Abstract
Atherosclerosis, chronic non-communicable diseases, and metabolic syndrome are highly interconnected and collectively contribute to global health concerns that reduce life expectancy and quality of life. These conditions arise from multiple risk factors, including inflammation, insulin resistance, impaired blood lipid profile, endothelial dysfunction, and increased cardiovascular risk. Adopting a plant-based diet has gained popularity as a viable alternative to promote health and mitigate the incidence of, and risk factors associated with, these three health conditions. Understanding the potential benefits of a plant-based diet for human health is crucial, particularly in the face of the rising prevalence of chronic diseases like diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Thus, this review focused on the plausible advantages of consuming a type of food pattern for the prevention and/or treatment of chronic diseases, emphasizing the dietary aspects that contribute to these conditions and the evidence supporting the benefits of a plant-based diet for human health. To facilitate a more in-depth analysis, we present separate evidence for each of these three concepts, acknowledging their intrinsic connection while providing a specific focus on each one. This review underscores the potential of a plant-based diet to target the underlying causes of these chronic diseases and enhance health outcomes for individuals and populations.
1. Introduction
Many people consider a plant-based diet (PBD), which includes only plant sources with the absence or occasionally minimal presence of processed food, a novel and even risky eating choice. This concern is heightened by the potential deficiencies in micronutrients, such as vitamin B12 and D, calcium, omega 3, or iron, compared to a traditional diet [1,2]. However, over the past decades, a wealth of scientific evidence has accumulated that provides strong support for the potential health benefits of a PBD. These benefits include the prevention of various chronic non-communicable diseases, such as type 2 diabetes [3,4], hypertension [5,6,7], dyslipidemia [8,9], atherosclerosis, and cancer [10,11]. Studies on supplementation and natural consumption approaches have demonstrated the practical advantages of a PBD, which are attributed to the bioactive compounds present in plants, such as catechins [12,13], anthocyanins [14,15], polyphenols [16], and phytosterols [17,18], among others.
Metabolic syndrome (MetS) is associated with several adverse effects on human health. Although there are various definitions, components, and criteria for MetS, they all include visceral obesity, insulin resistance, hypertension, and dyslipidemia [19]. According to National Cholesterol Educations Program Adult Treatment Panel ATP III, MetS is considered present when an individual meets at least three of the following five criteria: waist circumference over 40 inches (men, or >102 cm) or 35 inches (women, or >88 cm), blood pressure over 130/85 mmHg, fasting triglyceride level over 150 mg/dL, fasting HDL cholesterol level less than 40 mg/dL (men) or 50 mg/dL (women), and fasting blood sugar over 100 mg/dL [20]. Other entities even consider more demanding cut-off points [21]. Individuals with MetS have higher cardiovascular disease and all-cause mortality risk compared to whose without MetS [22,23].
In this line, a PBD has been found to have numerous positive and protective effects on metabolic health, significantly reducing the associated risks. For instance, Jovanovic et al. [24] concluded that a 1-unit increase in daily servings of a healthy plant-based diet, which excludes added sugars, refined grains, and oils, was associated with a 4% lower risk prevalence of elevated waist circumference and MetS risk. However, not all evidence supports these outcomes. Shang et al. [25] found that a vegan diet alone did not decrease the risk of MetS, but the study only assessed the absence of animal foods (meat, dairy, and eggs), not the quality of the diet. Previous research suggests that adherence to a healthful plant-based diet, which includes increased fiber intake, plant bioactive compounds, and lower consumption of ultra-processed foods, is associated with benefits related to MetS [26,27]. It is worth noting that there is a vast difference in quality and health outcomes between a healthy and an unhealthy PBD. Indeed, Li et al. [28] in 2022 established that a healthy PBD is associated with lower mortality risk than an unhealthy PBD.
A vegan diet is often used as an equivalent to a PBD, although considerable differences exist between these two concepts, both nutritionally and ethically [29]. While the first is exclusively related to selecting food for ethical reasons (animal empathy), the second is related to health and/or environmental protection. Additionally, a vegan diet does not necessarily prioritize food quality. In contrast, a PBD emphasizes consuming whole foods and minimally processed products, focusing on legumes, whole grains, fruits, vegetables, seeds, and nuts [30].
As defined by some authors, plant-based eating patterns include fish, poultry, and yogurt [31,32,33]. However, this definition is more accurately described as a pescatarian diet, including other seafood, or as a lacto-vegetarian diet. Other authors have included a low frequency of animal sources as a definition of a PBD [34], while other sources explicitly highlight that a PBD does not necessarily mean being vegetarian or vegan [30]. It is central to recognize these differences to fully understand the potential benefits and limitations of a PBD and make informed dietary choices.
This review aimed to highlight the benefits of diverse plant bioactive compounds and emphasize the significance of including plant-origin macronutrients, vitamins, and minerals in our diets to prevent and/or treat the pathogenesis of chronic non-communicable diseases, metabolic syndrome indicators, and atherosclerosis due to increased prevalence and incidence globally.
2. Plant-Based Diet and Atherosclerosis
2.1. Brief Summary of the Pathophysiology and Confounding Outcomes
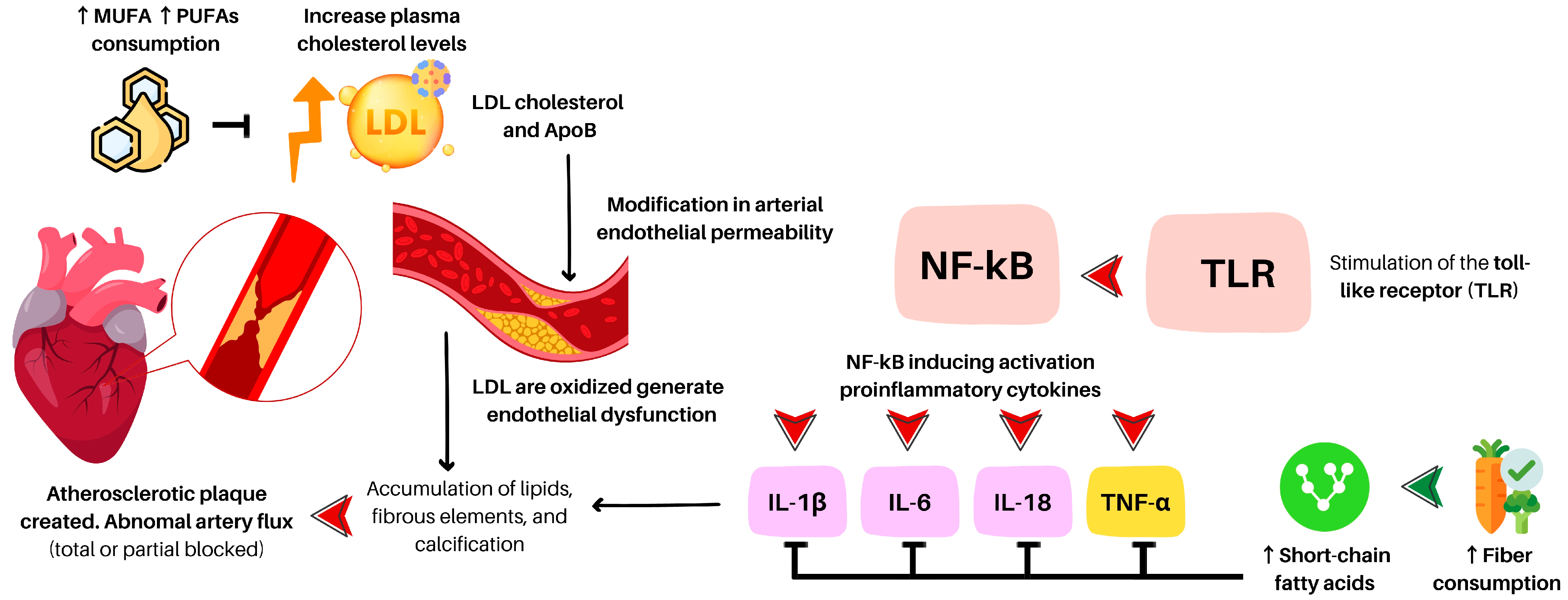
Atherosclerosis (ATE) is considered a principal cause of different coronary heart diseases. It is characterized by the accumulation of lipids, fibrous elements, and calcification within the large arteries, similar to a chronic inflammatory process [35]. This involves the stimulation of the toll-like receptor (TLR), which activates the transcription factor (nuclear factor-kappa beta), inducing the activation of proinflammatory components such as interleukin 1β (IL-1β), IL-6, IL-18, and tumor necrosis factor-alpha (TNF-α) [36] (see Figure 1).
Figure 1.
A brief description of the atherosclerosis process. MUFA: monounsaturated fatty acids; PUFA: polyunsaturated fatty acids; LDL: low-density lipoprotein; Apo-B: apolipoprotein B; NF-kB: nuclear factor-κB; IL: interleukin; TNF-α: tumor necrosis factor alpha.
An increase in plasma cholesterol levels can result in changes in arterial endothelial permeability, leading to the migration of lipids, particularly low-density lipoprotein cholesterol particles, into the arterial wall [37]. This process can be upregulated by certain proinflammatory conditions such as advanced glycation end-products (AGEs) [38], hypercholesterolemia, hypertension [39], or type 1 diabetes [40]. The trapped lipoproteins are oxidized, leading to endothelial dysfunction [36] and forming foam cells. LDL particles transport different components, including apolipoprotein B100 (Apo-B100) [41]. Recent evidence [42,43] suggests that Apo-B content is linked to ATE as the primary cause of atherogenic pathology.
Previous studies have identified that Apo-B, not LDL cholesterol, is strongly associated with coronary artery calcification [44,45]. However, a recent Mendelian randomized analysis showed that, in individuals with equal levels of non-HDL cholesterol, the development of coronary artery disease is not influenced by the number of Apo-B particles carried, suggesting that the clinical impact of lipid-lowering therapies is expected to be proportional to the reduction in non-HDL cholesterol rather than the reduction in Apo-B [46]. Thus, the LDL particle oxidation process is also a critical factor that should be considered, as it has been strongly linked to coronary atherosclerosis, arterial dysfunction, and mortality, affecting elasticity and vasodilatory endothelial vascular function [47,48]. In this line, it has been suggested that the regulation of oxidative stress could be one of the major strategies to reduce the trapping of Apo-B in the intima, decreasing atheroma formation, inflammation, and atherosclerosis pathogenesis [36].
In parallel, it has been established that people who do not develop atherosclerosis have an optimal and normal LDL cholesterol range of 50–70 mg/dL [49]. In a Consensus Statement from the European Atherosclerosis Society Consensus Panel (EAS), Ference et al. [50] emphasized the consistent evidence from clinical and genetic studies that unequivocally establishes the role of LDL in causing atherosclerotic cardiovascular disease (ASCVD). Despite that, not all the evidence supported this statement [51], which could be explained by reverse causation [52,53]. While observational studies in middle-aged individuals have reported a positive association between cardiovascular disease and cholesterol levels, the role of high cholesterol as a cardiovascular risk factor in individuals above 75 years old is controversial [54].
Overall, LDL cholesterol levels in plasma may not reflect lifetime LDL cholesterol levels due to comorbidities [55]. To address this issue, the authors of the study mentioned above [54] used LDL-GRS (genetic risk score) and found that the genetic predisposition to high LDL cholesterol levels contributes to mortality throughout life, including in the oldest individuals. Finally, the coexistence of coronary artery disease and malnutrition may reflect the intriguing phenomenon known as the “cholesterol paradox.” The last concept refers to a disparity in some of the results found and what the literature explains. In this line, a previous study [56] concluded that the worse mortality prognosis observed in patients with low LDL cholesterol group (<1.8 mmol/L) is mainly mediated by their higher prevalence of malnutrition. After adjustment for malnutrition, patients with coronary artery disease who had low baseline serum LDL cholesterol concentrations had a low risk of long-term all-cause mortality.
2.2. Saturated and Unsaturated Fat
A diet high in sugar, salt, cholesterol, and fat—commonly called a Western diet—has been linked to various health issues, including diabetes mellitus, high blood pressure, hyperlipidemia, obesity, and coronary artery disease. These conditions can promote atherogenesis, atherosclerosis, and atherothrombotic coronary artery disease [57]. However, a recent animal study by Huang et al. [58] showed that the adverse effects of Western diet-induced atherosclerosis could be mitigated by down-regulating obesity, inflammation, and chemotaxis signaling. These factors are modulated by the microbiota and derived short-chain fatty acids (SCFAs).
In contrast, a diet rich in extra-virgin oil and nuts has been shown to have beneficial effects. Compared to a Western diet, a diet high in unsaturated fats can lead to lower plasma cholesterol and triglyceride levels, as well as reduced inflammation and atherosclerosis in animal models (inhibited foamy monocyte formation, inflammation, adhesion, and reduced atherosclerosis in Ldlr -/- mice) [59]. Previous human research has shown that a high-unsaturated fat diet and a very low-fat diet can lead to a more significant decrease in LDL cholesterol than a high-saturated fat diet [60]. Increasing poly and mono-unsaturated fatty acids (PUFAs and MUFAs, respectively) reduce cardiovascular disease events mainly due to the degree of cholesterol-lowering. The cardiovascular effects of reducing saturated fat rely on changes in atherosclerosis via serum cholesterol [61], influencing pathways affecting inflammation, cardiac rhythm homeostasis, apolipoprotein-C III production, and high-density lipoprotein (HDL) function [62].
Notwithstanding the above, some authors [63] consider that American guidelines and recommendations may be biased and that saturated fat in certain foods, such as whole fat dairy or dark chocolate, can benefit health and are not associated with cardiovascular disease or diabetes. While Gershuni [64] supported this general conclusion, he also indicated that the saturated fatty acid found in meat, eggs, cacao, and nuts is primarily composed of triglycerides containing palmitic acid and stearic acid, covering 90% of fatty acid in the standard American diet. However, exogenous palmitic acid can exert a different effect depending on the source. In both animal and human in vivo and in vitro studies [65,66], palmitic acid has been associated with promoting atherosclerosis development due to cholesterol accumulation in LDL particles and macrophages activating the inflammatory process [67]. Further, elevated palmitic acid levels enhanced the uptake of oxidized LDL via the upregulation of lectin-like oxidized LDL receptors in macrophages, mediated by ROS-p38 pathways rather than TLRs [68].
A vast study involving 76,364 women observed that the consumption of high-fat foods such as peanuts and tree nuts (two or more times a week) and walnuts (one or more times a week) was associated with a 13–19% lower risk of total cardiovascular risk disease and a 15–23% lower risk of coronary heart disease [69,70]. In an animal study [71], a high-fat diet rich in walnuts was found to cause a 55% reduction in atherosclerotic plaque development in the aortic arch compared to the control diet. Urpi-Sarda et al. [72] found that a Mediterranean diet with virgin olive oils and nuts can down-regulate cellular inflammatory biomarkers associated with atherogenesis [73] and modify the process of the firm adhesion of circulating monocytes and lymphocytes T to endothelial cells during inflammation [74]. These findings suggest that adding nuts to a Mediterranean diet or adopting a whole-food vegan diet can reverse the atherosclerotic process of coronary artery disease [75].
In this line, a meta-analysis [76] demonstrated that replacing 1% of the dietary carbohydrate with MUFAs or PUFAs resulted in increased HDL cholesterol, decreased triacylglycerol concentration, and attenuated increases in LDL and total cholesterol levels. In contrast, a study on coconut oil found that reducing saturated fat without changing the polyunsaturated/saturated fatty acid ratio (P/S) did not lower total or LDL cholesterol but significantly reduced HDL cholesterol. However, a diet high in MUFAs and PUFAs resulted in a greater reduction in LDL cholesterol, lower LDL/HDL cholesterol, and an improved Apo-B/Apo-A ratio [77]. This last conclusion is fundamental, considering LDL/HDL ratio is suggested as a sensitive predictor of coronary atherosclerotic heart disease (CADH) [78]. Overall, although nutritional evidence has not convincingly shown that plant-based fats alone significantly improve HDL cholesterol, there is evidence that they can lower LDL cholesterol and maintain unchanged HDL cholesterol, thus improving the LDL/HDL ratio [79,80].
2.3. Trimethylamine N-Oxide and Gut Microbiota
Trimethylamine N-oxide (TMAO) is a compound that has gained interest due to its potential mechanistic links to atherosclerosis heart disease [81]. Trimethylamine (TMA) is generated by gut microbiota in response to nutrients, with eggs and meat being major dietary sources of the TMA precursor. In the liver, TMA is transformed into TMAO by flavin-containing monooxygenase 3 [82]. While a considerable body of evidence strongly supports the detrimental impact of TMAO on health, some results are inconsistent, with stronger relations observed in patients with preexisting medical conditions compared to healthy subjects [83]. However, in a previous Mendelian randomization analysis [84], the authors found that some chronic non-communicable diseases like type 2 diabetes mellitus and kidney disease increase TMAO levels, and such observational evidence for cardiovascular disease may be due to confounding or reverse causality. Despite the above, different non-modifiable factors increase plasma TMAO levels, such as age, sex, and genetic factors. However, various components found in animal and plant food can also potentially increase it [83]. Choline, phosphatidylcholine, l-carnitine, betaine, crono-betaine, and γ-butyrobetaine [85,86] can be used as precursors by gut microbiota to generate TMAO.
Kühn et al. [87] established that TMAO levels could be more affected by intra-individual variation, which could mediate the result due to physical activity or intestinal microbiota. Depending on the context, these factors can positively or negatively modify the type of intestinal bacteria present. For instance, betaine, mainly found in plants, can potentially affect TMAO levels and be synthesized from dietary choline. Betaine also serves as an osmoprotectant in the kidney and plays a crucial role in the methionine-homocysteine cycle, maintaining the s-adenosylmethionine/s-adenosyl-homocysteine ratio in the liver, especially when folate is insufficient [88]. However, some studies have shown no relationship between dietary betaine and the incidence of cardiovascular disease, even though TMAO increased cardiovascular mortality in some populations [89,90]. A possible explanation lies in the composition of the gut microbiota.
In a previous study [82], a carnitine challenge test was conducted on omnivorous and vegetarian/vegan participants using steak and/or veggie caps containing 250 mg of a stable isotope-labeled d3-L-carnitine to measure TMAO levels. The results showed that vegetarians/vegans challenged with d3-carnitine had a significantly reduced synthetic capacity to produce TMAO from oral carnitine compared to omnivorous individuals. Some authors have suggested that the richness, expressed in the number of species, of the intestinal microbiota may impact the host’s health, although this is still subject to debate [91].
However, De Filippo et al. [92] conducted previous research comparing the human intestinal microbiota among children from Burkina Faso (rural context) with a diet low in fat and animal protein, rich in starch, fiber, and plant polysaccharides, legumes, sorghum, and millet grain being predominantly vegetarian, versus children from Italy (urban context) with a diet high in animal protein, sugar, starch, fat, and low in fiber. The authors found that diet plays a primary role influencing the composition and diversity of the microbiota, suggesting that diet has a dominant influence over other variables such as hygiene, sanitation, ethnicity, geography, and climate. Gut bacteria can generate SCFA byproduct formation, named butyrate, propionate, and acetate, which help maintain normal large bowel function, prevent pathologies through their influence in the gut lumen, the colonic musculature and vasculature and through their metabolism by colonocytes [93]. At the same time, increased SCFA from plant-based sources has been associated with reduced TMAO levels and atherosclerotic risk.
Concerning protein consumption, an early culture-based study on gut microbiota [94] demonstrated lower counts of Bifidobacterium adolescentis and increased counts of Bacteroides and Clostridia in subjects consuming a high beef diet compared to those on a meatless diet. In a more recent study, Singh et al. [95] analyzed the effect of different types of protein (animal-based protein and plant-based protein) and found that pea protein increased intestinal SCFAs levels, which are considered anti-inflammatory and essential to the maintenance of the mucosal barrier [96]. This increase in SCFAs was associated with an increased prostaglandin E1/prostaglandin E2 ratio produced by subepithelial myofibroblasts enhancing mucin-2 (MUC-2) expression in epithelial cells [97]. These positive effects on the gut barrier, especially in MUC-2, help to reduce bacterial translocation (endotoxemia), gut permeability, inflammation, and TMAO levels [98,99,100,101].
Contrary to what has been stated, recent studies have shown that TMAO levels are positively correlated with the intake of vegetables and whole-grain cereal, contradicting the notion that a healthy diet may help reduce TMAO levels [102]. Similarly, Griffin et al. [103] found that a Mediterranean diet intervention over six months did not significantly mitigate TMAO concentration in a healthy population. These findings call into question the effectiveness of a high plant-based diet in reducing TMAO levels and, consequently, the risk of cardiovascular and coronary heart disease.
2.4. Plant-Based Diet, LDL Cholesterol, TMAO, and Atherosclerotic Risk
In a recent randomized cross-over study [104], the authors observed that a PBD that includes whole eggs might maintain or improve dyslipidemia, oxidative stress, and inflammation biomarkers over vegan or lactovegetarian diets in individuals with MetS. The study suggested that egg consumption may have theoretical benefits, such as increasing HDL cholesterol, without causing any adverse effects on LDL cholesterol, triglycerides, or glucose levels. These findings were consistent with previous research by Zhu et al. [105], who found that two eggs/day in overweight postmenopausal women significantly increased plasma choline and betaine levels but did not alter TMAO levels or gut microbiome composition.
In a previous study (2005) related to this topic [106], it was established that individuals over 60 years old with a healthy lipoprotein profile might consume eggs as part of their regular diet due to eggs consumption possibly increasing LDL cholesterol, but an increase in HDL cholesterol offsets this elevation. However, a meta-analysis [107] conducted earlier (2001) indicated that the beneficial rise in HDL cholesterol by consuming eggs is insufficient to offset the negative rise in total LDL cholesterol concentrations, implying that an increase in dietary cholesterol intake may increase the risk of coronary heart disease. Further, several decades ago, some studies [108,109,110] clearly and unequivocally established that egg consumption leads to an increase in total and LDL cholesterol, although the extent of the increase depends on baseline cholesterol levels [111]. However, and related to TMAO, a more recent study [112] concluded that egg consumption did not increase its levels of plasma. Notwithstanding, in this study, the authors used a 12-h fasting protocol to measure TMAO levels, and previous evidence has indicated that the kidneys efficiently eliminate TMAO to maintain a steady state of circulating choline levels in a couple of hours [113,114].
2.5. Endothelial Vascular Function
Endothelial vascular dysfunction is among the risk factors involved in coronary artery disease [57]. High salt consumption has presented strong evidence of the adverse effect on endothelial function [115,116] by stiffening human endothelial cells and reducing nitric oxide (NO) production [115]. NO is a key signaling molecule that regulates blood flow and tissue oxygenation, and its bioavailability reduction augments atherosclerosis risk [117], associated with an impairment of endothelium-dependent relaxation. In addition, a single high-fat (50 g) meal reduces blood flow-mediated vasoactivity in the 2- to 4-h postprandial period [118]. This previous outcome has also been reported by Keogh et al. [119], who detailed that a high-saturated fat diet causes deterioration in flow-mediated dilation (FMD) compared with a high PUFA, MUFA, or even CARB (carbohydrate) diet.
Contrary to what has been previously described, a singular food and/or full PBD has been recognized as a healthy eating pattern by improving FMD. An 8-week cross-over feeding trial demonstrated that walnuts consumption as a substitute for 32% of the energy from MUFAs in a cholesterol-lowering Mediterranean diet improves vascular endothelial function [120]. A hazelnut-enriched diet for four weeks has also shown a significantly improved FMD in hypercholesterolemic subjects, besides improving TC (total cholesterol), TG (triglycerides), LDL, and HDL as well oxidized LDL, CRP (c-reactive protein), and soluble vascular cell adhesion molecule-1 compared with the control diet [121].
Another study found that daily walnut consumption (56 g) improves endothelial function in overweight adults with visceral adiposity [122]. Daily consumption of high-flavanol cocoa drinks has been shown to lead to a sustained reversal of endothelial dysfunction in approximately five days. Interestedly, the magnitude of this positive effect observed with a high-flavanol cocoa drink was similar to that observed in a long-term pharmacological approach with statins [123,124]. Another study using daily inorganic nitrate as beetroot juice for six weeks in hypercholesterolemic individuals showed a ∼24% improvement in FMD through the rising in nitrate circulation. This beneficial effect was related to a reduction in platelet-monocyte aggregate numbers and reduced platelet P-selectin expression [125], associated with cardiovascular disease progression, which can initiate the release of atherogenic proinflammatory and adhesive molecules and induce procoagulant microparticle formation, respectively [126,127].
In this context, nitrate and nitrite have been widely recognized as nitric oxide precursors. In the case of nitrate, certain plant foods can improve vascular function through this content and enhance NO formation. Spinach, watercress, chervil, chard, arugula, beets, celery, and lettuce are some plant foods with high nitrate concentrations [128]. In this line, Bondonno et al. [129], in a study of older women, observed an inverse association between the intake of vegetable nitrates with CCA-IMT (intima-media thickness of the common carotid artery) and the risk of an ischemic cerebrovascular event for 15 years. This result was not observed with non-vegetable nitrates consumption. Although some evidence has demonstrated an insignificant change in a single and specific indicator, such as systolic blood pressure, after five weeks of supplementation of leafy green vegetables or pills containing the same amount of inorganic nitrate [130], most of the literature corroborates the benefits of PBD for the improvement of vascular function.
Prolonged consumption of soy nuts as part of a healthy diet improves endothelial function, LDL cholesterol concentration, and mean arterial pressure [131]. In this recent study, the enhancement is more related to restoring a condition of nitric oxide impairment rather than enhancing a normal physiological condition [132]. In contrast, previous evidence has shown that fish, with green tea and a lower consumption of saturated fat as part of a traditional Chinese diet, also improves endothelial function in older Chinese people’s arteries [133]. However, in later studies, fish oil supplementation or whole-fish consumption showed no significant effect on endothelial function [134,135,136]. Additionally, in a comparative study between lacto-ovo-vegetarians and omnivores on the measurement of vascular dilator function, the authors found that the vegetarian diet, by itself, has a direct beneficial effect on the vascular endothelium and the function of the smooth muscle, and may help explain the lower incidence of atherosclerosis and cardiovascular mortality [137].
2.6. Short-Chain Fatty Acids, Gut Microbiota, and Atheroma Formation
Gut microbiota, mainly through probiotics, bioactive compounds intake, and SCFA formation, exerts multiple health benefits, having a significantly positive role in the reduction of atherosclerosis. Soybeans, such as tempeh, are rich in bioactive compounds like genistein and daidzein. These isoflavones are present in different legumes but are markedly higher in soybean. The growing body of evidence around isoflavones in the last 15 years suggests their beneficial effects on preventing breast and prostate cancer, cardiovascular disease, and chronic non-communicable diseases. In animal models, type 2 diabetes has been demonstrated to aggravate colonic damage and inflammation response and decrease levels of SCFA [4] leading to dysbiosis. Gut dysbiosis can alter different homeostatic functions increasing the pathophysiology risk of several complications like diabetes or atherosclerosis, among others [138,139].
Previous evidence has observed different compositions of bacteria between patients with or without symptomatic atherosclerosis [140]. The production of SCFAs can inhibit foam cell formation by stimulating the expression of IL-10 and reducing the production of proinflammatory cytokines by the endothelium, contributing to the recovery of endothelial dysfunction and reducing atherosclerotic risk [141].
Inflammation can promote alteration in the gut barrier. Increasing gut permeability is associated with inflammation and reduced expression of specific tight junction proteins, such as zonula occludens-1, claudin-1, and occluding [142,143,144]. The reduction of its expression increases bacteria translocation of LPS (lipopolysaccharides) and proinflammatory cytokines. Therefore, SCFAs can reduce gut permeability by decreasing nuclear factor-kappa B (NF-kB) activation and, consequently, reduce proinflammatory cytokines such as IL-1b, IL-6, IL-8, and TNF-α [145]. Additionally, SCFA has proved to attenuate NF-kB and peroxisome proliferator-activated receptors’ (PPARγ) activities and, consequently, suppress adhesion molecules expression like vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 [146]. Accompanying these effects, they showed increased anti-inflammatory actions. For instance, in macrophages, butyrate had anti-inflammatory effects by decreasing inducible nitric oxide synthase, TNF-α, monocyte chemo-attractant protein-1, and IL-6 production through the activation of free fatty acids 3 receptors, which has been implicated in obesity and metabolic diseases [147]. In a mouse study [148], genistein has been shown to increase SCFAs concentration and modulate gut microbiota in mice.
In a regular omnivorous diet, the average daily fiber consumption is lower than recommended [149]. Additionally, Davies et al. [150] observed that omnivorous, vegetarian, and vegans reported different fiber intakes, with 23, 37, and 47 g/d, respectively. In both genders, the highest versus lowest fiber intake was associated with a 22% decrease in mortality [151] and reduced long-term ASCVD [152]. Nowadays, fiber is known as a microbiota-accessible carbohydrate (MAC) and represents the primary energy source for colonic bacteria. When fiber intake reaches 50–120 g/day, it is associated with a more diverse gut microbiota than people in Western countries. In the latter, it has been correlated with highly prevalent diseases [153].
Even so, the connection between MAC and TMAO formation is still debatable. Some authors propose that a high non-digestible carbohydrate diet may reduce TMAO formation by modulating the gut microbiota, but conflicting findings have been reported [154]. However, a previous study to determine the impact of fiber deprivation over four generations on the gut microbiota in mice colonized with human microbiota from a Westerner diet showed that the consumption of a regular Western low-fiber diet contributes to the loss of taxa over generations and may be responsible for the lower diversity of microbiota observed. The re-introduction of dietary MAC was insufficient to recover taxa [155].
In the context of human health, consuming plant-based foods promotes the development of a more diverse gut microbial community and may also impact the distribution of different species within it [156]. The difference in gut microbiota composition between omnivorous and vegetarians/vegans has been well documented, and fiber consumption has shown an inverse relationship with cardiovascular disease, including atherosclerosis [152,157]. A soy-based diet has been shown to reduce the risk of atherosclerosis by inhibiting the formation of foam cells in macrophages. Downregulating scavenger receptors achieve this effect in a cell culture model using THP-1 macrophages, which is attributed to the presence of soy pinitol, which could inhibit oxidized LDL formation [158]. Later, evaluating the consumption of a single high-fat meal, this study found that a high-fat meal resulted in a transient increase in acLDL (acetyl low-density lipoprotein) endocytosis and adhesion molecule expression in both classical and nonclassical monocytes, increasing the susceptibility to foam cell formation [159]. However, this study did not clarify the specific content of every meal, which varied between subjects. Therefore, it is essential to differentiate the effects of different diets and fat sources.
2.7. Fermented Plant-Food and Atherosclerosis
Rabbits subjected to a high-cholesterol diet experienced significant health complications. The progression was retarded by the administration of 3-(4’-hydroxyl-3’,5’-dimethoxyphenyl) propionic acid (HDMPPA), an active compound of kimchi, which can suppress TC and LDL cholesterol elevation, reducing the thickness of the aortic arch and antioxidant activity [160]. Yun et al. (2014) [161] also found that HDMPPA had a protective effect on the cell viability of THP-1-derived macrophages through the inhibition of lipid peroxidation, regulating cluster of differentiation 36 and ABCA1 expression, both of which at least partially participate in cholesterol influx and efflux. This context could regulate foam cell formation by attenuating cholesterol accumulation in macrophages.
Additionally, it is well documented that whole grains can improve health in different contexts. For instance, a 12-week whole-grain wheat-based diet increases fasting plasma propionate, a type of SCFA, in individuals with metabolic syndrome [162]. Interestingly, Lisosan G (LG), a fermented powder obtained from whole grain, has demonstrated an antioxidant and anti-inflammatory capacity [163]. Moreover, LG protects EPCs exposed to LPS, reducing intracellular reactive oxygen species (ROS), and is capable of inducing vascular damage and lowering or normalizing cytokine [164].
A study found that fermented plant beverages, in this case kombucha with pollen, led to a significant increase in SCFAs, probably depending on a mixture of microorganisms called the symbiotic culture of bacteria and yeast (SCOBY) [165]. In another beverage study, fermented Korean tea (chungtaejeon) was proven to scavenge oxidation and inhibit the cytokine-induced proliferation and migration of human aortic smooth muscle cells. Vascular smooth muscle cells secrete TNF-α, activating ERK1/2, a crucial mediator of signals that promote cell growth and motility. This pathway plays a pivotal role in the development of vascular lesions. Also, the chungtaejeon beverage inhibited the enzymatic action and protein expression of TNF-α-induce matrix-metalloproteinase (facilitates migration of vascular smooth muscle cells via matrix disruption contributing to the pathogenesis of atherosclerosis) in human aortic smooth muscle cells [166].
Moreover, red yeast rice has been reported to confer multiple health improvements, including atherosclerosis and lipid profile. Monacolin K is believed to be the key factor responsible for these positive effects, as recently reported by Rahmani et al. [167]. In an 8-week intervention study, a daily dose of 200 mg red yeast rice containing 2 mg of monacolin K significantly reduced LDL cholesterol, blood pressure, and Apo-B levels compared to the control group [168]. The mechanisms involved in these modifications are generated through various pathways, including cholesterol biosynthesis, LDL receptor metabolism, inhibiting acyl-coenzyme A-cholesterol acyltransferase, decreasing the conversion of cholesterol-to-cholesterol esters and the secretion of Apo-B, enhancing endothelial cell function due to NO activity, reducing ROS, preventing a connection between Lox-1 and ox-LDL, and reducing proinflammatory cytokines, among others [169].
2.8. Bioactive Compounds
Plant-derived bioactive components have auspicious therapeutic attributes, especially antioxidative properties [170]. Thus, both independently and in combination with other bioactive compounds found in plant foods, they have been shown to contribute to reducing plasma cholesterol. For instance, a pilot study [171] using supplemented pasta with 6% of β-glucan showed, after 30 days, a significant reduction in LDL cholesterol, IL-6, AGEs levels, and oxidative stress. In addition, it has been suggested by other researchers [172] that β-glucan exhibits significant physicochemical properties, including its antioxidant capability to scavenge reactive oxygen species, its role as a dietary fiber to inhibit cholesterol absorption, and its ability to promote the production of short-chain fatty acids (SCFAs). A previous meta-analysis has supported most of these results, indicating that barley β-glucan can significantly lower LDL and non-HDL cholesterol [173]. In another study, it was observed that a daily intake of 3 g of oat β-glucan safely reduced total, LDL, and non-HDL cholesterol in a large cohort of adults with mild hypercholesterolemia and a low cardiovascular risk profile [174].
In addition, berries as whole fruits, juice, or extract, decrease plasma LDL cholesterol and triglycerides and/or increase HDL cholesterol in individuals who exhibit elevated lipid biomarkers [175]. The modulating lipid metabolism primarily involves increasing the hepatic synthesis of apolipoprotein A-I, downregulating the activity of genes related to fatty acid synthesis, inducing the regression of aortic lesions, and decreasing inflammation and oxidative damage in experimental animals [175]. Although previous evidence suggested no significant change in serum total cholesterol, LDL cholesterol was significantly lower in the berries-consumed than in the placebo-treated subjects [176]. To complement this, a meta-analysis and trial sequential analysis of randomized controlled human trials was evaluated [177]. The authors established the potential role of CRP in cardiovascular disease since CRP can bind to LDL cholesterol and is present in atherosclerotic plaques. In this line, berry consumption markedly diminished CRP and TNF-α levels, decreasing inflammation and preventing the development of cardiovascular disease.
In a recent study [178], the authors established that proanthocyanidins regulate blood pressure due to antioxidative scavenging of oxidized LDL and LDL cholesterol and the removal of carotid atherosclerosis plaque. Oxidative stress may be reduced by protecting the blood–brain barrier during arteriosclerosis by inhibiting oxidized LDL docking to its receptor LOX-1 to prevent cerebrovascular diseases. Proanthocyanidin extract (0.1–1%) incorporated into rabbit diets ameliorated cholesterol-induced aortic lesions and atherosclerosis [179] while also decreasing oxidized LDL activation and foam cells via the antioxidative mechanism.
The benefits and importance of proper daily fruit consumption for health are widely known. In this line, mulberry has been demonstrated to inhibit the oxidation of LDL and reduce the intracellular ROS generation of macrophages. Mulberry leaf extract and mulberry leaf polyphenolic extract exhibit strong antioxidant properties, effectively neutralizing free radicals and lipid peroxides. Both improved the expression of antioxidant enzymes (superoxide dismutase-1, catalase, and glutathione peroxidase), lowered the expression of scavenger receptors via downregulating the transcription factor PPAR-γ, inhibiting the oxidized LDL uptake, foam cell formation, and intracellular lipid accumulation [180].
On the other hand, polyphenols can be implicated in a bidirectional relationship with gut microbiota affecting each other. According to Filosa et al. [181], polyphenols undergo enzymatic transformation by the microbiota, leading to enhanced bioavailability and improved health. In turn, polyphenols also influence the composition of the microbiota, preventing the proliferation of pathogens. Specific polyphenols can inhibit/increase the development of particular bacteria resulting in modulation of gut microbial composition [182]. Polyphenols can enhance the abundance of beneficial bacteria, such as Bifidobacterium and Lactobacillus, which contribute to gut barrier protection, Faecalibacterium prausnitzii, which presents anti-inflammatory action by blocking NF-kB activation, and Roseburia sp., which are butyrate producers.
In this line, probiotics administered in appropriate doses offer positive effects for the host [183]. Some bifidobacteria and lactobacilli prevent the adhesion of pathogenic bacteria by secreting lectin-like bacteriocins. The barrier protective effect involves the release of metabolic or other molecules, which, in turn, regulates tight junction integrity [184]. According to Gou et al. [185], lactobacillus plantarum MB452 increases the gene and protein expression of zonula occludens-1, zonula occludens-2, occludin, and cingulin. It also regulates the expression of tight junctions’ protein-degrading genes, stabilizing tight junctions and improving intestinal barrier function. As well, Bifidobacterium infantis and Lactobacillus acidophilus normalize the expression of the tight junctions’ proteins, occludin and claudin1, in an in vitro Caco-2 intestinal epithelial cell model, preventing barrier damage due to IL-1 stimulation. In this line, a six-week wild blueberry powder drink intake can positively modulate intestinal microbiota composition by increasing bifidobacterium [186].
Another bioactive compound with multiple health benefits is present in coffee. Previous evidence has investigated the relationship between coffee consumption and oxylipin, a biomarker related to cardiovascular disease, inflammation, and lipid peroxidation produced during foam cell formation in atherogenesis. The authors found that, after coffee consumption, urinary oxylipin was reduced. The phenolic compounds in coffee were implied to have anti-inflammatory and antioxidant activities. It is interesting to highlight that the participants received two coffees with different amounts of chlorogenic acid in this study. Those with a higher chlorogenic acid intake demonstrated higher oxylipin reduction, suggesting the protection of coffee against cardiovascular disease progression and development [187]. Finally, Table 1 displays a summary of findings on this matter and Figure 2 provides a comprehensive overview addressing the overall advantages of adopting a plant-based diet.

Table 1.
Influence of certain plant components on markers linked to atherosclerosis.
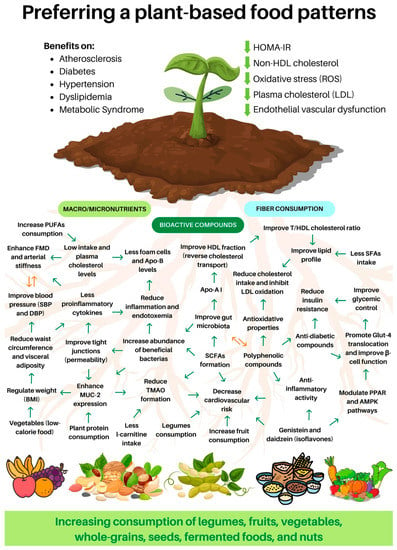
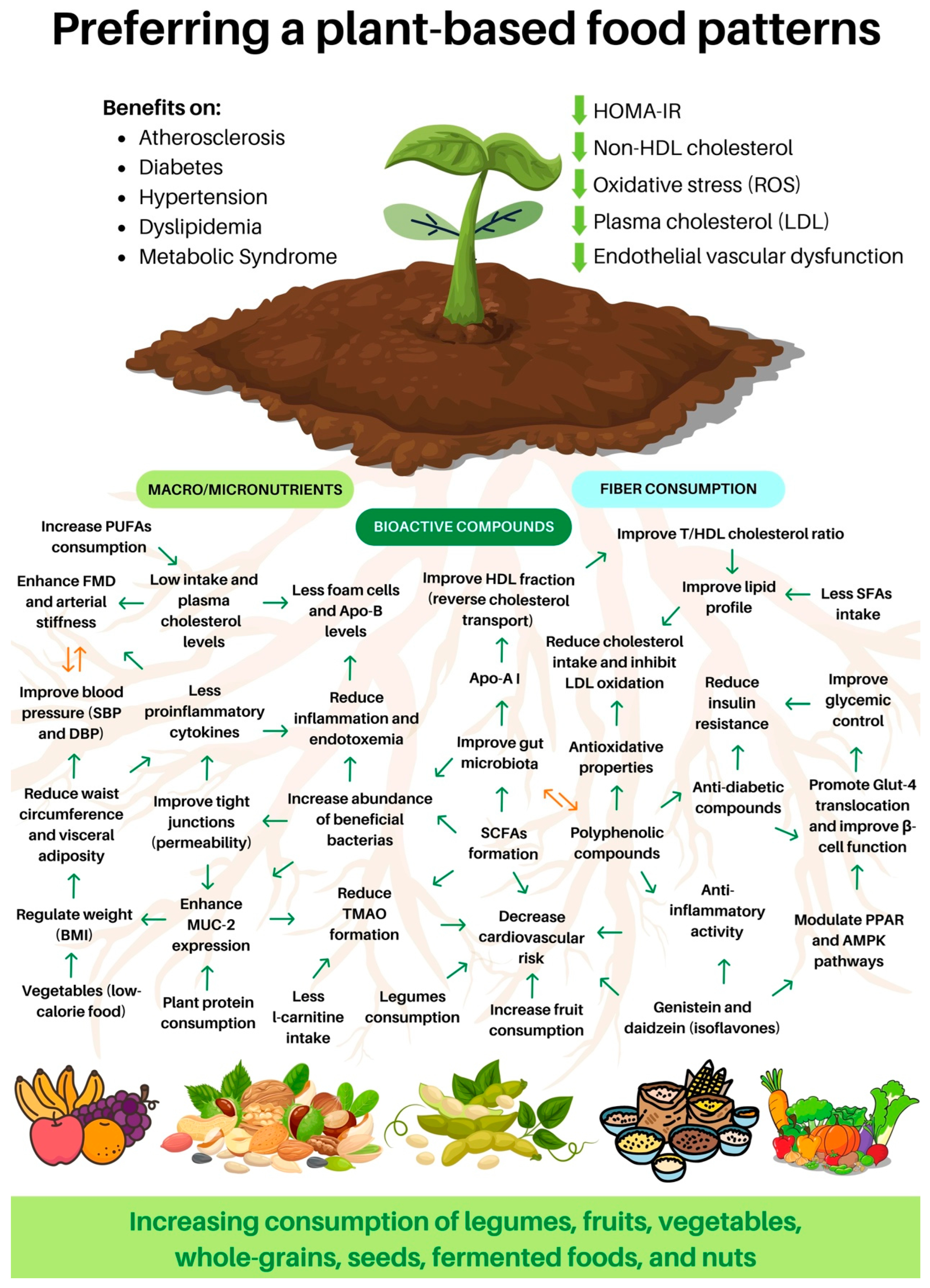
Figure 2.
Benefits of consuming a plant-based diet. TMAO: trimethylamine N-oxide; FMD: flow-mediated dilatation; SFAs: saturated fatty acids; PUFAs: polyunsaturated fatty acids; HDL: high-density lipoprotein; LDL: low-density lipoprotein; T/HDL cholesterol: total cholesterol and HDL cholesterol ratio; Apo: apolipoprotein; SBP: systolic blood pressure; DBP: diastolic blood pressure; BMI: body mass index; Glut-4: glucose transporter; SCFAs: short-chain fatty acids; PPAR: peroxisome proliferator-activated receptors; AMPK: AMP-activated protein kinase; ROS: reactive oxygen species; HOMA-IR: Homeostasis model assessment of insulin resistance; MUC-2: mucin-2.
3. A Plant-Based Diet and Chronic Non-Communicable Diseases
3.1. Diabetes
Diabetes is a prevalent common chronic condition affecting the human population. According to The World Health Organization (WHO), diabetes is projected to rank as the seventh leading cause of mortality globally by the year 2030 [188]. The macro and microvascular complications associated with diabetes are more common in older adults than middle-aged people. According to this, macrovascular complications can lead to an impaired platelet function and, thus, an increased risk for thrombus formation, atherosclerosis progression, and plaque rupture [189]. This has been supported by Huang et al. [190] who found that some microvascular complications, such as nephropathy and retinopathy, are a negative consequence but are usually not detected until late in the course of cardiovascular disease. In a 5-year follow-up observational study that included nearly 3000 participants adhering to a vegetarian diet while abstaining from smoking and alcohol consumption, a 35% lower risk of the incidence of diabetes was observed. The transition to a vegetarian diet also decreased the incidence of diabetes by 53% [191].
In this line, a PBD represents an appropriate option by which to enhance antioxidant consumption [192]. These outcomes have been supported by a recent meta-analysis [193]. The strength of this relationship was amplified when the definition of plant-based patterns encompassed fruits, vegetables, whole grains, legumes, and nuts. Similar conclusions have been drawn elsewhere [194].
The above-mentioned foods share a common feature: bioactive compounds. Among these, flavonoids are known as anti-diabetic bioactive compounds [188]. This property is related to the modulatory effects on blood sugar transporters by enhancing insulin secretion, reducing apoptosis, promoting the proliferation of pancreatic β-cells, reducing insulin resistance, inflammation, oxidative stress in muscle, and promoting Glut-4 translocation via PI3K/AKT and AMP-activated protein kinase (AMPK) pathways [195]. One of the most common flavonoids, quercetin, induces activities similar to those of metformin in muscle cells by activating AMPK pathways and thereby causing Glut-4 translocation [196]. However, quercetin also reduce proinflammatory cytokines, such as TNF-α, IL-6, and IL-1b, and modulates certain transcriptional factors like NRf2 and NF-kB [197,198]. The latter is essential, considering that accumulative evidence suggests that chronic activating proinflammatory pathways in target cells of insulin action may contribute to obesity, insulin resistance, and related metabolic disorders, including diabetes [199].
On the other hand, polyphenols are mostly found in tea, cocoa, and fruits, such as apples, berries, and citrus, among other plant-derived foods. Polyphenols act in an insulin-dependent manner by reducing β-cell apoptosis and oxidative stress, whilst stimulating β-cell proliferation, insulin signaling, and pancreatic insulin secretion [200]. They also act in an insulin-independent manner by inhibiting glucose absorption, digestive enzymes, and the formation of advanced glycation end-products, whilst regulating intestinal microbiota and modifying the inflammatory response. Moreover, dietary polyphenols ameliorate diabetic complications, such as vascular dysfunction and coronary diseases, among others [200].
A previous meta-analysis found that a 5% decrease in type 2 diabetes risk was obtained by a daily increase in anthocyanidin intake (7.5 mg) [201]. However, some studies present inconclusive results. One possible explanation of their beneficial effects lies in PPARγ activation. Some plant phytochemicals, such as quercetin, resveratrol, genistein, or curcumin affect inflammatory cascades by activating AMPK signaling via proteasomal activation and by inactivating crucial transcription factors, which may explain most of the properties attributed to PPARγ interaction. PPARγ represses inflammatory gene expression as inducible nitric oxide synthase suppresses transcriptional factors AP-1 and NF-κB, modulates mitogen-activated protein kinase (MAPK) activity, and influences glucose uptake [202].
Genistein, daidzein, and glycitein are the most active isoflavones and are mainly found in soybeans. In fact, these bioactive compounds have been proven to reduce cancer and decrease the risk of some chronic diseases like type 2 diabetes. Evidence on isoflavones reducing type 2 diabetes risk is mainly derived from animal and cell culture studies [203,204]. Extrapolating these findings to humans should be done cautiously due to the inherent limitations and differences of the biological context. Nonetheless, isoflavones have also shown a positive effect in human trials. For instance, two months of genistein consumption (50 mg/d) reduced insulin resistance in obese individuals, accompanied by a favorable modulation of the gut microbiota composition. Furthermore, subjects showed reduced metabolic endotoxemia and increased AMPK phosphorylation and genes expression involved in fatty acid oxidation in skeletal muscle [205].
Additionally, a one-year intervention with flavan-3-ols and isoflavones (850 mg/d and 100 mg/d, respectively) markedly reduced estimated peripheral insulin resistance (HOMA-IR) and enhanced insulin sensitivity due to a notable decrease in insulin levels in post-menopausal women with type 2 diabetes [206]. Moreover, genistein improved insulin sensitivity, serum triglyceride concentrations, and delayed the onset of type 2 diabetes [207]. Previous evidence proposes that genistein may help delay the onset of type 2 diabetes and improve different associated symptoms [207].
Finally, the whole-food plant-based diet has repeatedly shown benefits in this context [193]. Three prospective cohort studies found an inverse association between a healthy plant-based diet and type 2 diabetes, with 16,162 incident cases observed over 4,102,360 person-years of follow-up. These positive associations are related to antioxidants, fiber, unsaturated fatty acids, magnesium, and low saturated fat content. This outcome remains unchanged after the authors adjusted for body mass index [208]. In another large sample, maintaining an overall PBD is linked with reduced longitudinal insulin resistance, prediabetes risk, and type 2 diabetes. Further, the authors also concluded that the protective role of this diet is beyond strict vegetarian or vegan diets and includes a high plant-based diet and fewer animal-option foods [209].
Other authors concluded that a PBD accompanied by educational intervention could significantly improve HbA1c levels, weight, and, therefore, diabetes management [210]. Jardine et al. [3] highlight that insulin resistance and the succeeding dysfunction in β-cell serve as the key characteristics of the pathophysiology underlying type 2 diabetes. Along with this, long-term intervention and even a single high-fat meal can cause postprandial elevations in plasma glucose that can remain high for an extended period. This result is similar to what was found by Parry et al. [211]. The authors specify that consuming a saturated fat diet had a strong effect, improving intrahepatic triacylglycerol and exaggerating postprandial glycemia. Thus, a PBD has the potential to reverse β-cell dysfunction and peripheral insulin resistance in patients with type 2 diabetes [212], partly by improving glycemic control, reducing lipid accumulation in muscle and liver, and/or improving insulin sensitivity. This is important since a multi-adjusted analysis revealed that baseline non-alcoholic fatty liver was associated with a 2.95 times higher risk of type 2 diabetes within 10 years. The evidence has shown that a healthy plant-based diet has an inverse association with non-alcoholic fatty liver. Conversely, an unhealthy plant-based diet, distinguished by low amounts of fiber, vitamins, or minerals, and more refined sugar or sodium consumption due to the increase in ultra-processed foods, showed the opposite results [213].
3.2. Hypertension
Hypertension may be considered a chronic disease and is a risk factor for other diseases. Its incidence depends on modifiable risk factors (e.g., smoking, diet, drinking, or sedentarism/physical inactivity) and non-modifiable risk factors (e.g., genetic predisposition). As such, a diet or following a healthy foods pattern can be crucial for prevention and treatment. A healthy plant-based diet has shown positive results in both hypertensive and non-hypertensive people. In a 3-year prospective study with 1546 non-hypertensive individuals spanning the age range of 20 to 70, higher phytochemicals-rich foods consumption was linked with a lower risk of developing hypertension [214]. In a large study with 13,771 participants, the authors demonstrated that only in male individuals was an increase in antioxidants, vitamins, and phytochemicals correlated significantly with a reduction in CRP, systolic, and diastolic blood pressure (SBP/DBP, respectively) [215].
Previous studies showed that vegetarians, especially vegans, have lower SBP and DBP, and less hypertension than omnivores [216,217]. In a 4-week study, participants changed their diets to a PBD. The authors observed changes in nutrient intake, reducing saturated fat, cholesterol, protein, and some vitamins and minerals such as sodium, vitamin D, or vitamin B12. However, after four weeks, vitamin C, folate, dietary fiber, magnesium, vitamin A, and potassium significantly increased. Different measured biomarkers changed significantly, such as total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, insulin, HbA1c, and hs-CRP. However, glucose levels and total cholesterol/HDL ratio remained unchanged. The main findings highlight that SBP and DBP clinically and significantly changed after four weeks in a PBD. Interestingly, there were notable and marked declines in both blood pressure medication usage and serum lipids [218].
Berries can positively affect vascular function and reduce hypertension via their phytochemical anthocyanins. Previous studies have reported that a higher intake of anthocyanins and flavones is inversely associated with lower arterial stiffness in women through the significant reduction in central SBP, suggesting that incorporating one to two portions of berries daily (44 mg anthocyanins) could be relevant to reducing cardiovascular disease risk [219]. Fairlie-Jones et al. [220] stated that anthocyanins consumption from foods or extracts significantly enhanced vascular health. FMD, as an indicator of vascular function, is considered the gold-standard non-invasive vascular reactivity measure and has improved after acute or chronic anthocyanidins consumption.
Supporting this, other authors have highlighted that anthocyanin is an inexpensive, accessible, and effective approach to controlling atherosclerosis, cardiovascular risk, and cardiovascular aging. They can exert their effects mainly by improving endothelial function, managing oxidative stress, and inhibiting certain enzymes such as cyclooxygenase-1 and cyclooxygenase-2, which exert antihypertensive, antiatherogenic, antithrombotic, antiglycation, and anti-inflammatory activities, and ameliorate dyslipidemia and arterial stiffness [221]. Furthermore, a recent study [178] claimed that the main mechanism is an increase in endothelial-derived nitric oxide, which enhances endothelium-dependent vasorelaxation and prevents calcium-induced vascular smooth muscle contraction induced by endothelial nitric oxide synthase.
Conversely, high dietary sodium consumption is one of the significant harmful agents that impairs blood pressure homeostasis. According to the WHO, an intake of 5 g or more of sodium per day is considered excessive. This has been strongly connected to elevated blood pressure and the start of hypertension and its related cardiovascular complications [222].
The International Society of Hypertension Global Hypertension Practice Guidelines recommend reducing dietary salt intake and increasing the availability of fresh fruits and vegetables. The support for this lies in the physiological function of potassium in sodium homeostasis [223,224]. However, not all evidence supports this association [225]. An earlier study explained the theoretical mechanism referring to the capacity of potassium to modify central or peripheral neural mechanisms that regulate blood pressure. Additionally, high-potassium diets can lower blood pressure by relaxing the vascular smooth muscle and directly decreasing peripheral vascular resistance [226].
Furthermore, the “Renal Potassium Switch” (RPS) can be activated or inhibited depending on dietary potassium consumption. When potassium consumption is low, RPS activates the NaCl cotransporter (NCC) in the distal convoluted tubule. Interestingly, increased potassium intake decreases blood pressure by ~10 mmHg, which coincides with NCC inactivation. A high salt intake in animals significantly elevated blood pressure but not when the RPS was inactive [227,228]. Along with this, another study supported the positive function of potassium in regulating blood pressure and/or hypertension [229].
It was explained previously that small changes in serum potassium can cause endothelium-dependent vasodilation by hyperpolarizing the endothelial and vascular smooth muscle cells. A high-potassium diet may also enhance vascular integrity on increased tension due to hypertension. A high-potassium diet substantially reduced wall thickening in the very large or small arteries of spontaneous hypertension rats (SHR). In a previous analysis, a four-week increase in potassium consumption potentiates AngII-stimulated aldosterone secretion without affecting systemic cardiovascular hemodynamics in healthy normotensive men. This indicates that the antihypertensive effect of potassium is mediated by its diuretic effect [230]. In addition to maintaining normal plasma potassium levels, these natriuretic effects contribute to the blood pressure-lowering effect of high potassium intake. The result of a potassium-deficient diet at the expense of increased sodium retention has been linked to the pathogenesis of salt-sensitive hypertension [231].
A PBD may reduce blood pressure, body mass index, and lower sodium levels whilst increasing potassium content in foods. Also, its function has been analyzed in terms of improving NO bioavailability and the beneficial effect on the microbiome [5]. A recent study highlights the relevance of opting for a healthy plant-based diet in preventing hypertension. The authors found that 2244 individuals, based on a community cohort of 5636 men and women between 40–69 years, developed hypertension in a 14-year follow-up period. However, according to a healthy plant-based diet, the highest versus lowest quintiles exhibited significant differences. Those in the highest quintile of a healthy plant-based diet had a 35% lower incidence of hypertension, while an unhealthy plant-based diet showed a 44% greater hypertension incidence [232].
3.3. Dyslipidemia
Koh et al. [233] identified dyslipidemia as a prominent risk factor for ASCVD, a condition marked by the imbalance of atherogenic and protective lipids, such as triglycerides, LDL cholesterol, and HDL cholesterol. Statins are standard treatment, as they inhibit the critical step in cholesterol synthesis: the conversion of 3-hydroxy-3-methylglutaryl coenzyme A (HMGC) to mevalonate by HMGC reductase. This gives statins a potent lipid-lowering effect that reduces cardiovascular risk and decreases mortality (reducing LDL cholesterol by ≥50%). However, statins have many common side effects ranging from musculoskeletal symptoms, increased risk of diabetes, and higher rates of hemorrhagic stroke [234].
Considering that mentioned above, a PBD has shown multiple beneficial effects on dyslipidemia without adverse side effects. In a recent pilot, dietitian-led, vegan 12-week program, the results showed a significant reduction in LDL cholesterol and LDL particles, with a decreasing trend in very low lipoprotein density and chylomicron particles. These beneficial changes have been attributed to the observed decrease in inflammation, as measured by GlycA [235]. GlycA is considered a novel marker related to systemic and subclinical vascular inflammation [236], a significant consequence of dyslipidemia that affects others’ pathogenesis. In a study of 38 Romanian subjects who adopted a PBD for at least one year, the authors observed that 75% of subjects with elevated TG succeeded in normalizing them, as well as individuals with high LDL cholesterol levels, where 72.7% from the borderline elevated level became optimal. The total cholesterol/HDL ratio shifted from elevated to optimum levels in 78.6% of the cases [237].
One of the plant-based source components that plays an essential role in health is fiber. Different studies have evaluated dietary fiber’s benefits for reducing LDL cholesterol. In 1999, with 67 controlled clinical trials, it was reported that other soluble fibers could reduce total and LDL cholesterol to a similar extent. For instance, the consumption of 3 g of soluble oat fiber reduces LDL cholesterol by <0.13 mmol/L [238].
A recent meta-analysis found that foods high in unsaturated and low in saturated and trans fatty acids with added plant sterols/stanols and high in soluble fiber at least moderately reduce LDL cholesterol [239]. This has also been published in a previous study concluding that a low glycemic index and at least 23 g of fiber a day can help to improve dyslipidemia in subjects with type 2 diabetes. A particular finding in this study was that the changes were not dependent on altering energy intake or body composition. This is important because most beneficial effects could eventually be overshadowed or mixed with improving body composition, particularly the decrease in fat mass or improvement in the percentage of total body fat [240].
The mechanism by which fiber improves cardiovascular health is not fully understood. It has been proposed that fiber could modify cholesterol metabolism by directly interacting with pancreatic lipase, binding to bile acids, increasing intraluminal viscosity, intestinal microbiota secreting fermentation products that modulate hepatic fatty acid synthesis, changes in intestinal motility, and increasing satiety that results in to lower overall energy intake [238,241]. Soluble fiber directly affects serum cholesterol and LDL cholesterol levels by binding bile acids in the small intestine and increasing their excretion in the feces [242]. This trapping of cholesterol and bile acids in the small intestine reduces absorption/reabsorption [243]. SCFA complements this effect.
Soluble fiber is resistant to hydrolysis in the small intestine but is fermented by gut microbiota in the large intestine. In this context, a previous study observed that SCFA reduces cholesterol levels by negating the counteractive induction of hepatic cholesterol synthesis caused by increased bile acid excretion [244]. Also, it appears that SCFA plays a role in the production of Apo-A I, which could consequently improve the functionality of the serum HDL fraction [245]. This is relevant as HDL performs the opposite action to LDL (reverse cholesterol transport). The absorption of SCFAs such as propionic acid has been shown to decrease cholesterol synthesis in the liver, thus reducing plasma cholesterol and increasing sodium and water absorption into the colonic mucosal cells.
Further, dietary saponins directly inhibit cholesterol absorption in the small intestine and indirectly inhibit the reabsorption of bile acids to lower plasma cholesterol. Additionally, phytochemicals have been shown to decrease LDL levels, which signal cholesterol build-up, thus indicating that phytochemicals can also be used to reduce blood cholesterol levels [246]. Therefore, if cholesterol absorption from the diet is reduced, physiological adaptations must be generated to maintain levels within normal ranges. Lütjohann et al. [247] observed that lacto-vegetarians absorbed 44% less dietary cholesterol but synthesized 22% more cholesterol, while vegans absorbed 90% less dietary cholesterol, synthesized 35% more cholesterol, and had a similar plasma total cholesterol but a 13% lower plasma LDL cholesterol than omnivores. The authors concluded that the reduction of LDL cholesterol was significant only in vegans.
On the other hand, not all studies found a PBD to be beneficial for hypertriglyceridemia [9]. However, the outcomes were positive when specific foods such as walnuts were tested. Indeed, walnut consumption has shown notable improvements in triglyceride levels, particularly among overweight/obese individuals, with men experiencing more significant results than women. Nonetheless, a subgroup analysis reflects much-lowering effects, comparing individuals with comorbidities versus healthy subjects [248]. Thus, the different or negative impacts could be related to the “type of diet”.
As we mentioned at the beginning, according to the literature, two types of PBD exist: healthy and unhealthy. In this line, a plant-based diet index (PDI), which separates healthy plant-based diet options (i.e., fruits, whole grains, vegetables, legumes, nuts, coffee, and tea) from less healthy plant-based diet options (i.e., refined grains, potatoes, sugar-sweetened beverages, sweets and desserts, salty foods), was evaluated. The authors found that those in the highest quintile of PDI and consuming a healthy plant-based diet consumed more carbohydrates (including fiber), vitamins, and minerals but less cholesterol, protein, and fat. In this study’s follow-up of 29,313 person-years, the incidence of dyslipidemia was significantly lower in healthy plant-based diets than in unhealthy plant-based diets, comparing the highest and lowest quintiles. In fact, one standard deviation of PDI and healthy PDI was associated with a 9% and 16% lower risk of incident dyslipidemia, respectively, and one standard deviation of unhealthy PDI was associated with a 16% higher risk of dyslipidemia after adjustment for confounders effects. While PDI and a healthy plant-based diet were inversely connected with hypertriglyceridemia, an unhealthy plant-based diet was associated with all lipid disorders. Interestingly, this strong association remained after adjustment for anti-dyslipidemia medication [249].
Additionally, previous evidence has shown that quinoa could decrease weight gain, improve lipid profile, and improve capacity to respond to oxidative stress, mainly due to saponins content [250]. Another study also showed decreased LDL cholesterol and TG after 30 days of quinoa bar consumption [251]. This has been supported previously [252]. Here, the authors found a 36% reduction in TG levels after 12 weeks of 50 g of quinoa consumption. The mechanism by which quinoa exhibits these benefits is not fully comprehended. However, their effects are related to fiber content, reduction in dietary fat absorption due to increased lipid content in feces, and/or bile acid activity. One crucial detail is that these changes in triglyceride reduction levels were comparable to the reduction evidenced in pharmacologic therapy that used 40% nicotinic acid, 35% fibrates, and 20% statins. Finally, Table 2 displays a summary of findings on NCCD and MetS, Figure 2 provides a comprehensive overview addressing the overall advantages of adopting a plant-based diet, and Figure 3 provides a synthesis addressing the general effects of adopting a plant-based food pattern against specific chronic non-communicable diseases.

Table 2.
NCCD and MetS (pre-clinical, clinical, prospective, or follow-up human studies summary).
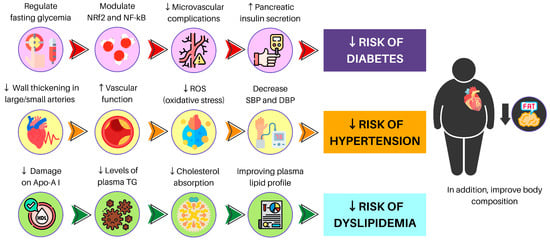
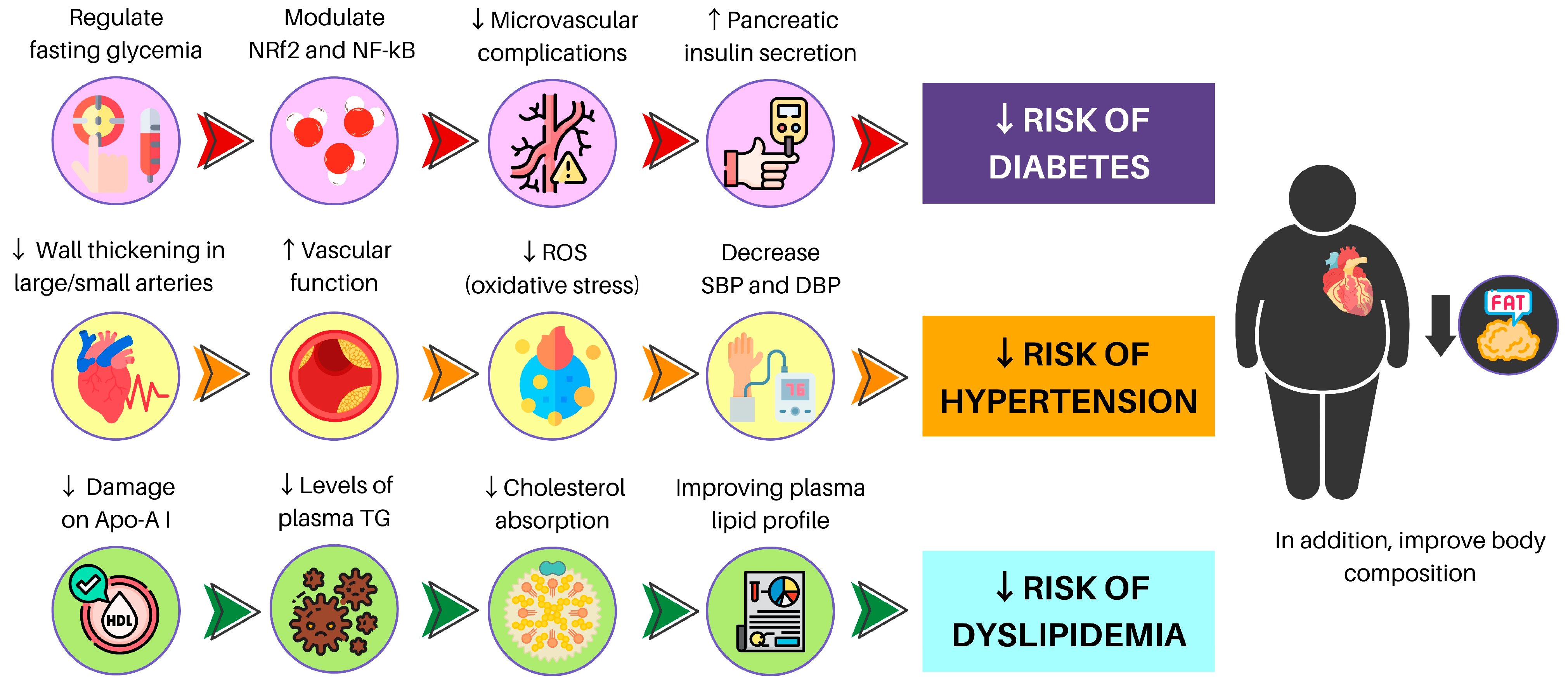
Figure 3.
A brief description of PBD benefits for NCCD and MetS. NRf2: nuclear factor erythroid 2-related factor 2; NF-kB: nuclear factor-κB; ROS: reactive oxygen species; SBP: systolic blood pressure; DBP: diastolic blood pressure; Apo-A I: apolipoprotein A I; TG: triglycerides.
4. A Plant-Based Diet and Metabolic Syndrome
4.1. Insulin Resistance (Fasting Blood Sugar)
The relationship between high fasting blood sugar (hyperglycemia) and insulin resistance (IR) is consistent. IR is a pathological condition in which cells fail to respond normally to insulin. Consequently, this leads to elevated blood glucose levels, often accompanied by hyperinsulinemia and hyperglycemia. In this condition, pancreatic β-cells secrete excessive insulin to maintain normal blood sugar levels.
The principal function of insulin in skeletal muscle is to stimulate glucose uptake by inducing the translocation of the specific glucose transporter GLUT4 from the cytoplasm to the plasma membrane. Upon binding to GLUT4, insulin initiates a signaling cascade to phosphorylate and activates the Insulin Receptor Substrate (IRS), leading to the activation of various protein kinases (PI3 kinase, Pdk1, and Akt). Activated Akt moves to the plasma membrane and phosphorylates Akt Substrate of 160 kDa (AS160). AS160 initiates GLUT4 translocation, allowing for glucose influx into skeletal muscle. This glucose is then undergoing glycolysis and thus blood glucose levels are lowered.
A previous study has demonstrated that both acute blueberry consumption and short-term blueberry supplementation have beneficial effects on glucose regulation and insulin balance in sedentary individuals, presumably mediated through gastrointestinal enzyme inhibition and incretins secretion [271]. Prioritizing plant sources to the detriment of traditional animal alternatives results in lower IR and a lower risk of prediabetes and type 2 diabetes [272]. Similarly, Kahleova et al. [212] found in a 16-week randomized clinical intervention that β-cell function and fasting insulin resistance were improved by a qualitative change in macronutrient composition with no limit on energy intake in overweight individuals with no history of diabetes. HOMA-IR is considered an effective indicator of IR since it provides an estimate of fasting glucose and insulin serum concentrations [273].
Further, previous studies have extensively demonstrated that AMPK stimulation can improve health in different contexts. The AMPK signaling pathway provides an alternative to the insulin-dependent glucose uptake pathway in muscle by activating phosphatidyl-inositol-3 kinase (PI3-K) and PKB/Akt [253]. Data support that AMPK inhibits Rab-GTPase activating proteins AS160 (TBC1D4) and TBC1D1, which triggers Glut-4 trafficking to the plasma membrane [274]. Firstly, related to the stimulation of Glut-4 translocation and AMPK activation, a previous study demonstrated that anthocyanin-rich extract from black rice promotes glucose uptake by increasing Glut-4 expression in the plasma membrane in C2C12 myotubes via activation of the PI3K/Akt and AMPK/p38 MAPK pathways [275]. Furthermore, resveratrol, a naturally occurring phytochemical, increases glucose uptake in insulin-resistant 3T3-L1 adipocytes by increasing pAkt phosphorylation and downstream AMPK activation. In another study, berberine, a natural plant-derived compound, can indirectly activate AMPK. Furthermore, a berberine derivative demonstrated enhanced insulin sensitivity and reduced adiposity in vivo in high-fat diet rats [254].
On the other hand, in recent years, PPAR has gained significant interest due to its potential role as an essential regulator of glucose metabolism and insulin sensitivity. PPAR-α activation stimulates pancreatic islet β-cells, potentiating glucose-stimulated insulin secretion. On the contrary, PPAR-α deficiency in a mouse model of obesity-related insulin resistance leads to reduced insulin secretion by pancreatic β-cells in response to glucose [255]. This improvement in insulin sensitivity has been theorized in the modification of signaling due to a decrease in ectopic lipids in non-adipose tissues and a decrease in circulating fatty acids and triglycerides, seen in animal models [256]. Furthermore, PPARγ activation in type 2 diabetic patients results in a marked improvement in insulin and glucose parameters by modifying whole-body insulin sensitivity.
Given this, diet-induced PPAR downregulation can be viewed as a negative effect. For instance, a high-fat diet can negatively affect the activity or expression of PPAR, favoring several complications, such as IR. However, some bioactive plant components, like the polyphenols present in coffee, rice, berries, and others, can modulate this pathway, increasing PPARα and γ mRNA [257]. Polyphenols have a positive effect on insulin sensitivity and improve IR by way of several mechanisms, including lowering postprandial glucose, modulating glucose transport, affecting insulin signaling pathways, and protecting against damage to insulin-secreting pancreatic β-cells [276]. In fact, synergistic metabolic action between exercise and polyphenols consumption from grapes counteracts anthropometric and metabolic impairments. It increases insulin sensitivity, probably via lipid oxidation enhancement and glycogen utilization reduction. Although this study was performed in animals, the amount of polyphenols consumed by the specific grape is equivalent to nutritional amounts ingested daily by humans [258].
Moreover, in a study of IR nondiabetic adults, the 6-week consumption of strawberry and cranberry polyphenols showed that the consumption of 333 mg polyphenols might improve insulin sensitivity and prevent an increase in compensatory insulin secretion without affecting plasma lipids, CRP, proinflammatory cytokines, or antioxidant capacity [270]. This result is in line with a previous study that evaluated daily dietary bioactive supplementation in freeze-dried whole blueberry powder. The authors found an improvement in insulin sensitivity in obese, nondiabetic, and insulin-resistant participants after six weeks, independent of any changes in inflammatory biomarkers or adiposity [277]. Different other studies have found similar results [278,279].
Inflammatory signaling pathways are associated with the activation of TLRs. TLRs are transmembrane proteins that regulate the innate immune response in various pathophysiological states like sepsis and cardiovascular disease [280]. TLR signaling triggers transforming growth factor β activating kinase 1 activation and, subsequently, also MAPK and NF-ĸB [281]. NF-ĸB regulates the transcription of inflammatory cytokines such as TNF, IL-6, and IL-1 [282]. TLR2 and TLR4 are involved in inflammation and insulin resistance [283] in human skeletal muscle cells [284]. Interestingly, in mice, the absence of TLR2 and TLR4 from the plasma membrane protects against obesity and IR [285], which generated a lot of interest in the TLRs as possible therapeutic targets in the fight against obesity and IR. A recent review identified some plant extracts as potential modulators of TLRs controlling inflammation and the immune response [259]. Counteracting the negative effects of chronic low-grade inflammation may result in beneficial effects in different pathological states such as insulin resistance.
4.2. Visceral Obesity and Waist Circumference
A recent study reported that greater adherence to a healthy plant-based pattern, but not an unhealthy one, was linked with lower visceral adipose tissue, accounting for several potential confounding variables [260,286]. This outcome aligns with a Dutch study comparing sweet snacks against fruit and vegetable consumption. While the first was associated with hepatic triglyceride content, consuming fruits and vegetables was negatively related to visceral fat and liver fat content (triglycerides) [261]. The theoretical mechanism of these positive results is partly in energy consumption, considering that a PBD is high in fiber [287] and higher in antioxidants. In the case of fiber, it can help increase satiety with little or no calorie intake.
On the other hand, antioxidants can reduce inflammation related to visceral adiposity. A healthy and unhealthy plant-based diet have marked differences in the amounts of sugar, glycemic index, and energy intake with the theoretical capacity to increase weight and, consequently, waist circumference.
A recent study [263] performed on older Australian women showed that body weight, body mass index, and waist circumference were significantly lower in pesco-vegetarian and vegetarians than meat-eaters. Moreover, among meat-eaters, subjects that consume meat regularly or several times a week, compared with those consuming meat two or fewer times a week, present higher body weight, body mass index, and waist circumference. To be detailed, in a dose-dependent meat intake, for every increase in the category of weekly meat intake, an associated 2.6 kg increase in body weight, a 0.9 kg/m2 increase in body mass index, and a 2–3 cm increase in waist circumference were reported. Although this evidence does not qualify as a PBD, it is essential to highlight that meat consumption frequency was the primary factor involved in the main results. Another study that included 9633 participants showed that greater adherence to a PBD was associated with lower body mass index, waist circumference, fat mass index, and body fat percentage across a median follow-up period of 7.1 years [262]. This result has been previously supported by other authors [288].
4.3. High-Blood Pressure
High blood pressure is universally known as a risk factor for several chronic diseases, such as hypertension. In a recent meta-analysis [6], the authors concluded that a PBD reduced SBP and DBP. The authors established that a high fruit and vegetable diet was associated with a mean reduction in SBP. Although some studies did not observe a consistent association between fruit and vegetable consumption and improved blood pressure in older age [264], the central body of evidence has found positive results. For instance, healthy PDI is associated with lower blood pressure, while unhealthy PDI is adversely related to blood pressure. A PBD rich in vegetables and whole grains and limited in refined grains, sugar-sweetened beverages, and total meat may contribute to these associations [289]. In a study by Stefler et al. [264], the preference for vegetable consumption involves preserved or cooked vegetables instead of having them raw, and including salt during both types of procedures might counteract the positive benefits of these foods. Additionally, the authors mentioned some study limitations that could explain the lack of clear association. Nevertheless, other studies have found the benefits of fruit and/or vegetable intake for blood pressure.
In this line, it has been hypothesized that fiber, different nutrients, bioactive components, and plant-sourced foods, such as fruits and/or vegetables, can exert their benefits on blood pressure due to the improvement in baroreflex sensitivity [290], endothelial-dependent vasodilatation related to high-flavonoid intake [291], and the inhibition of inflammation and oxidative stress response [265,266]. In a study on cranberry juice and vascular function, patients with coronary artery disease registered a reduction in carotid-femoral pulse wave velocity, a clinically relevant measure of arterial stiffness [292].
4.4. Hypertriglyceridemia
A PBD has widely demonstrated several benefits related to MetS. For instance, in a recent South Korean prospective cohort study [293] comparing the highest with the lowest quintiles of an unhealthy plant-based diet linked to greater intake versus lower intake, in a follow-up of 8 years, the highest quintile had a 50% higher risk of developing MetS. After adjusting for body mass index, those in the highest quintile of an unhealthy plant-based diet had a 24% to 46% higher risk of four out of five individual components of MetS, including hypertriglyceridemia. These results have been recently supported, detailing that greater adherence to PDI or healthy PDI was associated with a lower risk of incident dyslipidemia. In contrast, greater adherence to unhealthy PDI was associated with a higher risk.
Moreover, the authors detailed that PDI was inversely associated with low HDL cholesterol among women, while among men a greater adherence to PDI was inversely associated with hypertriglyceridemia [249]. Based on Korean food patterns, the authors reported that the lipid composition from a PBD leads to less absorption and conversion to blood cholesterol and reduced triglyceride concentration. This is because plant foods are rich in compounds for preventing dyslipidemia, such as dietary fiber, phytosterols, antioxidants, and polyphenols.
On the other hand, although some studies did not refer to a PBD, the reduction in animal-based food has also shown positive effects. Teixeira et al. [294], in a comparison between vegetarians and omnivores, found that different indicators such as blood pressure, fasting plasma glucose, total cholesterol, LDL cholesterol, and triglycerides were lower among vegetarians. In fact, the authors complemented the results, highlighting that an unbalanced omnivorous diet with excess animal protein and fat may be implicated, to a great extent, in the development of non-communicable diseases and conditions, especially cardiovascular risk.
The consumption of dietary fiber of those who opt for a PBD and those who follow a traditional Western diet differs significantly. In this context, dietary fiber has been evaluated in the reduction of postprandial triglycerides response [295]. The beneficial mechanism of this property is strongly attributed to its viscosity, which effectively slows down gastric emptying and disrupts the emulsification of fats and the formation of micelles in the gastrointestinal tract. This is achieved by reducing the availability of circulating bile acids. Also, soluble fiber can be fermented by the gut microbiota to release metabolites such as SCFA, which upregulate genes PPARα and PPAR-γ coactivator-1α (PGC-1α) involved in lipid metabolism and the regulation of postprandial triglycerides response.
4.5. Dyslipidemia (Low HDL Cholesterol)
Generally, HDL cholesterol is considered a healthy biomarker due to its ability to reverse cholesterol transport and reduce cardiovascular risk. This cardioprotective function is supported by theoretical mechanisms, such as the regulation of cholesterol efflux, where apolipoprotein A-I (Apo-A I), the main component of HDL, is mainly responsible for reverse cholesterol transport through the macrophage ATP-binding cassette transporter ABCA. Another mechanism lies in the modulation of inflammation, antioxidant, and vasoprotective effects [296]. However, according to Kosmas et al. [297], HDL functionality is more critical in atheroprotection than circulating HDL cholesterol levels. In fact, plasma HDL constitutes a heterogeneous group of particles with diverse structures and biological activity and, under certain conditions, may become proinflammatory. Further, Apo-A I can be damaged by oxidative mechanisms, which render the protein less able to promote cholesterol efflux. Navab et al. [296] complement this information by pointing out that a modification of the protein components of HDL can convert it from an anti-inflammatory to a proinflammatory particle.
Very high HDL cholesterol could be more harmful than harmless; several previous investigations have reported that the elevation of HDL cholesterol could result in a cardiovascular risk factor [298,299]. This relationship between high HDL cholesterol levels and mortality risk has been observed even at >80 mg/100 mL, although the authors reported that it was related to men, not women [300]. Both extremely high and low HDL cholesterol are associated with an increased mortality risk [301].
These paradoxical results’ mechanisms and theoretical explanations can be attributed to two main factors. Firstly, some genetic disorders raise HDL cholesterol, such as primary familial or secondary hyperalphalipoproteinemia, mainly resulting in the overproduction or variants of apolipoprotein C-III (Apo-C III) due to a mutation in Apo-A I. These complications promote HDL cholesterol dysfunction, stimulate smooth muscle cell proliferation, facilitate the interaction between monocytes and endothelial cells, and alter platelet activity, all triggering atherosclerosis [298,299,301]. Secondly, a previous study [302] has shown that a moderate to high HDL concentration impairs human endothelial progenitor cells and related angiogenesis by activating rho-associated kinase pathways in healthy subjects. The authors found that, although oxidized LDL reduces the cell viability of late-growing EPCs derived from healthy human peripheral blood in a dose-dependent relationship, HDL alone might not be enough to counteract this negative effect and might even be paradoxically impaired at high concentrations (400–800 μg/mL, equivalent to 40–80 mg/dL in humans).
Some evidence has indicated that vegans, vegetarians, or PBD reduce total, LDL, and HDL cholesterol compared to omnivores in observational and clinical studies [9]. Along with this, some authors have found that a short-term, very low carbohydrate diet has been associated with an increase in HDL cholesterol in normal-weight normolipidemic women [303]. However, a low carbohydrate plant-based diet (eco-atkins) was evaluated in hyperlipidemic subjects. The result demonstrated a greater reduction in Apo-B, Apo-A I, LDL, and HDL cholesterol compared to a high carbohydrate diet, resulting in better context in which to improve heart disease risk factors [304]. This discrepancy of findings might generate a confounding analysis in evaluating a PBD, cardiovascular risk, and HDL cholesterol.
Regarding these findings, some authors have evaluated the total cholesterol/HDL cholesterol ratio (T/H-r) as a predictor of cardiovascular disease. Previous studies established that this ratio has a better predictive value than the isolated parameters in vascular risk. In fact, when there is no reliable calculation of LDL cholesterol, it is preferable to use the T/H-r [305]. An earlier study has supported this and indicated that T/H-r is a superior measure of risk for coronary heart disease compared with either total cholesterol or LDL cholesterol levels [306]. A recent large study [267] has sustained this finding. Although non-HDL cholesterol is linearly related to ischemic heart disease and may be easier to calculate, T/H-r represents a higher predictability as a clinical predictor than non-HDL cholesterol. In addition, Quispe et al. [307] reported that the potential clinical utility of T/H-r is most apparent among individuals in whom TC/HDL-C levels are inconsistent with their other lipid parameters (i.e., LDL cholesterol and non-HDL cholesterol). Discordance was more pronounced in those with high triglycerides and low HDL cholesterol levels, characteristic of patients with insulin resistance, diabetes, and MetS, who also have a higher prevalence of LDL and Apo-B particles that are cholesterol depleted.
Considering the information above, the lower T/H-r, the better. Bleda et al. [308] found that improvement of nitrite levels is associated with decreased T/H-r values in peripheral artery disease patients, resulting in endothelial dysfunction recovery. In this line, a previous study demonstrated that a low fat, plant-based lifestyle intervention reduced HDL levels but this was not as great as the decrease in LDL and TC, resulting in improvements in the T/H-r of −3.2% and the LDL/HDL ratio of −5.3% [309]. According to the authors, even when HDL levels decreased, other indicators of cardiovascular risk improved, raising the question of whether HDL levels are a suitable predictor of cardiovascular risk in this population.
In subjects who do not consume a typical Western diet or have a diagnosis of MetS, it may not be appropriate in clinical practice or research to apply lifestyle interventions that promote a plant-based eating pattern. It is relevant to mention that, although 323 participants classified as having MetS at program entry no longer had this status after 30 days, 112 participants acquired the MetS classification because of reduced HDL levels. Regarding HDL levels, the mean value changed by −8.7% from 54.84 (baseline) to 50.07 (post-intervention), considering the average value within the normal ranges. Supporting this, a previous meta-analysis suggested that the T/H-r was more predictive than HDL or non-HDL cholesterol sub-fractions and two times more predictive than total cholesterol [310].
Taking this into account, HDL has components with anti-inflammatory functions, such as Apo-A I and paraoxonase 1. At the same time, the proinflammatory action lies mainly in Apo-A II and Apo-C III. Regarding the latter, four prospective cohort studies indicated that Apo-C III might mark a subfraction of HDL associated with a higher risk of coronary heart disease [311]. This finding raises the question of whether the reduction in HDL may decrease in part the proinflammatory component of this particle.
The presence or absence of Apo-C III in HDL could be responsible for opposite outcomes related to cardiovascular risk. For instance, HDL that lacks Apo-C III inhibits the monocyte-endothelial cell interaction, leading to a lowered inflammatory response. In contrast, HDL with Apo-C III did not diminish this interaction [268]. Previous studies have provided supporting evidence that certain drugs aimed at improving health can increase HDL cholesterol levels but do not effectively reduce coronary atherosclerosis [269]. In this study, using the CEPT (cholesteryl ester transfer protein) inhibitor after one-year of treatment increased HDL cholesterol by approximately 60%, while LDL cholesterol decreased by about 20%. However, there was no significant reduction in the progression of coronary atherosclerosis according to the percent atheroma volume, the primary efficacy measure. Another study has found similar results with a different drug [312]. Given that, for people who follow a healthy plant-based diet, reducing HDL cholesterol could not be as harmful as those who suffer from a chronic disease. The decrease in HDL cholesterol is likely less of a concern and the need to increase these levels is less critical.
Nonetheless, some plant foods have shown a positive relationship with improving HDL cholesterol levels. In a randomized, single-blinded, controlled clinical trial [313], tomato consumption showed an independent positive association with HDL cholesterol. In an isocaloric diet, subjects were randomized to receive 300 g of cucumber (control group) or two uncooked Roma tomatoes daily for four weeks. The results indicated that subjects with initial low HDL cholesterol who ate tomato changed their levels from 36.5 ± 7.5 mg/dL to 41.6 ± 6.9 mg/dL. Another study evaluating a specific plant food also found a positive association [314]. The authors found that 10 g/day consumed before breakfast can increase HDL cholesterol and improve other markers of abnormal lipid metabolism in coronary artery disease patients with low initial HDL cholesterol levels. At weeks 6 and 12, HDL cholesterol was 12–14% and 14–16% higher, referring to Pakistani and American almonds, respectively.
Furthermore, a more recent meta-analysis [315] reported that avocado consumption significantly increased HDL cholesterol with significant heterogeneity. This remained consistent in sensitivity and subgroup analyses. Therefore, although HDL cholesterol has been declared for decades as an essential indicator to protect our health, in some contexts where different health factors are present, such as physical activity or healthy eating habits, the subtle reduction of this component might not be a problem because a whole-food plant-based diet is considered more protective. Finally, Table 2 displays a summary of findings on NCCD and MetS, while Figure 2 shows a brief description of PBD benefits for NCCD and MetS, and Figure 3 provides a comprehensive overview addressing the overall advantages of adopting a plant-based diet.
5. Conclusions
Over the past two decades, a substantial body of consistent evidence has emerged at the cellular and molecular level, elucidating the numerous benefits of a plant-based diet (PBD) for preventing and mitigating conditions such as atherosclerosis, chronic noncommunicable diseases, and metabolic syndrome. It is paramount to prioritize the consumption of quality, natural, and fermented foods to fully harness the health potential of this dietary approach. With guidance from qualified professionals to ensure optimal nutrition, any concerns regarding potential nutritional deficiencies can be effectively addressed through diverse and well-planned food choices. This specialist support enables individuals to adopt a PBD at any stage of life, allowing them to reap its benefits while minimizing potential risks. Consequently, a plant-based diet offers a promising outlook for improving the health and well-being of the global population, with its protective effects mediated by bioactive compounds. It is crucial for both the public and researchers to recognize the significance of this evidence and its implications for nutrition science and public health. As our understanding of the underlying mechanisms of a PBD continues to expand, there remain exciting areas within this field of study to explore and uncover.
Author Contributions
H.P.-J. conceived and designed the review and wrote the initial draft. C.C.-M. contributed to the conceptualization of the design and critically reviewed the manuscript. All other authors provided valuable insights and feedback to enhance the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AMPK | AMP-activated protein kinase |
| DHA | docosahexaenoic acid |
| EPA | eicosapentaenoic acid |
| FMD | flow-mediated dilatation |
| HDL | high-density lipoprotein |
| LDL | low-density lipoprotein |
| MetS | metabolic syndrome |
| PBD | plant-based diet |
| PDI | plant-based diet index |
| PUFAs | polyunsaturated fatty acids |
| TMAO | trimethylamine N-oxide |
| SCFAs | short-chain fatty acids |
| TNF-α | tumor necrosis factor-alpha |
| ABC | ATP-binding cassette |
| ASCVD | atherosclerotic cardiovascular disease |
References
- Bakaloudi, D.R.; Halloran, A.; Rippin, H.L.; Oikonomidou, A.C.; Dardavesis, T.I.; Williams, J.; Wickramasinghe, K.; Breda, J.; Chourdakis, M. Intake and adequacy of the vegan diet. A systematic review of the evidence. Clin. Nutr. 2021, 40, 3503–3521. [Google Scholar] [CrossRef] [PubMed]
- Neufingerl, N.; Eilander, A. Nutrient intake and status in adults consuming plant-based diets compared to meat-eaters: A systematic review. Nutrients 2022, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Jardine, M.A.; Kahleova, H.; Levin, S.M.; Ali, Z.; Trapp, C.B.; Barnard, N.D. Perspective: Plant-Based Eating Pattern for Type 2 Diabetes Prevention and Treatment: Efficacy, Mechanisms, and Practical Considerations. Adv. Nutr. 2021, 12, 2045. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Wang, C.; Mao, Z.; Chen, Y.; Ren, P.; Fan, M.; Cui, S.; Niu, K.; Gu, R.; et al. Association of plant-based diet and type 2 diabetes mellitus in Chinese rural adults: The Henan Rural Cohort Study. J. Diabetes Investig. 2021, 12, 1569–1576. [Google Scholar] [CrossRef]
- Joshi, S.; Ettinger, L.; Liebman, S.E. Plant-Based Diets and Hypertension. Am. J. Lifestyle Med. 2020, 14, 397. [Google Scholar] [CrossRef]
- Gibbs, J.; Gaskin, E.; Ji, C.; Miller, M.A.; Cappuccio, F.P. The effect of plant-based dietary patterns on blood pressure: A systematic review and meta-analysis of controlled intervention trials. J. Hypertens. 2021, 39, 23–37. [Google Scholar] [CrossRef]
- Butnariu, M.; Fratantonio, D.; Herrera-Bravo, J.; Sukreet, S.; Martorell, M.; Ekaterina Robertovna, G.; Les, F.; López, V.; Kumar, M.; Pentea, M.; et al. Plant-food-derived bioactives in managing hypertension: From current findings to upcoming effective pharmacotherapies. Curr. Top. Med. Chem. 2023, 23, 589–617. [Google Scholar] [CrossRef]
- Wang, F.; Zheng, J.; Yang, B.; Jiang, J.; Fu, Y.; Li, D. Effects of Vegetarian Diets on Blood Lipids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2015, 4, e002408. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Levin, S.M.; Barnard, N.D. Association between plant-based diets and plasma lipids: A systematic review and meta-analysis. Nutr. Rev. 2017, 75, 683–698. [Google Scholar] [CrossRef]
- Tantamango-Bartley, Y.; Jaceldo-Siegl, K.; Fan, J.; Fraser, G. Vegetarian diets and the incidence of cancer in a low-risk population. Cancer Epidemiol. Biomarkers Prev. 2013, 22, 286–294. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhan, J.; Wang, Y.; Wang, D. The Relationship Between Plant-Based Diet and Risk of Digestive System Cancers: A Meta-Analysis Based on 3,059,009 Subjects. Front. Public Health 2022, 10, 892153. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.Y.; Sang, L.X.; Jiang, M.; McPhee, D.J. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef] [PubMed]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef]
- Wang, X.; Yang, D.Y.; Yang, L.Q.; Zhao, W.Z.; Cai, L.Y.; Shi, H.P. Anthocyanin Consumption and Risk of Colorectal Cancer: A Meta-Analysis of Observational Studies. J. Am. Coll. Nutr. 2019, 38, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Briata, I.M.; Paleari, L.; Rutigliani, M.; Petrera, M.; Caviglia, S.; Romagnoli, P.; Libera, M.D.; Oppezzi, M.; Puntoni, M.; Siri, G.; et al. A Presurgical Study of Curcumin Combined with Anthocyanin Supplements in Patients with Colorectal Adenomatous Polyps. Int. J. Mol. Sci. 2021, 22, 11024. [Google Scholar] [CrossRef]
- Della Pepa, G.; Vetrani, C.; Vitale, M.; Bozzetto, L.; Costabile, G.; Cipriano, P.; Mangione, A.; Patti, L.; Riccardi, G.; Rivellese, A.A.; et al. Effects of a diet naturally rich in polyphenols on lipid composition of postprandial lipoproteins in high cardiometabolic risk individuals: An ancillary analysis of a randomized controlled trial. Eur. J. Clin. Nutr. 2020, 74, 183–192. [Google Scholar] [CrossRef]
- Ghaedi, E.; Foshati, S.; Ziaei, R.; Beigrezaei, S.; Kord-Varkaneh, H.; Ghavami, A.; Miraghajani, M. Effects of phytosterols supplementation on blood pressure: A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 2702–2710. [Google Scholar] [CrossRef]
- Bao, X.; Zhang, Y.; Zhang, H.; Xia, L. Molecular Mechanism of β-Sitosterol and its Derivatives in Tumor Progression. Front. Oncol. 2022, 12, 926975. [Google Scholar] [CrossRef]
- DAYI, T.; OZGOREN, M. Effects of the Mediterranean diet on the components of metabolic syndrome. J. Prev. Med. Hyg. 2022, 63, E56. [Google Scholar] [CrossRef]
- Huang, P.L. A comprehensive definition for metabolic syndrome. DMM Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef]
- Ford, E.S. Prevalence of the Metabolic Syndrome Defined by the International Diabetes Federation Among Adults in the U.S. Diabetes Care 2005, 28, 2745–2749. [Google Scholar] [CrossRef] [PubMed]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Hui, W.S.; Liu, Z.; Ho, S.C. Metabolic syndrome and all-cause mortality: A meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2010, 25, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, C.E.S.; Hoelscher, D.M.; Chen, B.; Ranjit, N.; van den Berg, A.E. The associations of plant-based food and metabolic syndrome using NHANES 2015-16 data. J. Public Health 2022, 45, e22–e29. [Google Scholar] [CrossRef]
- Shang, P.; Shu MPH, Z.; Wang, Y.; Li, N.; Du, S.; Sun, F.; Xia, Y.; Zhan, S. Veganism does not reduce the risk of the metabolic syndrome in a Taiwanese cohort. Asia Pac. J. Clin. Nutr. 2011, 20, 404–410. [Google Scholar]
- Noce, A.; Di Lauro, M.; Di Daniele, F.; Zaitseva, A.P.; Marrone, G.; Borboni, P.; Di Daniele, N. Natural Bioactive Compounds Useful in Clinical Management of Metabolic Syndrome. Nutrients 2021, 13, 630. [Google Scholar] [CrossRef]
- McGrath, L.; Fernandez, M.L. Plant-based diets and metabolic syndrome: Evaluating the influence of diet quality. J. Agric. Food Res. 2022, 9, 100322. [Google Scholar] [CrossRef]
- Li, H.; Zeng, X.; Wang, Y.; Zhang, Z.; Zhu, Y.; Li, X.; Hu, A.; Zhao, Q.; Yang, W. A prospective study of healthful and unhealthful plant-based diet and risk of overall and cause-specific mortality. Eur. J. Nutr. 2022, 61, 387–398. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Parra-Soto, S.; Gray, S.; Anderson, J.; Welsh, P.; Gill, J.; Sattar, N.; Ho, F.K.; Celis-Morales, C.; Pell, J.P. Vegetarians, fish, poultry, and meat-eaters: Who has higher risk of cardiovascular disease incidence and mortality? A prospective study from UK Biobank. Eur. Heart J. 2021, 42, 1136–1143. [Google Scholar] [CrossRef]
- Storz, M.A. What makes a plant-based diet? a review of current concepts and proposal for a standardized plant-based dietary intervention checklist. Eur. J. Clin. Nutr. 2022, 76, 789. [Google Scholar] [CrossRef]
- Akinyemiju, T.; Moore, J.X.; Pisu, M.; Lakoski, S.G.; Shikany, J.; Goodman, M.; Judd, S.E. A prospective study of dietary patterns and cancer mortality among Blacks and Whites in the REGARDS cohort. Int. J. cancer 2016, 139, 2221. [Google Scholar] [CrossRef] [PubMed]
- Shikany, J.M.; Safford, M.M.; Newby, P.K.; Durant, R.W.; Brown, T.M.; Judd, S.E. Southern Dietary Pattern is Associated with Hazard of Acute Coronary Heart Disease in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circulation 2015, 132, 804. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, H.; Larpin, C.; De Mestral, C.; Guessous, I.; Reny, J.L.; Stringhini, S. Vegetarian, pescatarian and flexitarian diets: Sociodemographic determinants and association with cardiovascular risk factors in a Swiss urban population. Br. J. Nutr. 2020, 124, 844. [Google Scholar] [CrossRef] [PubMed]
- Satija, A.; Hu, F.B. Plant-based diets and cardiovascular health. Trends Cardiovasc. Med. 2018, 28, 437–441. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Bale, B.F.; Doneen, A.L.; Leimgruber, P.P.; Vigerust, D.J. The critical issue linking lipids and inflammation: Clinical utility of stopping oxidative stress. Front. Cardiovasc. Med. 2022, 9, 3281. [Google Scholar]
- Bergheanu, S.C.; Bodde, M.C.; Jukema, J.W. Pathophysiology and treatment of atherosclerosis: Current view and future perspective on lipoprotein modification treatment. Neth. Heart J. 2017, 25, 231. [Google Scholar] [CrossRef]
- Basta, G.; Schmidt, A.M.; De Caterina, R. Advanced glycation end products and vascular inflammation: Implications for accelerated atherosclerosis in diabetes. Cardiovasc. Res. 2004, 63, 582–592. [Google Scholar] [CrossRef]
- Nielsen, L.B. Transfer of low density lipoprotein into the arterial wall and risk of atherosclerosis. Atherosclerosis 1996, 123, 1–15. [Google Scholar] [CrossRef]
- Cazzaniga, A.; Scrimieri, R.; Giani, E.; Zuccotti, G.V.; Maier, J.A.M. Endothelial Hyper-Permeability Induced by T1D Sera Can be Reversed by iNOS Inactivation. Int. J. Mol. Sci. 2020, 21, 2798. [Google Scholar] [CrossRef]
- Matsuura, E.; Hughes, G.R.V.; Khamashta, M.A. Oxidation of LDL and its clinical implication. Autoimmun. Rev. 2008, 7, 558–566. [Google Scholar] [CrossRef]
- Trompet, S.; Packard, C.J.; Jukema, J.W. Plasma apolipoprotein-B is an important risk factor for cardiovascular disease, and its assessment should be routine clinical practice. Curr. Opin. Lipidol. 2018, 29, 51–52. [Google Scholar] [CrossRef] [PubMed]
- Behbodikhah, J.; Ahmed, S.; Elyasi, A.; Kasselman, L.J.; De Leon, J.; Glass, A.D.; Reiss, A.B. Apolipoprotein B and Cardiovascular Disease: Biomarker and Potential Therapeutic Target. Metabolites 2021, 11, 690. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, L.; Paredes, S.; Ramos, H.; Oliveira, J.C.; Palma, I. Apolipoprotein B and non-high-density lipoprotein cholesterol reveal a high atherogenicity in individuals with type 2 diabetes and controlled low-density lipoprotein-cholesterol. Lipids Health Dis. 2020, 19, 127. [Google Scholar] [CrossRef] [PubMed]
- Johannesen, C.D.L.; Mortensen, M.B.; Langsted, A.; Nordestgaard, B.G. Apolipoprotein B and Non-HDL Cholesterol Better Reflect Residual Risk Than LDL Cholesterol in Statin-Treated Patients. J. Am. Coll. Cardiol. 2021, 77, 1439–1450. [Google Scholar] [CrossRef]
- Helgadottir, A.; Thorleifsson, G.; Snaebjarnarson, A.; Stefansdottir, L.; Sveinbjornsson, G.; Tragante, V.; Björnsson, E.; Steinthorsdottir, V.; Gretarsdottir, S.; Helgason, H.; et al. Cholesterol not particle concentration mediates the atherogenic risk conferred by apolipoprotein B particles: A Mendelian randomization analysis. Eur. J. Prev. Cardiol. 2022, 29, 2374–2385. [Google Scholar] [CrossRef]
- Toikka, J.O.; Niemi, P.; Ahotupa, M.; Niinikoski, H.; Viikari, J.S.A.; Rönnemaa, T.; Hartiala, J.J.; Raitakari, O.T. Large-artery elastic properties in young men: Relationships to serum lipoproteins and oxidized low-density lipoproteins. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 436–441. [Google Scholar] [CrossRef]
- Linna, M.; Ahotupa, M.; Löppönen, M.K.; Irjala, K.; Vasankari, T. Circulating oxidised LDL lipids, when proportioned to HDL-c, emerged as a risk factor of all-cause mortality in a population-based survival study. Age Ageing 2013, 42, 110–113. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Cordain, L.; Harris, W.H.; Moe, R.M.; Vogel, R. Optimal low-density lipoprotein is 50 to 70 mg/dL: Lower is better and physiologically normal. J. Am. Coll. Cardiol. 2004, 43, 2142–2146. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459. [Google Scholar] [CrossRef]
- Kawamoto, R.; Kikuchi, A.; Akase, T.; Ninomiya, D.; Kumagi, T. Low density lipoprotein cholesterol and all-cause mortality rate: Findings from a study on Japanese community-dwelling persons. Lipids Health Dis. 2021, 20, 105. [Google Scholar] [CrossRef]
- Jacobs, D.R.; Blackburn, H.; Higgins, M.; Reed, D.; Iso, H.; McMillan, G.; Neaton, J.; Nelson, J.; Potter, J.; Rifkind, B.; et al. Report of the Conference on Low Blood Cholesterol: Mortality Associations. Circulation 1992, 86, 1046–1060. [Google Scholar] [CrossRef]
- Kritchevsky, S.B.; Wilcosky, T.C.; Morris, D.L.; Truong, K.N.; Tyroler, H.A. Changes in Plasma Lipid and Lipoprotein Cholesterol and Weight Prior to the Diagnosis of Cancer1 | Cancer Research | American Association for Cancer Research. Available online: https://aacrjournals.org/cancerres/article/51/12/3198/496777/Changes-in-Plasma-Lipid-and-Lipoprotein (accessed on 1 January 2023).
- Postmus, I.; Deelen, J.; Sedaghat, S.; Trompet, S.; De Craen, A.J.M.; Heijmans, B.T.; Franco, O.H.; Hofman, A.; Dehghan, A.; Slagboom, P.E.; et al. LDL cholesterol still a problem in old age? A Mendelian randomization study. Int. J. Epidemiol. 2015, 44, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Weverling-Rijnsburger, A.W.E.; Blauw, G.J.; Lagaay, A.M.; Knook, D.L.; Meinders, A.E.; Westendorp, R.G.J. Total cholesterol and risk of mortality in the oldest old. Lancet 1997, 350, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, J.; Chen, S.; Ying, M.; Chen, G.; Liu, L.; Lun, Z.; Li, H.; Huang, H.; Li, Q.; et al. Malnutrition affects cholesterol paradox in coronary artery disease: A 41,229 Chinese cohort study. Lipids Health Dis. 2021, 20, 36. [Google Scholar] [CrossRef]
- Tuso, P.; Stoll, S.R.; Li, W.W. A Plant-Based Diet, Atherogenesis, and Coronary Artery Disease Prevention. Perm. J. 2015, 19, 62. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Tung, C.L.; Yang, Y.C.S.H.; Lin, I.H.; Ng, X.E.; Tung, Y.T. Endurance exercise ameliorates Western diet-induced atherosclerosis through modulation of microbiota and its metabolites. Sci. Rep. 2022, 12, 3612. [Google Scholar] [CrossRef]
- Lian, Z.; Perrard, X.Y.D.; Peng, X.; Raya, J.L.; Hernandez, A.A.; Johnson, C.G.; Lagor, W.R.; Pownall, H.J.; Hoogeveen, R.C.; Simon, S.I.; et al. Replacing Saturated Fat With Unsaturated Fat in Western Diet Reduces Foamy Monocytes and Atherosclerosis in Male Ldlr-/- Mice. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 72–85. [Google Scholar] [CrossRef]
- Noakes, M.; Clifton, P.M. Changes in plasma lipids and other cardiovascular risk factors during 3 energy-restricted diets differing in total fat and fatty acid composition. Am. J. Clin. Nutr. 2000, 71, 706–712. [Google Scholar] [CrossRef]
- Hooper, L.; Martin, N.; Jimoh, O.F.; Kirk, C.; Foster, E.; Abdelhamid, A.S. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst. Rev. 2020, 8, CD011737. [Google Scholar] [CrossRef]
- Maki, K.C.; Dicklin, M.R.; Kirkpatrick, C.F. Saturated fats and cardiovascular health: Current evidence and controversies. J. Clin. Lipidol. 2021, 15, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Magkos, F.; Bier, D.M.; Brenna, J.T.; de Oliveira Otto, M.C.; Hill, J.O.; King, J.C.; Mente, A.; Ordovas, J.M.; Volek, J.S.; et al. Saturated Fats and Health: A Reassessment and Proposal for Food-Based Recommendations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Gershuni, V.M. Saturated Fat: Part of a Healthy Diet. Curr. Nutr. Rep. 2018, 7, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Eguchi, K.; Kono, N.; Fujiu, K.; Matsumoto, S.; Shibata, M.; Oishi-Tanaka, Y.; Komuro, I.; Arai, H.; Nagai, R.; et al. Saturated fatty acid palmitate aggravates neointima formation by promoting smooth muscle phenotypic modulation. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2596–2607. [Google Scholar] [CrossRef]
- Mills, C.E.; Harding, S.V.; Bapir, M.; Mandalari, G.; Salt, L.J.; Gray, R.; Fielding, B.A.; Wilde, P.J.; Hall, W.L.; Berry, S.E. Palmitic acid–rich oils with and without interesterification lower postprandial lipemia and increase atherogenic lipoproteins compared with a MUFA-rich oil: A randomized controlled trial. Am. J. Clin. Nutr. 2021, 113, 1221. [Google Scholar] [CrossRef] [PubMed]
- Afonso, M.S.; Lavrador, M.S.F.; Koike, M.K.; Cintra, D.E.; Ferreira, F.D.; Nunes, V.S.; Castilho, G.; Gioielli, L.A.; Paula Bombo, R.; Catanozi, S.; et al. Dietary interesterified fat enriched with palmitic acid induces atherosclerosis by impairing macrophage cholesterol efflux and eliciting inflammation. J. Nutr. Biochem. 2016, 32, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, J.; Taguchi, R.; Yamamoto, A.; Murakami, K. Palmitic acid enhances lectin-like oxidized LDL receptor (LOX-1) expression and promotes uptake of oxidized LDL in macrophage cells. Atherosclerosis 2010, 209, 118–124. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Liu, X.; Malik, V.S.; Sun, Q.; Willett, W.C.; Manson, J.A.E.; Rexrode, K.M.; Li, Y.; Hu, F.B.; Bhupathiraju, S.N. Nut Consumption and Risk of Cardiovascular Disease. J. Am. Coll. Cardiol. 2017, 70, 2519. [Google Scholar] [CrossRef]
- Riccardi, G.; Giosuè, A.; Calabrese, I.; Vaccaro, O. Dietary recommendations for prevention of atherosclerosis. Cardiovasc. Res. 2022, 118, 1188–1204. [Google Scholar] [CrossRef]
- Nergiz-Ünal, R.; Kuijpers, M.J.E.; De Witt, S.M.; Heeneman, S.; Feijge, M.A.H.; Garcia Caraballo, S.C.; Biessen, E.A.L.; Haenen, G.R.M.M.; Cosemans, J.M.E.M.; Heemskerk, J.W.M. Atheroprotective effect of dietary walnut intake in ApoE-deficient mice: Involvement of lipids and coagulation factors. Thromb. Res. 2013, 131, 411–417. [Google Scholar] [CrossRef]
- Urpi-Sarda, M.; Casas, R.; Chiva-Blanch, G.; Romero-Mamani, E.S.; Valderas-Martínez, P.; Arranz, S.; Andres-Lacueva, C.; Llorach, R.; Medina-Remón, A.; Lamuela-Raventos, R.M.; et al. Virgin olive oil and nuts as key foods of the Mediterranean diet effects on inflammatory biomakers related to atherosclerosis. Pharmacol. Res. 2012, 65, 577–583. [Google Scholar] [CrossRef]
- Estruch, R. Anti-inflammatory effects of the Mediterranean diet: The experience of the PREDIMED study. Proc. Nutr. Soc. 2010, 69, 333–340. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Sanchez, A.; Mejia, A.; Sanchez, J.; Runte, E.; Brown-Fraser, S.; Bivens, R.L. Diets with customary levels of fat from plant origin may reverse coronary artery disease. Med. Hypotheses 2019, 122, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; Katan, M.B. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler. Thromb. A J. Vasc. Biol. 1992, 12, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.; Lindman, A.S.; Brantsætert, A.L.; Pedersen, J.I. The Serum LDL/HDL Cholesterol Ratio Is Influenced More Favorably by Exchanging Saturated with Unsaturated Fat Than by Reducing Saturated Fat in the Diet of Women. J. Nutr. 2003, 133, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Chen, M.; Shen, H.; PingYin; Fan, L.; Chen, X.; Wu, J.; Xu, Z.; Zhang, J. Predictive value of LDL/HDL ratio in coronary atherosclerotic heart disease. BMC Cardiovasc. Disord. 2022, 22, 273. [Google Scholar] [CrossRef]
- Toscano, L.T.; Toscano, L.T.; Tavares, R.L.; da Silva, C.S.O.; Silva, A.S. Chia induces clinically discrete weight loss and improves lipid profile only in altered previous values. Nutr. Hosp. 2014, 31, 1176–1182. [Google Scholar] [CrossRef]
- Pacheco, L.S.; Li, Y.; Rimm, E.B.; Manson, J.E.; Sun, Q.; Rexrode, K.; Hu, F.B.; Guasch-Ferré, M. Avocado Consumption and Risk of Cardiovascular Disease in US Adults. J. Am. Heart Assoc. 2018, 11, e024014. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bergeron, N.; Levison, B.S.; Li, X.S.; Chiu, S.; Xun, J.; Koeth, R.A.; Lin, L.; Wu, Y.; Tang, W.H.W.; et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Heart J. 2019, 40, 583. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576. [Google Scholar] [CrossRef] [PubMed]
- Andraos, S.; Lange, K.; Clifford, S.A.; Jones, B.; Thorstensen, E.B.; Kerr, J.A.; Wake, M.; Saffery, R.; Burgner, D.P.; O’Sullivan, J.M. Plasma Trimethylamine N-Oxide and Its Precursors: Population Epidemiology, Parent–Child Concordance, and Associations with Reported Dietary Intake in 11- to 12-Year-Old Children and Their Parents. Curr. Dev. Nutr. 2020, 4, nzaa103. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Dou, P.; Gao, M.; Kong, X.; Li, C.; Liu, Z.; Huang, T. Assessment of Causal Direction Between Gut Microbiota-Dependent Metabolites and Cardiometabolic Health: A Bidirectional Mendelian Randomization Analysis. Diabetes 2019, 68, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.J.; Bostwick, B.L.; Kennedy, A.D.; Donti, T.R.; Sun, Q.; Sutton, V.R.; Elsea, S.H. Chronic Oral l-Carnitine Supplementation Drives Marked Plasma TMAO Elevations in Patients with Organic Acidemias Despite Dietary Meat Restrictions. JIMD Rep. 2016, 30, 39. [Google Scholar] [CrossRef]
- Yu, Z.L.; Zhang, L.Y.; Jiang, X.M.; Xue, C.H.; Chi, N.; Zhang, T.T.; Wang, Y.M. Effects of dietary choline, betaine, and L-carnitine on the generation of trimethylamine-N-oxide in healthy mice. J. Food Sci. 2020, 85, 2207–2215. [Google Scholar] [CrossRef] [PubMed]
- Kühn, T.; Rohrmann, S.; Sookthai, D.; Johnson, T.; Katzke, V.; Kaaks, R.; Von Eckardstein, A.; Müller, D. Intra-individual variation of plasma trimethylamine-N-oxide (TMAO), betaine and choline over 1 year. Clin. Chem. Lab. Med. 2017, 55, 261–268. [Google Scholar] [CrossRef]
- Ilyas, A.; Wijayasinghe, Y.S.; Khan, I.; El Samaloty, N.M.; Adnan, M.; Dar, T.A.; Poddar, N.K.; Singh, L.R.; Sharma, H.; Khan, S. Implications of trimethylamine N-oxide (TMAO) and Betaine in Human Health: Beyond Being Osmoprotective Compounds. Front. Mol. Biosci. 2022, 9, 818. [Google Scholar] [CrossRef]
- Meyer, K.A.; Shea, J.W. Dietary Choline and Betaine and Risk of CVD: A Systematic Review and Meta-Analysis of Prospective Studies. Nutrients 2017, 9, 711. [Google Scholar] [CrossRef]
- Samulak, J.J.; Sawicka, A.K.; Hartmane, D.; Grinberga, S.; Pugovics, O.; Lysiak-Szydlowska, W.; Olek, R.A. L-Carnitine Supplementation Increases Trimethylamine-N-Oxide but not Markers of Atherosclerosis in Healthy Aged Women. Ann. Nutr. Metab. 2019, 74, 11–17. [Google Scholar] [CrossRef]
- Losasso, C.; Eckert, E.M.; Mastrorilli, E.; Villiger, J.; Mancin, M.; Patuzzi, I.; Di Cesare, A.; Cibin, V.; Barrucci, F.; Pernthaler, J.; et al. Assessing the influence of vegan, vegetarian and omnivore oriented westernized dietary styles on human gut microbiota: A cross sectional study. Front. Microbiol. 2018, 9, 317. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Hentges, D.J.; Maier, B.R.; Burton, G.C.; Flynn, M.A.; Tsutakawa, R.K. Effect of a High-Beef Diet on the Fecal Bacterial Flora of Humans1 | Cancer Research | American Association for Cancer Research. Available online: https://aacrjournals.org/cancerres/article/37/2/568/481691/Effect-of-a-High-Beef-Diet-on-the-Fecal-Bacterial (accessed on 1 January 2023).
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Park, J.; Kim, M. Gut Microbiota-Derived Short-Chain Fatty Acids, T Cells, and Inflammation. Immune Netw. 2014, 14, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, L.E.M.; Koetsier, M.A.; Van Deventer, S.J.H.; Van Tol, E.A.F. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut 2003, 52, 1442. [Google Scholar] [CrossRef]
- Bergstrom, K.S.B.; Kissoon-Singh, V.; Gibson, D.L.; Ma, C.; Montero, M.; Sham, H.P.; Ryz, N.; Huang, T.; Velcich, A.; Finlay, B.B.; et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010, 6, 1000902. [Google Scholar] [CrossRef]
- Grondin, J.A.; Kwon, Y.H.; Far, P.M.; Haq, S.; Khan, W.I. Mucins in Intestinal Mucosal Defense and Inflammation: Learning From Clinical and Experimental Studies. Front. Immunol. 2020, 11, 2054. [Google Scholar] [CrossRef]
- Yao, D.; Dai, W.; Dong, M.; Dai, C.; Wu, S. MUC2 and related bacterial factors: Therapeutic targets for ulcerative colitis. EBioMedicine 2021, 74, 103751. [Google Scholar] [CrossRef]
- Kang, Y.; Park, H.; Choe, B.H.; Kang, B. The Role and Function of Mucins and Its Relationship to Inflammatory Bowel Disease. Front. Med. 2022, 9, 1312. [Google Scholar] [CrossRef]
- Costabile, G.; Vetrani, C.; Bozzetto, L.; Giacco, R.; Bresciani, L.; Del Rio, D.; Vitale, M.; Della Pepa, G.; Brighenti, F.; Riccardi, G.; et al. Plasma TMAO increase after healthy diets: Results from 2 randomized controlled trials with dietary fish, polyphenols, and whole-grain cereals. Am. J. Clin. Nutr. 2021, 114, 1342–1350. [Google Scholar] [CrossRef]
- Griffin, L.E.; Djuric, Z.; Angiletta, C.J.; Mitchell, C.M.; Baugh, M.E.; Davy, K.P.; Neilson, A.P. A Mediterranean diet does not alter plasma trimethylamine N-oxideconcentrations in healthy adults at risk for colon cancer. Food Funct. 2019, 10, 2138. [Google Scholar] [CrossRef]
- Thomas, M.S.; Puglisi, M.; Malysheva, O.; Caudill, M.A.; Sholola, M.; Cooperstone, J.L.; Fernandez, M.L. Eggs Improve Plasma Biomarkers in Patients with Metabolic Syndrome Following a Plant-Based Diet—A Randomized Crossover Study. Nutrients 2022, 14, 2138. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Sawrey-Kubicek, L.; Bardagjy, A.S.; Houts, H.; Tang, X.; Sacchi, R.; Randolph, J.M.; Steinberg, F.M.; Zivkovic, A.M. Whole egg consumption increases plasma choline and betaine without affecting TMAO levels or gut microbiome in overweight postmenopausal women. Nutr. Res. 2020, 78, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.M.; Zern, T.L.; Wood, R.J.; Shrestha, S.; Aggarwal, D.; Sharman, M.J.; Volek, J.S.; Fernandez, M.L. Maintenance of the LDL cholesterol:HDL cholesterol ratio in an elderly population given a dietary cholesterol challenge. J. Nutr. 2005, 135, 2793–2798. [Google Scholar] [CrossRef] [PubMed]
- Weggemans, R.M.; Zock, P.L.; Katan, M.B. Dietary cholesterol from eggs increases the ratio of total cholesterol to high-density lipoprotein cholesterol in humans: A meta-analysis. Am. J. Clin. Nutr. 2001, 73, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Connor, W.E.; Hodges, R.E.; Bleiler, R.E. The serum lipids in men receiving high cholesterol and cholesterol-free diets. J. Clin. Investig. 1961, 40, 894. [Google Scholar] [CrossRef]
- Wells, V.M.; Bronte-Stewart, B. Egg Yolk and Serum-cholesterol Levels: Importance of Dietary Cholesterol Intake. Br. Med. J. 1963, 1, 577. [Google Scholar] [CrossRef]
- Rouhani, M.H.; Rashidi-Pourfard, N.; Salehi-Abargouei, A.; Karimi, M.; Haghighatdoost, F. Effects of Egg Consumption on Blood Lipids: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Am. Coll. Nutr. 2018, 37, 99–110. [Google Scholar] [CrossRef]
- Hopkins, P.N. Effects of dietary cholesterol on serum cholesterol: A meta-analysis and review. Am. J. Clin. Nutr. 1992, 55, 1060–1070. [Google Scholar] [CrossRef]
- West, A.A.; Shih, Y.; Wang, W.; Oda, K.; Jaceldo-Siegl, K.; Sabaté, J.; Haddad, E.; Rajaram, S.; Caudill, M.A.; Burns-Whitmore, B. Egg n-3 fatty acid composition modulates biomarkers of choline metabolism in free-living lacto-ovo-vegetarian women of reproductive age. J. Acad. Nutr. Diet. 2014, 114, 1594–1600. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, W.H.W.; Buffa, J.A.; Fu, X.; Britt, E.B.; Koeth, R.A.; Levison, B.S.; Fan, Y.; Wu, Y.; Hazen, S.L. Editor’s choice: Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur. Heart J. 2014, 35, 904. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, K.M.; Clifton, P.M.; Keogh, J.B. Endothelial function is impaired after a high-salt meal in healthy subjects–. Am. J. Clin. Nutr. 2011, 93, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Lennon-Edwards, S.; Ramick, M.G.; Matthews, E.L.; Brian, M.S.; Farquhar, W.B.; Edwards, D.G. Salt loading has a more deleterious effect on flow-mediated dilation in salt-resistant men than women. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 990–995. [Google Scholar] [CrossRef]
- Kawashima, S.; Yokoyama, M. Dysfunction of Endothelial Nitric Oxide Synthase and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 998–1005. [Google Scholar] [CrossRef]
- Vogel, R.A.; Corretti, M.C.; Plotnick, G.D. Effect of a single high-fat meal on endothelial function in healthy subjects. Am. J. Cardiol. 1997, 79, 350–354. [Google Scholar] [CrossRef]
- Keogh, J.B.; Grieger, J.A.; Noakes, M.; Clifton, P.M. Flow-Mediated Dilatation Is Impaired by a High–Saturated Fat Diet but Not by a High-Carbohydrate Diet. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1274–1279. [Google Scholar] [CrossRef]
- Ros, E.; Núñez, I.; Pérez-Heras, A.; Serra, M.; Gilabert, R.; Casals, E.; Deulofeu, R. A Walnut Diet Improves Endothelial Function in Hypercholesterolemic Subjects. Circulation 2004, 109, 1609–1614. [Google Scholar] [CrossRef]
- Mateos, R.; Salvador, M.D.; Fregapane, G.; Goya, L. Why Should Pistachio Be a Regular Food in Our Diet? Nutrients 2022, 14, 3207. [Google Scholar] [CrossRef]
- Katz, D.L.; Davidhi, A.; Ma, Y.; Kavak, Y.; Bifulco, L.; Njike, V.Y. Effects of Walnuts on Endothelial Function in Overweight Adults with Visceral Obesity: A Randomized, Controlled, Crossover Trial. J. Am. Coll. Nutr. 2013, 31, 415–423. [Google Scholar] [CrossRef]
- Drexler, H.; Hornig, B. Endothelial dysfunction in human disease. J. Mol. Cell. Cardiol. 1999, 31, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.; Finis, D.; Kleinbongard, P.; Hoffmann, A.; Rassaf, T.; Kelm, M.; Sies, H. Sustained increase in flow-mediated dilation after daily intake of high-flavanol cocoa drink over 1 week. J. Cardiovasc. Pharmacol. 2007, 49, 74–80. [Google Scholar] [CrossRef]
- Velmurugan, S.; Gan, J.M.; Rathod, K.S.; Khambata, R.S.; Ghosh, S.M.; Hartley, A.; Van Eijl, S.; Sagi-Kiss, V.; Chowdhury, T.A.; Curtis, M.; et al. Dietary nitrate improves vascular function in patients with hypercholesterolemia: A randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 2016, 103, 25. [Google Scholar] [CrossRef] [PubMed]
- Haynes, A.; Linden, M.D.; Robey, E.; Naylor, L.H.; Cox, K.L.; Lautenschlager, N.T.; Green, D.J. Relationship between monocyte-platelet aggregation and endothelial function in middle-aged and elderly adults. Physiol. Rep. 2017, 5, 13189. [Google Scholar] [CrossRef] [PubMed]
- Burger, P.C.; Wagner, D.D. Platelet P-selectin facilitates atherosclerotic lesion development. Blood 2003, 101, 2661–2666. [Google Scholar] [CrossRef]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef]
- Bondonno, C.P.; Blekkenhorst, L.C.; Prince, R.L.; Ivey, K.L.; Lewis, J.R.; Devine, A.; Woodman, R.J.; Lundberg, J.O.; Croft, K.D.; Thompson, P.L.; et al. Association of Vegetable Nitrate Intake with Carotid Atherosclerosis and Ischemic Cerebrovascular Disease in Older Women. Stroke 2017, 48, 1724–1729. [Google Scholar] [CrossRef]
- Sundqvist, M.L.; Larsen, F.J.; Carlström, M.; Bottai, M.; Pernow, J.; Hellénius, M.L.; Weitzberg, E.; Lundberg, J.O. A randomized clinical trial of the effects of leafy green vegetables and inorganic nitrate on blood pressure. Am. J. Clin. Nutr. 2020, 111, 749–756. [Google Scholar] [CrossRef]
- Tischmann, L.; Adam, T.C.; Mensink, R.P.; Joris, P.J. Longer-term soy nut consumption improves vascular function and cardiometabolic risk markers in older adults: Results of a randomized, controlled cross-over trial. Clin. Nutr. 2022, 41, 1052–1058. [Google Scholar] [CrossRef]
- Tucci, M.; Marino, M.; Martini, D.; Porrini, M.; Riso, P.; Del Bo’, C. Plant-Based Foods and Vascular Function: A Systematic Review of Dietary Intervention Trials in Older Subjects and Hypothesized Mechanisms of Action. Nutrients 2022, 14, 2615. [Google Scholar] [CrossRef]
- Woo, K.S.; McCrohon, J.A.; Chook, P.; Adams, M.R.; Robinson, J.T.C.; McCredie, R.J.; Lam, C.W.K.; Feng, J.Z.; Celermajer, D.S. Chinese adults are less susceptible than whites to age-related endothelial dysfunction. J. Am. Coll. Cardiol. 1997, 30, 113–118. [Google Scholar] [CrossRef]
- Anderson, J.S.; Nettleton, J.A.; Herrington, D.M.; Johnson, W.C.; Tsai, M.Y.; Siscovick, D. Relation of omega-3 fatty acid and dietary fish intake with brachial artery flow-mediated vasodilation in the Multi-Ethnic Study of Atherosclerosis. Am. J. Clin. Nutr. 2010, 92, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.A.B.; Hall, W.L.; Maniou, Z.; Lewis, F.; Seed, P.T.; Chowienczyk, P.J. Effect of low doses of long-chain n-3 PUFAs on endothelial function and arterial stiffness: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 94, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Wei, W.; Li, X. Effect of Fish Oil Supplementation on Fasting Vascular Endothelial Function in Humans: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2012, 7, 46028. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Fang, T.C.; Gueng, M.K. Vascular dilatory functions of ovo-lactovegetarians compared with omnivores. Atherosclerosis 2001, 158, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.; Srivatsav, V.; Rizwan, A.; Nashed, A.; Liu, R.; Shen, R.; Akhtar, M. Bridging the Gap between Gut Microbial Dysbiosis and Cardiovascular Diseases. Nutrition 2017, 9, 859. [Google Scholar] [CrossRef]
- Ma, J.; Li, H. The role of gut microbiota in atherosclerosis and hypertension. Front. Pharmacol. 2018, 9, 1082. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Fåk, F.; Nookaew, I.; Tremaroli, V.; Fagerberg, B.; Petranovic, D.; Bäckhed, F.; Nielsen, J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012, 3, 1245. [Google Scholar] [CrossRef]
- Richards, L.B.; Li, M.; van Esch, B.C.A.M.; Garssen, J.; Folkerts, G. The effects of short-chain fatty acids on the cardiovascular system. Pharma Nutrition 2016, 4, 68–111. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, Y.; Wang, Y.; Wang, P.; Song, H.; Wang, F. Antibiotics induced intestinal tight junction barrier dysfunction is associated with microbiota dysbiosis, activated NLRP3 inflammasome and autophagy. PLoS ONE 2019, 14, e0218384. [Google Scholar] [CrossRef]
- Edelblum, K.L.; Turner, J.R. The Tight Junction in Inflammatory Disease: Communication Breakdown. Curr. Opin. Pharmacol. 2009, 9, 715. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Wang, M.; Zhang, J.; Barve, S.S.; McClain, C.J.; Joshi-Barve, S. Acrolein Disrupts Tight Junction Proteins and Causes Endoplasmic Reticulum Stress-Mediated Epithelial Cell Death Leading to Intestinal Barrier Dysfunction and Permeability. Am. J. Pathol. 2017, 187, 2686–2697. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Giudici, F.; Fiorindi, C.; Ficari, F.; Scaringi, S.; Amedei, A. Immunomodulating Activity and Therapeutic Effects of Short Chain Fatty Acids and Tryptophan Post-biotics in Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 2754. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of Inflammation by Short Chain Fatty Acids. Nutrients 2011, 3, 858. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; van Esch, B.C.A.M.; Wagenaar, G.T.M.; Garssen, J.; Folkerts, G.; Henricks, P.A.J. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 2018, 831, 52–59. [Google Scholar] [CrossRef]
- Yang, R.; Jia, Q.; Mehmood, S.; Ma, S.; Liu, X. Genistein ameliorates inflammation and insulin resistance through mediation of gut microbiota composition in type 2 diabetic mice. Eur. J. Nutr. 2021, 60, 2155–2168. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Davies, G.J.; Crowder, M.; Dickerson, J.W.T. Dietary fibre intakes of individuals with different eating patterns. Hum. Nutr. Appl. Nutr. 1985, 39, 139–148. [Google Scholar]
- Park, Y.; Subar, A.F.; Hollenbeck, A.; Schatzkin, A. Dietary Fiber Intake and Mortality in the NIH-AARP Diet and Health Study. Arch. Intern. Med. 2011, 171, 1061–1068. [Google Scholar] [CrossRef]
- Zhang, S.; Tian, J.; Lei, M.; Zhong, C.; Zhang, Y. Association between dietary fiber intake and atherosclerotic cardiovascular disease risk in adults: A cross-sectional study of 14,947 population based on the National Health and Nutrition Examination Surveys. BMC Public Health 2022, 22, 1076. [Google Scholar] [CrossRef]
- Daïen, C.I.; Pinget, G.V.; Tan, J.K.; Macia, L. Detrimental impact of microbiota-accessible carbohydrate-deprived diet on gut and immune homeostasis: An overview. Front. Immunol. 2017, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinction in the gut microbiota compounds over generations. Nature 2016, 529, 212. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The effects of vegetarian and vegan diets on gut microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef]
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef]
- Nagarajan, S. Mechanisms of anti-atherosclerotic functions of soy-based diets. J. Nutr. Biochem. 2010, 21, 255–260. [Google Scholar] [CrossRef]
- Henning, A.L.; Venable, A.S.; Vingren, J.L.; Hill, D.W.; McFarlin, B.K. Consumption of a high-fat meal was associated with an increase in monocyte adhesion molecules, scavenger receptors, and Propensity to Form Foam Cells. Cytom. Part B Clin. Cytom. 2018, 94, 606–612. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, K.Y. Clinical trials of kimchi intakes on the regulation of metabolic parameters and colon health in healthy Korean young adults. J. Funct. Foods 2018, 47, 325–333. [Google Scholar] [CrossRef]
- Yun, Y.R.; Kim, H.J.; Song, Y.O. Kimchi Methanol Extract and the Kimchi Active Compound, 3′-(4′-Hydroxyl-3′,5′-Dimethoxyphenyl)Propionic Acid, Downregulate CD36 in THP-1 Macrophages Stimulated by oxLDL. J. Med. Food 2014, 17, 886. [Google Scholar] [CrossRef]
- Vetrani, C.; Costabile, G.; Luongo, D.; Naviglio, D.; Rivellese, A.A.; Riccardi, G.; Giacco, R. Effects of whole-grain cereal foods on plasma short chain fatty acid concentrations in individuals with the metabolic syndrome. Nutrition 2016, 32, 217–221. [Google Scholar] [CrossRef]
- Amato, R.; Rossino, M.G.; Cammalleri, M.; Locri, F.; Pucci, L.; Dal Monte, M.; Casini, G. Lisosan G Protects the Retina from Neurovascular Damage in Experimental Diabetic Retinopathy. Nutrients 2018, 10, 1932. [Google Scholar] [CrossRef] [PubMed]
- Giusti, L.; Gabriele, M.; Penno, G.; Garofolo, M.; Longo, V.; Del Prato, S.; Lucchesi, D.; Pucci, L. A Fermented Whole Grain Prevents Lipopolysaccharides-Induced Dysfunction in Human Endothelial Progenitor Cells. Oxid. Med. Cell. Longev. 2017, 2017, 1026268. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Arnone, A.; Ciampaglia, R.; Tenore, G.C.; Novellino, E. Fermentation of Foods and Beverages as a Tool for Increasing Availability of Bioactive Compounds. Focus on Short-Chain Fatty Acids. Foods 2020, 9, 999. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Sahi, N.; Jeon, E.R.; Park, Y.S.; Kim, D.W. Chungtaejeon, a Korean fermented tea, scavenges oxidation and inhibits cytokine induced proliferation and migration of human aortic smooth muscle cells. Plant Foods Hum. Nutr. 2011, 66, 27–33. [Google Scholar] [CrossRef]
- Rahmani, P.; Melekoglu, E.; Tavakoli, S.; Malekpour Alamdari, N.; Rohani, P.; Sohouli, M.H. Impact of red yeast rice supplementation on lipid profile: A systematic review and meta-analysis of randomized-controlled trials. Expert Rev. Clin. Pharmacol. 2023, 16, 73–81. [Google Scholar] [CrossRef]
- Minamizuka, T.; Koshizaka, M.; Shoji, M.; Yamaga, M.; Hayashi, A.; Ide, K.; Ide, S.; Kitamoto, T.; Sakamoto, K.; Hattori, A.; et al. Low dose red yeast rice with monacolin K lowers LDL cholesterol and blood pressure in Japanese with mild dyslipidemia: A multicenter, randomized trial. Asia Pac. J. Clin. Nutr. 2021, 30, 424–435. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J.; Gan, Q.X.; Ran, Q.; Lou, G.H.; Xiong, H.J.; Peng, C.Y.; Sun, J.L.; Yao, R.C.; Huang, Q.W. Impact of Red Yeast Rice on Metabolic Diseases: A Review of Possible Mechanisms of Action. J. Agric. Food Chem. 2020, 68, 10441–10455. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Barera, A.; Buscemi, S.; Monastero, R.; Caruso, C.; Caldarella, R.; Ciaccio, M.; Vasto, S. β-glucans: Ex vivo inflammatory and oxidative stress results after pasta intake. Immun. Ageing 2016, 13, 14. [Google Scholar] [CrossRef]
- Nakashima, A.; Yamada, K.; Iwata, O.; Sugimoto, R.; Atsuji, K.; Ogawa, T.; Ishibashi-Ohgo, N.; Suzuki, K. β-Glucan in Foods and Its Physiological Functions. J. Nutr. Sci. Vitaminol. 2018, 64, 8–17. [Google Scholar] [CrossRef]
- Ho, H.V.T.; Sievenpiper, J.L.; Zurbau, A.; Mejia, S.B.; Jovanovski, E.; Au-Yeung, F.; Jenkins, A.L.; Vuksan, V. A systematic review and meta-analysis of randomized controlled trials of the effect of barley β-glucan on LDL-C, non-HDL-C and apoB for cardiovascular disease risk reductioni-iv. Eur. J. Clin. Nutr. 2016, 70, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Veronesi, M.; Strocchi, E.; Grandi, E.; Rizzoli, E.; Poli, A.; Marangoni, F.; Borghi, C. A Randomized Placebo-Controlled Clinical Trial to Evaluate the Medium-Term Effects of Oat Fibers on Human Health: The Beta-Glucan Effects on Lipid Profile, Glycemia and inTestinal Health (BELT) Study. Nutrients 2020, 12, 686. [Google Scholar] [CrossRef] [PubMed]
- Basu, A. Role of Berry Bioactive Compounds on Lipids and Lipoproteins in Diabetes and Metabolic Syndrome. Nutrients 2019, 11, 1983. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, G.; Liao, D.; Zhu, Y.; Xue, X. Effects of Berries Consumption on Cardiovascular Risk Factors: A Meta-analysis with Trial Sequential Analysis of Randomized Controlled Trials. Sci. Rep. 2016, 6, 23625. [Google Scholar] [CrossRef] [PubMed]
- Luís, Â.; Domingues, F.; Pereira, L. Association between berries intake and cardiovascular diseases risk factors: A systematic review with meta-analysis and trial sequential analysis of randomized controlled trials. Food Funct. 2018, 9, 740–757. [Google Scholar] [CrossRef]
- Vendrame, S.; Adekeye, T.E.; Klimis-Zacas, D. The Role of Berry Consumption on Blood Pressure Regulation and Hypertension: An Overview of the Clinical Evidence. Nutrients 2022, 14, 2701. [Google Scholar] [CrossRef]
- Yamakoshi, J.; Kataoka, S.; Koga, T.; Ariga, T. Proanthocyanidin-rich extract from grape seeds attenuates the development of aortic atherosclerosis in cholesterol-fed rabbits. Atherosclerosis 1999, 142, 139–149. [Google Scholar] [CrossRef]
- Yang, M.Y.; Huang, C.N.; Chan, K.C.; Yang, Y.S.; Peng, C.H.; Wang, C.J. Mulberry leaf polyphenols possess antiatherogenesis effect via inhibiting LDL oxidation and foam cell formation. J. Agric. Food Chem. 2011, 59, 1985–1995. [Google Scholar] [CrossRef]
- Filosa, S.; Di Meo, F.; Crispi, S. Polyphenols-gut microbiota interplay and brain neuromodulation. Neural Regen. Res. 2018, 13, 2055. [Google Scholar] [CrossRef]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef]
- Tang, M.; Cheng, L.; Liu, Y.; Wu, Z.; Zhang, X.; Luo, S. Plant Polysaccharides Modulate Immune Function via the Gut Microbiome and May Have Potential in COVID-19 Therapy. Molecules 2022, 27, 2773. [Google Scholar] [CrossRef] [PubMed]
- Krishna Rao, R.; Samak, G. Protection and Restitution of Gut Barrier by Probiotics: Nutritional and Clinical Implications. Curr. Nutr. Food Sci. 2013, 9, 99. [Google Scholar] [CrossRef]
- Gou, H.Z.; Zhang, Y.L.; Ren, L.F.; Li, Z.J.; Zhang, L. How do intestinal probiotics restore the intestinal barrier? Front. Microbiol. 2022, 13, 929346. [Google Scholar] [CrossRef]
- Vendrame, S.; Guglielmetti, S.; Riso, P.; Arioli, S.; Klimis-Zacas, D.; Porrini, M. Six-week consumption of a wild blueberry powder drink increases bifidobacteria in the human gut. J. Agric. Food Chem. 2011, 59, 12815–12820. [Google Scholar] [CrossRef]
- Lara-Guzmán, O.J.; Medina, S.; Álvarez, R.; Oger, C.; Durand, T.; Galano, J.M.; Zuluaga, N.; Gil-Izquierdo, Á.; Muñoz-Durango, K. Oxylipin regulation by phenolic compounds from coffee beverage: Positive outcomes from a randomized controlled trial in healthy adults and macrophage derived foam cells. Free Radic. Biol. Med. 2020, 160, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Al-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Cade, W.T. Diabetes-Related Microvascular and Macrovascular Diseases in the Physical Therapy Setting. Phys. Ther. 2008, 88, 1322. [Google Scholar] [CrossRef]
- Huang, D.; Refaat, M.; Mohammedi, K.; Jayyousi, A.; Al Suwaidi, J.; Abi Khalil, C. Macrovascular Complications in Patients with Diabetes and Prediabetes. Biomed Res. Int. 2017, 2017, 7839101. [Google Scholar] [CrossRef]
- Olfert, M.D.; Wattick, R.A. Vegetarian Diets and the Risk of Diabetes. Curr. Diab. Rep. 2018, 18, 101. [Google Scholar] [CrossRef]
- Schiattarella, A.; Lombardo, M.; Morlando, M.; Rizzo, G. The Impact of a Plant-Based Diet on Gestational Diabetes: A Review. Antioxidants 2021, 10, 557. [Google Scholar] [CrossRef]
- Qian, F.; Liu, G.; Hu, F.B.; Bhupathiraju, S.N.; Sun, Q. Association Between Plant-Based Dietary Patterns and Risk of Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2019, 179, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Drouin-Chartier, J.P.; Li, Y.; Baden, M.Y.; Manson, J.E.; Willett, W.C.; Voortman, T.; Hu, F.B.; Bhupathiraju, S.N. Changes in Plant-Based Diet Indices and Subsequent Risk of Type 2 Diabetes in Women and Men: Three U.S. Prospective Cohorts. Diabetes Care 2021, 44, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, R.; Xu, B. Antidiabetic properties of dietary flavonoids: A cellular mechanism review. Nutr. Metab. 2015, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Martineau, L.C.; Saleem, A.; Muhammad, A.; Vallerand, D.; Benhaddou-Andaloussi, A.; Nistor, L.; Afshar, A.; Arnason, J.T.; Haddad, P.S. Stimulation of AMP-activated protein kinase and enhancement of basal glucose uptake in muscle cells by quercetin and quercetin glycosides, active principles of the antidiabetic medicinal plant Vaccinium vitis-idaea. Mol. Nutr. Food Res. 2010, 54, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Leyva-López, N.; Gutierrez-Grijalva, E.P.; Ambriz-Perez, D.L.; Basilio Heredia, J. Flavonoids as Cytokine Modulators: A Possible Therapy for Inflammation-Related Diseases. Int. J. Mol. Sci. 2016, 17, 921. [Google Scholar] [CrossRef]
- Yan, L.; Vaghari-Tabari, M.; Malakoti, F.; Moein, S.; Qujeq, D.; Yousefi, B.; Asemi, Z. Quercetin: An effective polyphenol in alleviating diabetes and diabetic complications. Crit. Rev. Food Sci. Nutr. 2022, 1–24. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. Rev. 2019, 14, 50. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, C.; Guven, E.C.; Paoli, P.; Simal-Gandara, J.; Ramkumar, K.M.; Wang, S.; Buleu, F.; Pah, A.; Turi, V.; et al. Dietary polyphenols as antidiabetic agents: Advances and opportunities. Food Front. 2020, 1, 18–44. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Castro-Barquero, S.; Becerra-Tomás, N.; Babio, N.; Martínez-González, M.Á.; Corella, D.; Fitó, M.; Romaguera, D.; Vioque, J.; Alonso-Gomez, A.M.; et al. Adopting a High-Polyphenolic Diet Is Associated with an Improved Glucose Profile: Prospective Analysis within the PREDIMED-Plus Trial. Antioxidants 2022, 11, 316. [Google Scholar] [CrossRef]
- Aryaeian, N.; Sedehi, S.K.; Arablou, T. Polyphenols and their effects on diabetes management: A review. Med. J. Islam. Repub. Iran 2017, 31, 134. [Google Scholar] [CrossRef]
- Gilbert, E.R.; Liu, D. Anti-diabetic functions of soy isoflavone genistein: Mechanisms underlying effects on pancreatic β-cell function. Food Funct. 2013, 4, 200. [Google Scholar] [CrossRef]
- Duru, K.C.; Kovaleva, E.G.; Danilova, I.G.; van der Bijl, P.; Belousova, A.V. The potential beneficial role of isoflavones in type 2 diabetes mellitus. Nutr. Res. 2018, 59, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Cruz, M.; Godinez-Salas, E.T.; Sanchez-Tapia, M.; Torres-Villalobos, G.; Pichardo-Ontiveros, E.; Guizar-Heredia, R.; Arteaga-Sanchez, L.; Gamba, G.; Mojica-Espinosa, R.; Schcolnik-Cabrera, A.; et al. Genistein stimulates insulin sensitivity through gut microbiota reshaping and skeletal muscle AMPK activation in obese subjects. BMJ Open Diabetes Res. Care 2020, 8, e000948. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.J.; Dhatariya, K.; Sampson, M.; Kroon, P.A.; Potter, J.; Cassidy, A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: A 1-year, double-blind, randomized, controlled trial. Diabetes Care 2012, 35, 226–232. [Google Scholar] [CrossRef]
- Jain, R.; Bolch, C.; Al-Nakkash, L.; Sweazea, K.L. Systematic review of the impact of genistein on diabetes-related outcomes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 323, R279–R288. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.E.; Borgi, L.; Willett, W.C.; Manson, J.A.E.; Sun, Q.; Hu, F.B. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef]
- Chen, Z.; Zuurmond, M.G.; van der Schaft, N.; Nano, J.; Wijnhoven, H.A.H.; Ikram, M.A.; Franco, O.H.; Voortman, T. Plant versus animal based diets and insulin resistance, prediabetes and type 2 diabetes: The Rotterdam Study. Eur. J. Epidemiol. 2018, 33, 883–893. [Google Scholar] [CrossRef]
- Toumpanakis, A.; Turnbull, T.; Alba-Barba, I. Effectiveness of plant-based diets in promoting well-being in the management of type 2 diabetes: A systematic review. BMJ Open Diabetes Res. Care 2018, 6, 534. [Google Scholar] [CrossRef]
- Parry, S.A.; Rosqvist, F.; Mozes, F.E.; Cornfield, T.; Hutchinson, M.; Piche, M.E.; Hülsmeier, A.J.; Hornemann, T.; Dyson, P.; Hodson, L. Intrahepatic Fat and Postprandial Glycemia Increase After Consumption of a Diet Enriched in Saturated Fat Compared With Free Sugars. Diabetes Care 2020, 43, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Tura, A.; Hill, M.; Holubkov, R.; Barnard, N.D. A Plant-Based Dietary Intervention Improves Beta-Cell Function and Insulin Resistance in Overweight Adults: A 16-Week Randomized Clinical Trial. Nutrients 2018, 10, 189. [Google Scholar] [CrossRef]
- Kouvari, M.; Tsiampalis, T.; Kosti, R.I.; Naumovski, N.; Chrysohoou, C.; Skoumas, J.; Pitsavos, C.S.; Panagiotakos, D.B.; Mantzoros, C.S. Quality of plant-based diets is associated with liver steatosis, which predicts type 2 diabetes incidence ten years later: Results from the ATTICA prospective epidemiological study. Clin. Nutr. 2022, 41, 2094–2102. [Google Scholar] [CrossRef]
- Golzarand, M.; Bahadoran, Z.; Mirmiran, P.; Sadeghian-Sharif, S.; Azizi, F. Dietary phytochemical index is inversely associated with the occurrence of hypertension in adults: A 3-year follow-up (the Tehran Lipid and Glucose Study). Eur. J. Clin. Nutr. 2015, 69, 392–398. [Google Scholar] [CrossRef]
- Pounis, G.; Costanzo, S.; Di Giuseppe, R.; De Lucia, F.; Santimone, I.; Sciarretta, A.; Barisciano, P.; Persichillo, M.; De Curtis, A.; Zito, F.; et al. Consumption of healthy foods at different content of antioxidant vitamins and phytochemicals and metabolic risk factors for cardiovascular disease in men and women of the Moli-sani study. Eur. J. Clin. Nutr. 2013, 67, 207–213. [Google Scholar] [CrossRef]
- Pettersen, B.J.; Anousheh, R.; Fan, J.; Jaceldo-Siegl, K.; Fraser, G.E. Vegetarian diets and blood pressure among white subjects: Results from the Adventist Health Study-2 (AHS-2). Public Health Nutr. 2012, 15, 1909. [Google Scholar] [CrossRef]
- Fraser, G.; Katuli, S.; Anousheh, R.; Knutsen, S.; Herring, P.; Fan, J. Vegetarian diets and cardiovascular risk factors in black members of the Adventist Health Study-2. Public Health Nutr. 2015, 18, 537. [Google Scholar] [CrossRef]
- Najjar, R.S.; Moore, C.E.; Montgomery, B.D. A defined, plant-based diet utilized in an outpatient cardiovascular clinic effectively treats hypercholesterolemia and hypertension and reduces medications. Clin. Cardiol. 2018, 41, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; Welch, A.A.; Fairweather-Tait, S.J.; Kay, C.; Minihane, A.M.; Chowienczyk, P.; Jiang, B.; Cecelja, M.; Spector, T.; Macgregor, A.; et al. Higher anthocyanin intake is associated with lower arterial stiffness and central blood pressure in women. Am. J. Clin. Nutr. 2012, 96, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Fairlie-Jones, L.; Davison, K.; Fromentin, E.; Hill, A.M. The Effect of Anthocyanin-Rich Foods or Extracts on Vascular Function in Adults: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2017, 9, 908. [Google Scholar] [CrossRef]
- Mozos, I.; Flangea, C.; Vlad, D.C.; Gug, C.; Mozos, C.; Stoian, D.; Luca, C.T.; Horbańczuk, J.O.; Horbańczuk, O.K.; Atanasov, A.G. Effects of Anthocyanins on Vascular Health. Biomolecules 2021, 11, 811. [Google Scholar] [CrossRef] [PubMed]
- Grillo, A.; Salvi, L.; Coruzzi, P.; Salvi, P.; Parati, G. Sodium Intake and Hypertension. Nutrients 2019, 11, 1970. [Google Scholar] [CrossRef]
- Geleijnse, J.M.; Kok, F.J.; Grobbee, D.E. Blood pressure response to changes in sodium and potassium intake: A metaregression analysis of randomised trials. J. Hum. Hypertens. 2003, 17, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Binia, A.; Jaeger, J.; Hu, Y.; Singh, A.; Zimmermann, D. Daily potassium intake and sodium-to-potassium ratio in the reduction of blood pressure: A meta-analysis of randomized controlled trials. J. Hypertens. 2015, 33, 1509–1520. [Google Scholar] [CrossRef]
- Dickinson, H.O.; Nicolson, D.; Campbell, F.; Beyer, F.R.; Mason, J. Potassium supplementation for the management of primary hypertension in adults. Cochrane Database Syst. Rev. 2006, 19, CD004641. [Google Scholar] [CrossRef] [PubMed]
- Treasure, J.; Ploth, D. Role of dietary potassium in the treatment of hypertension. Hypertension 1983, 5, 864–872. [Google Scholar] [CrossRef]
- Poulsen, S.B.; Fenton, R.A.; Petersen, O.; Brown, D.; Poulsen, S.B.; Fenton, R.A. K+ and the renin–angiotensin–aldosterone system: New insights into their role in blood pressure control and hypertension treatment. J. Physiol. 2019, 597, 4451–4464. [Google Scholar] [CrossRef]
- Grimm, P.R.; Delpire, E.; Welling, P.A. A Renal Potassium-Switch Prioritizes Dietary Potassium Over Sodium, Driving Salt-Sensitive Hypertension. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Staruschenko, A. Beneficial effects of high potassium: Contribution of renal basolateral k+ channels. Hypertension 2018, 71, 1015–1022. [Google Scholar] [CrossRef]
- Dreier, R.; Andersen, U.B.; Forman, J.L.; Sheykhzade, M.; Egfjord, M.; Jeppesen, J.L. Effect of Increased Potassium Intake on Adrenal Cortical and Cardiovascular Responses to Angiotensin II: A Randomized Crossover Study. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2021, 10, 18716. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Clegg, D.J. Blood pressure lowering and potassium intake. J. Hum. Hypertens. 2020, 34, 671–672. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Giovannucci, E.L. Quality of plant-based diets and risk of hypertension: A Korean genome and examination study. Eur. J. Nutr. 2021, 60, 3841–3851. [Google Scholar] [CrossRef] [PubMed]
- Koh, N.; Ference, B.A.; Nicholls, S.J.; Navar, A.M.; Chew, D.P.; Kostner, K.; He, B.; Tse, H.F.; Dalal, J.; Santoso, A.; et al. Asian Pacific Society of Cardiology Consensus Recommendations on Dyslipidaemia. Eur. Cardiol. Rev. 2021, 16, e54. [Google Scholar] [CrossRef] [PubMed]
- Pinal-Fernandez, I.; Casal-Dominguez, M.; Mammen, A.L. Statins: Pros and cons. Med. Clin. 2018, 150, 398. [Google Scholar] [CrossRef]
- Chiu, T.H.T.; Kao, Y.C.; Wang, L.Y.; Chang, H.R.; Lin, C.L. A Dietitian-Led Vegan Program May Improve GlycA, and Other Novel and Traditional Cardiometabolic Risk Factors in Patients With Dyslipidemia: A Pilot Study. Front. Nutr. 2022, 9, 152. [Google Scholar] [CrossRef]
- Connelly, M.A.; Otvos, J.D.; Shalaurova, I.; Playford, M.P.; Mehta, N.N. GlycA, a novel biomarker of systemic inflammation and cardiovascular disease risk. J. Transl. Med. 2017, 15, 219. [Google Scholar] [CrossRef]
- Zugravu, C.A.; Otelea, M.R.; Vladareanu, R.; Grigoriu, C.; Salmen, T.; Manolache, F.A.; Bohiltea, R.E. The Effect of Plant-Based Nutrition Diets on Plasma Lipids Profile—A Study Case in Romania. Sustainability 2022, 14, 1008. [Google Scholar] [CrossRef]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Schoeneck, M.; Iggman, D. The effects of foods on LDL cholesterol levels: A systematic review of the accumulated evidence from systematic reviews and meta-analyses of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1325–1338. [Google Scholar] [CrossRef]
- Jiménez-Cruz, A.; Turnbull, W.H.; Bacardi-Gascón, M.; Rosales-Garay, P. A high-fiber, moderate-glycemic-index, Mexican style diet improves dyslipidemia in individuals with type 2 diabetes. Nutr. Res. 2004, 24, 19–27. [Google Scholar] [CrossRef]
- Tovar, A.R.; Guevara-Cruz, M.; Serralde Zúñiga, A.E.; Torres, N. Dietary Fiber and Hyperlipidemia and Cardiovascular Disease. In Science and Technology of Fibers in Food Systems; Springer: Cham, Switzerland, 2020; pp. 219–239. [Google Scholar]
- Anderson, J.W.; Baird, P.; Davis, R.H.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef]
- Jacobson, T.A.; Maki, K.C.; Orringer, C.E.; Jones, P.H.; Kris-Etherton, P.; Sikand, G.; La Forge, R.; Daniels, S.R.; Wilson, D.P.; Morris, P.B.; et al. National Lipid Association Recommendations for Patient-Centered Management of Dyslipidemia: Part 2. J. Clin. Lipidol. 2015, 9, S1–S122.e1. [Google Scholar] [CrossRef]
- Hara, H.; Haga, S.; Aoyama, Y.; Kiriyama, S. Short-chain fatty acids suppress cholesterol synthesis in rat liver and intestine. J. Nutr. 1999, 129, 942–948. [Google Scholar] [CrossRef]
- Popeijus, H.E.; Zwaan, W.; Tayyeb, J.Z.; Plat, J. Potential Contribution of Short Chain Fatty Acids to Hepatic Apolipoprotein A-I Production. Int. J. Mol. Sci. 2021, 22, 5986. [Google Scholar] [CrossRef]
- Laka, K.; Makgoo, L.; Mbita, Z. Cholesterol-Lowering Phytochemicals: Targeting the Mevalonate Pathway for Anticancer Interventions. Front. Genet. 2022, 13, 628. [Google Scholar] [CrossRef]
- Lütjohann, D.; Meyer, S.; von Bergmann, K.; Stellaard, F. Cholesterol Absorption and Synthesis in Vegetarians and Omnivores. Mol. Nutr. Food Res. 2018, 62, 1700689. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, S.M.; Mashat, R.M.; Almutairi, D.; Mathkour, A.; Alqahtani, S.S.; Alasmari, A.; Alzahrani, A.H.; Ayed, R.; Asiri, M.Y.; Elsherif, A.; et al. The Effect of Walnut Intake on Lipids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 4460. [Google Scholar] [CrossRef]
- Lee, K.; Kim, H.; Rebholz, C.M.; Kim, J. Association between Different Types of Plant-Based Diets and Risk of Dyslipidemia: A Prospective Cohort Study. Nutrients 2021, 13, 220. [Google Scholar] [CrossRef]
- Simnadis, T.G.; Tapsell, L.C.; Beck, E.J. Physiological Effects Associated with Quinoa Consumption and Implications for Research Involving Humans: A Review. Plant Foods Hum. Nutr. 2015, 70, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Maria, F.; Farinazzi-Machado, V.; Barbalho, S.M.; Oshiiwa, M.; Goulart, R.; Pessan Junior, O. Use of cereal bars with quinoa (Chenopodium quinoa W.) to reduce risk factors related to cardiovascular diseases. Food Sci. Technol. 2012, 32, 239–244. [Google Scholar] [CrossRef]
- Navarro-Perez, D.; Radcliffe, J.; Tierney, A.; Jois, M. Quinoa Seed Lowers Serum Triglycerides in Overweight and Obese Subjects: A Dose-Response Randomized Controlled Clinical Trial. Curr. Dev. Nutr. 2017, 1, e001321. [Google Scholar] [CrossRef] [PubMed]
- Webster, I.; Friedrich, S.O.; Lochner, A.; Huisamen, B. AMP kinase activation and glut4 translocation in isolated cardiomyocytes. Cardiovasc. J. Afr. 2010, 21, 72. [Google Scholar]
- Lyons, C.L.; Roche, H.M. Nutritional Modulation of AMPK-Impact upon Metabolic-Inflammation. Int. J. Mol. Sci. 2018, 19, 3092. [Google Scholar] [CrossRef] [PubMed]
- Chiazza, F.; Collino, M. Peroxisome Proliferator-Activated Receptors (PPARs) in Glucose Control. Mol. Nutr. Diabetes A Vol. Mol. Nutr. Ser. 2016, 105–114. [Google Scholar] [CrossRef]
- Haluzík, M.M.; Haluzík, M. PPAR-alpha and insulin sensitivity. Physiol. Res. 2006, 55, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Abraham Domínguez-Avila, J.; González-Aguilar, G.A.; Alvarez-Parrilla, E.; de la Rosa, L.A. Modulation of PPAR Expression and Activity in Response to Polyphenolic Compounds in High Fat Diets. Int. J. Mol. Sci. 2016, 17, 1002. [Google Scholar] [CrossRef] [PubMed]
- Lambert, K.; Hokayem, M.; Thomas, C.; Fabre, O.; Cassan, C.; Bourret, A.; Bernex, F.; Feuillet-Coudray, C.; Notarnicola, C.; Mercier, J.; et al. Combination of nutritional polyphenols supplementation with exercise training counteracts insulin resistance and improves endurance in high-fat diet-induced obese rats. Sci. Rep. 2018, 8, 2885. [Google Scholar] [CrossRef]
- Chahal, D.S.; Sivamani, R.K.; Rivkah Isseroff, R.; Dasu, M.R. Plant-based modulation of Toll-like receptors: An emerging therapeutic model. Phytother. Res. 2013, 27, 1423–1438. [Google Scholar] [CrossRef]
- Shahavandi, M.; Djafari, F.; Shahinfar, H.; Davarzani, S.; Babaei, N.; Ebaditabar, M.; Djafarian, K.; Clark, C.C.T.; Shab-Bidar, S. The association of plant-based dietary patterns with visceral adiposity, lipid accumulation product, and triglyceride-glucose index in Iranian adults. Complement. Ther. Med. 2020, 53, 102531. [Google Scholar] [CrossRef]
- Van Eekelen, E.; Geelen, A.; Alssema, M.; Lamb, H.J.; De Roos, A.; Rosendaal, F.R.; De Mutsert, R. Sweet Snacks Are Positively and Fruits and Vegetables Are Negatively Associated with Visceral or Liver Fat Content in Middle-Aged Men and Women. J. Nutr. 2019, 149, 304–313. [Google Scholar] [CrossRef]
- Chen, Z.; Schoufour, J.D.; Rivadeneira, F.; Lamballais, S.; Ikram, M.A.; Franco, O.H.; Voortman, T. Plant-based Diet and Adiposity Over Time in a Middle-aged and Elderly Population: The Rotterdam Study. Epidemiology 2019, 30, 303–310. [Google Scholar] [CrossRef]
- Ferguson, J.J.A.; Oldmeadow, C.; Mishra, G.D.; Garg, M.L. Plant-based dietary patterns are associated with lower body weight, BMI and waist circumference in older Australian women. Public Health Nutr. 2022, 25, 18–31. [Google Scholar] [CrossRef]
- Stefler, D.; Malyutina, S.; Nikitin, Y.; Nikitenko, T.; Rodriguez-Artalejo, F.; Peasey, A.; Pikhart, H.; Sabia, S.; Bobak, M. Fruit, vegetable intake and blood pressure trajectories in older age. J. Hum. Hypertens. 2019, 33, 671–678. [Google Scholar] [CrossRef]
- Holt, E.M.; Steffen, L.M.; Moran, A.; Basu, S.; Steinberger, J.; Ross, J.A.; Hong, C.P.; Sinaiko, A.R. Fruit and vegetable consumption and its relation to markers of inflammation and oxidative stress in adolescents. J. Am. Diet. Assoc. 2009, 109, 414. [Google Scholar] [CrossRef]
- Krzemińska, J.; Wronka, M.; Młynarska, E.; Franczyk, B.; Rysz, J. Arterial Hypertension—Oxidative Stress and Inflammation. Antioxidants 2022, 11, 172. [Google Scholar] [CrossRef]
- Calling, S.; Johansson, S.E.; Wolff, M.; Sundquist, J.; Sundquist, K. Total cholesterol/HDL-C ratio versus non-HDL-C as predictors for ischemic heart disease: A 17-year follow-up study of women in southern Sweden. BMC Cardiovasc. Disord. 2021, 21, 163. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, A.; Aikawa, M.; Alcaide, P.; Luscinskas, F.W.; Libby, P.; Sacks, F.M. Apolipoprotein CIII induces expression of vascular cell adhesion molecule-1 in vascular endothelial cells and increases adhesion of monocytic cells. Circulation 2006, 114, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Tardif, J.-C.; Nicholls, S.J.; Revkin, J.H.; Shear, C.L.; Duggan, W.T.; Ruzyllo, W.; Bachinsky, W.B.; Lasala, G.P.; Tuzcu, E.M. Effect of torcetrapib on the progression of coronary atherosclerosis. N. Engl. J. Med. 2007, 356, 1304–1316. [Google Scholar] [CrossRef]
- Paquette, M.; Medina Larqué, A.S.; Weisnagel, S.J.; Desjardins, Y.; Marois, J.; Pilon, G.; Dudonné, S.; Marette, A.; Jacques, H. Strawberry and cranberry polyphenols improve insulin sensitivity in insulin-resistant, non-diabetic adults: A parallel, double-blind, controlled and randomised clinical trial. Br. J. Nutr. 2017, 117, 519–531. [Google Scholar] [CrossRef]
- Palma, X.; Thomas-Valdés, S.; Cruz, G. Acute Consumption of Blueberries and Short-Term Blueberry Supplementation Improve Glucose Management and Insulin Levels in Sedentary Subjects. Nutrients 2021, 13, 1458. [Google Scholar] [CrossRef]
- Banaszak, M.; Górna, I.; Przysławski, J. Non-Pharmacological Treatments for Insulin Resistance: Effective Intervention of Plant-Based Diets—A Critical Review. Nutrients 2022, 14, 1400. [Google Scholar] [CrossRef]
- Matli, B.; Schulz, A.; Koeck, T.; Falter, T.; Lotz, J.; Rossmann, H.; Pfeiffer, N.; Beutel, M.; Münzel, T.; Strauch, K.; et al. Distribution of HOMA-IR in a population-based cohort and proposal for reference intervals. Clin. Chem. Lab. Med. 2021, 59, 1844–1851. [Google Scholar] [CrossRef] [PubMed]
- Habegger, K.M.; Hoffman, N.J.; Ridenour, C.M.; Brozinick, J.T.; Elmendorf, J.S. AMPK Enhances Insulin-Stimulated GLUT4 Regulation via Lowering Membrane Cholesterol. Endocrinology 2012, 153, 2130. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.Y.; Wu, S.J.; Chang, Y.C.; Ng, L.T.; Chang, S.J. Stimulation of GLUT4 Glucose Uptake by Anthocyanin-Rich Extract from Black Rice (Oryza sativa L.) via PI3K/Akt and AMPK/p38 MAPK Signaling in C2C12 Cells. Metabolites 2022, 12, 856. [Google Scholar] [CrossRef]
- Williamson, G.; Sheedy, K. Effects of Polyphenols on Insulin Resistance. Nutrients 2020, 12, 3135. [Google Scholar] [CrossRef] [PubMed]
- Stull, A.J.; Cash, K.C.; Johnson, W.D.; Champagne, C.M.; Cefalu, W.T. Bioactives in Blueberries Improve Insulin Sensitivity in Obese, Insulin-Resistant Men and Women. J. Nutr. 2010, 140, 1764–1768. [Google Scholar] [CrossRef]
- Hokayem, M.; Blond, E.; Vidal, H.; Lambert, K.; Meugnier, E.; Feillet-Coudray, C.; Coudray, C.; Pesenti, S.; Luyton, C.; Lambert-Porcheron, S.; et al. Grape Polyphenols Prevent Fructose-Induced Oxidative Stress and Insulin Resistance in First-Degree Relatives of Type 2 Diabetic Patients. Diabetes Care 2013, 36, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Edirisinghe, I.; Banaszewski, K.; Cappozzo, J.; Sandhya, K.; Ellis, C.L.; Tadapaneni, R.; Kappagoda, C.T.; Burton-Freeman, B.M. Strawberry anthocyanin and its association with postprandial inflammation and insulin. Br. J. Nutr. 2011, 106, 913–922. [Google Scholar] [CrossRef]
- Agnese, D.M.; Calvano, J.E.; Hahm, S.J.; Coyle, S.M.; Corbett, S.A.; Calvano, S.E.; Lowry, S.F. Human toll-like receptor 4 mutations but not CD14 polymorphisms are associated with an increased risk of gram-negative infections. J. Infect. Dis. 2002, 186, 1522–1525. [Google Scholar] [CrossRef]
- Wang, C.; Deng, L.; Hong, M.; Akkaraju, G.R.; Inoue, J.I.; Chen, Z.J. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 2001, 412, 346–351. [Google Scholar] [CrossRef]
- Carmody, R.J.; Chen, Y.H. Nuclear Factor-κB: Activation and Regulation during Toll-Like Receptor Signaling. Cell. Mol. Immunol. 2007, 4, 31–41. [Google Scholar]
- Senn, J.J. Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J. Biol. Chem. 2006, 281, 26865–26875. [Google Scholar] [CrossRef]
- Reyna, S.M.; Ghosh, S.; Tantiwong, P.; Meka, C.S.R.M.; Eagan, P.; Jenkinson, C.P.; Cersosimo, E.; Defronzo, R.A.; Coletta, D.K.; Sriwijitkamol, A.; et al. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes 2008, 57, 2595–2602. [Google Scholar] [CrossRef]
- Himes, R.W.; Smith, C.W. Tlr2 is critical for diet-induced metabolic syndrome in a murine model. FASEB J. 2010, 24, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Ratjen, I.; Morze, J.; Enderle, J.; Both, M.; Borggrefe, J.; Müller, H.P.; Kassubek, J.; Koch, M.; Lieb, W. Adherence to a plant-based diet in relation to adipose tissue volumes and liver fat content. Am. J. Clin. Nutr. 2020, 112, 354–363. [Google Scholar] [CrossRef]
- Kristensen, M.D.; Bendsen, N.T.; Christensen, S.M.; Astrup, A.; Raben, A. Meals based on vegetable protein sources (beans and peas) are more satiating than meals based on animal protein sources (veal and pork)—A randomized cross-over meal test study. Food Nutr. Res. 2016, 60, 32634. [Google Scholar] [CrossRef]
- Austin, G.; Ferguson, J.J.A.; Garg, M.L. Effects of plant-based diets on weight status in type 2 diabetes: A systematic review and meta-analysis of randomised controlled trials. Nutrients 2021, 13, 4099. [Google Scholar] [CrossRef]
- Aljuraiban, G.; Chan, Q.; Gibson, R.; Stamler, J.; Daviglus, M.L.; Dyer, A.R.; Miura, K.; Wu, Y.; Ueshima, H.; Zhao, L.; et al. Association between plant-based diets and blood pressure in the INTERMAP study. BMJ Nutr. Prev. Health 2020, 3, 133. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.N.; Meng, X.; Li, Y.; Li, S.; Liu, Q.; Tang, G.Y.; Li, H. Bin Fruits for Prevention and Treatment of Cardiovascular Diseases. Nutrients 2017, 9, 598. [Google Scholar] [CrossRef]
- Macready, A.L.; George, T.W.; Chong, M.F.; Alimbetov, D.S.; Jin, Y.; Vidal, A.; Spencer, J.P.E.; Kennedy, O.B.; Tuohy, K.M.; Minihane, A.M.; et al. Flavonoid-rich fruit and vegetables improve microvascular reactivity and inflammatory status in men at risk of cardiovascular disease—FLAVURS: A randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Dohadwala, M.M.; Holbrook, M.; Hamburg, N.M.; Shenouda, S.M.; Chung, W.B.; Titas, M.; Kluge, M.A.; Wang, N.; Palmisano, J.; Milbury, P.E.; et al. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am. J. Clin. Nutr. 2011, 93, 934–940. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.; Rebholz, C.M.; Kim, J. Plant-based diets and incident metabolic syndrome: Results from a South Korean prospective cohort study. PLoS Med. 2020, 17, 1003371. [Google Scholar] [CrossRef]
- Teixeira, R.D.C.M.D.A.; Molina, M.D.C.B.; Zandonade, E.; Mill, J.G. Cardiovascular risk in vegetarians and omnivores: A comparative study. Arq. Bras. Cardiol. 2007, 89, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.P.S.; Low, J.H.M.; Chen, J.R.; Zimmermann, D.; Actis-Goretta, L.; Kim, J.E. The Influence of Different Foods and Food Ingredients on Acute Postprandial Triglyceride Response: A Systematic Literature Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2020, 11, 1529. [Google Scholar] [CrossRef]
- Navab, M.; Reddy, S.T.; Van Lenten, B.J.; Fogelman, A.M. HDL and cardiovascular disease: Atherogenic and atheroprotective mechanisms. Nat. Rev. Cardiol. 2011, 8, 222–232. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Martinez, I.; Sourlas, A.; Bouza, K.V.; Campos, F.N.; Torres, V.; Montan, P.D.; Guzman, E. High-density lipoprotein (HDL) functionality and its relevance to atherosclerotic cardiovascular disease. Drugs Context 2018, 7, 212525. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: Two prospective cohort studies. Eur. Heart J. 2017, 38, 2478–2486. [Google Scholar] [CrossRef]
- Franczyk, B.; Rysz, J.; Ławiński, J.; Rysz-Górzyńska, M.; Gluba-Brzózka, A. Is a High HDL-Cholesterol Level Always Beneficial? Biomedicines 2021, 9, 1083. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Dhindsa, D.; Almuwaqqat, Z.; Sun, Y.V.; Quyyumi, A.A. Very High High-Density Lipoprotein Cholesterol Levels and Cardiovascular Mortality. Am. J. Cardiol. 2022, 167, 43–53. [Google Scholar] [CrossRef]
- Zhong, G.C.; Huang, S.Q.; Peng, Y.; Wan, L.; Wu, Y.Q.L.; Hu, T.Y.; Hu, J.J.; Hao, F.B. HDL-C is associated with mortality from all causes, cardiovascular disease and cancer in a J-shaped dose-response fashion: A pooled analysis of 37 prospective cohort studies. Eur. J. Prev. Cardiol. 2020, 27, 1187–1203. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Lin, F.Y.; Shih, C.M.; Au, H.K.; Chang, Y.J.; Nakagami, H.; Morishita, R.; Chang, N.C.; Shyu, K.G.; Chen, J.W. Moderate to high concentrations of high-density lipoprotein from healthy subjects paradoxically impair human endothelial progenitor cells and related angiogenesis by activating rho-associated kinase pathways. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2405–2417. [Google Scholar] [CrossRef]
- Volek, J.S.; Sharman, M.J.; Gómez, A.L.; Scheett, T.P.; Kraemer, W.J. An isoenergetic very low carbohydrate diet improves serum HDL cholesterol and triacylglycerol concentrations, the total cholesterol to HDL cholesterol ratio and postprandial pipemic responses compared with a low fat diet in normal weight, normolipidemic women. J. Nutr. 2003, 133, 2756–2761. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Wong, J.M.W.; Kendall, C.W.C.; Esfahani, A.; Ng, V.W.Y.; Leong, T.C.K.; Faulkner, D.A.; Vidgen, E.; Greaves, K.A.; Paul, G.; et al. The effect of a plant-based low-carbohydrate (“Eco-Atkins”) diet on body weight and blood lipid concentrations in hyperlipidemic subjects. Arch. Intern. Med. 2009, 169, 1046–1054. [Google Scholar] [CrossRef]
- Millán, J.; Pintó, X.; Muñoz, A.; Zúñiga, M.; Rubiés-Prat, J.; Pallardo, L.F.; Masana, L.; Mangas, A.; Hernández-Mijares, A.; González-Santos, P.; et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc. Health Risk Manag. 2009, 5, 757. [Google Scholar] [CrossRef] [PubMed]
- Kinosian, B.; Glick, H.; Garland, G. Cholesterol and coronary heart disease: Predicting risks by levels and ratios. Ann. Intern. Med. 1994, 121, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Quispe, R.; Elshazly, M.B.; Zhao, D.; Toth, P.P.; Puri, R.; Virani, S.S.; Blumenthal, R.S.; Martin, S.S.; Jones, S.R.; Michos, E.D. TC/HDL-C Ratio Discordance with LDL-C and non-HDL-C and Incidence of Atherosclerotic Cardiovascular Disease in Primary Prevention: The ARIC Study. Eur. J. Prev. Cardiol. 2020, 27, 1597. [Google Scholar] [CrossRef]
- Bleda, S.; De Haro, J.; Varela, C.; Esparza, L.; Rodriguez, J.; Acin, F. Improving Total-Cholesterol/HDL-Cholesterol Ratio Results in an Endothelial Dysfunction Recovery in Peripheral Artery Disease Patients. Cholesterol 2012, 2012, 895326. [Google Scholar] [CrossRef]
- Kent, L.; Morton, D.; Rankin, P.; Ward, E.; Grant, R.; Gobble, J.; Diehl, H. The effect of a low-fat, plant-based lifestyle intervention (CHIP) on serum HDL levels and the implications for metabolic syndrome status—A cohort study. Nutr. Metab. 2013, 10, 58. [Google Scholar] [CrossRef]
- MacMahon, S.; Duffy, S.; Rodgers, A.; Tominaga, S.; Chambless, L.; De Backer, G.; De Bacquer, D.; Kornitzer, M.; Whincup, P.; Wannamethee, S.G.; et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: A meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007, 370, 1829–1839. [Google Scholar] [CrossRef]
- Jensen, M.K.; Aroner, S.A.; Mukamal, K.J.; Furtado, J.D.; Post, W.S.; Tsai, M.Y.; Tjønneland, A.; Polak, J.F.; Rimm, E.B.; Overvad, K.; et al. HDL subspecies defined by presence of apolipoprotein C-III and incident coronary heart disease in four cohorts. Circulation 2018, 137, 1364. [Google Scholar] [CrossRef]
- The AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 2011, 365, 2255–2267. [Google Scholar] [CrossRef]
- Cuevas-Ramos, D.; Almeda-Valdés, P.; Chávez-Manzanera, E.; Meza-Arana, C.E.; Brito-Córdova, G.; Mehta, R.; Pérez-Méndez, O.; Gómez-Pérez, F.J. Effect of tomato consumption on high-density lipoprotein cholesterol level: A randomized, single-blinded, controlled clinical trial. Diabetes Metab. Syndr. Obes. Targets Ther. 2013, 6, 263. [Google Scholar] [CrossRef]
- Jamshed, H.; Sultan, F.A.T.; Iqbal, R.; Gilani, A.H. Dietary Almonds Increase Serum HDL Cholesterol in Coronary Artery Disease Patients in a Randomized Controlled Trial. J. Nutr. 2015, 145, 2287–2292. [Google Scholar] [CrossRef] [PubMed]
- Mahmassani, H.A.; Avendano, E.E.; Raman, G.; Johnson, E.J. Avocado consumption and risk factors for heart disease: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 107, 523–536. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).