Dietary and Serum Antioxidants Associated with Prostate-Specific Antigen for Middle-Aged and Older Men
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection and Measurements
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Webber, M.M.; Waghray, A.; Bello, D. Prostate-specific antigen, a serine protease, facilitates human prostate cancer cell invasion. Clin. Cancer Res. 1995, 1, 1089–1094. [Google Scholar]
- American Cancer Society: Screening Tests for Prostate Cancer. Available online: https://www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/tests.html#:~:text=Men%20with%20a%20PSA%20level,prostate%20cancer%20is%20over%2050%25 (accessed on 27 April 2023).
- Minciullo, P.L.; Inferrera, A.; Navarra, M.; Calapai, G.; Magno, C.; Gangemi, S. Oxidative stress in benign prostatic hyperplasia: A systematic review. Urol. Int. 2015, 94, 249–254. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Allkanjari, O.; Busetto, G.M.; Cai, T.; Largana, G.; Magri, V.; Perletti, G.; Robustelli Della Cuna, F.S.; Russo, G.I.; Stamatiou, K.; et al. Nutraceutical treatment and prevention of benign prostatic hyperplasia and prostate cancer. Arch. Ital. Urol. Androl. 2019, 91, 139–152. [Google Scholar] [CrossRef]
- Reza, H.S.; Ali, Z.; Tara, H.; Ali, B. Age-specific reference ranges of prostate-specific antigen in the elderly of Amirkola: A population-based study. Asian J. Urol. 2021, 8, 183–188. [Google Scholar] [CrossRef]
- Chen, C.H.; Yao, H.H.; Huang, S.W.; Chuang, C.K.; Hsu, H.S.; Wang, C.J.; Pu, Y.S. Using age-referenced prostate-specific antigen percentile to predict survival outcomes in screened Taiwanese men. Int. J. Cancer 2013, 132, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Safarinejad, M.R. Population-based screening for prostate cancer by measuring free and total serum prostate-specific antigen in Iran. Ann. Oncol. 2006, 17, 1166–1171. [Google Scholar] [CrossRef]
- He, D.; Wang, M.; Chen, X.; Gao, Z.; He, H.; Zhau, H.E.; Wang, W.; Chung, L.W.; Nan, X. Ethnic differences in distribution of serum prostate-specific antigen: A study in a healthy Chinese male population. Urology 2004, 63, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Salinas, C.A.; Tsodikov, A.; Ishak-Howard, M.; Cooney, K.A. Prostate cancer in young men: An important clinical entity. Nat. Rev. Urol. 2014, 11, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Bleyer, A.; Spreafico, F.; Barr, R. Prostate cancer in young men: An emerging young adult and older adolescent challenge. Cancer 2020, 126, 46–57. [Google Scholar] [CrossRef]
- Lin, D.W.; Porter, M.; Montgomery, B. Treatment and survival outcomes in young men diagnosed with prostate cancer: A Population-based Cohort Study. Cancer 2009, 115, 2863–2871. [Google Scholar] [CrossRef]
- Checconi, P.; De Angelis, M.; Marcocci, M.E.; Fraternale, A.; Magnani, M.; Palamara, A.T.; Nencioni, L. Redox-Modulating Agents in the Treatment of Viral Infections. Int. J. Mol. Sci. 2020, 21, 4084. [Google Scholar] [CrossRef]
- Tavassolifar, M.J.; Vodjgani, M.; Salehi, Z.; Izad, M. The Influence of Reactive Oxygen Species in the Immune System and Pathogenesis of Multiple Sclerosis. Autoimmune Dis. 2020, 2020, 5793817. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid. Med. Cell. Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Oxidative Stress, Diet and Prostate Cancer. World J. Men’s Health 2021, 39, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Udensi, U.K.; Tchounwou, P.B. Oxidative stress in prostate hyperplasia and carcinogenesis. J. Exp. Clin. Cancer Res. 2016, 35, 139. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Guarnido, O.; Urquiza-Salvat, N.; Saiz, M.; Lozano-Paniagua, D.; Rodrigo, L.; Pascual-Geler, M.; Lorente, J.A.; Alvarez-Cubero, M.J.; Rivas, A. Bioactive compounds of the Mediterranean diet and prostate cancer. Aging Male 2018, 21, 251–260. [Google Scholar] [CrossRef]
- Meyer, F.; Galan, P.; Douville, P.; Bairati, I.; Kegle, P.; Bertrais, S.; Estaquio, C.; Hercberg, S. Antioxidant vitamin and mineral supplementation and prostate cancer prevention in the SU.VI.MAX trial. Int. J. Cancer 2005, 116, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Willis, M.S.; Wians, F.H. The role of nutrition in preventing prostate cancer: A review of the proposed mechanism of action of various dietary substances. Clin. Chim. Acta 2003, 330, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Kaya, E.; Ozgok, Y.; Zor, M.; Eken, A.; Bedir, S.; Erdem, O.; Ebiloglu, T.; Ergin, G. Oxidative stress parameters in patients with prostate cancer, benign prostatic hyperplasia and asymptomatic inflammatory prostatitis: A prospective controlled study. Adv. Clin. Exp. Med. 2017, 26, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.; Martin, R.M.; Beynon, R.; Harris, R.; Savovic, J.; Zuccolo, L.; Bekkering, G.E.; Fraser, W.D.; Sterne, J.A.; Metcalfe, C. Associations of circulating and dietary vitamin D with prostate cancer risk: A systematic review and dose-response meta-analysis. Cancer Causes Control 2011, 22, 319–340. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.A.; Oliver, S.E.; Appleby, P.N.; Lentjes, M.A.; Emmett, P.; Kuh, D.; Stephen, A.; Brunner, E.J.; Shipley, M.J.; Hamdy, F.C.; et al. Prostate cancer risk related to foods, food groups, macronutrients and micronutrients derived from the UK Dietary Cohort Consortium food diaries. Eur. J. Clin. Nutr. 2017, 71, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Sarre, S.; Maattanen, L.; Tammela, T.L.; Auvinen, A.; Murtola, T.J. Postscreening follow-up of the Finnish Prostate Cancer Screening Trial on putative prostate cancer risk factors: Vitamin and mineral use, male pattern baldness, pubertal development and non-steroidal anti-inflammatory drug use. Scand. J. Urol. 2016, 50, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Moman, R.N.; Gupta, N.; Varacallo, M. Physiology, Albumin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.M.; et al. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef]
- Sejima, T.; Iwamoto, H.; Masago, T.; Morizane, S.; Yao, A.; Isoyama, T.; Kadowaki, H.; Takenaka, A. Low pre-operative levels of serum albumin predict lymph node metastases and ultimately correlate with a biochemical recurrence of prostate cancer in radical prostatectomy patients. Cent. Eur. J. Urol. 2013, 66, 126–132. [Google Scholar]
- Wang, Y.; Chen, W.; Hu, C.; Wen, X.; Pan, J.; Xu, F.; Zhu, Y.; Shao, X.; Shangguan, X.; Fan, L.; et al. Albumin and Fibrinogen Combined Prognostic Grade Predicts Prognosis of Patients with Prostate Cancer. J. Cancer 2017, 8, 3992–4001. [Google Scholar] [CrossRef]
- Sohlberg, E.M.; Thomas, I.C.; Yang, J.; Kapphahn, K.; Velaer, K.N.; Goldstein, M.K.; Wagner, T.H.; Chertow, G.M.; Brooks, J.D.; Patel, C.J.; et al. Laboratory-wide association study of survival with prostate cancer. Cancer 2021, 127, 1102–1113. [Google Scholar] [CrossRef]
- Xu, K.; Yan, Y.; Cheng, C.; Li, S.; Liao, Y.; Zeng, J.; Chen, Z.; Zhou, J. The relationship between serum albumin and prostate-specific antigen: A analysis of the National Health and Nutrition Examination Survey, 2003–2010. Front. Public Health 2023, 11, 1078280. [Google Scholar] [CrossRef]
- Arthur, R.; Williams, R.; Garmo, H.; Holmberg, L.; Stattin, P.; Malmstrom, H.; Lambe, M.; Hammar, N.; Walldius, G.; Robinsson, D.; et al. Serum inflammatory markers in relation to prostate cancer severity and death in the Swedish AMORIS study. Int. J. Cancer 2018, 142, 2254–2262. [Google Scholar] [CrossRef]
- Kristal, A.R.; Arnold, K.B.; Schenk, J.M.; Neuhouser, M.L.; Goodman, P.; Penson, D.F.; Thompson, I.M. Dietary patterns, supplement use, and the risk of symptomatic benign prostatic hyperplasia: Results from the prostate cancer prevention trial. Am. J. Epidemiol. 2008, 167, 925–934. [Google Scholar] [CrossRef]
- Guo, Y.; Shi, D.; Zhang, J.; Mao, S.; Wang, L.; Zhang, W.; Zhang, Z.; Jin, L.; Yang, B.; Ye, L.; et al. The Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) Score is a Novel Significant Prognostic Factor for Patients with Metastatic Prostate Cancer Undergoing Cytoreductive Radical Prostatectomy. J. Cancer 2019, 10, 81–91. [Google Scholar] [CrossRef]
- Kaya, C.; Caliskan, S.; Sungur, M.; Aydin, C. HALP score and albumin levels in men with prostate cancer and benign prostate hyperplasia. Int. J. Clin. Pract. 2021, 75, e13766. [Google Scholar] [CrossRef]
- Buzby, G.P.; Mullen, J.L.; Matthews, D.C.; Hobbs, C.L.; Rosato, E.F. Prognostic nutritional index in gastrointestinal surgery. Am. J. Surg. 1980, 139, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Pan, M.; Nie, J.; Xiao, F.; Zhang, Y. Evaluation of the prognostic nutritional index for the prognosis of Chinese patients with high/extremely high-risk prostate cancer after radical prostatectomy. World J. Clin. Cases 2022, 10, 8863–8871. [Google Scholar] [CrossRef]
- Kucukarda, A.; Gokyer, A.; Gokmen, I.; Ozcan, E.; Hacioglu, M.B.; Erdogan, B.; Uzunoglu, S.; Cicin, I. Prognostic nutritional index is an independent prognostic factor for treatment response, survival and drug choice in metastatic castration-resistant prostate cancer treated with abiraterone acetate or enzalutamide. Actas Urológicas Españolas 2022, 46, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Wang, J.; Wei, Y.; Zhang, T.; Zhang, S.; Ye, D.; Zhu, Y. Combination of body mass index and albumin predicts the survival in metastatic castration-resistant prostate cancer patients treated with abiraterone: A post hoc analysis of two randomized trials. Cancer Med. 2021, 10, 6697–6704. [Google Scholar] [CrossRef] [PubMed]
- Verstuyf, A.; Carmeliet, G.; Bouillon, R.; Mathieu, C. Vitamin D: A pleiotropic hormone. Kidney Int. 2010, 78, 140–145. [Google Scholar] [CrossRef]
- Cooke, N.E.; Haddad, J.G. Vitamin D binding protein (Gc-globulin). Endocr. Rev. 1989, 10, 294–307. [Google Scholar] [CrossRef]
- Bikle, D.D.; Halloran, B.P.; Gee, E.; Ryzen, E.; Haddad, J.G. Free 25-hydroxyvitamin D levels are normal in subjects with liver disease and reduced total 25-hydroxyvitamin D levels. J. Clin. Investig. 1986, 78, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Elieh Ali Komi, D.; Vaisi-Raygani, A.; Kiani, A.; Moradi, M.; Aliyari, M.; Rahimi, Z.; Mohammadi-Noori, E.; Bashiri, H. Association Between Vitamin D Binding Protein Gene Polymorphism (rs7041), Vitamin D Receptor, and 25-Hydroxyvitamin D Serum Levels With Prostate Cancer in Kurdish Population in West of Iran. Pathol. Oncol. Res. 2022, 28, 1610246. [Google Scholar] [CrossRef]
- Campbell, R.A.; Li, J.; Malone, L.; Levy, D.A. Correlative Analysis of Vitamin D and Omega-3 Fatty Acid Intake in Men on Active Surveillance for Prostate Cancer. Urology 2021, 155, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Grammatikopoulou, M.G.; Gkiouras, K.; Papageorgiou, S.T.; Myrogiannis, I.; Mykoniatis, I.; Papamitsou, T.; Bogdanos, D.P.; Goulis, D.G. Dietary Factors and Supplements Influencing Prostate Specific-Antigen (PSA) Concentrations in Men with Prostate Cancer and Increased Cancer Risk: An Evidence Analysis Review Based on Randomized Controlled Trials. Nutrients 2020, 12, 2985. [Google Scholar] [CrossRef] [PubMed]
| Middle-Aged Men (Age: 40–64.9, n = 3651) | Older Men (Age: ≥65, n = 2119) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | PSA ≤ 4 | PSA: 4.01–10 | PSA > 10 | p-Value b | PSA ≤ 4 | PSA: 4.01–10 | PSA > 10 | p-Value b | |
| N (wt %) a | N (wt %) a | N (wt %) a | N (wt %) a | N (wt %) a | N (wt %) a | N (wt %) a | |||
| Total | 5770 | 3515 (97.0) | 120 (2.8) | 16 (0.3) | 1716 (82.5) | 316 (14.1) | 87 (3.4) | ||
| Age | 56.2 ± 0.2 | 50.7 ± 0.2 | 57.6 ± 0.7 | 53.0 ± 1.9 | <0.001 | 72.5 ± 0.2 | 74.0 ± 0.5 | 74.8 ± 1.0 | 0.001 |
| Race | |||||||||
| Non-Hispanic white | 3138 (77.3) | 1701 (97.1) | 55 (2.8) | 3 (0.1) | 0.023 | 1128 (83.1) | 205 (13.9) | 46 (3.0) | 0.002 |
| Non-Hispanic Black | 1057 (9.2) | 722 (96.6) | 26 (2.5) | 7 (0.9) | 222 (72.0) | 58 (20.5) | 22 (7.5) | ||
| Other | 1575 (13.5) | 1092 (96.6) | 39 (2.9) | 6 (0.5) | 366 (84.5) | 53 (11.3) | 19 (4.3) | ||
| Income to poverty ratio c | |||||||||
| ≤1 | 864 (9.5) | 556 (96.9) | 14 (1.8) | 6 (1.3) | <0.001 | 225 (78.2) | 53 (19.3) | 10 (2.4) | 0.494 |
| 1.1–4 | 2876 (46.2) | 1586 (97.9) | 46 (2.0) | 5 (0.1) | 1003 (81.9) | 190 (14.7) | 46 (3.4) | ||
| >4 | 1633 (44.3) | 1140 (96.4) | 50 (3.4) | 4 (0.2) | 361 (83.9) | 58 (12.5) | 20 (3.5) | ||
| Body Mass Index (BMI) | |||||||||
| <25 | 1320 (21.3) | 750 (97.1) | 24 (2.8) | 2 (0.1) | 0.218 | 415 (77.7) | 95 (17.7) | 34 (4.5) | 0.053 |
| 25–29.9 | 2397 (43.1) | 1442 (96.3) | 54 (3.4) | 9 (0.3) | 728 (83.0) | 133 (14.2) | 31 (2.8) | ||
| ≥30 | 1963 (35.6) | 1287 (97.8) | 41 (2.0) | 5 (0.2) | 533 (86.1) | 79 (10.8) | 18 (3.1) | ||
| Smoking d | |||||||||
| Never | 2196 (40.0) | 1458 (96.6) | 54 (3) | 10 (0.4) | 0.190 | 524 (79.3) | 114 (16.2) | 36 (4.5) | 0.236 |
| Former | 2274 (37.7) | 1040 (96.7) | 42 (3.2) | 3 (0.1) | 988 (84.3) | 162 (12.8) | 39 (2.9) | ||
| Current | 1297 (22.3) | 1016 (97.8) | 24 (1.8) | 3 (0.3) | 203 (82.7) | 39 (14.6) | 12 (2.8) | ||
| Days of alcohol usage in the past 12 months | |||||||||
| ≤3 | 815 (17.1) | 449 (94.3) | 21 (5.0) | 4 (0.7) | 280 (84.6) | 49 (12.7) | 12 (2.7) | ||
| 4–50 | 1094 (27.3) | 767 (97.6) | 18 (2.0) | 6 (0.3) | 248 (85.5) | 44 (11.7) | 11 (2.8) | ||
| >50 | 2130 (55.6) | 1424 (97.1) | 51 (2.7) | 4 (0.2) | 0.069 | 531 (81.1) | 96 (15.9) | 24 (2.9) | 0.578 |
| Factors | Total Mean ± SE a (n = 3651) | PSA ≤ 4 Mean ± SE a (n = 3515) | PSA: 4.01–10 Mean ± SE a (n = 120) | PSA: >10 Mean ± SE a (n = 16) | p-Value b |
|---|---|---|---|---|---|
| Endogenous antioxidants | |||||
| Bilirubin (umol/L) | 14.28 ± 0.12 | 14.27 ± 0.1 | 14.7 ± 0.7 | 12.5 ± 0.8 | 0.077 |
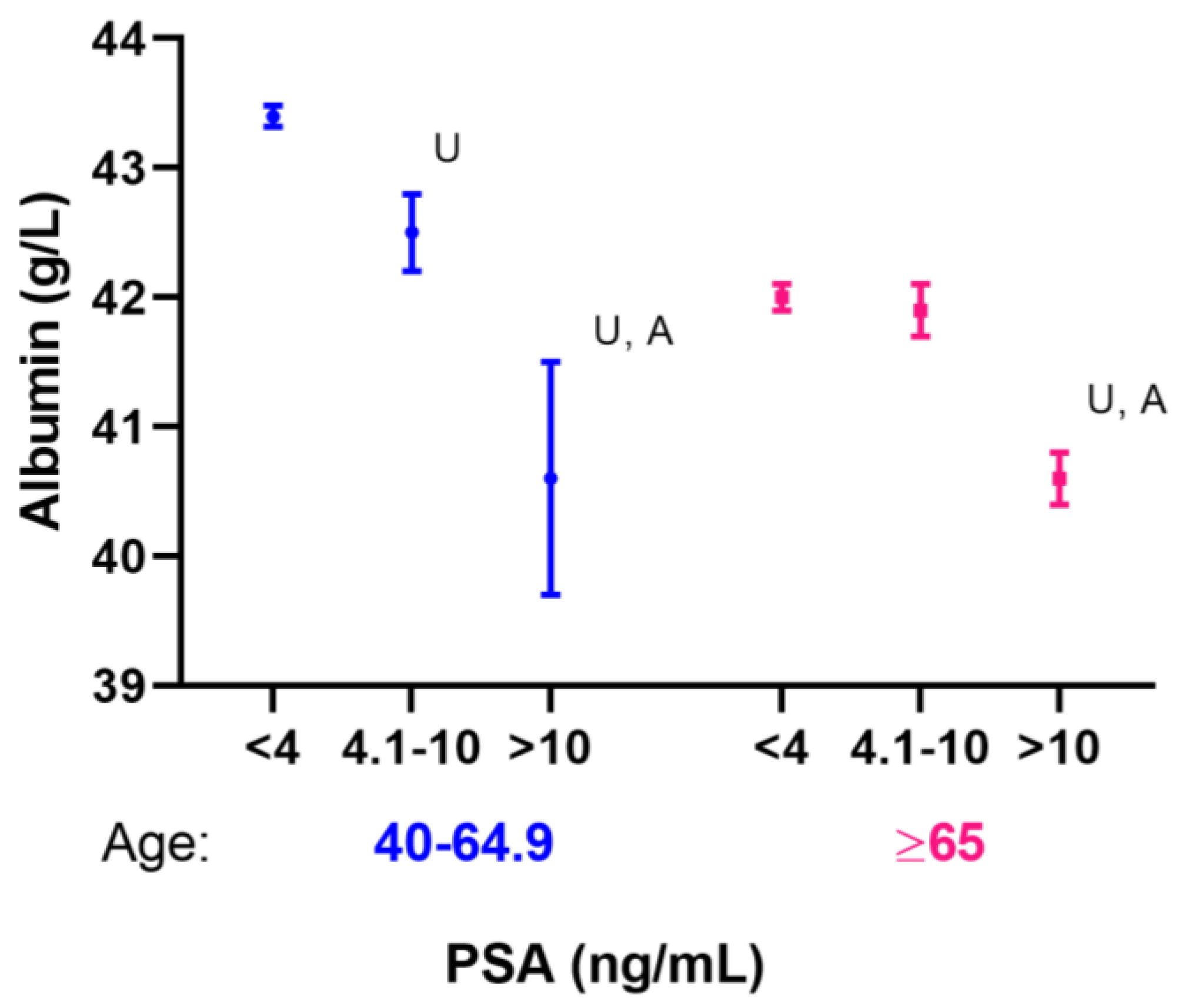
| Albumin (g/L) | 43.39 ± 0.08 | 43.4 ± 0.08 | 42.5 ± 0.3 | 40.6 ± 0.9 | <0.001 |
| Uric acid(umol/L) | 363.78 ± 1.94 | 364.00 ± 2.0 | 357.9 ± 10.5 | 343.9 ± 21.5 | 0.514 |
| Dietary antioxidants | |||||
| Vitamin A, RAE c (mcg) | 687.9 ± 12.2 | 686.9 ± 12.3 | 720.1 ± 52.8 | 707.9 ± 170 | 0.815 |
| Vitamin B2 (mg) | 2.6 ± 0.03 | 2.6 ± 0.03 | 2.5 ± 0.2 | 2.5 ± 0.4 | 0.589 |
| Vitamin C (mg) | 92.4 ± 2.1 | 92.0 ± 2.1 | 103.5 ± 9.5 | 112.3 ± 43.6 | 0.443 |
| Vitamin D (mcg) | 5.6 ± 0.2 (n = 2131) | 5.5 ± 0.2 (n = 2051) | 7.2 ± 1.7 (n = 71) | 3 ± 0.7 (n = 9) | <0.001 |
| Vitamin E (mg) | 8.6 ± 0.1 | 8.6 ± 0.1 | 8.4 ± 0.4 | 9.6 ± 2 | 0.759 |
| Alpha carotene (mcg) | 405.3 ± 18.1 | 403.6 ± 18.4 | 468.7 ± 97.7 | 367.7 ± 168.9 | 0.789 |
| Selenium (mcg) | 132.1 ± 1.4 | 132.1 ± 1.4 | 131.3 ± 5.7 | 126.3 ± 13 | 0.869 |
| Lycopene (mcg) | 6767.1 ± 227.2 | 6761.9 ± 242.8 | 6869.7 ± 1052.5 | 7631 ± 2463.2 | 0.939 |
| Lutein + zeaxanthin (mcg) | 1581.4 ± 66 | 1582.1 ± 68.1 | 1501.8 ± 149.8 | 2174.9 ± 854.5 | 0.656 |
| Beta-cryptoxanthin (mcg) | 115.9 ± 5.0 | 115.4 ± 5.1 | 132.2 ± 20.6 | 113.7 ± 44.4 | 0.750 |
| Folate, DFE c (mcg) | 467.8 ± 6.6 | 467.4 ± 6.7 | 473.7 ± 22 | 544.7 ± 78.2 | 0.570 |
| Unadjusted a | Adjusted a | |||
|---|---|---|---|---|
| Factors (Unit) | PSA 4–10 vs. PSA < 4 OR (95% CI) | PSA ≥ 10 vs. PSA < 4 OR (95% CI) | PSA 4–10 vs. PSA < 4 OR (95% CI) | PSA ≥ 10 vs. PSA < 4 OR (95% CI) |
| Endogenous antioxidants | ||||
| Bilirubin (umol/L) | 1.02 (0.97, 1.07) | 0.91 (0.82, 1.01) | 1.03 (0.98, 1.08) | 0.91 (0.82, 1.01) |
| Albumin (g/L) | 0.91 (0.87, 0.96) *** | 0.82 (0.75, 0.88) *** | 0.96 (0.91, 1.01) | 0.82 (0.76, 0.89) *** |
| Uric acid, per 10 umol/L | 0.99 (0.95, 1.03) | 0.96 (0.89, 1.05) | 0.99 (0.95, 1.03) | 0.96 (0.89, 1.05) |
| Dietary antioxidants | ||||
| Vitamin A, RAE b, per 100 mcg | 1.01 (0.98, 1.04) | 1.01 (0.92, 1.1) | 1.01 (0.98, 1.04) | 1.01 (0.92, 1.1) |
| Vitamin B2 (mg) | 0.90 (0.69, 1.17) | 0.91 (0.52, 1.59) | 0.97 (0.75, 1.27) | 0.93 (0.52, 1.64) |
| Vitamin C (mg), per 10 mg | 1.01 (0.99, 1.03) | 1.02 (0.96, 1.09) | 1.02 (0.99, 1.04) | 1.02 (0.96, 1.09) |
| Vitamin D (mcg) | 1.04 (0.98, 1.1) | 0.8 (0.63, 1.01) # | 1.04 (0.99, 1.10) | 0.80 (0.63, 1.02)# |
| Vitamin E (mg) | 0.99 (0.96, 1.02) | 1.03 (0.93, 1.14) | 1.01 (0.98, 1.04) | 1.03 (0.94, 1.14) |
| Alpha-carotene, per 100 mcg | 1.01 (0.99, 1.03) | 0.99 (0.93, 1.06) | 1.01 (0.98, 1.03) | 0.99 (0.93, 1.06) |
| Selenium (mcg), per 10 mcg | 1.00 (0.96, 1.04) | 0.98 (0.89, 1.07) | 1.03 (0.99, 1.07) | 0.99 (0.89, 1.1) |
| Lycopene, per 1000 mcg | 1.00 (0.97, 1.03) | 1.01 (0.96, 1.06) | 1.01 (0.98, 1.04) | 1.01 (0.96, 1.06) |
| Lutein + zeaxanthin, per 1000 mcg | 0.99 (0.94, 1.04) | 1.06 (0.95, 1.18) | 0.98 (0.92, 1.04) | 1.06 (0.94, 1.18) |
| Beta-cryptoxanthin, per 100 mcg | 1.03 (0.97, 1.1) | 1.00 (0.79, 1.25) | 1.02 (0.96, 1.08) | 0.99 (0.81, 1.23) |
| Folate, DFE b, per 100 mcg | 1.01 (0.93, 1.11) | 1.13 (0.94, 1.35) | 1.05 (0.97, 1.15) | 1.14 (0.96, 1.35) |
| Factors (Unit) | Total Mean ± SE a (n = 2119) | PSA: 1–4 Mean ± SE a (n = 1716) | PSA: 4.01–10 Mean ± SE a (n = 316) | PSA: >10 Mean ± SE a (n = 87) | p-Value b |
|---|---|---|---|---|---|
| Endogenous antioxidants | |||||
| Bilirubin (umol/L) | 14.9 ± 0.2 | 15.0 ± 0.2 | 14.9 ± 0.4 | 14.3 ± 0.4 | 0.298 |
| Albumin (g/L) | 41.9 ± 0.1 | 42.0 ± 0.1 | 41.9 ± 0.2 | 40.6 ± 0.2 | <0.001 |
| Uric acid(umol/L) | 368.2 ± 2.3 | 366.7 ± 2.4 | 373.7 ± 6.2 | 381.5 ± 20.3 | 0.392 |
| Dietary antioxidants | |||||
| Vitamin A, RAE c (mcg) | 753.7 ± 20.4 | 757.7 ± 22.6 | 766.2 ± 57.4 | 606 ± 48.2 | 0.011 |
| Vitamin B2 (mg) | 2.4 ± 0.04 | 2.4 ± 0.04 | 2.3 ± 0.1 | 2.2 ± 0.2 | 0.626 |
| Vitamin C (mg) | 92.4 ± 2.0 | 92.0 ± 2.4 | 96.9 ± 6.4 | 82.9 ± 9.9 | 0.519 |
| Vitamin D (mcg) | 5.6 ± 0.2 (n = 1116) | 5.6 ± 0.2 (n = 905) | 5.7 ± 0.6 (n = 161) | 4.6 ± 0.5 (n = 50) | 0.141 |
| Vitamin E (mg) | 7.4 ± 0.1 | 7.5 ± 0.2 | 7.3 ± 0.3 | 6.6 ± 0.4 | 0.117 |
| Alpha carotene (mcg) | 472.5 ± 28.2 | 486.9 ± 29.6 | 431.5 ± 66.4 | 294.7 ± 77.7 | 0.072 |
| Selenium (mcg) | 105.3 ± 1.3 | 105.6 ± 1.3 | 103.8 ± 2.8 | 106.9 ± 7 | 0.679 |
| Lycopene (mcg) | 5447.5 ± 291.4 | 5452.1 ± 309.1 | 5538.8 ± 698.8 | 4964.3 ± 819.1 | 0.824 |
| Lutein + zeaxanthin (mcg) | 1548.9 ± 81.6 | 1559.6 ± 89.0 | 1504.2 ± 154.5 | 1473.2 ± 233.5 | 0.886 |
| Beta-cryptoxanthin (mcg) | 133.5 ± 5.6 | 132.8 ± 6.7 | 147.9 ± 23.2 | 92.9 ± 15.1 | 0.057 |
| Folate, DFE c (mcg) | 427.7 ± 6.3 | 429.2 ± 7.3 | 430.5 ± 24.2 | 377.8 ± 20.5 | 0.055 |
| Unadjusted a | Adjusted a | |||
|---|---|---|---|---|
| Factors (Unit) | PSA 4–10 vs. PSA < 4 OR (95% CI) | PSA ≥ 10 vs. PSA < 4 OR (95% CI) | PSA 4–10 vs. PSA < 4 OR (95% CI) | PSA ≥ 10 vs. PSA < 4 OR (95% CI) |
| Endogenous antioxidants | ||||
| Bilirubin (umol/L) | 1.00 (0.98, 1.02) | 0.98 (0.98, 1.02) | 1.00 (0.98, 1.03) | 0.99 (0.95, 1.02) |
| Albumin (g/L) | 0.99 (0.94, 1.05) | 0.88 (0.84, 0.92) *** | 1.01 (0.95, 1.07) | 0.90 (0.85, 0.96) *** |
| Uric acid, per 10 umol/L | 1.01 (0.99, 1.03) | 1.02 (0.97, 1.08) | 1.01 (0.99, 1.03) | 1.02 (0.97, 1.07) |
| Dietary antioxidants | ||||
| Vitamin A, RAE b, per 100 mcg | 1.00 (0.98, 1.02) | 0.93 (0.87, 1.00) # | 1.00 (0.98, 1.02) | 0.94 (0.88, 1.01) |
| Vitamin B2 (mg) | 0.98 (0.79, 1.21) | 0.85 (0.61, 1.21) | 1.02 (0.82, 1.26) | 0.96 (0.71, 1.31) |
| Vitamin C (mg), per 10 mg | 1.01 (0.98, 1.04) | 0.98 (0.93, 1.03) | 1.01 (0.98, 1.04) | 0.98 (0.94, 1.03) |
| Vitamin D (mcg) | 1.00 (0.96, 1.05) | 0.94 (0.87, 1.02) | 1.01 (0.96, 1.05) | 0.96 (0.89, 1.03) |
| Vitamin E (mg) | 0.99 (0.95, 1.03) | 0.94 (0.88, 1.01) | 1.00 (0.96, 1.04) | 0.97 (0.90, 1.04) |
| Alpha-carotene, per 100 mcg | 0.99 (0.97, 1.02) | 0.95 (0.89, 1.02) | 0.99 (0.97, 1.02) | 0.96 (0.90, 1.02) |
| Selenium (mcg), per 10 mcg | 0.99 (0.95, 1.02) | 1.01 (0.94, 1.08) | 1.00 (0.97, 1.03) | 1.03 (0.97, 1.10) |
| Lycopene, per 1000 mcg | 1.00 (0.98, 1.02) | 0.99 (0.96, 1.02) | 1.01 (0.99, 1.03) | 1.00 (0.97, 1.03) |
| Lutein + zeaxanthin, per 1000 mcg | 0.99 (0.94, 1.05) | 0.99 (0.91, 1.07) | 0.99 (0.94, 1.04) | 0.98 (0.91, 1.06) |
| Beta-cryptoxanthin, per 100 mcg | 1.03 (0.94, 1.13) | 0.83 (0.66, 1.05) | 1.03 (0.94, 1.14) | 0.83 (0.66, 1.04) |
| Folate, DFE b, per 100 mcg | 1.00 (0.89, 1.13) | 0.87 (0.75, 1.00) * | 1.03 (0.91, 1.15) | 0.91 (0.79, 1.04) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-Y.; Zhu, X.; Aucoin, A.J.; Fu, Q.; Park, J.Y.; Tseng, T.-S. Dietary and Serum Antioxidants Associated with Prostate-Specific Antigen for Middle-Aged and Older Men. Nutrients 2023, 15, 3298. https://doi.org/10.3390/nu15153298
Lin H-Y, Zhu X, Aucoin AJ, Fu Q, Park JY, Tseng T-S. Dietary and Serum Antioxidants Associated with Prostate-Specific Antigen for Middle-Aged and Older Men. Nutrients. 2023; 15(15):3298. https://doi.org/10.3390/nu15153298
Chicago/Turabian StyleLin, Hui-Yi, Xiaodan Zhu, Alise J. Aucoin, Qiufan Fu, Jong Y. Park, and Tung-Sung Tseng. 2023. "Dietary and Serum Antioxidants Associated with Prostate-Specific Antigen for Middle-Aged and Older Men" Nutrients 15, no. 15: 3298. https://doi.org/10.3390/nu15153298
APA StyleLin, H.-Y., Zhu, X., Aucoin, A. J., Fu, Q., Park, J. Y., & Tseng, T.-S. (2023). Dietary and Serum Antioxidants Associated with Prostate-Specific Antigen for Middle-Aged and Older Men. Nutrients, 15(15), 3298. https://doi.org/10.3390/nu15153298







