bahi-Buisson, N.; et al. Updating the profileof C-terminal MECP2 deletions in Rett syndrome. J. Med. Genet. 2010, 47, 242–248. [Google Scholar] [CrossRef]
- Newman, R.; Vilardell, N.; Clavé, P.; Speyer, R. Effect of Bolus Viscosity on the Safety and Efficacy of Swallowing and the Kinematics of the Swallow Response in Patients with Oropharyngeal Dysphagia: White Paper by the European Society for Swallowing Disorders (ESSD). Dysphagia 2016, 31, 232–249, Erratum in Dysphagia 2016, 31, 719. [Google Scholar] [CrossRef] [PubMed]
- Bianco, E.; Rota, D. Oral findings in Rett syndrome: An update and review of the literature. Dent. Med. Probl. 2018, 55, 441–445. [Google Scholar] [CrossRef]
- Ramirez, J.-M.; Karlen-Amarante, M.; Wang, J.-D.J.; Huff, A.; Burgraff, N. Breathing disturbances in Rett syndrome. Handb. Clin. Neurol. 2022, 189, 139–151. [Google Scholar]
- Yagi, N.; Oku, Y.; Nagami, S.; Yamagata, Y.; Kayashita, J.; Ishikawa, A.; Domen, K.; Takahashi, R. Inappropriate Timing of Swallow in the Respiratory Cycle Causes Breathing–Swallowing Discoordination. Front. Physiol. 2017, 8, 676. [Google Scholar] [CrossRef]
- Cocca, S.; Viviano, M.; Loglisci, M.; Parrini, S.; Monciatti, G.; Paganelli, I.I.; Livi, W.; Mezzedimi, C. Correlation Between Dysphagia and Malocclusion in Rett Syndrome: A preliminary study. Sultan Qaboos Univ. Med. J. 2018, 18, 489–493. [Google Scholar] [CrossRef]
- Cichero, J.A. Thickening agents used for dysphagia management: Effect on bioavailability of water, medication and feelings of satiety. Nutr. J. 2013, 12, 54. [Google Scholar] [CrossRef]
- Garon, B.R.; Sierzant, T.; Ormiston, C. Silent aspiration: Results of 2,000 video fluoroscopic evaluations. J. Neurosci. Nurs. 2009, 41, 178–185, quiz 186–187. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.T.; Dodrill, P.; Ward, E.C. Interventions for oropharyngeal dysphagia in children with neurological impairment. Cochrane Database Syst. Rev. 2012, 10, CD009456. [Google Scholar] [CrossRef] [PubMed]
| Consumption Frequency Categories | Ranks Assigned to Frequency Categories | Daily Frequency (Times/Day) |
|---|---|---|
| Never or almost never | 1 | 0.0 |
| Once a month or less frequently | 2 | 0.025 |
| Several times a month | 3 | 0.100 |
| Several times a week | 4 | 0.571 |
| Every day | 5 | 1.000 |
| Several times a day | 6 | 2.000 |
| Parameters | Study Group (n = 49) | Control Group (n = 22) | p | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Q0 (Min) | Q1 | Q2 (Median) | Q3 | Q4 (Max) | IQR | Mean | SD | Q0 (Min) | Q1 | Q2 (Median) | Q3 | Q4 (Max) | IQR | ||
| Age (years) | 8.7 | 4.9 | 2.1 | 5.2 | 8.2 | 11.4 | 27.6 | 6.2 | 10.7 | 3.5 | 4.5 | 8.7 | 10.5 | 12.3 | 18.6 | 3.6 | 0.09 |
| Body mass (kg) | 22.3 | 11.4 | 9.4 | 15.7 | 19.4 | 26.4 | 57.5 | 10.7 | 38.4 | 13.5 | 16.3 | 27.9 | 38.6 | 47.0 | 73.5 | 19.0 | 0.00 |
| Height (cm) | 114.2 | 19.5 | 69.0 | 100.0 | 115.0 | 127.0 | 160.0 | 27.0 | 145.2 | 17.0 | 105.5 | 135.7 | 147.2 | 154.9 | 169.5 | 19.1 | 0.00 |
| BMI (kg/m2) | 16.7 | 6.9 | 9.8 | 12.8 | 14.8 | 17.2 | 52.9 | 4.3 | 17.7 | 3.4 | 13.9 | 15.1 | 16.9 | 19.1 | 27.0 | 3.9 | 0.15 |
| BMI z-score | −0.8 | 2.2 | −6.4 | −2.3 | −0.9 | 0.4 | 6.3 | 2.8 | −0.1 | 1.1 | −1.9 | −0.7 | −0.2 | 0.5 | 2.0 | 1.1 | 0.07 |
| Waist circumference (cm) | 56.3 | 10.5 | 39.0 | 49.0 | 54.0 | 65.0 | 78.0 | 16.0 | 63.4 | 14.9 | 47.0 | 55.2 | 60.0 | 64.7 | 118.0 | 9.5 | 0.03 |
| Hip circumference (cm) | 63.3 | 13.8 | 43.0 | 54.0 | 62.0 | 73.0 | 96.0 | 19.0 | 77.4 | 12.0 | 57.0 | 69.2 | 76.7 | 87.0 | 104.0 | 17.7 | 0.00 |
| WHR | 0.9 | 0.1 | 0.5 | 0.8 | 0.9 | 0.9 | 1.1 | 0.1 | 0.8 | 0.2 | 0.7 | 0.7 | 0.8 | 0.8 | 1.7 | 0.1 | 0.00 |
| WHtR | 0.5 | 0.1 | 0.3 | 0.4 | 0.5 | 0.5 | 1.1 | 0.1 | 0.4 | 0.1 | 0.4 | 0.4 | 0.4 | 0.4 | 1.0 | 0.0 | 0.01 |
| Arm circumference (cm) | 17.4 | 4.1 | 11.0 | 14.5 | 16.0 | 19.0 | 31.0 | 4.5 | 21.7 | 3.9 | 16.5 | 18.4 | 21.5 | 23.7 | 32.0 | 5.4 | 0.00 |
| Muscle mass (kg) | 13.7 | 2.9 | 7.0 | 11.8 | 13.2 | 15.8 | 23.1 | 3.9 | 17.1 | 3.0 | 12.7 | 15.5 | 16.3 | 18.0 | 25.1 | 2.5 | 0.00 |
| Suprailiac skinfold * (mm) | 8.5 | 7.5 | 2.0 | 4.0 | 6.0 | 12.0 | 45.0 | 8.0 | 12.0 | 12.9 | 4.0 | 6.2 | 10.0 | 12.0 | 67.0 | 5.7 | 0.06 |
| Subscapular skinfold ** (mm) | 8.5 | 4.9 | 3.0 | 5.0 | 7.0 | 11.0 | 24.0 | 6.0 | 9.5 | 5.5 | 5.0 | 5.2 | 8.5 | 10.0 | 26.0 | 4.7 | 0.38 |
| Triceps skinfold *** (mm) | 11.6 | 5.3 | 2.0 | 8.0 | 10.5 | 16.0 | 25.0 | 8.0 | 14.4 | 5.5 | 6.0 | 11.0 | 14.0 | 17.5 | 26.0 | 6.5 | 0.04 |
| FAT (%) | 22.9 | 6.7 | 10.0 | 17.6 | 20.9 | 28.3 | 36.0 | 10.7 | 25.6 | 5.7 | 15.6 | 21.8 | 24.3 | 28.8 | 36.5 | 6.9 | 0.09 |
| Bioimpedance Analysis (BIA) | |||||||||||||||||
| BMR (kcal/d) | 828.0 | 99.6 | 701.0 | 733.0 | 843.0 | 866.0 | 1016.0 | 133.0 | 1326.2 | 205.8 | 887.0 | 1159.0 | 1375.0 | 1453.0 | 1685.0 | 294.0 | 0.00 |
| FAT (%) | 21.2 | 13.8 | 8.4 | 10.2 | 19.4 | 24.6 | 53.5 | 14.4 | 19.7 | 6.8 | 3.0 | 15.2 | 18.4 | 23.8 | 32.2 | 8.5 | 0.89 |
| LBM (%) | 79.4 | 14.0 | 46.5 | 75.4 | 83.1 | 89.8 | 91.6 | 14.4 | 79.6 | 6.8 | 67.8 | 75.5 | 80.9 | 83.2 | 96.9 | 7.7 | 0.44 |
| TBW (l) | 64.2 | 11.0 | 43.9 | 59.0 | 62.2 | 75.7 | 78.0 | 16.7 | 58.7 | 4.1 | 49.7 | 56.7 | 58.2 | 61.5 | 67.0 | 4.8 | 0.06 |
| Cell mass (kg) | 6.4 | 2.8 | 4.3 | 4.4 | 6.1 | 6.3 | 13.6 | 1.9 | 15.3 | 5.1 | 6.6 | 10.9 | 15.7 | 18.2 | 24.0 | 7.2 | 0.00 |
| Muscle mass (kg) | 3.8 | 0.9 | 2.8 | 2.9 | 4.3 | 4.4 | 4.6 | 1.6 | 12.1 | 4.2 | 4.7 | 8.4 | 12.1 | 13.8 | 19.9 | 5.4 | 0.00 |
| Study Group n (%) | Control Group n (%) | |
|---|---|---|
| Significantly underweight | 19 (38.78) | 0 |
| Underweight | 5 (10.2) | 3 (13.64) |
| Normal | 18 (36.73) | 16 (72.73) |
| Overweight | 2 (4.08) | 2 (9.09) |
| Obese | 3 (6.12) | 1 (4.55) |
| Extremely obese | 2 (4.08) | 0 (0.0) |
| Total | 49 (100) | 22 (100) |
| Group of Products | Average Times Per Day | p | |
|---|---|---|---|
| Study Group | Control Group | ||
| 1. Sugar for sweetening drinks | 0.56 | 0.30 | 0.27 |
| 2. Honey for sweetening drinks | 0.41 | 0.23 | 0.35 |
| 3. Chocolates (chocolate candies and chocolate bars) | 0.10 | 0.39 | 0.00 |
| 4. Non-chocolate candies (e.g., fruit candies, caramels, gummies, fudge, toffees) | 0.03 | 0.15 | 0.00 |
| 5. Biscuits and cookies | 0.14 | 0.23 | 0.01 |
| 6. Ice cream and pudding | 0.07 | 0.21 | 0.00 |
| 7. Salty snacks | 0.09 | 0.18 | 0.00 |
| 8. Milk and natural milk drinks | 0.56 | 0.68 | 0.40 |
| 9. Sweetened milk drinks | 0.34 | 0.26 | 0.99 |
| 10. Cocoa | 0.07 | 0.18 | 0.07 |
| 11. Natural cottage cheeses | 0.18 | 0.25 | 0.62 |
| 12. Cottage cheese with flavor additives | 0.12 | 0.04 | 0.09 |
| 13. Cheese (yellow) | 0.29 | 0.46 | 0.69 |
| 14. Other cheeses | 0.08 | 0.09 | 0.92 |
| 15. Eggs and egg dishes where eggs are the main ingredient of the dish | 0.42 | 0.26 | 0.07 |
| 16. Wholegrain bread or bread with grains (so-called dark) | 0.48 | 0.46 | 0.39 |
| 17. Refined bread (so-called white) | 0.64 | 0.58 | 0.90 |
| 18. Unrefined coarse grains, brown rice, wholegrain pasta | 0.18 | 0.22 | 0.67 |
| 19. Refined fine grain groats | 0.16 | 0.09 | 0.42 |
| 20. White rice | 0.15 | 0.21 | 0.66 |
| 21. Pasta | 0.22 | 0.22 | 0.85 |
| 22. Breakfast cereals | 0.24 | 0.24 | 0.26 |
| 23. Canola oil | 0.37 | 0.46 | 0.17 |
| 24. Soybean oil | 0.01 | 0.00 | 1.00 |
| 25. Flaxseed oil | 0.08 | 0.01 | 0.39 |
| 26. Olive oil | 0.20 | 0.27 | 0.11 |
| 27. Other vegetable oils | 0.09 | 0.02 | 0.33 |
| 28. Butter | 1.01 | 0.71 | 0.13 |
| 29. Margarine cubes | 0.06 | 0.01 | 0.81 |
| 30. Margarine in cups | 0.07 | 0.03 | 0.32 |
| 31. Cream | 0.28 | 0.22 | 0.82 |
| 32. Other animal fats | 0.02 | 0.01 | 0.15 |
| 33. Mayonnaise and dressings | 0.07 | 0.02 | 0.18 |
| 34. Fruits | 0.88 | 0.94 | 0.67 |
| 35. Stone fruits | 0.25 | 0.36 | 0.32 |
| 36. Kiwi and citrus | 0.21 | 0.43 | 0.02 |
| 37. Other tropical fruits | 0.13 | 0.24 | 0.13 |
| 38. Berry fruit | 0.21 | 0.41 | 0.10 |
| 39. Bananas | 0.49 | 0.26 | 0.01 |
| 40. Apples and pears | 0.45 | 0.52 | 0.32 |
| 41. Avocado | 0.08 | 0.12 | 0.92 |
| 42. Olives | 0.03 | 0.15 | 0.02 |
| 43. Dried fruits | 0.11 | 0.06 | 0.57 |
| 44. Sweet fruit preserves | 0.19 | 0.12 | 0.31 |
| 45. Vegetables | 1.02 | 0.89 | 0.2 |
| 46. Cruciferous vegetables | 0.28 | 0.29 | 0.76 |
| 47. Yellow-orange vegetables | 0.62 | 0.47 | 0.21 |
| 48. Leafy green vegetables | 0.20 | 0.27 | 0.83 |
| 49. Tomatoes | 0.43 | 0.52 | 0.43 |
| 50. Vegetables such as cucumber | 0.34 | 0.51 | 0.04 |
| 51. Root and other vegetables | 0.48 | 0.34 | 0.04 |
| 52. Fresh legumes and canned seeds | 0.13 | 0.22 | 0.48 |
| 53. Dry legume seeds | 0.03 | 0.13 | 0.94 |
| 54. Soy | 0.00 | 0.11 | 0.55 |
| 55. Dry legumes in dishes | 0.03 | 0.14 | 0.75 |
| 56. Potatoes | 0.56 | 0.38 | 0.04 |
| 57. Walnuts | 0.07 | 0.08 | 0.12 |
| 58. Peanuts | 0.02 | 0.04 | 0.38 |
| 59. Other nuts | 0.05 | 0.14 | 0.00 |
| 60. Nut creams | 0.05 | 0.06 | 0.16 |
| 61. Seeds | 0.06 | 0.08 | 0.40 |
| 62. Flax (Flax seeds) | 0.06 | 0.03 | 0.02 |
| 63. Sausages | 0.32 | 0.16 | 0.12 |
| 64. High-quality sausage (cold cuts) | 0.56 | 0.35 | 0.20 |
| 65. Sausage products and offal | 0.10 | 0.08 | 0.06 |
| 66. Red meat | 0.24 | 0.19 | 0.33 |
| 67. Poultry and rabbit meat | 0.42 | 0.35 | 0.26 |
| 68. Venison | 0.01 | 0.06 | 0.71 |
| 69. Lean fish | 0.08 | 0.09 | 0.88 |
| 70. Fatty fish | 0.06 | 0.08 | 0.48 |
| 71. Fruit juices and fruit nectars | 0.28 | 0.45 | 0.08 |
| 72. Vegetable and vegetable-fruit juices | 0.08 | 0.18 | 0.56 |
| 73. Energy drinks | 0.00 | 0.09 | 0.56 |
| 74. Sweetened drinks | 0.00 | 0.04 | 0.00 |
| Nutrients (Daily Intake) | Study Group (n = 49) | Control Group (n = 22) | p | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Q0 (Min) | Q1 | Q2 (Median) | Q3 | Q4 (Max) | IQR | Mean | SD | Q0 (Min) | Q1 | Q2 (Median) | Q3 | Q4 (Max) | IQR | ||
| Energy (kcal) | 1352.5 | 476.4 | 615.4 | 1101.8 | 1248.5 | 1572.1 | 3577.0 | 470.3 | 1611.8 | 361.8 | 1019.9 | 1398.2 | 1683.9 | 1778.6 | 2218.4 | 380.4 | 0.04 |
| (%EAR) | 87.6 | 26.6 | 43.9 | 70.1 | 80.1 | 101.5 | 160.3 | 31.4 | 88.2 | 26.3 | 50.9 | 66.2 | 84.7 | 106.3 | 143.1 | 40.1 | 0.91 |
| (kcal/kg b.w) | 70.1 | 28.8 | 25.4 | 53.1 | 63.3 | 90.8 | 135.5 | 37.7 | 47.0 | 19.1 | 14.8 | 33.7 | 46.4 | 54.6 | 96.6 | 20.9 | 0.00 |
| Protein (g) | 56.3 | 23.2 | 26.6 | 42.8 | 52.6 | 67.3 | 176.2 | 24.5 | 60.6 | 16.7 | 36.2 | 46.0 | 60.9 | 70.1 | 93.2 | 24.0 | 0.19 |
| (%RDA) | 213.8 | 106.6 | 81.7 | 130.1 | 183.9 | 257.2 | 513.6 | 127.1 | 172.3 | 76.6 | 72.0 | 107.6 | 153.5 | 221.5 | 348.6 | 113.9 | 0.10 |
| (g/kg b.w) | 2.9 | 1.3 | 0.9 | 2.1 | 2.5 | 3.8 | 6.7 | 1.8 | 1.8 | 0.8 | 0.5 | 1.0 | 1.7 | 2.3 | 3.6 | 1.2 | 0.00 |
| Fats (g) | 44.5 | 20.8 | 16.5 | 31.5 | 41.6 | 51.1 | 141.2 | 19.6 | 44.9 | 14.1 | 23.2 | 34.5 | 45.5 | 51.8 | 75.4 | 17.3 | 0.47 |
| (%RDA) | 82.3 | 28.0 | 29.5 | 64.6 | 79.9 | 95.7 | 163.1 | 31.1 | 72.7 | 24.2 | 37.5 | 59.5 | 72.1 | 89.1 | 145.0 | 29.6 | 0.12 |
| (g/kg b.w) | 2.3 | 1.0 | 0.6 | 1.6 | 2.0 | 2.8 | 5.3 | 1.1 | 1.3 | 0.4 | 0.4 | 1.1 | 1.2 | 1.6 | 2.1 | 0.5 | 0.00 |
| Carbohydrates (g) | 188.3 | 67.2 | 88.9 | 137.7 | 181.6 | 212.4 | 411.7 | 74.7 | 249.7 | 60.5 | 128.3 | 198.1 | 257.0 | 295.5 | 353.5 | 97.5 | 0.00 |
| (g/kg b.w) | 9.8 | 4.3 | 3.3 | 7.1 | 8.9 | 12.3 | 20.1 | 5.3 | 7.4 | 3.4 | 2.2 | 4.8 | 6.9 | 8.5 | 16.3 | 3.6 | 0.02 |
| Amino acids | |||||||||||||||||
| Isoleucine (mg) | 2532.9 | 11,422 | 64.7 | 1917.0 | 2366.6 | 2868.8 | 8095.3 | 951.8 | 2810.1 | 829.8 | 1644.0 | 2138.5 | 2806.2 | 3298.4 | 4521.3 | 1159.9 | 0.13 |
| Leucine (mg) | 4066.9 | 1900.3 | 111.3 | 3078.7 | 3823.2 | 4706.9 | 13,629.4 | 1628.2 | 4630.2 | 1397.1 | 2578.9 | 3636.3 | 4717.0 | 5230.2 | 7398.5 | 1593.9 | 0.07 |
| Lysine (mg) | 3588.6 | 1662.7 | 61.8 | 2609.9 | 3308.3 | 4273.7 | 11,444.2 | 1663.8 | 3807.3 | 1139.5 | 1987.7 | 2862.3 | 3829.4 | 4518.0 | 6453.6 | 1 65.7 | 0.23 |
| Methionine (mg) | 1264.1 | 562.4 | 36.9 | 966.5 | 1189.2 | 1437.3 | 3982.5 | 470.8 | 1402.4 | 421.4 | 746.1 | 1124.6 | 1423.4 | 1650.3 | 2234.7 | 525.7 | 0.12 |
| Cystine (mg) | 731.5 | 275.8 | 43.8 | 602.1 | 701.6 | 799.1 | 1894.9 | 197.0 | 933.7 | 267.8 | 496.9 | 741.2 | 930.6 | 1117.8 | 1508.8 | 376.6 | 0.00 |
| Phenylalanine (mg) | 2299.7 | 1047.3 | 76.96 | 1819.5 | 2148.9 | 2663.9 | 7588.0 | 844.5 | 2646.7 | 763.6 | 1554.8 | 2029.3 | 2708.8 | 3009.2 | 4115.9 | 979.9 | 0.06 |
| Tryptophan (mg) | 670.2 | 311.5 | 15.9 | 510.5 | 638.8 | 773.5 | 2236.4 | 262.9 | 746.6 | 218.0 | 443.0 | 547.4 | 733.3 | 868.5 | 1258.4 | 321.1 | 0.09 |
| Minerals | |||||||||||||||||
| Sodium (mg) | 2275.7 | 1021.4 | 773.2 | 1661.8 | 2074.4 | 2658.6 | 6555.1 | 996.8 | 2500.8 | 629.2 | 1439.0 | 2049.9 | 2585.1 | 2812.8 | 3731.2 | 762.9 | 0.08 |
| (%AI) | 201.3 | 73.6 | 68.3 | 142.7 | 197.2 | 239.2 | 437.0 | 96.5 | 196.6 | 57.8 | 95.9 | 170.0 | 191.1 | 248.1 | 286.2 | 78.1 | 0.90 |
| (mg/kg b.w) | 115.5 | 51.8 | 29.0 | 86.0 | 107.5 | 134.9 | 248.4 | 48.9 | 74.9 | 34.9 | 20.3 | 47.8 | 79.1 | 94.4 | 163.5 | 46.6 | 0.00 |
| Potassium (mg) | 2198.9 | 894.0 | 957.1 | 1625.8 | 1983.4 | 2496.8 | 5596.0 | 871.0 | 2250.8 | 611.2 | 1337.4 | 1800.8 | 2235.1 | 2552.3 | 4106.9 | 751.7 | 0.29 |
| (%AI) | 146.5 | 74.5 | 48.9 | 91.9 | 124.2 | 189.9 | 383.7 | 98.0 | 110.2 | 52.4 | 43.4 | 78.5 | 90.6 | 138.7 | 232.7 | 60.2 | 0.03 |
| (mg/kg b.w) | 112.9 | 50.5 | 44.8 | 71.9 | 105.7 | 140.2 | 254.2 | 68.3 | 66.2 | 29.6 | 22.1 | 50.0 | 57.2 | 75.5 | 129.9 | 25.5 | 0.00 |
| Calcium (mg) | 581.8 | 299.5 | 175.5 | 366.7 | 519.2 | 731.6 | 1773.0 | 364.9 | 596.3 | 218.8 | 266.1 | 457.1 | 611.4 | 681.9 | 1210.0 | 224.8 | 0.59 |
| (%RDA) | 58.5 | 27.4 | 17.6 | 33.6 | 58.4 | 74.3 | 136.4 | 40.8 | 51.7 | 21.4 | 20.5 | 36.8 | 47.6 | 65.6 | 121.0 | 28.8 | 0.35 |
| (mg/kg b.w) | 30.4 | 17.5 | 6.6 | 19.1 | 26.9 | 37.9 | 87.4 | 18.9 | 17.6 | 8.8 | 4.6 | 9.9 | 17.3 | 22.1 | 40.9 | 12.2 | 0.00 |
| Phosphorus (mg) | 870.2 | 359.5 | 398.9 | 664.3 | 813.9 | 1002.2 | 2649.5 | 337.9 | 960.1 | 240.8 | 558.2 | 780.4 | 967.1 | 1115.7 | 1391.3 | 335.3 | 0.06 |
| (%RDA) | 134.7 | 53.5 | 51.7 | 91.7 | 121.9 | 176.5 | 304.1 | 84.8 | 115.9 | 58.9 | 50.0 | 69.3 | 93.0 | 159.1 | 231.9 | 89.8 | 0.12 |
| (mg/kg b.w) | 45.6 | 21.8 | 12.9 | 29.6 | 38.4 | 59.6 | 100.4 | 30.0 | 28.3 | 12.5 | 9.1 | 16.4 | 29.0 | 34.4 | 58.9 | 17.9 | 0.00 |
| Magnesium (mg) | 204.0 | 80.3 | 88.6 | 147.6 | 185.3 | 237.3 | 525.8 | 89.7 | 226.2 | 47.3 | 152.9 | 193.6 | 228.7 | 255.9 | 356.4 | 62.3 | 0.04 |
| (%RDA) | 138.9 | 67.0 | 35.7 | 81.1 | 128.2 | 177.8 | 275.8 | 96.8 | 113.6 | 59.5 | 54.1 | 68.2 | 83.1 | 148.5 | 274.1 | 80.3 | 0.13 |
| (mg/kg b.w) | 10.5 | 4.8 | 3.8 | 6.9 | 9.4 | 13.6 | 22.5 | 6.6 | 6.6 | 2.5 | 2.6 | 4.9 | 6.6 | 7.4 | 12.1 | 2.4 | 0.00 |
| Iron (mg) | 9.4 | 5.9 | 3.5 | 6.0 | 8.2 | 10.5 | 39.9 | 4.5 | 9.3 | 3.6 | 6.0 | 6.9 | 8.5 | 9.6 | 21.6 | 2.7 | 0.54 |
| (%RDA) | 92.8 | 57.5 | 35.0 | 61.9 | 83.2 | 106.5 | 398.6 | 44.6 | 78.8 | 29.5 | 43.3 | 62.6 | 69.3 | 88.8 | 146.4 | 26.2 | 0.35 |
| (mg/kg b.w) | 0.5 | 0.3 | 0.2 | 0.3 | 0.4 | 0.5 | 2.3 | 0.3 | 0.3 | 0.1 | 0.1 | 0.2 | 0.2 | 0.3 | 0.5 | 0.2 | 0.00 |
| Zinc (mg) | 7.3 | 3.6 | 3.4 | 4.9 | 6.3 | 7.9 | 22.6 | 2.9 | 6.8 | 1.4 | 4.2 | 5.8 | 6.9 | 7.4 | 10.2 | 1.6 | 0.55 |
| (%RDA) | 135.8 | 66.4 | 55.0 | 95.4 | 120.4 | 166.7 | 459.5 | 71.4 | 101.3 | 43.6 | 52.1 | 69.1 | 84.0 | 137. | 203.8 | 68.3 | 0.01 |
| (mg/kg b.w) | 0.4 | 0.2 | 0.1 | 0.2 | 0.3 | 0.5 | 1.4 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.4 | 0.1 | 0.00 |
| Copper (mg) | 0.8 | 0.5 | 0.4 | 0.6 | 0.7 | 0.9 | 2.8 | 0.3 | 0.9 | 0.3 | 0.5 | 0.8 | 0.9 | 1.15 | 1.6 | 0.4 | 0.01 |
| (%RDA) | 147.9 | 66.3 | 54.8 | 96.6 | 124.5 | 183.3 | 311.2 | 86.6 | 137.9 | 44.8 | 74.3 | 110.1 | 129.3 | 160.1 | 243.6 | 50.0 | 0.97 |
| (mg/kg b.w) | 0.04 | 0.02 | 0.02 | 0.03 | 0.04 | 0.05 | 0.09 | 0.02 | 0.03 | 0.01 | 0.01 | 0.02 | 0.03 | 0.03 | 0.05 | 0.01 | 0.00 |
| Manganese (mg) | 3.1 | 1.4 | 0.9 | 2.3 | 2.9 | 3.5 | 7.0 | 1.3 | 2.8 | 0.8 | 1.5 | 2.3 | 2.6 | 3.2 | 4.4 | 1.0 | 0.46 |
| (%AI) | 197.4 | 74.7 | 77.6 | 151.5 | 191.2 | 232.5 | 391.7 | 80.9 | 171.1 | 56.4 | 92.5 | 129.0 | 154.6 | 204.8 | 293.8 | 75.8 | 0.16 |
| (mg/kg b.w) | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.3 | 0.1 | 0.1 | 0.04 | 0.02 | 0.1 | 0.1 | 0.1 | 0.2 | 0.04 | 0.00 |
| Vitamins | |||||||||||||||||
| Retinol (ug) | 1171.4 | 4362.1 | 61.9 | 198.1 | 267.9 | 485.2 | 30,013.1 | 287.0 | 240.4 | 108.5 | 101.9 | 164.0 | 210.3 | 267.7 | 512.8 | 103.7 | 0.06 |
| (ug/kg b.w) | 46.1 | 127.3 | 4.3 | 9.4 | 13.9 | 25.7 | 850.2 | 16.3 | 7.1 | 3.9 | 1.8 | 3.8 | 6.7 | 10.2 | 14.7 | 6.4 | 0.00 |
| B1 (mg) | 2.3 | 7.3 | 0.3 | 0.7 | 0.9 | 1.4 | 52.4 | 0.7 | 0.9 | 0.3 | 0.4 | 0.7 | 0.8 | 0.9 | 1.7 | 0.2 | 0.17 |
| (%RDA) | 258.7 | 668.0 | 52.9 | 90.2 | 111.1 | 213.4 | 4759.9 | 123.3 | 93.3 | 33.3 | 38.3 | 67.7 | 81.4 | 113.2 | 153.3 | 45.6 | 0.00 |
| (mg/kg b.w) | 0.1 | 0.2 | 0.02 | 0.03 | 0.05 | 0.1 | 1.5 | 0.05 | 0.02 | 0.01 | 0.01 | 0.02 | 0.02 | 0.03 | 0.05 | 0.01 | 0.00 |
| B2 (mg) | 2.7 | 7.3 | 0.5 | 1.1 | 1.4 | 1.9 | 52.5 | 0.9 | 1.4 | 0.5 | 0.7 | 0.9 | 1.3 | 1.6 | 3.0 | 0.6 | 0.26 |
| (%RDA) | 310.2 | 661.8 | 98.0 | 121.8 | 173.5 | 264.1 | 4771.2 | 142.3 | 145.8 | 57.8 | 65.4 | 101.0 | 127.8 | 186.3 | 275.3 | 85.3 | 0.01 |
| (mg/kg b.w) | 0.1 | 0.2 | 0.02 | 0.05 | 0.1 | 0.1 | 1.5 | 0.1 | 0.04 | 0.02 | 0.01 | 0.02 | 0.04 | 0.1 | 0.1 | 0.04 | 0.00 |
| Niacin (mg) | 22.6 | 73.9 | 3.6 | 8.5 | 11.7 | 13.9 | 528.1 | 5.5 | 13.7 | 6.1 | 5.2 | 9.7 | 12.5 | 15.2 | 33.2 | 5.5 | 0.26 |
| (%RDA) | 199.1 | 524.6 | 35.2 | 87.1 | 109.7 | 137.5 | 3771.9 | 50.3 | 115.3 | 53.6 | 37.4 | 83.5 | 101.7 | 130.2 | 261.3 | 46.7 | 0.55 |
| (mg/kg b.w) | 0.9 | 2.1 | 0.1 | 0.4 | 0.5 | 0.7 | 14.9 | 0.3 | 0.4 | 0.3 | 0.11 | 0.2 | 0.4 | 0.5 | 1.0 | 0.3 | 0.00 |
| C (mg) | 275.4 | 785.6 | 21.2 | 38.0 | 63.9 | 135.1 | 5225.0 | 97.0 | 85.9 | 61.3 | 8.4 | 42.3 | 77.8 | 111.4 | 299.5 | 69.2 | 0.98 |
| (%RDA) | 494.3 | 1261.3 | 42.4 | 76.1 | 141.2 | 262.0 | 8038.5 | 185.9 | 155.4 | 123.5 | 16.9 | 83.7 | 130.9 | 169.5 | 598.9 | 85.8 | 0.63 |
| (mg/kg b.w) | 12.9 | 29.2 | 0.9 | 2.1 | 3.16 | 6.3 | 148.0 | 4.2 | 2.5 | 2.2 | 0.2 | 1.2 | 1.9 | 3.2 | 9.5 | 2.0 | 0.00 |
| A (ug) | 1826.8 | 4472.3 | 271.7 | 606.9 | 793.1 | 1268.8 | 31,278.2 | 661.8 | 946.9 | 681.7 | 195.1 | 440.9 | 764.1 | 1154.6 | 2818.9 | 713.7 | 0.18 |
| (%RDA) | 327.1 | 643.8 | 54.3 | 120.8 | 166.3 | 298.5 | 4468.3 | 177.8 | 171.5 | 119.5 | 32.5 | 68.0 | 149.9 | 265.5 | 469.8 | 197.5 | 0.12 |
| (ug/kg b.w) | 81.8 | 132.4 | 12.2 | 30.3 | 39.9 | 80.8 | 886.1 | 50.5 | 28.3 | 23.0 | 4.8 | 10.6 | 21.8 | 38.5 | 92.9 | 27.8 | 0.00 |
| E (mg) | 35.5 | 111.4 | 2.2 | 3.8 | 6.1 | 12.8 | 753.2 | 8.9 | 6.0 | 2.5 | 2.5 | 4.6 | 5.5 | 7.1 | 12.1 | 2.4 | 0.58 |
| (%AI) | 503.9 | 1594.2 | 34.2 | 56.5 | 83.6 | 154.8 | 10,760.5 | 98.3 | 79.0 | 35.6 | 31.2 | 55.8 | 73.5 | 95.1 | 170.5 | 39.3 | 0.22 |
| (mg/kg b.w) | 1.9 | 0.3 | 0.1 | 0.2 | 0.3 | 0.5 | 29.5 | 0.3 | 0.2 | 0.1 | 0.03 | 0.1 | 0.2 | 0.2 | 0.4 | 0.1 | 0.00 |
| B6 (mg) | 2.8 | 7.6 | 0.6 | 1.02 | 1.5 | 2.1 | 54.7 | 1.0 | 1.4 | 0.5 | 0.8 | 1.1 | 1.3 | 1.5 | 3.2 | 0.4 | 0.36 |
| (%RDA) | 311.5 | 646.8 | 72.1 | 130.3 | 170.5 | 260.5 | 4558.8 | 130.2 | 142.1 | 60.1 | 67.5 | 102.1 | 122.2 | 162.2 | 279.8 | 60.1 | 0.01 |
| (mg/kg b.w) | 0.1 | 0.2 | 0.02 | 0.05 | 0.1 | 0.1 | 1.5 | 0.05 | 0.04 | 0.02 | 0.01 | 0.03 | 0.04 | 0.05 | 0.1 | 0.02 | 0.00 |
| B12 (ug) | 8.4 | 36.5 | 0.5 | 1.9 | 2.4 | 2.9 | 256.4 | 1.0 | 2.2 | 1.0 | 1.0 | 1.5 | 1.9 | 2.7 | 5.3 | 1.2 | 0.10 |
| (%RDA) | 429.7 | 1514.4 | 30.1 | 96.9 | 144.9 | 265.2 | 10,684.5 | 168.3 | 122.6 | 58.9 | 41.2 | 74.2 | 114.9 | 169.3 | 222.9 | 95.1 | 0.02 |
| (ug/kg b.w) | 0.3 | 1.0 | 0.03 | 0.1 | 0.1 | 0.2 | 7.3 | 0.1 | 0.1 | 0.04 | 0.02 | 0.04 | 0.05 | 0.09 | 0.2 | 0.05 | 0.00 |
| D (ug) | 47.5 | 86.1 | 0.5 | 12.9 | 28.0 | 51.6 | 511.1 | 38.7 | 2.1 | 1.8 | 0.6 | 1.2 | 1.8 | 2.09 | 9.4 | 0.9 | 0.00 |
| (%RDA) | 316.6 | 574.1 | 3.0 | 86.1 | 186.8 | 344.3 | 3407.3 | 258.2 | 14.0 | 12.3 | 3.8 | 8.1 | 12.4 | 13.9 | 62.5 | 5.9 | 0.00 |
| (ug/kg b.w) | 2.3 | 3.1 | 0.02 | 0.9 | 1.6 | 2.6 | 16.2 | 1.9 | 0.1 | 0.05 | 0.01 | 0.04 | 0.05 | 0.1 | 0.2 | 0.03 | 0.00 |
| Folate (ug) | 247.6 | 163.8 | 96.6 | 152.9 | 203.8 | 265.1 | 971.7 | 112.1 | 218.3 | 91.3 | 115.7 | 161.1 | 190.5 | 244.1 | 452.1 | 83.0 | 0.72 |
| (%RDA) | 95.9 | 54.1 | 37.2 | 63.5 | 76.9 | 116.3 | 248.6 | 52.8 | 69.9 | 29.2 | 28.9 | 46.8 | 63.3 | 80.2 | 150.7 | 33.4 | 0.05 |
| (ug/kg b.w) | 12.4 | 7.4 | 3.9 | 7.4 | 9.8 | 14.6 | 36.8 | 7.2 | 6.3 | 3.1 | 1.6 | 4.5 | 5.9 | 8.1 | 14.3 | 3.6 | 0.00 |
| Other | |||||||||||||||||
| Glucose (g) | 7.5 | 5.2 | 1.1 | 3.9 | 5.8 | 9.4 | 23.9 | 5.4 | 7.3 | 4.7 | 0.8 | 4.5 | 6.6 | 8.8 | 22.4 | 4.3 | 0.89 |
| Saccharose (g) | 44.1 | 25.3 | 4.5 | 28.7 | 39.2 | 51.8 | 139.8 | 23.0 | 50.8 | 16.8 | 17.1 | 41.3 | 52.4 | 61.2 | 93.6 | 19.9 | 0.04 |
| (g/kg b.w) | 2.3 | 1.4 | 0.1 | 1.4 | 2.2 | 2.9 | 6.2 | 1.5 | 1.5 | 0.8 | 0.4 | 1.0 | 1.2 | 2.0 | 3.2 | 1.0 | 0.02 |
| Fructose (g) | 9.8 | 6.8 | 0.9 | 4.8 | 8.2 | 12.9 | 28.7 | 8.1 | 10.9 | 8.1 | 0.7 | 5.9 | 9.3 | 14.3 | 36.6 | 8.4 | 0.57 |
| Lactose (g) | 8.4 | 7.9 | 0.2 | 2.7 | 6.0 | 10.3 | 32.4 | 7.6 | 8.9 | 5.5 | 0.6 | 4.9 | 7.7 | 12.9 | 21.3 | 7.9 | 0.40 |
| Starch (g) | 75.6 | 35.6 | 6.6 | 58.3 | 72.1 | 91.8 | 189.5 | 33.5 | 125.0 | 37.5 | 48.9 | 102.5 | 132.3 | 139.0 | 213.7 | 36.5 | 0.00 |
| Dietary fiber (g) | 13.2 | 5.6 | 5.4 | 9.8 | 11.7 | 16.1 | 29.3 | 6.2 | 16.7 | 4.9 | 8.6 | 13.5 | 16.4 | 20.8 | 26.8 | 7.3 | 0.00 |
| Dietary fiber (%AI) | 86.9 | 32.8 | 34.6 | 63.9 | 84.6 | 103.4 | 183.2 | 39.5 | 96.6 | 31.6 | 47.3 | 72.5 | 107.1 | 112.9 | 167.5 | 40.5 | 0.20 |
| (g/kg b.w) | 0.7 | 0.3 | 0.2 | 0.4 | 0.6 | 0.8 | 1.7 | 0.3 | 0.5 | 0.2 | 0.1 | 0.3 | 0.4 | 0.6 | 1.0 | 0.2 | 0.01 |
| Cholesterol (mg) | 234.8 | 135.0 | 19.7 | 155.2 | 209.9 | 277.2 | 749.8 | 121.9 | 196.6 | 84.5 | 64.2 | 138.8 | 177.1 | 264.1 | 374.5 | 125.3 | 0.33 |
| (mg/kg b.w) | 12.8 | 8.9 | 0.6 | 6.9 | 9.6 | 17.6 | 42.6 | 10.7 | 5.8 | 3.3 | 1.6 | 3.2 | 5.1 | 7.9 | 12.6 | 4.7 | 0.00 |
| SFA (g) | 18.9 | 11.4 | 6.4 | 12.3 | 15.7 | 23.1 | 63.4 | 10.8 | 17.3 | 7.3 | 8.1 | 12.1 | 15.3 | 21.2 | 36.3 | 9.1 | 0.73 |
| SFA (%AI) | 132.4 | 81.9 | 40.5 | 85.4 | 116.9 | 140.6 | 506.9 | 55.3 | 120.5 | 52.4 | 64.6 | 83.6 | 99.3 | 143.4 | 283.9 | 59.8 | 0.81 |
| (g/kg b.w) | 0.9 | 0.5 | 0.2 | 0.6 | 0.9 | 1.2 | 2.6 | 0.5 | 0.5 | 0.2 | 0.1 | 0.4 | 0.5 | 0.7 | 1.0 | 0.3 | 0.00 |
| MUFA (g) | 14.5 | 7.6 | 0.8 | 10.2 | 13.8 | 18.9 | 50.7 | 8.8 | 16.7 | 5.7 | 6.8 | 12.2 | 16.4 | 19.9 | 27.9 | 7.7 | 0.11 |
| 18:2 | 3.8 | 2.2 | 0.2 | 2.5 | 3.2 | 4.6 | 13.2 | 2.1 | 5.8 | 2.7 | 2.5 | 3.3 | 5.5 | 7.5 | 10.7 | 4.1 | 0.00 |
| 18:3 | 0.8 | 0.5 | 0.1 | 0.4 | 0.6 | 0.8 | 2.6 | 0.4 | 0.9 | 0.6 | 0.4 | 0.5 | 0.7 | 1.3 | 2.5 | 0.8 | 0.16 |
| PUFA (g) | 5.1 | 2.7 | 1.4 | 3.2 | 4.3 | 6.7 | 14.9 | 3.5 | 7.2 | 3.3 | 3.6 | 4.3 | 6.4 | 9.1 | 12.8 | 4.7 | 0.00 |
| n-3 | 0.9 | 0.8 | 0.2 | 0.5 | 0.8 | 1.2 | 4.6 | 0.7 | 1.2 | 0.8 | 0.4 | 0.6 | 0.9 | 1.6 | 3.5 | 1.0 | 0.20 |
| n-6 | 3.9 | 2.2 | 0.2 | 2.5 | 3.3 | 4.7 | 13.2 | 2.2 | 5.9 | 2.7 | 2.6 | 3.4 | 5.5 | 7.5 | 10.7 | 4.1 | 0.00 |
| Salt (g) | 5.6 | 2.6 | 1.9 | 4.2 | 5.1 | 6.5 | 16.4 | 2.3 | 6.0 | 1.6 | 3.6 | 4.9 | 5.9 | 7.0 | 9.3 | 2.2 | 0.15 |
| Water (mL) | 1361.4 | 499.6 | 624.8 | 999.2 | 1243.7 | 1550.8 | 3129.2 | 551.7 | 1434.8 | 520.2 | 599.9 | 1069.4 | 1383.8 | 1866.7 | 2359.5 | 797.2 | 0.48 |
| (%AI) | 81.7 | 26.6 | 39.0 | 64.5 | 75.8 | 95.5 | 164.7 | 30.9 | 78.5 | 28.9 | 31.8 | 56.5 | 74.9 | 105.4 | 121.0 | 48.9 | 0.83 |
| (ml/kg b.w) | 69.9 | 28.5 | 26.4 | 50.6 | 62.4 | 93.5 | 134.9 | 42.9 | 42.0 | 20.2 | 13.2 | 21.8 | 44.6 | 56.6 | 92.0 | 34.8 | 0.00 |
| %E from P | 16.7 | 2.6 | 10.3 | 15.1 | 16.6 | 18.6 | 22.1 | 3.5 | 15.0 | 2.0 | 10.8 | 13.4 | 15.6 | 16.2 | 18.4 | 2.8 | 0.02 |
| %E from F | 29.3 | 6.7 | 11.9 | 24.4 | 28.4 | 34.5 | 50.2 | 10.2 | 24.9 | 5.6 | 12.9 | 21.7 | 24.7 | 28.6 | 34.8 | 6.9 | 0.01 |
| %E from C | 52.1 | 7.2 | 33.7 | 46.7 | 51.4 | 56.9 | 73.4 | 10.2 | 58.0 | 5.4 | 47.3 | 54.2 | 57.9 | 61.1 | 71.7 | 6.9 | 0.00 |
| Nutrient [Type of Norms] | Study Group (Implementation of the Norm) | Control Group (Implementation of the Norm) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Energy (%EAR) | 13 | 26.5 | 7 | 31.8 |
| Protein (%RDA) | 46 | 93.9 | 15 | 68.2 |
| Fat (%RDA) | 9 | 18.4 | 1 | 4.5 |
| Sodium (%AI) | 48 | 97.9 | 21 | 95.4 |
| Potassium (%AI) | 36 | 73.5 | 7 | 31.8 |
| Calcium (%RDA) | 3 | 6.1 | 1 | 4.5 |
| Phosphosus (%RDA) | 35 | 71.4 | 10 | 45.4 |
| Magnesium (%RDA) | 33 | 67.3 | 10 | 45.4 |
| Iron (%RDA) | 15 | 30.6 | 4 | 18.2 |
| Zinc (%RDA) | 30 | 61.2 | 8 | 36.4 |
| Copper (%RDA) | 35 | 71.4 | 17 | 77.3 |
| Manganese (%AI) | 44 | 89.8 | 20 | 90.9 |
| B1 (%RDA) | 32 | 65.3 | 9 | 40.9 |
| B2 (%RDA) | 49 | 100.0 | 16 | 72.7 |
| Niacin (%RDA) | 30 | 61.2 | 13 | 59.1 |
| C (%RDA) | 31 | 63.3 | 14 | 63.6 |
| A (%RDA) | 47 | 95.9 | 13 | 59.1 |
| E (%AI) | 20 | 40.8 | 13 | 59.1 |
| B6 (%RDA) | 42 | 85.7 | 17 | 77.3 |
| B12 (%RDA) | 35 | 71.4 | 11 | 50.0 |
| D (%RDA) | 35 | 71.4 | 0 | 0.0 |
| Folates (%RDA) | 16 | 32.6 | 3 | 13.6 |
| Dietary fiber (%AI) | 17 | 34.7 | 11 | 50.0 |
| SFA (%AI) | 32 | 65.3 | 11 | 50.0 |
| Water (%AI) | 9 | 18.4 | 7 | 31.8 |
| Variables | Study Group | ||||||
|---|---|---|---|---|---|---|---|
| N (Important) | BMI z-Score | Arm Circumference | Muscle Mass | ||||
| R (Spearman) | p | R (Spearman) | p | R (Spearman) | p | ||
| Body mass | 49 | 0.57 | 0.00 | 0.82 | 0.00 | 0.76 | 0.00 |
| BMI z-score | 49 | - | - | 0.69 | 0.00 | 0.53 | 0.00 |
| Waist circumference | 49 | 0.67 | 0.00 | 0.79 | 0.00 | 0.72 | 0.00 |
| Hip circumference | 49 | 0.69 | 0.00 | 0.85 | 0.00 | 0.76 | 0.00 |
| Arm circumference | 49 | 0.69 | 0.00 | - | - | 0.92 | 0.00 |
| Muscle mass | 49 | 0.53 | 0.00 | 0.92 | 0.00 | - | - |
| Suprailiac skinfold | 49 | 0.66 | 0.00 | 0.63 | 0.00 | 0.59 | 0.00 |
| Subscapular skinfold | 49 | 0.71 | 0.00 | 0.77 | 0.00 | 0.70 | 0.00 |
| Triceps skinfold | 49 | 0.79 | 0.00 | 0.73 | 0.00 | 0.46 | 0.00 |
| % FAT | 49 | 0.79 | 0.00 | 0.79 | 0.00 | 0.61 | 0.00 |
| Variables | Control Study | ||||||
|---|---|---|---|---|---|---|---|
| N (Important) | BMI z-Score | Arm Circumference | Muscle Mass | ||||
| R (Spearman) | p | R (Spearman) | p | R (Spearman) | p | ||
| Body mass | 22 | 0.36 | 0.09 | 0.89 | 0.00 | 0.88 | 0.00 |
| BMI z-score | 22 | - | - | 0.63 | 0.00 | 0.32 | 0.14 |
| Waist circumference | 22 | 0.63 | 0.00 | 0.90 | 0.00 | 0.79 | 0.00 |
| Hip circumference | 22 | 0.33 | 0.12 | 0.87 | 0.00 | 0.88 | 0.00 |
| Arm circumference | 22 | 0.63 | 0.00 | - | - | 0.89 | 0.00 |
| Muscle mass | 22 | 0.32 | 0.14 | 0.89 | 0.00 | - | - |
| Suprailiac skinfold | 22 | 0.44 | 0.04 | 0.36 | 0.10 | 0.26 | 0.24 |
| Subscapular skinfold | 22 | 0.71 | 0.00 | 0.74 | 0.00 | 0.61 | 0.00 |
| Triceps skinfold | 22 | 0.85 | 0.00 | 0.67 | 0.00 | 0.32 | 0.14 |
| % FAT | 22 | 0.87 | 0.00 | 0.75 | 0.00 | 0.48 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czerwonogrodzka-Senczyna, A.; Milewska, M.; Kwiecień, P.; Szczałuba, K. Diet and Nutritional Status of Polish Girls with Rett Syndrome—A Case-Control Study. Nutrients 2023, 15, 3334. https://doi.org/10.3390/nu15153334
Czerwonogrodzka-Senczyna A, Milewska M, Kwiecień P, Szczałuba K. Diet and Nutritional Status of Polish Girls with Rett Syndrome—A Case-Control Study. Nutrients. 2023; 15(15):3334. https://doi.org/10.3390/nu15153334
Chicago/Turabian StyleCzerwonogrodzka-Senczyna, Aneta, Magdalena Milewska, Paweł Kwiecień, and Krzysztof Szczałuba. 2023. "Diet and Nutritional Status of Polish Girls with Rett Syndrome—A Case-Control Study" Nutrients 15, no. 15: 3334. https://doi.org/10.3390/nu15153334
APA StyleCzerwonogrodzka-Senczyna, A., Milewska, M., Kwiecień, P., & Szczałuba, K. (2023). Diet and Nutritional Status of Polish Girls with Rett Syndrome—A Case-Control Study. Nutrients, 15(15), 3334. https://doi.org/10.3390/nu15153334







