A Randomized, Double-Blind, Controlled Trial Assessing If Medium-Chain Triglycerides in Combination with Moderate-Intensity Exercise Increase Muscle Strength in Healthy Middle-Aged and Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Subjects
2.3. Target Number of Cases
2.4. Test Design
2.5. Test Foods and Dietary Surveys
2.6. Exercise Intervention
- (1)
- Walking on a treadmill or other walking machines at a normal walking speed for a designated period of time.
- (2)
- Foot stomping on a flat surface for a designated time at a tempo equivalent to normal walking without moving.
2.7. Life Surveys
2.8. Adverse Events
2.9. Measurements
- (1)
- The subject is asked to be in a standing position, with the upper limb in a drooping position and the forearm in a mid-rotational position.
- (2)
- The grasping bar of the grip strength meter should be adjusted so that it is half the length from the base of the subject’s thumb to the tip of the index finger.
- (3)
- The measurement of maximum grip strength involved two consecutive measurements in the following order: right hand, left hand, 1 min rest, right hand, left hand. The recorded representative value was the average value.
- (1)
- Have the subject sit deeply in a chair and start walking on the examiner’s cue.
- (2)
- The examiner instructs the subject as follows: “Please walk as quickly as possible, go around the pole located 3 m ahead, in any direction you prefer. Once you return, please sit down on the chair immediately”.
- (3)
- The time from when the subject’s body starts moving until their buttocks made contact with the chair was measured. The measurement was taken once.
2.10. Statistical Analysis
3. Results
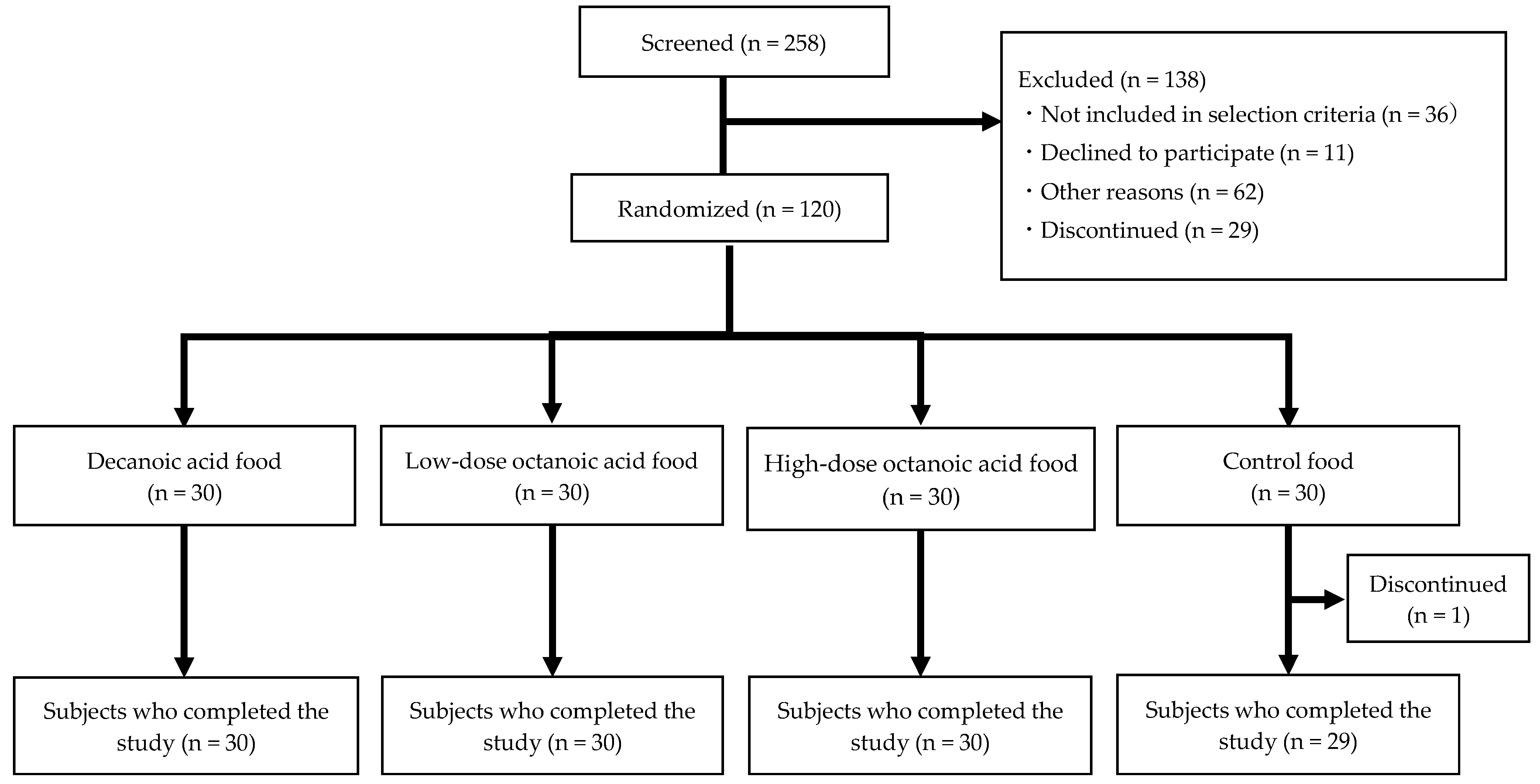
3.1. Subjects’ Eligibility, Registration, and Flowchart
3.2. Adverse Events
3.3. Nutrient Intake
3.4. Body Composition
3.5. Muscle Strength
3.6. Walking Ability (TUG)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Hashimoto, S.; Kawado, M.; Seko, R.; Murakami, Y.; Hayashi, M.; Kato, M.; Noda, T.; Ojima, T.; Nagai, M.; Tsuji, I. Trends in Disability-Free Life Expectancy in Japan, 1995-2004. J. Epidemiol. 2010, 20, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G. Increased Healthcare Costs Associated with Frailty among Community-dwelling Older People: A Systematic Review and Meta-Analysis. Arch. Gerontol. Geriatr. 2019, 84, 103898. [Google Scholar] [CrossRef]
- Murray, M.P.; Gardner, G.M.; Mollinger, L.A.; Sepic, S.B. Strength of Isometric and Isokinetic Contractions: Knee Muscles of Men Aged 20 to 86. Phys. Ther. 1980, 60, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.L.; Bandeen-Roche, K.; Varadhan, R.; Zhou, J.; Fried, L.P. Initial Manifestations of Frailty Criteria and the Development of Frailty Phenotype in the Women’s Health and Aging Study II. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Bartali, B.; Frongillo, E.A.; Bandinelli, S.; Lauretani, F.; Semba, R.D.; Fried, L.P.; Ferrucci, L. Low Nutrient Intake is an Essential Component of Frailty in Older Persons. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 589–593. [Google Scholar] [CrossRef]
- Smit, E.; Winters-Stone, K.M.; Loprinzi, P.D.; Tang, A.M.; Crespo, C.J. Lower Nutritional Status and Higher Food Insufficiency in Frail Older US Adults. Br. J. Nutr. 2013, 110, 172–178. [Google Scholar] [CrossRef]
- Vellas, B.J.; Hunt, W.C.; Romero, L.J.; Koehler, K.M.; Baumgartner, R.N.; Garry, P.J. Changes in Nutritional Status and Patterns of Morbidity among Free-Living Elderly Persons: A 10-Year Longitudinal Study. Nutrition 1997, 13, 515–519. [Google Scholar] [CrossRef]
- Japanese Association on Sarcopenia and Frailty. Guidelines for the Management of Sarcopenia 2017. In Clinical Guidelines for Sarcopenia 2017; Revised Edition; Clinical Guidelines for Sarcopenia Development Committee, Life Science Publishing Co., Ltd.: Tokyo, Japan, 2020; pp. 34–44. [Google Scholar]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.P. Prevalence of and Interventions for Sarcopenia in Ageing Adults: A Systematic Review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef]
- Pedersen, A.N.; Cederholm, T. Health Effects of Protein Intake in Healthy Elderly Populations: A Systematic Literature Review. Food Nutr. Res. 2014, 58, 23364–23402. [Google Scholar] [CrossRef]
- Morley, J.E. Anorexia of aging: Physiologic and Pathologic. Am. J. Clin. Nutr. 1997, 66, 760–773. [Google Scholar] [CrossRef]
- Morley, J.E. Anorexia of Ageing: A Key Component in the Pathogenesis of both Sarcopenia and Cachexia. J. Cachexia Sarcopenia Muscle 2017, 8, 523–526. [Google Scholar] [CrossRef]
- Papamandjaris, A.A.; MacDougall, D.E.; Jones, P.J. Medium-Chain Fatty Acid Metabolism and Energy Expenditure: Obesity Treatment Implications. Life Sci. 1998, 62, 1203–1215. [Google Scholar] [CrossRef]
- Abe, S.; Ezaki, O.; Suzuki, M. Medium-Chain Triglycerides in Combination with Leucine and Vitamin D Increase Muscle Strength and Function in Frail Elderly Adults in a Randomized Controlled Trial. J. Nutr. 2016, 146, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Nishi, Y.; Hiejima, H.; Hosoda, H.; Kaiya, H.; Mori, K.; Fukue, Y.; Yanase, T.; Nawata, H.; Kangawa, K.; Kojima, M. Ingested Medium-chain Fatty Acids are Directly Utilized for the Acyl Modification of Ghrelin. Endocrinology 2005, 146, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- Ashitani, J.; Matsumoto, N.; Nakazato, M. Effect of Octanoic Acid-Rich Formula on Plasma Ghrelin Levels in Cachectic Patients with Chronic Respiratory Disease. Nutr. J. 2009, 8, 25–28. [Google Scholar] [CrossRef]
- Pujol, J.B.; Christinat, N.; Ratinaud, Y.; Savoia, C.; Mitchell, S.E.; Dioum, E.H.M. Coordination of GPR40 and Ketogenesis Signaling by Medium-Chain Fatty Acids Regulates Beta Cell Function. Nutrients 2018, 10, 473. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Ezaki, O.; Suzuki, M. Medium-Chain Triglycerides (8:0 and 10:0) are Promising Nutrients for Sarcopenia: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2019, 110, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The Timed “Up & GO”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Hubbard, R.; Lang, I.A.; Llewellyn, D.J.; Rockwood, K. Frailty, Body Mass Index, and Abdominal Obesity in Older People. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 377–381. [Google Scholar] [CrossRef]
- Omori, G.; Koga, Y.; Tanaka, M.; Nawata, A.; Watanabe, H.; Narumi, K.; Endoh, K. Quadriceps Muscle Strength and its Relationship to Radiographic Knee Osteoarthritis in Japanese Elderly. J. Orthop. Sci. 2013, 18, 536–542. [Google Scholar] [CrossRef]
- Narumi, K.; Funaki, Y.; Yoshimura, N.; Muraki, S.; Omori, G.; Nawata, A.; Seki, R. Quadriceps Muscle Strength Reference Value as Index for Functional Deterioration of Locomotive Organs: Data from 3617 Men and Women in Japan. J. Orthop. Sci. 2017, 22, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Judge, J.O.; Underwood, M.; Gennosa, T. Exercise to Improve Gait Velocity in Older Persons. Arch. Phys. Med. Rehabil. 1993, 74, 400–406. [Google Scholar] [PubMed]
- Hunter, G.R.; Treuth, M.S.; Weinsier, R.L.; Kekes-Szabo, T.; Kell, S.H.; Roth, D.L.; Nicholson, C. The Effects of Strength Conditioning on Older Women’s Ability to Perform Daily Tasks. J. Am. Geriatr. Soc. 1995, 43, 756–760. [Google Scholar] [CrossRef]
- Rantanen, T.; Avela, J. Leg Extension Power and Walking Speed in Very Old People Living Independently. J. Gerontol. 1997, 52, M225–M231. [Google Scholar] [CrossRef]
- Asakawa, Y.; Ikezoe, T.; Hazaki, K.; Kawano, I.; Irie, S.; Kanzaki, H.; Aoki, N. Relationship between Falls and Knee Extension Strength in the Elderly. J. Phys. Ther. Sci. 1996, 8, 45–48. [Google Scholar] [CrossRef]
- Rantanen, T.; Guralnik, J.M.; Izmirlian, G.; Williamson, J.D.; Simonsick, E.M.; Ferrucci, L.; Fried, L.P. Association of Muscle Strength with Maximum Walking Speed in Disabled Older Women. Am. J. Phys. Med. Rehabil. 1998, 77, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.; Hug, S.; Dunlop, D.; Singer, R.; Chang, R.W. Predictors of Change in Walking Velocity in Older Adults. J. Am. Geriatr. Soc. 1996, 44, 126–132. [Google Scholar] [CrossRef]
- Tinetti, M.E.; Speechly, M.; Ginter, S.F. Risk Factor for Falls among Elderly Persons Living in the Community. N. Engl. J. Med. 1988, 319, 1701–1707. [Google Scholar] [CrossRef]
- Visser, M.; Goodpaster, B.H.; Kritchevsky, S.B.; Newman, A.B.; Nevitt, M.; Rubin, S.M.; Simonsick, E.M.; Harris, T.B. Muscle Mass, Muscle Strength, and Muscle Fat Infiltration as Predictors of Incident Mobility Limitations in Well-Functioning Older Persons. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 324–333. [Google Scholar] [CrossRef]
- Guralnik, J.M.; LaCroix, A.Z.; Abbott, R.D.; Berkman, L.F.; Satterfield, S.; Evans, D.A.; Wallace, R.B. Maintaining Mobility in Late Life. I. Demographic Characteristics and Chronic Conditions. Am. J. Epidemiol. 1993, 137, 845–857. [Google Scholar] [CrossRef]
- Fried, L.P.; Guralnik, J.M. Disability in Older Adults: Evidence Regarding Significance, Etiology, and Risk. J. Am. Geriatr. Soc. 1997, 45, 92–100. [Google Scholar] [CrossRef]
- Jylha, M.; Guralnik, J.M.; Balfour, J.; Fried, L.P. Walking Difficulty, Walking Speed, and Age as Predictors of Self-Rated Health: The Women’s Health and Aging Study. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M609–M617. [Google Scholar] [CrossRef] [PubMed]
- Manty, M.; Heinonen, A.; Viljanen, A.; Pajala, S.; Koskenvuo, M.; Kaprio, J.; Rantanen, T. Self-Reported Preclinical Mobility Limitation and Fall History as Predictors of Future Falls in Older Women: Prospective Cohort Study. Osteoporos. Int. 2010, 21, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Volpato, S.; Cavalieri, M.; Sioulis, F.; Guerra, G.; Maraldi, C.; Zuliani, G.; Fellin, R.; Guralnik, J.M. Predictive Value of the Short Physical Performance Battery Following Hospitalization in Older Patients. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Ferrucci, L.; Balfour, J.L.; Volpato, S.; Lorio, A.D. Progressive Versus Catastrophic Loss of the Ability to Walk: Implications for the Prevention of Mobility Loss. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 49, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.C.; Ribeiro, S.M.; Luna, N.M.S.; Peterson, M.D.; Bocalini, D.S.; Serra, M.M.; Brech, G.C.; Greve, J.M.D.; Garcez-Leme, L.E. Association between Handgrip Strength, Balance, and Knee Flexion/Extension Strength in Older Adults. PLoS ONE 2018, 13, e0198185. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.; Kuh, D.; Cooper, C.; Gale, C.R.; Lawlor, D.A.; Matthews, F.; Hardy, R. Objective Measures of Physical Capability and Subsequent Health: A Systematic Review. Age Ageing 2011, 40, 14–23. [Google Scholar] [CrossRef]
- Syddall, H.; Cooper, C.; Martin, Z.F.; Briggs, R.; Sayer, A.A. Is Grip Strength a Useful Single Marker of Frailty? Age Ageing 2003, 32, 650–656. [Google Scholar] [CrossRef]
- Sayer, A.A.; Syddall, H.E.; Martin, H.J.; Dennison, E.M.; Roberts, H.C.; Cooper, C. Is Grip Strength Associated with Health-Related Quality of Life? Findings from the Hertfordshire Cohort Study. Age Ageing 2006, 35, 409–415. [Google Scholar] [CrossRef]
- Snih, S.A.; Markides, K.S.; Ottenbacher, K.J.; Raji, M.A. Hand Grip Strength and Incident ADL Disability in Elderly Mexican Americans over a Seven-Year Period. Aging Clin. Exp. Res. 2004, 16, 481–486. [Google Scholar] [CrossRef]
- Rantanen, T.; Volpato, S.; Ferrucci, L.; Heikkinen, E.; Fried, L.P.; Guralnik, J.M. Handgrip Strength and Cause-Specific and Total Mortality in Older Disabled Women, Exploring the Mechanism. J. Am. Geriatr. Soc. 2003, 51, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Bueno, N.B.; De Melo, I.V.; Florencio, T.T.; Sawaya, A.L. Dietary Medium-Chain Triacylglycerols versus Long-Chain Triacylglycerols for Body Composition in Adults, Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Coll. Nutr. 2015, 34, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Mumme, K.; Stonehouse, W. Effects of Medium-Chain Triglycerides on Weight Loss and Body Composition: A Meta-Analysis of Randomized Controlled Trials. J. Acad. Nutr. Diet. 2015, 115, 249–263. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Bosarge, A. Weight-loss Diet that Includes Consumption of Medium-Chain Triacylglycerol Oil Leads to a Greater Rate of Weight and Fat Mass Loss than Does Olive Oil. Am. J. Clin. Nutr. 2008, 87, 621–626. [Google Scholar] [CrossRef]
- Phillips, S.M.; Tang, J.E.; Moore, D.R. The Role of Milk- and Soy-Based Protein in Support of Muscle Protein Synthesis and Muscle Protein Accretion in Young and Elderly Persons. J. Am. Coll. Nutr. 2009, 28, 343–354. [Google Scholar] [CrossRef]
- Kyle, U.G.; Genton, L.; Hans, D.; Karsegard, L.; Slosman, D.O.; Pichard, C. Age-Related Differences in Fat-Free Mass, Skeletal Muscle, Body Cell Mass and Fat Mass between 18 and 94 Years. Eur. J. Clin. Nutr. 2001, 55, 663–672. [Google Scholar] [CrossRef]
- Balagopal, P.; Rooyackers, O.E.; Adey, D.B.; Ades, P.A.; Nair, K.S. Effects of Aging on in vivo Synthesis of Skeletal Muscle Myosin Heavy-Chain and Sarcoplasmic Protein in Humans. Am. J. Physiol. 1997, 273, E790–E800. [Google Scholar] [CrossRef]
- Nishimura, S.; Inai, M.; Takagi, T.; Nonaka, Y.; Urashima, S.; Honda, K.; Aoyama, T.; Terada, S. Preventive Effects of the Dietary Intake of Medium-chain Triacylglycerols on Immobilization-induced Muscle Atrophy in Rats. J. Oleo Sci. 2017, 66, 917–924. [Google Scholar] [CrossRef]
- Ishizawa, R.; Masuda, K.; Sakata, S.; Nakatani, A. Effects of Different Fatty Acid Chain Lengths on Fatty Acid Oxidation-Related Protein Expression Levels in Rat Skeletal Muscles. J. Oleo Sci. 2015, 64, 415–421. [Google Scholar] [CrossRef]
- Fushiki, T.; Matsumoto, K.; Inoue, K.; Kawada, T.; Sugimoto, E. Swimming Endurance Capacity of Mice is Increased by Chronic Consumption of Medium-Chain Triglycerides. J. Nutr. 1995, 125, 531–539. [Google Scholar]
- Nagai, N.; Yagyu, S.; Hata, A.; Nirengi, S.; Kotani, K.; Moritani, T.; Sakane, N. Maslinic Acid Derived from Olive Fruit in Combination with Resistance Training Improves Muscle Mass and Mobility Functions in the Elderly. J. Clin. Biochem. Nutr. 2019, 64, 224–230. [Google Scholar] [CrossRef]
- Vukovich, M.D.; Stubbs, N.B.; Bohlken, R.M. Body Composition in 70-Year-Old Adults Responds to Dietary β-Hydroxy-β-Methylbutyrate Similarly to That of Young Adults. J. Nutr. 2001, 131, 2049–2052. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.R.; Smith-Ryan, A.E.; Fukuda, D.H.; Kendall, K.L.; Moon, J.R.; Hoffman, J.R.; Wilson, J.M.; Oliver, J.S.; Mustad, V.A. Effect of Calcium β-Hydroxy-β-Methylbutyrate (CaHMB) with and without Resistance Training in Men and Women 65+ yrs: A Randomized, Double-Blind Pilot Trial. Exp. Gerontol. 2013, 48, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Navarro, M.; Peragón, J.; Amores, V.; Higuera, M.D.L.; Lupiáñez, J.A. Maslinic Acid Added to the Diet Increases Growth and Protein-Turnover Rates in the White Muscle of Rainbow Trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008, 147, 158–167. [Google Scholar] [CrossRef]
- Murata, S.; Sasaki, T.; Yamauchi, Y.; Shimizu, M.; Sato, R. Maslinic Acid Activates mTORC1 and Human TGR5 and Induces Skeletal Muscle Hypertrophy. Biosci. Biotechnol. Biochem. 2021, 85, 2311–2321. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Ferdousi, F.; Fukumitsu, S.; Isoda, H. Maslinic Acid Attenuates Denervation-Induced Loss of Skeletal Muscle Mass and Strength. Nutrients 2021, 13, 2950. [Google Scholar] [CrossRef]
- Smith, H.J.; Mukerji, P.; Tisdale, M.J. Attenuation of Proteasome-Induced Proteolysis in Skeletal Muscle by β-Hydroxy-β-Methylbutyrate in Cancer-Induced Muscle Loss. Cancer Res. 2005, 65, 277–283. [Google Scholar] [CrossRef]
- Eley, H.L.; Russell, S.T.; Baxter, J.H.; Mukerji, P.; Tisdale, M.J. Signaling Pathways Initiated by β-Hydroxy-β-Methylbutyrate to Attenuate the Depression of Protein Synthesis in Skeletal Muscle in Response to Cachectic Stimuli. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E923–E931. [Google Scholar] [CrossRef]
- Aversa, Z.; Bonetto, A.; Costelli, P.; Minero, V.G.; Penna, F.; Baccino, F.M.; Lucia, S.; Fanelli, F.R.; Muscaritoli, M. β-Hydroxy-β-Methylbutyrate (HMB) Attenuates Muscle and Body Weight Loss in Experimental Cancer Cachexia. Int. J. Oncol. 2011, 38, 713–720. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of Leucine and its Metabolite β-Hydroxy-β-Hethylbutyrate on Human Skeletal Muscle Protein Metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar] [CrossRef]
- Gerlinger-Romero, F.; Guimarães-Ferreira, L.; Giannocco, G.; Nunes, M.T. Chronic Supplementation of beta-Hydroxy-beta Methylbutyrate (HMβ) Increases the Activity of the GH/IGF-I Axis and Induces Hyperinsulinemia in Rats. Growth Horm. IGF Res. 2011, 21, 57–62. [Google Scholar] [CrossRef]
- Kornasio, R.; Riederer, I.; Butler-Browne, G.; Mouly, V.; Uni, Z.; Halevy, O. β-Hydroxy-β-Methylbutyrate (HMB) Stimulates Myogenic Cell Proliferation, Differentiation and Survival via the MAPK/ERK and PI3K/Akt Pathways. Biochim. Biophys. Acta 2009, 1793, 755–763. [Google Scholar] [CrossRef]
- Townsend, J.R.; Hoffman, J.R.; Gonzalez, A.M.; Jajtner, A.R.; Boone, C.H.; Robinson, E.H.; Mangine, G.T.; Wells, A.J.; Fragala, M.S.; Fukuda, D.H.; et al. Effects of β-Hydroxy-β-Methylbutyrate Free Acid Ingestion and Resistance Exercise on the Acute Endocrine Response. Int. J. Endocrinol. 2015, 2015, 856708–856714. [Google Scholar] [CrossRef]
- American College of Sports Medicine. American College of Sports Medicine Position Stand. Progression Models in Resistance Training for Healthy Adults. Med. Sci. Sport. Exerc. 2009, 41, 687–708. [Google Scholar] [CrossRef]
- Balakrishnan, V.S.; Rao, M.; Menon, V.; Gordon, P.L.; Pilichowska, M.; Castaneda, F.; Castaneda-Sceppa, C. Resistance Training Increases Muscle Mitochondrial Biogenesis in Patients with Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 996–1002. [Google Scholar] [CrossRef]
- Wakabayashi, H. Rehabilitation and Clinical Nutrition. Jpn. J. Rehabil. Med. 2011, 48, 270–281. [Google Scholar] [CrossRef]
- Williams, M.A.; Haskell, W.L.; Ades, P.A.; Amsterdam, E.A.; Bittner, V.; Franklin, B.A.; Gulanick, M.; Laing, S.T.; Stewart, K.J. Resistance Exercise in Individuals with and without Cardiovascular Disease: 2007 update: A scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation 2007, 116, 572–584. [Google Scholar] [CrossRef]
- Mitchell, C.J.; Churchward-Venne, T.A.; West, D.W.D.; Burd, N.A.; Breen, L.; Baker, S.K.; Phillips, S.M. Resistance Exercise Load Does Not Determine Training-Mediated Hypertrophic Gains in Young Men. J. Appl. Physiol. 2012, 113, 71–77. [Google Scholar] [CrossRef]
- Roie, E.V.; Delecluse, C.; Coudyzer, W.; Boonen, S.; Bautmans, I. Strength Training at High versus Low External Resistance in Older Adults: Effects on Muscle Volume, Muscle Strength, and Force-Velocity Characteristics. Exp. Gerontol. 2013, 48, 1351–1361. [Google Scholar] [CrossRef]
- Miura, S.; Kawanaka, K.; Kai, Y.; Tamura, M.; Goto, M.; Shiuchi, T.; Minokoshi, Y.; Ezaki, O. An Increase in Murine Skeletal Muscle Peroxisome Proliferator-Activated Receptor-γ Coactivator-1α(PGC-1α) mRNA in Response to Exercise Is Mediated by β-Adrenergic Receptor Activation. Endocrinology 2007, 148, 3441–3448. [Google Scholar] [CrossRef]
- Pahlavani, H.A.; Laher, I.; Knechtle, B.; Zouhal, H. Exercise and Mitochondrial Mechanisms in Patients with Sarcopenia. Front. Physiol. 2022, 13, 1040381. [Google Scholar] [CrossRef]
- Yamada, M.; Arai, H.; Yoshimura, K.; Kajiwara, Y.; Sonoda, T.; Nishiguchi, S.; Aoyama, T. Nutritional Supplementation During Resistance Training Improved Skeletal Muscle Mass in Community-Dwelling Frail Older Adults. J. Frailty Ageing 2012, 1, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, A.; Koike, A.; Karasawa, T.; Tsutsui, M.; Kondo, S.; Terada, S. Effects of a Ketogenic Diet Containing Medium-Chain Triglycerides and Endurance Training on Metabolic Enzyme Adaptations in Rat Skeletal Muscle. Nutrients 2020, 12, 1269. [Google Scholar] [CrossRef] [PubMed]
- Koshinaka, K.; Toshinai, K.; Mohammad, A.; Noma, K.; Oshikawa, M.; Ueno, H.; Yamaguchi, H.; Nakazato, M. Therapeutic Potential of Ghrelin Treatment for Unloading-Induced Muscle Atrophy in Mice. Biochem. Biophys. Res. Commun. 2011, 412, 296–301. [Google Scholar] [CrossRef]
- Kawai, K.; Nakashima, M.; Kojima, M.; Yamashita, S.; Takakura, S.; Shimiz, M.; Kubo, C.; Sudo, N. Ghrelin Activation and Neuropeptide Y Elevation in Response to Medium-Chain Triglyceride Administration in Anorexia Nervosa Patients. Clin. Nutr. ESPEN 2017, 17, 100–104. [Google Scholar] [CrossRef]
- Forman, B.M.; Chen, J.; Evans, R.M. Hypolipidemic Drugs, Polyunsaturated Fatty Acids, and Eicosanoids are Ligands for Peroxisome Proliferator-Activated Receptors α and δ. Proc. Natl. Acad. Sci. USA 1997, 94, 4312–4317. [Google Scholar] [CrossRef] [PubMed]
- Malapaka, R.R.V.; Khoo, S.; Zhang, J.; Choi, J.H.; Zhou, X.E.; Xu, Y.; Gong, Y.; Li, J.; Yong, E.L.; Chalmers, M.J.; et al. Identification and Mechanism of 10-Carbon Fatty Acid as Modulating Ligand of Peroxisome Proliferator-activated Receptors. J. Biol. Chem. 2012, 287, 183–195. [Google Scholar] [CrossRef]
- Tsujino, S.; Nosaka, N.; Sadamitsu, S.; Kato, K. Effect of Continuous Ingestion of 2 g of Medium-Chain Triglycerides on Substrate Metabolism during Low-Intensity Physical Activity. Nutrients 2022, 14, 536. [Google Scholar] [CrossRef]
- Sekine, S.; Terada, S.; Aoyama, T. Medium-Chain Triacylglycerol Suppresses the Decrease of Plasma Albumin Level through the Insulin-Akt-mTOR Pathway in the Livers of Malnourished Rats. J. Nutr. Sci. Vitaminol. 2013, 59, 123–128. [Google Scholar] [CrossRef][Green Version]
- Nosaka, N.; Adachi, K.; Kawashima, Y.; Suzuki, H.; Hayashi, S.; Aoyama, T.; Nakamura, T. Effect of Ingestion of Medium-Chain Fatty Acids on Serum Albumin in the Elderly with Protein-Energy Malnutrition (PEM). J. Jpn. Soc. Clin. Nutr. 2010, 32, 52–61. [Google Scholar]
- Hirabayashi, T. (Kobe University Hospital, Kobe, Hyogo, Japan); Fujino, H. (Kobe University, Kobe, Hyogo, Japan). Personal communication, 2022.
- Kimura, M. (University of Yamanashi, Kofu, Yamanashi, Japan); Mochizuki, K. (University of Yamanashi, Kofu, Yamanashi, Japan). Personal communication, 2023.
- Bischoff, H.A.; Conzelmann, M.; Lindemann, D.; Singer-Lindpaintner, L.; Stucki, G.; Vonthein, R.; Dick, W.; Theiler, R.; Stähelin, H.B. Self-Reported Exercise before Age 40: Influence on Quantitative Skeletal Ultrasound and Fall Risk in the Elderly. Arch. Phys. Med. Rehabil. 2001, 82, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Samson, M.M.; Meeuwsen, I.B.; Crowe, A.; Dessens, J.A.; Duursma, S.A.; Verhaar, H.J. Relationships between Physical Performance Measures, Age, Height and Body Weight in Healthy Adults. Age Ageing 2000, 29, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Shumway-Cook, A.; Brauer, S. Predicting the Probability for Falls in Community-Dwelling Older Adults Using the Timed Up & Go Test. Phys. Ther. 2000, 80, 896–903. [Google Scholar] [CrossRef]
- Furuna, T.; Nagasaki, H.; Nishizawa, S.; Sugiura, M.; Okuzumi, H.; Ito, H.; Kinugasa, T.; Hashizume, K.; Maruyama, H. Longitudinal Change in the Physical Performance of Older Adults in the Community. J. Jpn. Phys. Ther. Assoc. 1998, 1, 1–5. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Ferrucci, L.; Simonsick, E.M.; Salive, M.E.; Wallace, R.B. Lower-Extremity Function in Persons over the Age of 70 Years as a Predictor of Subsequent Disability. N. Engl. J. Med. 1995, 332, 556–561. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Mutoh, T.; Kunitoki, K.; Tatewaki, Y.; Yamamoto, S.; Thyreau, B.; Matsudaira, I.; Kawashima, R.; Taki, Y. Impact of Medium-Chain Triglycerides on Gait Performance and Brain Metabolic Network in Healthy Older Adults: A Double-Blind, Randomized Controlled Study. Geroscience 2022, 44, 1325–1338. [Google Scholar] [CrossRef]
- Lin, C.H.; Faisal, A.A. Decomposing Sensorimotor Variability Changes in Ageing and Their Connection to Falls in Older People. Sci. Rep. 2018, 8, 14546–14558. [Google Scholar] [CrossRef]
- Kuhl, D.E.; Metter, E.J.; Riege, W.H.; Phelps, M.E. Effects of Human Aging on Patterns of Local Cerebral Glucose Utilization Determined by the [18F] Fluorodeoxyglucose Method. J. Cereb. Blood Flow. Metab. 1982, 2, 163–171. [Google Scholar] [CrossRef]
- Lying-Tunell, U.; Lindblad, B.S.; Malmlund, H.O.; Persson, B. Cerebral Blood Flow and Metabolic Rate of Oxygen, Glucose, Lactate, Pyruvate, Ketone Bodies and Amino Acids. Acta Neurol. Scand. 1981, 63, 337–350. [Google Scholar] [CrossRef]
- Kathleen, A.P.; Williamson, A.; Yu, N.; McNay, E.C.; Dzuira, J.; McCrimmon, R.J.; Sherwin, R.S. Medium-Chain Fatty Acids Improve Cognitive Function in Intensively Treated Type 1 Diabetic Patients and Support In Vitro Synaptic Transmission During Acute Hypoglycemia. Diabetes 2009, 58, 1237–1244. [Google Scholar] [CrossRef]
- Veneman, T.; Mitrakou, A.; Mokan, M.; Cryer, P.; Gerich, J. Effect of Hyperketonemia and Hyperlacticacidemia on Symptoms, Cognitive Dysfunction, and Counterregulatory Hormone Responses during Hypoglycemia in Normal Humans. Diabetes 1994, 43, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Reger, M.A.; Henderson, S.T.; Hale, C.; Cholerton, B.; Baker, L.D.; Watson, G.S.; Hyde, K.; Chapman, D.; Craft, S. Effects of β-Hydroxybutyrate on Cognition in Memory-Impaired Adults. Neurobiol. Aging 2004, 25, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.T.; Vogel, J.L.; Barr, L.J.; Garvin, F.; Jones, J.J.; Costantini, L.C. Study of the Ketogenic Agent AC-1202 in Mild to Moderate Alzheimer’s Disease: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. Nutr. Metab. 2009, 6, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Fujiwara, Y.; Chaves, P.H.M.; Motohashi, Y.; Shinkai, S. Frequency of Going Outdoors as a Good Predictors for Incident Disability of Physical Function as well as Disability Recovery in Community-Dwelling Older Adults in Rural Japan. J. Epidemiol. 2006, 16, 261–270. [Google Scholar] [CrossRef]
- Tsubokawa, T.; Shobugawa, Y.; Iguchi, S.; Suzuki, T.; Watanabe, M.; Saito, R.; Kondo, K. Do Community Social Capital and Built Environment Associate with Homebound in Older Adults? The JAGES Niigata Study. J. Epidemiol. 2022, 32, 254–269. [Google Scholar] [CrossRef]
| Control Food | Decanoic Acid Food | Low-Dose Octanoic Acid Food | High-Dose Octanoic Acid Food | ||
|---|---|---|---|---|---|
| LCT | g/day 1 | 6 | - | 4 | - |
| MCT | g/day 1 | - | 6 | 2 | 6 |
| Octanoic acid (8:0) | g/day 2 | (-) | (1.40, 1.95) | (1.24, 1.38) | (3.72, 4.14) |
| Decanoic acid (10:0) | g/day 2 | (-) | (3.47, 4.00) | (0.352, 0.487) | (1.06, 1.46) |
| PPS (n = 112) | ||||
|---|---|---|---|---|
| Control Food | Decanoic Acid Food | Low-Dose Octanoic Acid Food | High-Dose Octanoic Acid Food | |
| Number of subjects, M/F | 29 (14/15) | 27 (12/15) | 28 (13/15) | 28 (15/13) |
| Age, years | 67.6 ± 0.8 | 67.9 ± 0.8 | 68.2 ± 0.8 | 67.7 ± 0.8 |
| Height, cm | 160.2 ± 1.3 | 161.4 ± 1.5 | 159.6 ± 1.7 | 163.4 ± 1.5 |
| Blood albumin level, g/dL | 4.5 ± 0.0 | 4.6 ± 0.1 | 4.6 ± 0.1 | 4.5 ± 0.1 |
| PPS 3 (n = 112) | |||||
|---|---|---|---|---|---|
| Week | Control Food | Decanoic Acid Food | Low-Dose Octanoic Acid Food | High-Dose Octanoic Acid Food | |
| Energy, kcal/day | 0 | 1600.4 ± 104.7 | 1585.3 ± 75.7 | 1572.0 ± 80.7 | 1710.6 ± 92.5 |
| 4 | 1598.6 ± 106.3 | 1594.4 ± 76.3 | 1578.3 ± 74.3 | 1733.3 ± 95.6 | |
| 8 | 1583.3 ± 99.4 | 1570.1 ± 85.4 | 1553.3 ± 83.4 | 1776.7 ± 82.1 | |
| 12 | 1563.7 ± 101.5 | 1552.2 ± 76.5 | 1579.8 ± 70.1 | 1717.7 ± 91.2 | |
| Fat, g/day | 0 | 49.9 ± 3.6 | 51.9 ± 3.6 | 53.5 ± 2.6 | 53.4 ± 3.7 |
| 4 | 49.9 ± 3.7 | 52.8 ± 3.4 | 53.0 ± 2.8 | 54.1 ± 3.8 | |
| 8 | 47.9 ± 3.2 | 54.1 ± 3.6 | 52.5 ± 3.1 | 55.7 ± 3.8 | |
| 12 | 48.8 ± 3.5 | 51.0 ± 3.4 | 53.8 ± 2.7 | 54.4 ± 3.8 | |
| Saturated fatty acid, g/day 4 | 0 | 12.8 ± 1.1 | 13.5 ± 0.9 | 13.7 ± 0.7 | 14.0 ± 1.1 |
| 4 | 12.8 ± 1.1 | 13.7 ± 0.9 | 13.4 ± 0.8 | 14.2 ± 1.1 | |
| 8 | 12.5 ± 1.0 | 14.0 ± 1.0 | 13.4 ± 0.8 | 14.3 ± 1.1 | |
| 12 | 12.6 ± 1.1 | 13.8 ± 0.8 | 13.8 ± 0.8 | 14.2 ± 1.1 | |
| Monounsaturated fatty acid, g/day | 0 | 17.9 ± 1.3 | 18.5 ± 1.4 | 19.0 ± 1.0 | 18.9 ± 1.4 |
| 4 | 17.9 ± 1.3 | 18.9 ± 1.3 | 18.8 ± 1.1 | 19.2 ± 1.4 | |
| 8 | 17.0 ± 1.2 | 19.4 ± 1.4 | 18.6 ± 1.1 | 19.8 ± 1.5 | |
| 12 | 17.4 ± 1.3 | 18.1 ± 1.3 | 19.1 ± 1.0 | 19.3 ± 1.4 | |
| n-6 Polyunsaturated fatty acid, g/day | 0 | 9.9 ± 0.6 | 10.4 ± 0.7 | 10.7 ± 0.6 | 10.5 ± 0.7 |
| 4 | 9.9 ± 0.6 | 10.6 ± 0.7 | 10.8 ± 0.6 | 10.7 ± 0.7 | |
| 8 | 9.5 ± 0.6 | 10.9 ± 0.7 | 10.4 ± 0.6 | 11.1 ± 0.7 | |
| 12 | 9.7 ± 0.6 | 10.1 ± 0.7 | 10.8 ± 0.6 | 10.7 ± 0.7 | |
| n-3 Polyunsaturated fatty acid, g/day | 0 | 2.4 ± 0.2 | 2.5 ± 0.2 | 2.6 ± 0.2 | 2.6 ± 0.2 |
| 4 | 2.4 ± 0.2 | 2.5 ± 0.2 | 2.7 ± 0.2 | 2.6 ± 0.2 | |
| 8 | 2.2 ± 0.2 | 2.5 ± 0.2 | 2.7 ± 0.3 | 2.8 ± 0.2 | |
| 12 | 2.3 ± 0.2 | 2.5 ± 0.2 | 2.6 ± 0.2 | 2.7 ± 0.2 | |
| Medium chain fatty acid, mg/day 5 | 0 | 259.0 ± 43.8 | 316.9 ± 43.8 | 289.1 ± 38.0 | 309.0 ± 51.4 |
| 4 | 254.5 ± 43.9 | 316.8 ± 43.0 | 283.3 ± 29.3 | 318.2 ± 53.4 | |
| 8 | 265.5 ± 42.0 | 318.8 ± 44.4 | 296.5 ± 44.0 | 307.3 ± 44.5 | |
| 12 | 265.8 ± 43.4 | 334.4 ± 44.4 | 299.6 ± 39.1 | 313.4 ± 49.9 | |
| Protein, g/day | 0 | 61.5 ± 3.9 | 63.9 ± 3.9 | 66.9 ± 3.6 | 70.5 ± 3.9 |
| 4 | 61.9 ± 4.0 | 64.1 ± 3.8 | 66.5 ± 3.9 | 71.6 ± 4.4 | |
| 8 | 62.4 ± 3.7 | 63.3 ± 3.9 | 66.3 ± 4.2 | 71.9 ± 4.1 | |
| 12 | 59.6 ± 3.7 | 63.4 ± 3.9 | 66.7 ± 3.7 | 72.2 ± 4.4 | |
| Carbohydrate, g/day | 0 | 212.8 ± 15.0 | 197.2 ± 8.2 | 193.6 ± 12.2 | 217.0 ± 13.3 |
| 4 | 212.3 ± 15.2 | 196.2 ± 9.2 | 198.6 ± 10.2 | 220.0 ± 13.3 | |
| 8 | 212.6 ± 14.7 | 190.4 ± 9.9 | 190.5 ± 13.7 | 228.0 ± 11.4 | |
| 12 | 208.6 ± 14.6 | 194.9 ± 9.2 | 196.1 ± 9.9 | 215.6 ± 12.8 | |
| Cholesterol, mg/day | 0 | 372.4 ± 35.3 | 353.5 ± 31.3 | 403.9 ± 31.9 | 432.8 ± 44.6 |
| 4 | 369.2 ± 35.7 | 361.6 ± 29.9 | 400.0 ± 32.5 | 436.2 ± 46.4 | |
| 8 | 367.9 ± 33.6 | 368.2 ± 34.1 | 404.4 ± 38.4 | 431.9 ± 42.2 | |
| 12 | 354.0 ± 32.1 | 349.4 ± 31.1 | 398.3 ± 31.8 | 444.2 ± 46.1 | |
| Alcohol, g/day | 0 | 5.1 ± 1.5 | 8.4 ± 3.1 | 5.1 ± 2.2 | 8.9 ± 2.0 |
| 4 | 4.9 ± 1.4 | 8.9 ± 3.4 | 4.0 ± 1.7 | 8.8 ± 2.0 | |
| 8 | 5.1 ± 1.5 | 7.8 ± 2.8 | 5.4 ± 2.0 | 8.1 ± 2.1 | |
| 12 | 4.8 ± 1.4 | 6.5 ± 2.7 | 4.4 ± 1.9 | 8.3 ± 1.9 | |
| Control Food | Decanoic Acid Food | Low-Dose Octanoic Acid Food | High-Dose Octanoic Acid Food | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Week | Measurement Values | Change Values | Measurement Values | Change Values | Measurement Values | Change Values | Measurement Values | Change Values | |
| Body weight, kg | 0 | 56.4 ± 1.3 | 56.1 ± 1.6 | 55.3 ± 1.6 | 57.3 ± 1.3 | ||||
| 4 | 56.1 ± 1.4 | −0.2 ± 0.1 | 56.1 ± 1.5 | 0.0 ± 0.1 | 55.2 ± 1.6 | −0.1 ± 0.1 | 57.4 ± 1.3 | 0.1 ± 0.1 | |
| 8 | 56.0 ± 1.4 | −0.4 ± 0.2 | 55.8 ± 1.5 | −0.3 ± 0.1 | 55.2 ± 1.5 | −0.1 ± 0.1 | 57.2 ± 1.3 | −0.1 ± 0.2 | |
| 12 | 55.7 ± 1.3 | −0.7 ± 0.2 | 55.8 ± 1.5 | −0.3 ± 0.2 | 54.9 ± 1.6 | −0.5 ± 0.1 | 56.9 ± 1.3 | −0.4 ± 0.2 | |
| Protein mass, kg | 0 | 8.3 ± 0.2 | 8.2 ± 0.3 | 8.4 ± 0.3 | 8.8 ± 0.3 | ||||
| 4 | 8.3 ± 0.2 | 0.0 ± 0.0 | 8.2 ± 0.3 | 0.0 ± 0.0 | 8.4 ± 0.3 | 0.0 ± 0.0 | 8.8 ± 0.3 | 0.0 ± 0.0 | |
| 8 | 8.2 ± 0.2 | −0.1 ± 0.0 | 8.2 ± 0.3 | −0.1 ± 0.0 | 8.3 ± 0.3 | −0.1 ± 0.0 | 8.8 ± 0.2 | −0.1 ± 0.0 | |
| 12 | 8.2 ± 0.2 | −0.1 ± 0.0 | 8.2 ± 0.3 | 0.0 ± 0.0 | 8.3 ± 0.3 | −0.1 ± 0.0 | 8.8 ± 0.3 | 0.000 | |
| Body fat mass, kg | 0 | 14.0 ± 0.7 | 14.1 ± 0.7 | 12.4 ± 0.6 | 12.3 ± 0.7 | ||||
| 4 | 13.6 ± 0.7 | −0.4 ± 0.1 | 14.0 ± 0.6 | −0.1 ± 0.2 | 12.2 ± 0.6 | −0.2 ± 0.2 | 12.2 ± 0.7 | 0.0 ± 0.2 | |
| 8 | 14.0 ± 0.7 | 0.0 ± 0.1 | 14.0 ± 0.6 | −0.1 ± 0.2 | 12.6 ± 0.6 | 0.1 ± 0.1 | 12.3 ± 0.7 | 0.0 ± 0.2 | |
| 12 | 13.5 ± 0.6 | −0.5 ± 0.2 | 13.7 ± 0.6 | −0.3 ± 0.2 | 12.3 ± 0.6 | −0.2 ± 0.2 | 11.9 ± 0.7 | −0.3 ± 0.3 | |
| Muscle mass, kg | 0 | 39.9 ± 1.2 | 39.6 ± 1.5 | 40.3 ± 1.5 | 42.4 ± 1.2 | ||||
| 4 | 40.0 ± 1.2 | 0.1 ± 0.1 | 39.7 ± 1.4 | 0.1 ± 0.2 | 40.5 ± 1.5 | 0.1 ± 0.2 | 42.5 ± 1.2 | 0.1 ± 0.1 | |
| 8 | 39.5 ± 1.2 | −0.4 ± 0.2 | 39.4 ± 1.4 | −0.2 ± 0.1 | 40.1 ± 1.5 | −0.2 ± 0.2 | 42.3 ± 1.2 | −0.2 ± 0.2 | |
| 12 | 39.8 ± 1.2 | −0.1 ± 0.2 | 39.6 ± 1.5 | 0.0 ± 0.2 | 40.1 ± 1.5 | −0.2 ± 0.2 | 42.4 ± 1.2 | 0.0 ± 0.2 | |
| Lean body mass, kg | 0 | 42.4 ± 1.2 | 42.1 ± 1.5 | 42.9 ± 1.6 | 45.1 ± 1.3 | ||||
| 4 | 42.5 ± 1.2 | 0.1 ± 0.2 | 42.2 ± 1.5 | 0.1 ± 0.2 | 43.0 ± 1.6 | 0.1 ± 0.2 | 45.2 ± 1.3 | 0.1 ± 0.2 | |
| 8 | 41.9 ± 1.2 | −0.4 ± 0.2 | 41.9 ± 1.5 | −0.2 ± 0.1 | 42.6 ± 1.5 | −0.3 ± 0.2 | 44.9 ± 1.2 | −0.2 ± 0.2 | |
| 12 | 42.2 ± 1.2 | −0.2 ± 0.2 | 42.1 ± 1.5 | 0.0 ± 0.2 | 42.6 ± 1.6 | −0.3 ± 0.2 | 45.0 ± 1.3 | 0.0 ± 0.2 | |
| Skeletal muscle mass, kg | 0 | 22.9 ± 0.7 | 22.8 ± 0.9 | 23.2 ± 0.9 | 24.6 ± 0.8 | ||||
| 4 | 23.0 ± 0.7 | 0.1 ± 0.1 | 22.8 ± 0.9 | 0.0 ± 0.1 | 23.3 ± 0.9 | 0.1 ± 0.1 | 24.6 ± 0.8 | 0.0 ± 0.1 | |
| 8 | 22.7 ± 0.7 | −0.3 ± 0.1 | 22.7 ± 0.9 | −0.2 ± 0.1 | 23.0 ± 0.9 | −0.2 ± 0.1 | 24.4 ± 0.7 | −0.2 ± 0.1 | |
| 12 | 22.8 ± 0.7 | −0.1 ± 0.1 | 22.8 ± 0.9 | −0.1 ± 0.1 | 23.1 ± 0.9 | −0.2 ± 0.1 | 24.5 ± 0.8 | −0.1 ± 0.1 | |
| Segmental muscle mass | |||||||||
| Right arm, kg | 0 | 2.0 ± 0.1 | 2.0 ± 0.1 | 2.0 ± 0.1 | 2.2 ± 0.1 | ||||
| 4 | 2.0 ± 0.1 | 0.0 ± 0.0 | 2.0 ± 0.1 | −0.1 ± 0.0 | 2.0 ± 0.1 | 0.0 ± 0.0 | 2.2 ± 0.1 | 0.0 ± 0.0 | |
| 8 | 2.0 ± 0.1 | 0.0 ± 0.0 | 2.0 ± 0.1 | −0.1 ± 0.0 | 2.0 ± 0.1 | 0.0 ± 0.0 | 2.2 ± 0.1 | 0.0 ± 0.0 | |
| 12 | 2.0 ± 0.1 | 0.0 ± 0.0 | 2.0 ± 0.1 | 0.0 ± 0.0 | 2.0 ± 0.1 | 0.0 ± 0.0 | 2.2 ± 0.1 | 0.0 ± 0.0 | |
| Left arm, kg | 0 | 2.0 ± 0.1 | 2.0 ± 0.1 | 2.0 ± 0.1 | 2.2 ± 0.1 | ||||
| 4 | 2.0 ± 0.1 | 0.0 ± 0.0 | 2.0 ± 0.1 | −0.1 ± 0.0 | 2.0 ± 0.1 | 0.0 ± 0.0 | 2.2 ± 0.1 | 0.0 ± 0.0 | |
| 8 | 2.0 ± 0.1 | 0.0 ± 0.0 | 2.0 ± 0.1 | −0.1 ± 0.0 | 2.0 ± 0.1 | 0.0 ± 0.0 | 2.2 ± 0.1 | 0.0 ± 0.0 | |
| 12 | 2.0 ± 0.1 | 0.0 ± 0.0 | 2.0 ± 0.1 | −0.1 ± 0.0 | 2.0 ± 0.1 | 0.0 ± 0.0 | 2.2 ± 0.1 | 0.0 ± 0.0 | |
| Trunk, kg | 0 | 18.1 ± 0.6 | 18.2 ± 0.7 | 18.1 ± 0.7 | 19.4 ± 0.6 | ||||
| 4 | 18.1 ± 0.6 | 0.0 ± 0.1 | 17.9 ± 0.7 | −0.3 ± 0.1 | 18.0 ± 0.7 | −0.1 ± 0.1 | 19.3 ± 0.6 | −0.2 ± 0.1 | |
| 8 | 17.9 ± 0.6 | −0.2 ± 0.1 | 17.9 ± 0.7 | −0.3 ± 0.1 | 17.9 ± 0.6 | −0.2 ± 0.1 | 19.2 ± 0.6 | −0.3 ± 0.1 | |
| 12 | 17.9 ± 0.6 | −0.2 ± 0.1 | 17.9 ± 0.7 | −0.3 ± 0.1 | 17.8 ± 0.7 | −0.2 ± 0.1 | 19.2 ± 0.6 | −0.2 ± 0.1 | |
| Right leg, kg | 0 | 6.6 ± 0.2 | 6.5 ± 0.3 | 6.7 ± 0.3 | 7.0 ± 0.2 | ||||
| 4 | 6.6 ± 0.2 | 0.0 ± 0.0 | 6.7 ± 0.3 | 0.2 ± 0.0 | 6.8 ± 0.3 | 0.1 ± 0.0 | 7.2 ± 0.2 | 0.1 ± 0.0 | |
| 8 | 6.6 ± 0.2 | 0.1 ± 0.0 | 6.6 ± 0.3 | 0.1 ± 0.0 | 6.7 ± 0.3 | 0.0 ± 0.0 | 7.2 ± 0.2 | 0.1 ± 0.0 | |
| 12 | 6.7 ± 0.2 | 0.1 ± 0.0 | 6.7 ± 0.3 | 0.2 ± 0.0 | 6.7 ± 0.3 | 0.1 ± 0.0 | 7.1 ± 0.3 | 0.1 ± 0.0 | |
| Left leg, kg | 0 | 6.6 ± 0.2 | 6.5 ± 0.3 | 6.6 ± 0.3 | 7.0 ± 0.2 | ||||
| 4 | 6.6 ± 0.2 | 0.0 ± 0.0 | 6.6 ± 0.3 | 0.2 ± 0.0 | 6.7 ± 0.3 | 0.1 ± 0.0 | 7.1 ± 0.2 | 0.1 ± 0.0 | |
| 8 | 6.6 ± 0.2 | 0.0 ± 0.0 | 6.6 ± 0.3 | 0.1 ± 0.0 | 6.6 ± 0.3 | 0.0 ± 0.0 | 7.1 ± 0.2 | 0.1 ± 0.0 | |
| 12 | 6.6 ± 0.2 | 0.1 ± 0.0 | 6.6 ± 0.3 | 0.2 ± 0.0 | 6.7 ± 0.3 | 0.0 ± 0.0 | 7.1 ± 0.2 | 0.1 ± 0.0 | |
| Control food | Decanoic Acid Food | Low-Dose Octanoic Acid Food | High-Dose Octanoic Acid Food | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Week | Measurement Values | Change Values | Measurement Values | Change Values | Measurement values | Change Values | Measurement Values | Change Values | |
| Knee extension strength | |||||||||
| Right, N | 0 | 309.3 ± 21.0 | 306.4 ± 19.6 | 254.6 ± 19.9 | 326.3 ± 30.1 | ||||
| 4 | 321.3 ± 17.4 | 12.0 ± 11.2 | 333.9 ± 20.5 | 27.5 ± 14.3 | 292.9 ± 19.7 | 38.3 ± 14.6 | 361.5 ± 26.3 | 35.3 ± 16.2 | |
| 8 | 363.1 ± 20.3 | 53.8 ± 13.5 † | 400.2 ± 20.7 † | 93.8 ± 17.4 † | 354.7 ± 21.7 † | 100.1 ± 14.1 † | 418.1 ± 25.9 † | 91.8 ± 18.2 † | |
| 12 | 356.7 ± 16.5 | 47.3 ± 14.7† | 426.0 ± 20.3 † | 119.6 ± 17.3 *,† | 391.3 ± 23.0 † | 136.7 ± 19.1 *,† | 456.8 ± 26.3 *,† | 130.5 ± 18.3 *,† | |
| Left, N | 0 | 291.8 ± 19.9 | 308.0 ± 21.1 | 244.0 ± 18.1 | 323.0 ± 24.3 | ||||
| 4 | 315.4 ± 17.4 | 23.6 ± 11.5 | 329.2 ± 21.6 | 21.3 ± 14.3 | 281.9 ± 17.8 | 37.9 ± 16.4 | 345.9 ± 22.9 | 22.8 ± 11.3 | |
| 8 | 338.3 ± 21.9 | 46.5 ± 16.4 † | 410.1 ± 22.3 † | 102.1 ± 15.3 † | 357.1 ± 19.2 † | 113.1 ± 16.4 *,† | 413.4 ± 24.4 *,† | 90.3 ± 18.6 † | |
| 12 | 356.6 ± 20.1 | 64.8 ± 15.9 † | 433.0 ± 20.2 *,† | 125.1 ± 13.0 *,† | 379.6 ± 20.7 † | 135.6 ± 21.7 *,† | 441.6 ± 23.1 *,† | 118.6 ± 17.9 † | |
| Grip strength | |||||||||
| Right, kg | 0 | 28.6 ± 1.5 | 27.3 ± 1.6 | 28.6 ± 1.5 | 30.0 ± 1.6 | ||||
| 4 | 29.4 ± 1.4 | 0.8 ± 0.5 | 27.3 ± 1.6 | 0.1 ± 0.5 | 28.5 ± 1.5 | 0.0 ± 0.4 | 29.8 ± 1.5 | −0.2 ± 0.5 | |
| 8 | 29.4 ± 1.3 | 0.8 ± 0.5 | 28.2 ± 1.6 | 1.0 ± 0.7 | 29.1 ± 1.5 | 0.5 ± 0.5 | 30.3 ± 1.5 | 0.3 ± 0.4 | |
| 12 | 29.3 ± 1.2 | 0.7 ± 0.6 | 29.1 ± 1.5 | 1.9 ± 0.7 | 29.5 ± 1.6 | 0.9 ± 0.5 | 30.8 ± 1.4 | 0.8 ± 0.6 | |
| Left, kg | 0 | 27.4 ± 1.3 | 26.0 ± 1.5 | 27.3 ± 1.4 | 28.3 ± 1.5 | ||||
| 4 | 27.6 ± 1.3 | 0.1 ± 0.4 | 26.2 ± 1.5 | 0.2 ± 0.6 | 27.1 ± 1.4 | −0.1 ± 0.4 | 28.8 ± 1.6 | 0.5 ± 0.5 | |
| 8 | 27.7 ± 1.3 | 0.2 ± 0.4 | 27.3 ± 1.5 | 1.3 ± 0.6 † | 27.6 ± 1.5 | 0.3 ± 0.4 | 29.3 ± 1.4 | 1.0 ± 0.5 † | |
| 12 | 27.3 ± 1.2 | −0.1 ± 0.5 | 27.7 ± 1.5 | 1.7 ± 0.7 † | 28.0 ± 1.4 | 0.7 ± 0.4 | 29.7 ± 1.5 | 1.4 ± 0.5 † | |
| Control Food | Decanoic Acid Food | Low-Dose Octanoic Acid Food | High-Dose Octanoic Acid Food | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Week | Measurement Values | Change Values | Measurement Values | Change Values | Measurement Values | Change Values | Measurement Values | Change Values | |
| Timed up & go test, s | 0 | 6.7 ± 0.3 | 7.3 ± 0.3 | 6.9 ± 0.2 | 6.7 ± 0.2 | ||||
| 4 | 6.4 ± 0.2 | −0.4 ± 0.2 | 6.8 ± 0.2 | −0.5 ± 0.3 † | 6.7 ± 0.2 | −0.2 ± 0.1 † | 6.5 ± 0.2 | −0.2 ± 0.1 | |
| 8 | 6.2 ± 0.1 | −0.6 ± 0.2 † | 6.5 ± 0.2 † | −0.9 ± 0.2 † | 6.3 ± 0.2 | −0.6 ± 0.1 † | 6.3 ± 0.2 | −0.4 ± 0.1 | |
| 12 | 6.1 ± 0.1 † | −0.6 ± 0.2 † | 6.2 ± 0.1 † | −1.2 ± 0.2 † | 5.9 ± 0.2 † | −1.0 ± 0.1 † | 5.9 ± 0.1 † | −0.8 ± 0.1 † | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kojima, K.; Ishikawa, H.; Watanabe, S.; Nosaka, N.; Mutoh, T. A Randomized, Double-Blind, Controlled Trial Assessing If Medium-Chain Triglycerides in Combination with Moderate-Intensity Exercise Increase Muscle Strength in Healthy Middle-Aged and Older Adults. Nutrients 2023, 15, 3275. https://doi.org/10.3390/nu15143275
Kojima K, Ishikawa H, Watanabe S, Nosaka N, Mutoh T. A Randomized, Double-Blind, Controlled Trial Assessing If Medium-Chain Triglycerides in Combination with Moderate-Intensity Exercise Increase Muscle Strength in Healthy Middle-Aged and Older Adults. Nutrients. 2023; 15(14):3275. https://doi.org/10.3390/nu15143275
Chicago/Turabian StyleKojima, Keiichi, Haruna Ishikawa, Shinji Watanabe, Naohisa Nosaka, and Tatsushi Mutoh. 2023. "A Randomized, Double-Blind, Controlled Trial Assessing If Medium-Chain Triglycerides in Combination with Moderate-Intensity Exercise Increase Muscle Strength in Healthy Middle-Aged and Older Adults" Nutrients 15, no. 14: 3275. https://doi.org/10.3390/nu15143275
APA StyleKojima, K., Ishikawa, H., Watanabe, S., Nosaka, N., & Mutoh, T. (2023). A Randomized, Double-Blind, Controlled Trial Assessing If Medium-Chain Triglycerides in Combination with Moderate-Intensity Exercise Increase Muscle Strength in Healthy Middle-Aged and Older Adults. Nutrients, 15(14), 3275. https://doi.org/10.3390/nu15143275







