Mediation of BMI on 25-Hydroxyvitamin D Levels in U.S. Adults with Sugar-Sweetened Beverages Consumption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Questionnaires
2.3. Dietary Intake
2.4. SSB Intake
2.5. Body Mass Index
2.6. Clinical Examination
2.7. Statistical Analysis
3. Results
4. Discussion
5. Limitation and Strengths
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Institutes of Health. Vitamin D: Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/ (accessed on 16 January 2023).
- Reid, I.R.; Bolland, M.J.; Grey, A. Effects of vitamin D supplements on bone mineral density: A systematic review and meta-analysis. Lancet 2014, 383, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Veldurthy, V.; Wei, R.; Oz, L.; Dhawan, P.; Jeon, Y.H.; Christakos, S. Vitamin D, calcium homeostasis and aging. Bone Res. 2016, 4, 16041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemieux, P.; Weisnagel, S.J.; Caron, A.Z.; Julien, A.S.; Morisset, A.S.; Carreau, A.M.; Poirier, J.; Tchernof, A.; Robitaille, J.; Bergeron, J.; et al. Effects of 6-month vitamin D supplementation on insulin sensitivity and secretion: A randomised, placebo-controlled trial. Eur. J. Endocrinol. 2019, 181, 287–299. [Google Scholar] [CrossRef]
- Pilz, S.; Verheyen, N.; Grubler, M.R.; Tomaschitz, A.; Marz, W. Vitamin D and cardiovascular disease prevention. Nat. Rev. Cardiol. 2016, 13, 404–417. [Google Scholar] [CrossRef]
- Grant, W.B. Review of Recent Advances in Understanding the Role of Vitamin D in Reducing Cancer Risk: Breast, Colorectal, Prostate, and Overall Cancer. Anticancer. Res. 2020, 40, 491–499. [Google Scholar] [CrossRef] [Green Version]
- Vellekkatt, F.; Menon, V. Efficacy of vitamin D supplementation in major depression: A meta-analysis of randomized controlled trials. J. Postgrad. Med. 2019, 65, 74–80. [Google Scholar] [CrossRef]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and immune function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef] [Green Version]
- Munger, K.L.; Zhang, S.M.; O’Reilly, E.; Hernan, M.A.; Olek, M.J.; Willett, W.C.; Ascherio, A. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004, 62, 60–65. [Google Scholar] [CrossRef]
- Maroufi, N.F.; Pezeshgi, P.; Mortezania, Z.; Pourmohammad, P.; Eftekhari, R.; Moradzadeh, M.; Vahedian, V.; Nouri, M. Association between vitamin D deficiency and prevalence of metabolic syndrome in female population: A systematic review. Horm. Mol. Biol. Clin. Investig. 2020, 41, 20200033. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Giovannucci, E.; Willett, W.C.; Dietrich, T.; Dawson-Hughes, B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am. J. Clin. Nutr. 2006, 84, 18–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Baylin, A.; Levy, P.D. Vitamin D deficiency and insufficiency among US adults: Prevalence, predictors and clinical implications. Br. J. Nutr. 2018, 119, 928–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forrest, K.Y.; Stuhldreher, W.L. Prevalence and correlates of vitamin D deficiency in US adults. Nutr. Res. 2011, 31, 48–54. [Google Scholar] [CrossRef]
- Jaaskelainen, T.; Knekt, P.; Marniemi, J.; Sares-Jaske, L.; Mannisto, S.; Heliovaara, M.; Jarvinen, R. Vitamin D status is associated with sociodemographic factors, lifestyle and metabolic health. Eur. J. Nutr. 2013, 52, 513–525. [Google Scholar] [CrossRef]
- WHO. W.H.O. Fact Sheet Obesity and Overweight 2013. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/# (accessed on 5 March 2023).
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018; NCHS Data Brief, no 360.; National Center for Health Statistics: Hyattsville, MD, USA, 2020; pp. 1–8. [Google Scholar]
- Vimaleswaran, K.S.; Berry, D.J.; Lu, C.; Tikkanen, E.; Pilz, S.; Hiraki, L.T.; Cooper, J.D.; Dastani, Z.; Li, R.; Houston, D.K.; et al. Causal relationship between obesity and vitamin D status: Bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013, 10, e1001383. [Google Scholar] [CrossRef]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevinsky, J.R.; Lee, S.H.; Blanck, H.M.; Park, S. Prevalence of Self-Reported Intake of Sugar-Sweetened Beverages Among US Adults in 50 States and the District of Columbia, 2010 and 2015. Prev. Chronic. Dis. 2021, 18, E35. [Google Scholar] [CrossRef]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Despres, J.P.; Hu, F.B. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 2010, 121, 1356–1364. [Google Scholar] [CrossRef]
- Lin, W.T.; Kao, Y.H.; Sothern, M.S.; Seal, D.W.; Lee, C.H.; Lin, H.Y.; Chen, T.; Tseng, T.S. The association between sugar-sweetened beverages intake, body mass index, and inflammation in US adults. Int. J. Public Health 2020, 65, 45–53. [Google Scholar] [CrossRef]
- Malik, V.S.; Hu, F.B. Fructose and Cardiometabolic Health: What the Evidence From Sugar-Sweetened Beverages Tells Us. J. Am. Coll. Cardiol. 2015, 66, 1615–1624. [Google Scholar] [CrossRef] [Green Version]
- Duchaine, C.S.; Diorio, C. Association between intake of sugar-sweetened beverages and circulating 25-hydroxyvitamin D concentration among premenopausal women. Nutrients 2014, 6, 2987–2999. [Google Scholar] [CrossRef] [Green Version]
- Olson, M.L.; Maalouf, N.M.; Oden, J.D.; White, P.C.; Hutchison, M.R. Vitamin D deficiency in obese children and its relationship to glucose homeostasis. J. Clin. Endocrinol. Metab. 2012, 97, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Kalicanin, D.; Cvek, M.; Baric, A.; Skrabic, V.; Punda, A.; Boraska Perica, V. Associations between vitamin D levels and dietary patterns in patients with Hashimoto’s thyroiditis. Front. Nutr. 2023, 10, 1188612. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC); National Center for Health Statistics (NCHS). NHANES Survey. Methods and Analytic Guidelines. Available online: https://www.cdc.gov/nchs/nhanes/index.htm (accessed on 5 March 2023).
- The World Health Organization (WHO). Global Strategy on Diet, Physical Activity and Health. Available online: https://www.who.int/dietphysicalactivity/factsheet_adults/en/ (accessed on 5 March 2023).
- Institute of Medicine; Food and Nutrition Board; Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Apovian, C.M. Sugar-sweetened soft drinks, obesity, and type 2 diabetes. JAMA 2004, 292, 978–979. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.W.; Dumke, K.A.; Goran, M.I. Fructose content in popular beverages made with and without high-fructose corn syrup. Nutrition 2014, 30, 928–935. [Google Scholar] [CrossRef] [Green Version]
- Agricultural Research Service US & Department of Agriculture Beltsville Human Nutrition Research Center. Food and Nutrient Database for Dietary Studies (FNDDS). 2015. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsvillehuman-nutrition-research-center/food-surveys-research-group/docs/fndds-download-databases/ (accessed on 5 March 2023).
- Centers for Disease Control and Prevention (CDC). Defining Adult Overweight and Obesity. Available online: https://www.cdc.gov/obesity/adult/defining.html (accessed on 10 March 2023).
- Centers for Disease Control and Prevention (CDC). The National Health and Nutrition Examination Survey Laboratory Procedure Manual Serum 25-Hydroxyvitamin D Diasorin (Formerly Incstar) 25-OH-D Assay; CDC: Atlanta, GA, USA, 2008. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/VID_H.htm (accessed on 8 February 2023).
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [Green Version]
- Sharma, U.; Pal, D.; Prasad, R. Alkaline phosphatase: An overview. Indian J. Clin. Biochem. 2014, 29, 269–278. [Google Scholar] [CrossRef] [Green Version]
- National Institutes of Health (NIH). Office of Dietary Supplements. Phosphorus. Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Phosphorus-HealthProfessional/ (accessed on 22 March 2023).
- Bazydlo, L.A.L.; Needham, M.; Harris, N.S. Calcium, Magnesium, and Phosphate. Lab. Med. 2014, 45, e44–e50. [Google Scholar] [CrossRef]
- Garcia-Contreras, F.; Paniagua, R.; Avila-Diaz, M.; Cabrera-Munoz, L.; Martinez-Muniz, I.; Foyo-Niembro, E.; Amato, D. Cola beverage consumption induces bone mineralization reduction in ovariectomized rats. Arch. Med. Res. 2000, 31, 360–365. [Google Scholar] [CrossRef]
- Douard, V.; Asgerally, A.; Sabbagh, Y.; Sugiura, S.; Shapses, S.A.; Casirola, D.; Ferraris, R.P. Dietary fructose inhibits intestinal calcium absorption and induces vitamin D insufficiency in CKD. J. Am. Soc. Nephrol. 2010, 21, 261–271. [Google Scholar] [CrossRef] [Green Version]
- Lee, O.; Bruce, W.R.; Dong, Q.; Bruce, J.; Mehta, R.; O’Brien, P.J. Fructose and carbonyl metabolites as endogenous toxins. Chem. Biol. Interact. 2009, 178, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Portale, A.A.; Halloran, B.P.; Murphy, M.M.; Morris, R.C., Jr. Oral intake of phosphorus can determine the serum concentration of 1,25-dihydroxyvitamin D by determining its production rate in humans. J. Clin. Invest. 1986, 77, 7–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucker, K.L.; Morita, K.; Qiao, N.; Hannan, M.T.; Cupples, L.A.; Kiel, D.P. Colas, but not other carbonated beverages, are associated with low bone mineral density in older women: The Framingham Osteoporosis Study. Am. J. Clin. Nutr. 2006, 84, 936–942. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Massaro, J.M.; Fox, C.S.; Larson, M.G.; Keyes, M.J.; McCabe, E.L.; Robins, S.J.; O’Donnell, C.J.; Hoffmann, U.; Jacques, P.F.; et al. Adiposity, cardiometabolic risk, and vitamin D status: The Framingham Heart Study. Diabetes 2010, 59, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Kull, M.; Kallikorm, R.; Lember, M. Body mass index determines sunbathing habits: Implications on vitamin D levels. Intern. Med. J. 2009, 39, 256–258. [Google Scholar] [CrossRef]
- Heaney, R.P.; Horst, R.L.; Cullen, D.M.; Armas, L.A. Vitamin D3 distribution and status in the body. J. Am. Coll. Nutr. 2009, 28, 252–256. [Google Scholar] [CrossRef]
- Gallagher, J.C.; Yalamanchili, V.; Smith, L.M. The effect of vitamin D supplementation on serum 25(OH)D in thin and obese women. J. Steroid Biochem. Mol. Biol. 2013, 136, 195–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taksler, G.B.; Cutler, D.M.; Giovannucci, E.; Keating, N.L. Vitamin D deficiency in minority populations. Public Health Nutr. 2015, 18, 379–391. [Google Scholar] [CrossRef] [Green Version]
- Scragg, R.; Camargo, C.A., Jr. Frequency of leisure-time physical activity and serum 25-hydroxyvitamin D levels in the US population: Results from the Third National Health and Nutrition Examination Survey. Am. J. Epidemiol. 2008, 168, 577–586, discussion 587–591. [Google Scholar] [CrossRef] [Green Version]
- Tonnesen, R.; Hovind, P.H.; Jensen, L.T.; Schwarz, P. Determinants of vitamin D status in young adults: Influence of lifestyle, sociodemographic and anthropometric factors. BMC Public Health 2016, 16, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baggerly, C.A.; Cuomo, R.E.; French, C.B.; Garland, C.F.; Gorham, E.D.; Grant, W.B.; Heaney, R.P.; Holick, M.F.; Hollis, B.W.; McDonnell, S.L.; et al. Sunlight and Vitamin D: Necessary for Public Health. J. Am. Coll. Nutr. 2015, 34, 359–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Hypponen, E.; Berry, J.; Vieth, R.; et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 1357–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
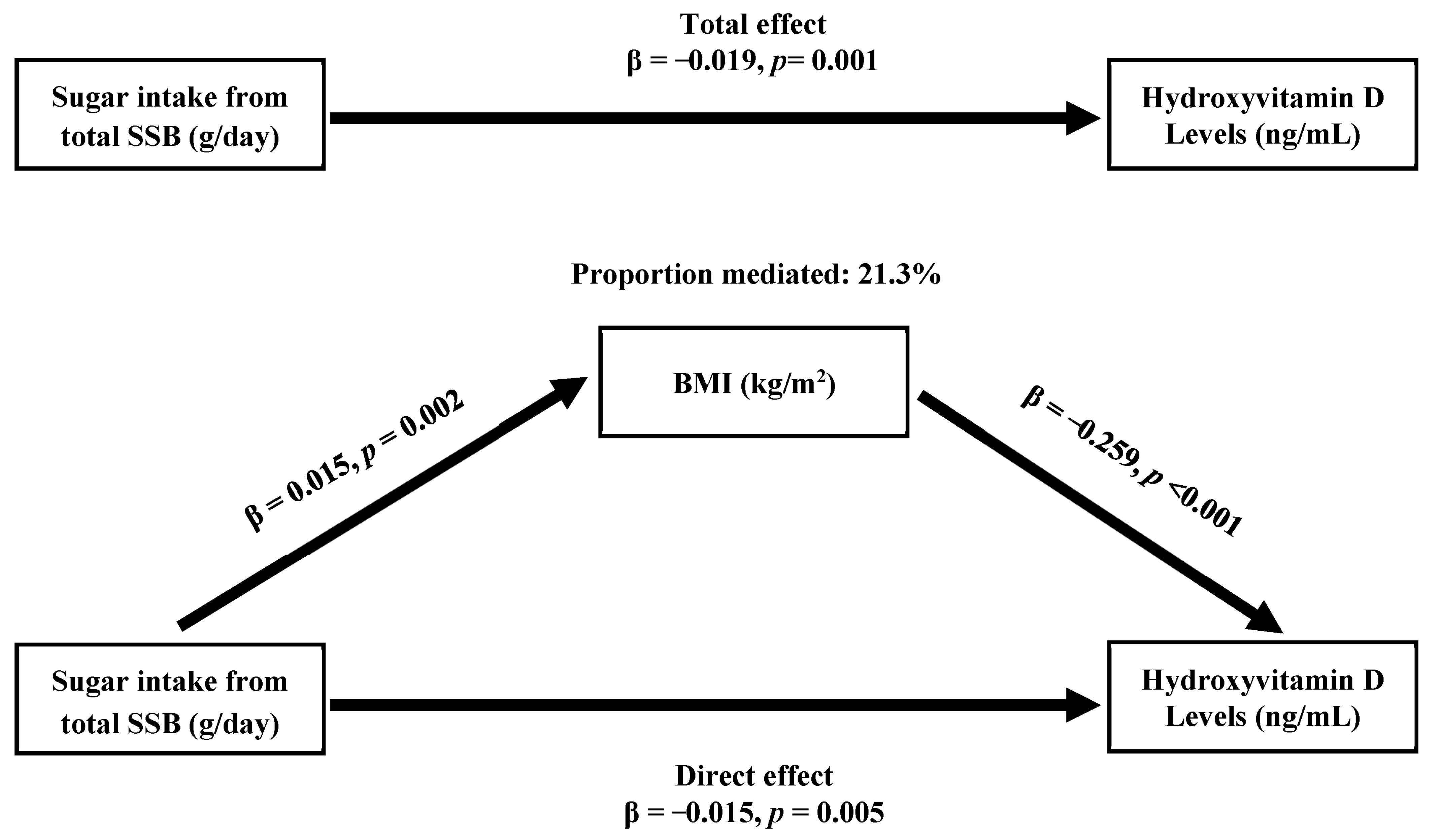
| Factors | 25-Hydroxyvitamin D (ng/mL) | |||||
|---|---|---|---|---|---|---|
| Mean ± se | p-Value | Normal | Insufficiency | Deficiency | p-Value | |
| Number of population 1 | N = 4505 | N = 1465 | N = 1687 | N = 1353 | ||
| Survey-weighted 2 | 28.0 ± 0.5 | 38.1% | 37.8% | 24.1% | ||
| Personal characteristics | ||||||
| Age, years (mean ± se) | -- | -- | 52.5 ± 0.7 | 44.9 ± 0.5 | 41.7 ± 0.9 | <0.001 |
| Gender | <0.001 | <0.001 | ||||
| Male | 26.6 ± 0.5 | 32.7% | 42.1% | 25.2% | ||
| Female | 29.4 ± 0.6 | 43.3% | 33.6% | 23.1% | ||
| Ethnicity | <0.001 | <0.001 | ||||
| Non-Hispanic White | 30.7 ± 0.6 | 47.5% | 38.1% | 14.4% | ||
| Non-Hispanic Black | 20.2 ± 0.6 | 15.4% | 26.0% | 58.6% | ||
| Mexican American | 21.8 ± 1.0 | 14.9% | 42.4% | 42.7% | ||
| Other Hispanic | 25.6 ± 0.6 | 25.0% | 50.4% | 24.6% | ||
| Other Race | 24.9 ± 0.6 | 28.5% | 37.3% | 34.2% | ||
| Ratio of family income to poverty | <0.001 | <0.001 | ||||
| Below poverty | 23.9 ± 0.7 | 24.7% | 36.9% | 38.3% | ||
| 1–1.99 | 26.2 ± 0.6 | 31.0% | 39.4% | 29.5% | ||
| 2–2.99 | 27.5 ± 0.9 | 32.4% | 40.2% | 27.4% | ||
| 3–3.00 | 28.5 ± 0.8 | 41.1% | 37.9% | 21.0% | ||
| ≥4 | 30.6 ± 0.8 | 48.6% | 36.4% | 15.0% | ||
| Lifestyle patterns | ||||||
| Cigarette use | <0.001 | 0.002 | ||||
| None | 28.1 ± 0.6 | 38.8% | 37.0% | 24.2% | ||
| Former | 29.6 ± 0.5 | 41.7% | 40.3% | 18.0% | ||
| Current | 25.6 ± 0.8 | 31.3% | 37.1% | 31.7% | ||
| Alcohol use | 0.211 | 0.187 | ||||
| None | 28.0 ± 0.7 | 35.4% | 26.5% | 28.1% | 0.187 | |
| Light | 28.3 ± 0.7 | 39.9% | 37.6% | 22.5% | ||
| Heavy | 27.0 ± 0.8 | 35.8% | 41.2% | 23.1% | ||
| Physical activity (hour/week) | 0.002 | <0.001 | ||||
| Low | 27.2 ± 0.4 | 35.0% | 37.6% | 27.3% | ||
| Adequate | 29.3 ± 0.8 | 43.2% | 38.0% | 18.8% | ||
| Medical conditions 3 | <0.001 | <0.001 | ||||
| No | 25.9 ± 0.6 | 28.7% | 42.2% | 29.2% | ||
| Yes | 29.7 ± 0.5 | 46.0% | 34.1% | 19.9% | ||
| 25-Hydroxyvitamin D (ng/mL) | ||||||
|---|---|---|---|---|---|---|
| Mean ± se | p-Value | Normal | Insufficiency | Deficiency | p-Value | |
| Number of population 1 | N = 4505 | N = 1465 | N = 1687 | N = 1353 | ||
| Survey-weighted 2 | 28.0 ± 0.5 | 38.1% | 37.8% | 24.1% | ||
| Daily dietary intake, mean± se | ||||||
| Total energy (kcal) | -- | -- | 2073 ± 19 | 2081 ± 37 | 2157 ± 34 | 0.136 |
| Total sugar (g) | -- | -- | 106 ± 2.1 | 106 ± 2.0 | 114 ± 3.8 | 0.094 |
| Total nutrients intake 3 | ||||||
| Vitamin D (D2 + D3) (μg) | <0.001 | <0.001 | ||||
| Sufficient | 34.8 ± 0.5 | 63.4% | 30.2% | 6.4% | ||
| Insufficient | 25.1 ± 0.5 | 27.4% | 41.0% | 31.6% | ||
| Calcium (mg) | <0.001 | <0.001 | ||||
| Sufficient | 29.7 ± 0.6 | 45.3% | 36.3% | 18.4% | ||
| Insufficient | 26.4 ± 0.5 | 31.6% | 39.1% | 29.3% | ||
| SSB-related factors | ||||||
| Sugar intake from SSB (g), mean ± se | -- | -- | 26.0 ± 2.9 | 36.1 ± 1.8 | 52.7 ± 3.7 | <0.001 |
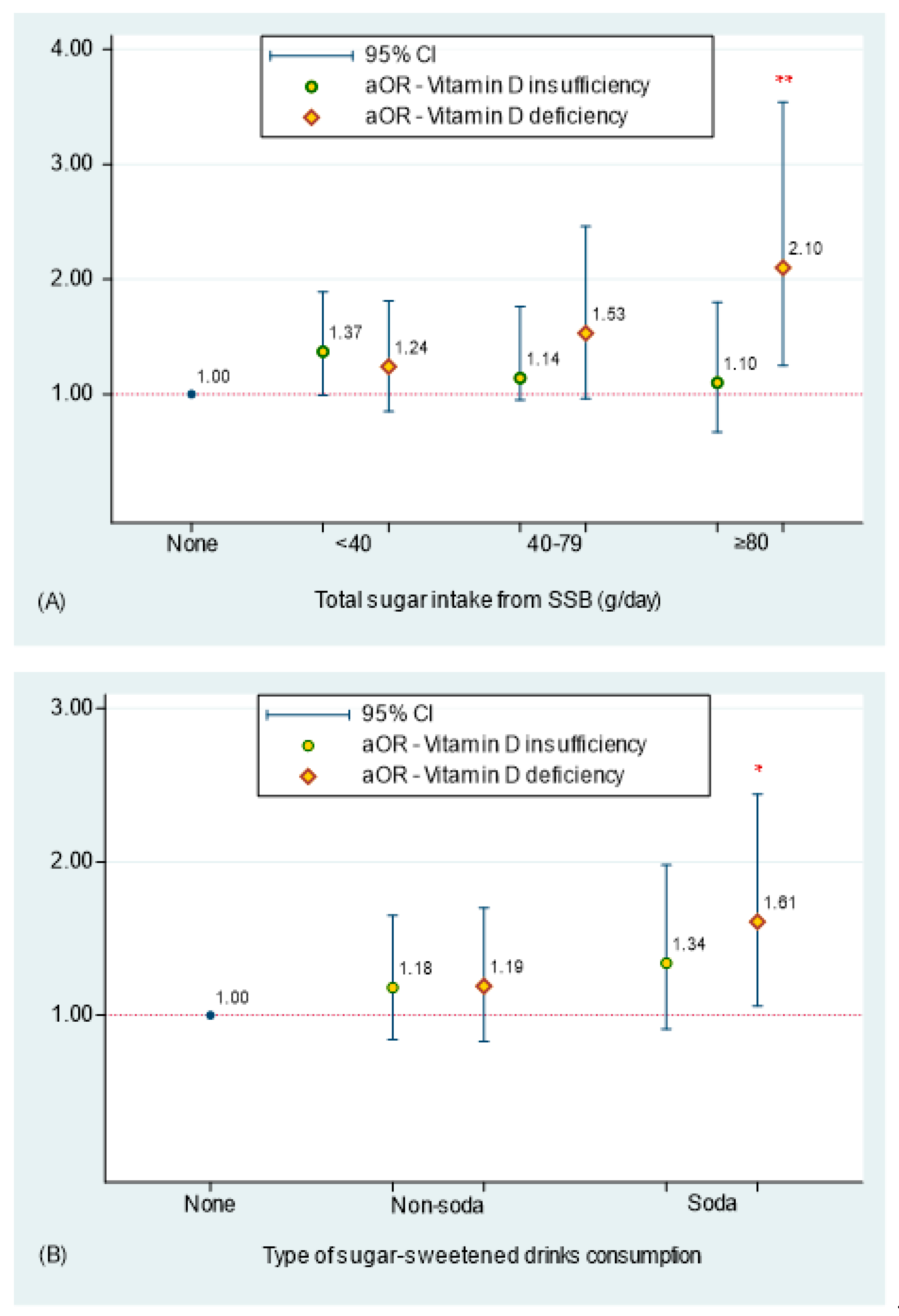
| Total sugar intake from SSB per day | <0.001 | <0.001 | ||||
| Non-SSB consumers | 30.3 ± 0.6 | 47.1% | 35.7% | 17.1% | ||
| <40 g | 28.1 ± 0.7 | 36.0% | 42.1% | 21.9% | ||
| 40–79 g | 25.9 ± 0.8 | 31.2% | 38.4% | 30.5% | ||
| ≥80 g | 23.7 ± 0.5 | 24.6% | 35.4% | 40.0% | ||
| Type of soda consumption (n = 2742) | <0.001 | 0.002 | ||||
| Non-soda SSB consumers 4 | 28.5 ± 0.7 | 38.8% | 37.8% | 23.3% | ||
| Soda consumers 5 | 25.2 ± 0.6 | 28.0% | 40.0% | 32.1% | ||
| Body mass index (kg/m2) | 0.003 | <0.001 | ||||
| Normal/under weight | 29.9 ± 0.8 | 46.9% | 32.4% | 20.7% | ||
| Overweight | 28.3 ± 0.6 | 38.7% | 40.4% | 20.9% | ||
| Obesity | 26.1 ± 0.6 | 30.5% | 39.8% | 29.7% | ||
| Clinical biomarker | ||||||
| Alkaline phosphatase (IU/L) | 0.880 | 0.582 | ||||
| Normal | 28.0 ± 0.5 | 38.1% | 37.9% | 24.0% | ||
| Abnormal | 27.4 ± 3.9 | 39.1% | 26.6% | 34.3% | ||
| Total triglycerides (mg/dL) | 0.123 | 0.379 | ||||
| Normal | 28.2 ± 0.6 | 39.2% | 37.0% | 23.8% | ||
| Higher | 27.6 ± 0.6 | 36.5% | 39.0% | 24.5% | ||
| Phosphorus (mg/dL) | 0.230 | 0.682 | ||||
| Normal | 27.8 ± 0.6 | 37.8% | 37.9% | 24.3% | ||
| Abnormal | 29.5 ± 1.2 | 40.8% | 36.9% | 22.4% | ||
| 25-Hydroxyvitamin D Levels (ng/mL) | ||||
|---|---|---|---|---|
| Model 1 | Model 2 | |||
| aDiff | (95%CI) | aDiff | (95%CI) | |
| Total sugar intake from SSB | ||||
| Non-SSB consumers | Ref | Ref | ||
| <40 g | −1.07 | (−2.42, 0.27) | −1.02 | (−2.32, 0.28) |
| 40–79 g | −1.83 | (−3.23, −0.43) * | −1.42 | (−2.88, 0.03) |
| ≥80 g | −2.88 | (−4.33, −1.44) *** | −2.53 | (−3.93, −1.12) ** |
| Body mass index (kg/m2) | ||||
| Normal/under weight | -- | -- | Ref | |
| Overweight | -- | -- | −1.58 | (−2.95, −0.21) * |
| Obesity | -- | -- | −3.68 | (−4.77, −2.59) *** |
| 25-Hydroxyvitamin D Levels (ng/mL) | ||||
|---|---|---|---|---|
| Model 1 | Model 2 | |||
| aDiff | (95%CI) | aDiff | (95%CI) | |
| Type of SSB intake | ||||
| Non-SSB consumers | Ref | Ref | ||
| Non-soda SSB 1 | −0.50 | (−1.88, 0.88) | −0.33 | (−1.78, 1.11) |
| Soda drinks 2 | −2.14 | (−3.37, −0.90) ** | −1.92 | (−3.14, −0.70) ** |
| Body mass index (kg/m2) | ||||
| Normal/under weight | -- | -- | Ref | |
| Overweight | -- | -- | −1.60 | (−3.03, −0.16) * |
| Obesity | -- | -- | −0.71 | (−4.84, −2.58) *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.-T.; Gonzalez, G.V.; Kao, Y.-H.; Lin, H.-Y.; Li, M.S.; Seal, D.W.; Lee, C.-H.; Hu, C.-y.; Chen, L.-S.; Tseng, T.-S. Mediation of BMI on 25-Hydroxyvitamin D Levels in U.S. Adults with Sugar-Sweetened Beverages Consumption. Nutrients 2023, 15, 3291. https://doi.org/10.3390/nu15153291
Lin W-T, Gonzalez GV, Kao Y-H, Lin H-Y, Li MS, Seal DW, Lee C-H, Hu C-y, Chen L-S, Tseng T-S. Mediation of BMI on 25-Hydroxyvitamin D Levels in U.S. Adults with Sugar-Sweetened Beverages Consumption. Nutrients. 2023; 15(15):3291. https://doi.org/10.3390/nu15153291
Chicago/Turabian StyleLin, Wei-Ting, Gabrielle V. Gonzalez, Yu-Hsiang Kao, Hui-Yi Lin, Mirandy S. Li, David W. Seal, Chien-Hung Lee, Chih-yang Hu, Lei-Shih Chen, and Tung-Sung Tseng. 2023. "Mediation of BMI on 25-Hydroxyvitamin D Levels in U.S. Adults with Sugar-Sweetened Beverages Consumption" Nutrients 15, no. 15: 3291. https://doi.org/10.3390/nu15153291
APA StyleLin, W.-T., Gonzalez, G. V., Kao, Y.-H., Lin, H.-Y., Li, M. S., Seal, D. W., Lee, C.-H., Hu, C.-y., Chen, L.-S., & Tseng, T.-S. (2023). Mediation of BMI on 25-Hydroxyvitamin D Levels in U.S. Adults with Sugar-Sweetened Beverages Consumption. Nutrients, 15(15), 3291. https://doi.org/10.3390/nu15153291








