Impact of Preparticipating Hypohydration on Cardiopulmonary Exercise Capacity in Ambitious Recreational Athletes
Abstract
:1. Background
2. Materials and Methods
3. Statistical Analysis
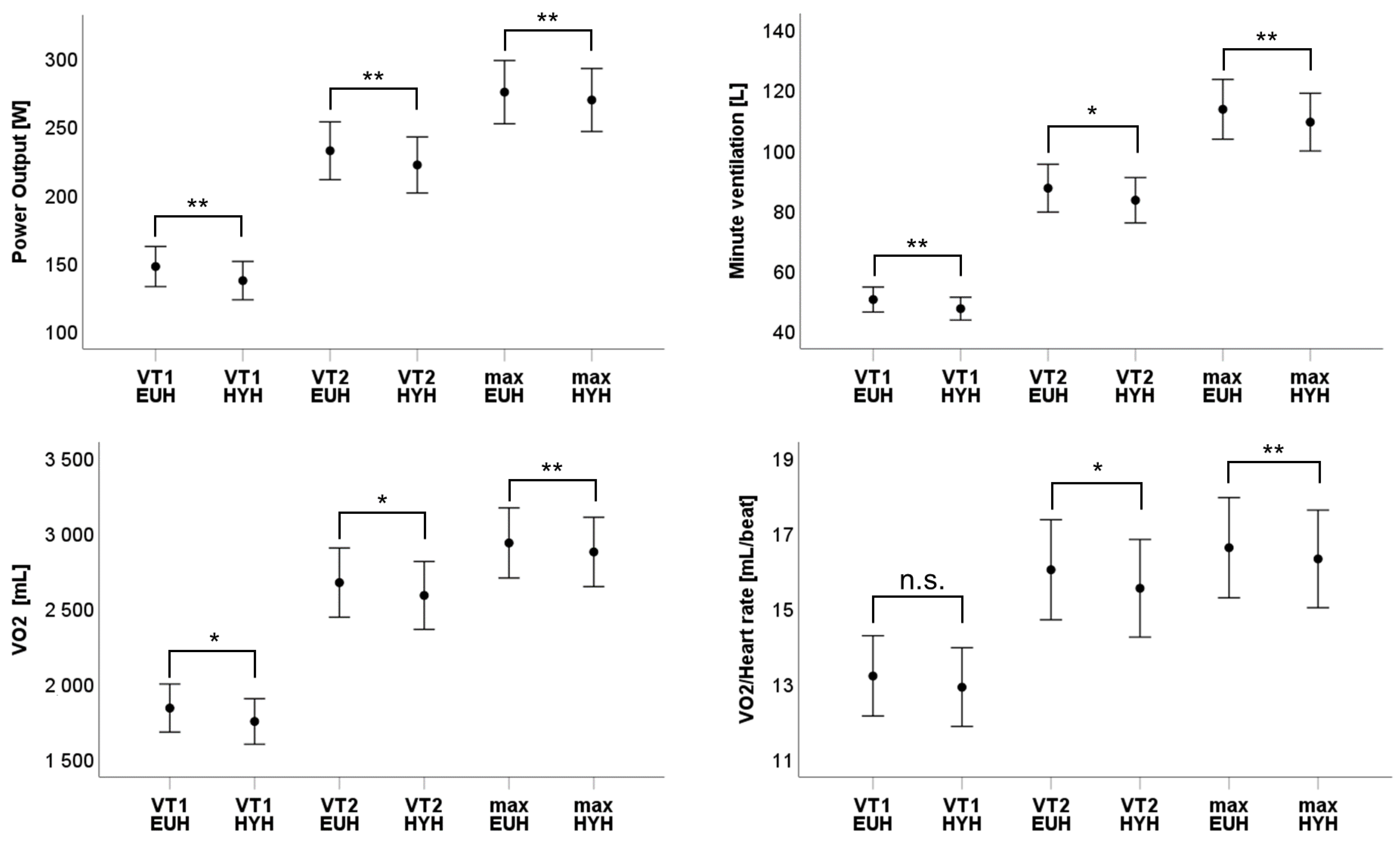
4. Results
5. Discussion
- -
- A loss of about 2 % of body water after 12 h of a standardised fluid deprivation test;
- -
- A significant reduction of ventilation (caused by a significant reduction of tidal volume at ventilatory threshold 1 and a significant reduction of breathing rate at maximum power);
- -
- A delayed increase in heart rate (with a significant reduction of heart rate at first ventilatory threshold) with a significant reduction of oxygen pulse (at second ventilatory threshold and maximum power);
- -
- A resulting significant reduction of oxygen uptake and power output (as well as at both ventilatory thresholds as maximum power).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nybo, L.; Rasmussen, P.; Sawka, M.N. Performance in the heat-physiological factors of importance for hyperthermia-induced fatigue. Compr. Physiol. 2014, 4, 657–689. [Google Scholar] [CrossRef]
- Racinais, S.; Alonso, J.M.; Coutts, A.J.; Flouris, A.D.; Girard, O.; Gonzalez-Alonso, J.; Hausswirth, C.; Jay, O.; Lee, J.K.; Mitchell, N.; et al. Consensus recommendations on training and competing in the heat. Br. J. Sports Med. 2015, 49, 1164–1173. [Google Scholar] [CrossRef] [Green Version]
- Racinais, S.; Alonso, J.M.; Coutts, A.J.; Flouris, A.D.; Girard, O.; Gonzalez-Alonso, J.; Hausswirth, C.; Jay, O.; Lee, J.K.; Mitchell, N.; et al. Consensus recommendations on training and competing in the heat. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. S1), 6–19. [Google Scholar] [CrossRef] [Green Version]
- Galloway, S.D.R.; Maughan, R.J. Effects of ambient temperature on the capacity to perform prolonged cycle exercise in man. Med. Sci. Sports Exerc. 1997, 29, 1240–1249. [Google Scholar] [CrossRef]
- Periard, J.D.; Cramer, M.N.; Chapman, P.G.; Caillaud, C.; Thompson, M.W. Cardiovascular strain impairs prolonged self-paced exercise in the heat. Exp. Physiol. 2011, 96, 134–144. [Google Scholar] [CrossRef]
- Rowell, L.B. Human cardiovascular adjustments to exercise and thermal stress. Physiol. Rev. 1974, 54, 75–159. [Google Scholar] [CrossRef]
- Ely, M.R.; Cheuvront, S.N.; Roberts, W.O.; Montain, S.J. Impact of weather on marathon-running performance. Med. Sci. Sports Exerc. 2007, 39, 487–493. [Google Scholar] [CrossRef]
- Morante, S.M.; Brotherhood, J.R. Autonomic and behavioural thermoregulation in tennis. Br. J. Sports Med. 2008, 42, 679–685; discussion 685. [Google Scholar] [CrossRef]
- Mohr, M.; Nybo, L.; Grantham, J.; Racinais, S. Physiological responses and physical performance during football in the heat. PLoS ONE 2012, 7, e39202. [Google Scholar] [CrossRef]
- Nassis, G.P.; Brito, J.; Dvorak, J.; Chalabi, H.; Racinais, S. The association of environmental heat stress with performance: Analysis of the 2014 FIFA World Cup Brazil. Br. J. Sports Med. 2015, 49, 609–613. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Stohr, E.J.; Akiyama, K.; Watanabe, S.; Gonzalez-Alonso, J. Dehydration reduces stroke volume and cardiac output during exercise because of impaired cardiac filling and venous return, not left ventricular function. Physiol. Rep. 2020, 8, e14433. [Google Scholar] [CrossRef]
- Gonzalez-Alonso, J.; Mora-Rodriguez, R.; Below, P.R.; Coyle, E.F. Dehydration reduces cardiac output and increases systemic and cutaneous vascular resistance during exercise. J. Appl. Physiol. 1995, 79, 1487–1496. [Google Scholar] [CrossRef]
- Logan-Sprenger, H.M.; Heigenhauser, G.J.; Jones, G.L.; Spriet, L.L. The effect of dehydration on muscle metabolism and time trial performance during prolonged cycling in males. Physiol. Rep. 2015, 3, e12483. [Google Scholar] [CrossRef]
- Campa, F.; Piras, A.; Raffi, M.; Trofe, A.; Perazzolo, M.; Mascherini, G.; Toselli, S. The Effects of Dehydration on Metabolic and Neuromuscular Functionality During Cycling. Int. J. Environ. Res. Public Health 2020, 17, 1161. [Google Scholar] [CrossRef] [Green Version]
- Hargreaves, M.; Dillo, P.; Angus, D.; Febbraio, M. Effect of fluid ingestion on muscle metabolism during prolonged exercise. J. Appl. Physiol. 1996, 80, 363–366. [Google Scholar] [CrossRef]
- Berry, C.W.; Wolf, S.T.; Cottle, R.M.; Kenney, W.L. Hydration Is More Important Than Exogenous Carbohydrate Intake during Push-to-the-Finish Cycle Exercise in the Heat. Front. Sports Act. Living 2021, 3, 742710. [Google Scholar] [CrossRef]
- Schuttler, D.; Weckbach, L.T.; Hamm, W.; Maier, F.; Kassem, S.; Schier, J.; Lackermair, K.; Brunner, S. Effect of acute altitude exposure on ventilatory thresholds in recreational athletes. Respir. Physiol. Neurobiol. 2021, 293, 103723. [Google Scholar] [CrossRef]
- Kellnar, A.; Hoppe, J.M.; Brunner, S.; Stremmel, C. Hospitalization for COVID-19 is associated with significant changes in body composition. Clin. Nutr. ESPEN 2021, 45, 499–502. [Google Scholar] [CrossRef]
- Gonzalez-Alonso, J.; Calbet, J.A.L.; Mora-Rodriguez, R.; Kippelen, P. Pulmonary ventilation and gas exchange during prolonged exercise in humans: Influence of dehydration, hyperthermia and sympathoadrenal activity. Exp. Physiol. 2023, 108, 188–206. [Google Scholar] [CrossRef]
- Marshall, H.; Gibson, O.R.; Romer, L.M.; Illidi, C.; Hull, J.H.; Kippelen, P. Systemic but not local rehydration restores dehydration-induced changes in pulmonary function in healthy adults. J. Appl. Physiol. 2021, 130, 517–527. [Google Scholar] [CrossRef]
- Adams, W.M.; Ferraro, E.M.; Huggins, R.A.; Casa, D.J. Influence of body mass loss on changes in heart rate during exercise in the heat: A systematic review. J. Strength Cond. Res. 2014, 28, 2380–2389. [Google Scholar] [CrossRef]
- Herdy, A.H.; Ritt, L.E.; Stein, R.; Araujo, C.G.; Milani, M.; Meneghelo, R.S.; Ferraz, A.S.; Hossri, C.; Almeida, A.E.; Fernandes-Silva, M.M.; et al. Cardiopulmonary Exercise Test: Background, Applicability and Interpretation. Arq. Bras. Cardiol. 2016, 107, 467–481. [Google Scholar] [CrossRef]
- Crisafulli, A.; Piras, F.; Chiappori, P.; Vitelli, S.; Caria, M.A.; Lobina, A.; Milia, R.; Tocco, F.; Concu, A.; Melis, F. Estimating stroke volume from oxygen pulse during exercise. Physiol. Meas. 2007, 28, 1201–1212. [Google Scholar] [CrossRef]
- Kinnear, W.; Blakey, J.; Kinnear, W.; Blakey, J. 2829 Oxygen pulse. In A Practical Guide to the Interpretation of Cardio-Pulmonary Exercise Tests; Oxford University Press: Oxford, UK, 2014. [Google Scholar] [CrossRef]
- Adams, J.D.; Scott, D.M.; Brand, N.A.; Suh, H.G.; Seal, A.D.; McDermott, B.P.; Ganio, M.S.; Kavouras, S.A. Mild hypohydration impairs cycle ergometry performance in the heat: A blinded study. Scand. J. Med. Sci. Sports 2019, 29, 686–695. [Google Scholar] [CrossRef]
- Deshayes, T.A.; Pancrate, T.; Goulet, E.D.B. Impact of dehydration on perceived exertion during endurance exercise: A systematic review with meta-analysis. J. Exerc. Sci. Fit 2022, 20, 224–235. [Google Scholar] [CrossRef]
- Sjostrom, A.; Bartuseviciene, I.; Hoybye, C. Simplified and improved fluid deprivation test for diagnosing diabetes insipidus. Eur. J. Endocrinol. 2021, 184, 123–131. [Google Scholar] [CrossRef]
- Trimpou, P.; Olsson, D.S.; Ehn, O.; Ragnarsson, O. Diagnostic value of the water deprivation test in the polyuria-polydipsia syndrome. Hormones (Athens) 2017, 16, 414–422. [Google Scholar] [CrossRef] [Green Version]
- Minshull, C.; James, L. The effects of hypohydration and fatigue on neuromuscular activation performance. Appl. Physiol. Nutr. Metab. 2013, 38, 21–26. [Google Scholar] [CrossRef]
- Zubac, D.; Simunic, B.; Buoite Stella, A.; Morrison, S.A. Neuromuscular performance after rapid weight loss in Olympic-style boxers. Eur J Sport Sci 2020, 20, 1051–1060. [Google Scholar] [CrossRef]
- Cheuvront, S.N.; Carter, R., 3rd; Sawka, M.N. Fluid balance and endurance exercise performance. Curr. Sports Med. Rep. 2003, 2, 202–208. [Google Scholar] [CrossRef]
- Shirreffs, S.M. Conference on "Multidisciplinary approaches to nutritional problems". Symposium on "Performance, exercise and health". Hydration, fluids and performance. Proc. Nutr. Soc. 2009, 68, 17–22. [Google Scholar] [CrossRef]
- McConell, G.K.; Burge, C.M.; Skinner, S.L.; Hargreaves, M. Influence of ingested fluid volume on physiological responses during prolonged exercise. Acta Physiol. Scand. 1997, 160, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Fallowfield, J.L.; Williams, C.; Booth, J.; Choo, B.H.; Growns, S. Effect of water ingestion on endurance capacity during prolonged running. J. Sports Sci. 1996, 14, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Bachle, L.; Eckerson, J.; Albertson, L.; Ebersole, K.; Goodwin, J.; Petzel, D. The effect of fluid replacement on endurance performance. J. Strength Cond. Res. 2001, 15, 217–224. [Google Scholar]
- McConell, G.K.; Stephens, T.J.; Canny, B.J. Fluid ingestion does not influence intense 1-h exercise performance in a mild environment. Med. Sci. Sports Exerc. 1999, 31, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.A.; Hawley, J.A.; Palmer, G.S.; Wilson, G.R.; Gray, D.A.; Noakes, T.D.; Dennis, S.C. Water ingestion does not improve 1-h cycling performance in moderate ambient temperatures. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 71, 153–160. [Google Scholar] [CrossRef]
- Adams, J.D.; Sekiguchi, Y.; Suh, H.G.; Seal, A.D.; Sprong, C.A.; Kirkland, T.W.; Kavouras, S.A. Dehydration Impairs Cycling Performance, Independently of Thirst: A Blinded Study. Med. Sci. Sports Exerc. 2018, 50, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.S.; McGarr, G.W.; Mallette, M.M.; Wallace, P.J.; Watson, C.L.; Kim, I.M.; Greenway, M.J. Separate and combined effects of dehydration and thirst sensation on exercise performance in the heat. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. 1), 104–111. [Google Scholar] [CrossRef]
- Sawka, M.N.; Noakes, T.D. Does dehydration impair exercise performance? Med. Sci. Sports Exerc. 2007, 39, 1209–1217. [Google Scholar] [CrossRef]
| Euhydrated [EUH] | Hypohydrated [HYH] | p-Value | |
|---|---|---|---|
| Blood | |||
| Na+ [mmol/L] | 139.4 [138.9; 140] | 139.9 [136; 144] | 0.2 |
| K+ [mmol/L] | 4.2 [4.1: 4.3] | 4.2 [4.1; 4.3] | 0.5 |
| Cl− [mmol/L] | 102.2 [101.6; 102.8] | 103 [102.5; 103.5] | 0.05 |
| Mg2+ [mmol/L] | 0.81 [0.8; 0.83] | 0.83 [0.82; 0.85] | 0.1 |
| Ca2+ [mmol/L] | 2.4 [2.4; 2.5] | 2.4 [2.4; 2.4] | 0.2 |
| osmolality [mosm/kg] | 289 [288; 290] | 291 [290; 291] | 0.06 |
| creatinine [mg/dL] | 0.94 [0.89; 1] | 0.91 [0.86; 0.96] | 0.04 |
| hematocrit | 0.42 [0.41; 0.43] | 0.42 [0.41; 0.43] | 0.6 |
| Urine | |||
| Na+ [mmol/L] | 94.4 [76.3; 112.5] | 143.6 [128.3; 159] | <0.01 |
| osmolality [mosm/kg] | 537 [445; 629] | 767 [694; 839] | <0.01 |
| specific weight [kg/L] | 1.017 [1.016; 1.019] | 1.021 [1.020; 1.023] | <0.01 |
| Euhydrated [EUH] | Hypohydrated [HYH] | Δ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| @ VT1 | @ VT2 | @ V02 max | @ VT1 | @ VT2 | @ V02 Max | @ VT1 | @ VT2 | @ V02 Max | |
| Power output [W] | 148 [133; 163] | 233 [212; 254] | 276 [253; 299] | 138 [124; 152] | 222 [202; 243] | 270 [247; 293] | −6.8% p < 0.01 | −4.7% p < 0.01 | −2.2% p = 0.01 |
| Heart rate [min−1] | 140 [135; 144] | 167 [163; 171] | 177 [174; 180] | 136 [131; 141] | 166 [162; 170] | 176 [173; 180] | −2.3% p = 0.04 | −0.1% p = 0.8 | −0.4% p = 0.4 |
| Minute ventilation [L] | 50.9 [46.7; 55.1] | 88.1 [80.1; 96.2] | 114.4 [104.4; 124.5] | 48 [44.1; 51.8] | 84.1 [76.6; 91.8] | 110.5 [100.9; 120] | −4% p = 0.01 | −3.1% p = 0.03 | −3.3% p < 0.01 |
| VO2 [mL] | 1858 [1697; 2019] | 2695 [2463; 2927] | 2958 [2723; 3193] | 1769 [1616; 1922] | 2609 [2381; 2837] | 2900 [2667; 3131] | −4.8% p = 0.02 | −3.2% p = 0.03 | −2.0% p < 0.01 |
| VO2 [mLxkg1xmin−1] | 25.5 [23.8; 27.3] | 36.9 [34.5; 39.3] | 41.1 [38.7; 43.4] | 24.6 [22.8; 26.4] | 36 [33.5; 38.5] | 40.2 [37.9; 42.6] | −3.5% p = 0.07 | −2.4% p = 0.1 | −1.9% p = 0.02 |
| VO2/Heart rate [mL/beat] | 13.2 [12.2; 14.2] | 16.1 [14.7; 17.4] | 16.6 [15.3; 18] | 12.9 [11.9; 14] | 15.6 [14.3; 16.9] | 16.3 [15; 17.6] | −2.3% p = 0.1 | −3.1% p = 0.02 | −1.8% p < 0.01 |
| Breathing rate [min−1] | 26 [25; 28] | 35 [33; 37] | 43 [40; 46] | 26 [24; 27] | 34 [32; 36] | 42 [39; 44] | −2.4% p = 0.2 | −2.5% p = 0.1 | −3.5% p = 0.03 |
| Tidal volume [mL] | 1987 [1815; 2159] | 2526 [2336; 2715] | 2670 [2482; 2857] | 1896 [1750; 2042] | 2482 [2294; 2668] | 2657 [2467; 2846] | −4.6% p = 0.02 | −1.7% p = 0.3 | −0.5% p = 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strüven, A.; Brunner, S.; Weis, G.; Stremmel, C.; Teupser, D.; Schlichtiger, J.; Lackermair, K. Impact of Preparticipating Hypohydration on Cardiopulmonary Exercise Capacity in Ambitious Recreational Athletes. Nutrients 2023, 15, 3333. https://doi.org/10.3390/nu15153333
Strüven A, Brunner S, Weis G, Stremmel C, Teupser D, Schlichtiger J, Lackermair K. Impact of Preparticipating Hypohydration on Cardiopulmonary Exercise Capacity in Ambitious Recreational Athletes. Nutrients. 2023; 15(15):3333. https://doi.org/10.3390/nu15153333
Chicago/Turabian StyleStrüven, Anna, Stefan Brunner, Georges Weis, Christopher Stremmel, Daniel Teupser, Jenny Schlichtiger, and Korbinian Lackermair. 2023. "Impact of Preparticipating Hypohydration on Cardiopulmonary Exercise Capacity in Ambitious Recreational Athletes" Nutrients 15, no. 15: 3333. https://doi.org/10.3390/nu15153333
APA StyleStrüven, A., Brunner, S., Weis, G., Stremmel, C., Teupser, D., Schlichtiger, J., & Lackermair, K. (2023). Impact of Preparticipating Hypohydration on Cardiopulmonary Exercise Capacity in Ambitious Recreational Athletes. Nutrients, 15(15), 3333. https://doi.org/10.3390/nu15153333






