Bitter Is Better: Wild Greens Used in the Blue Zone of Ikaria, Greece
Abstract
1. Introduction
2. Materials and Methods
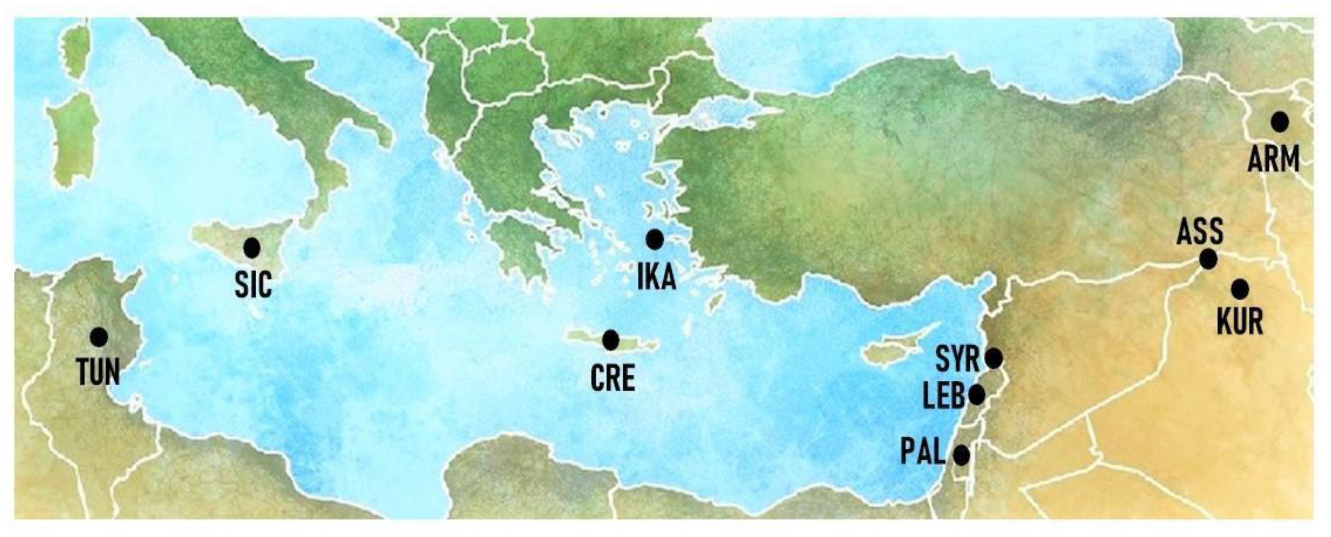
2.1. Study Area
2.2. Ethnobotanical Field Study
2.3. Cross-Cultural Data Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Ikarian Chorta
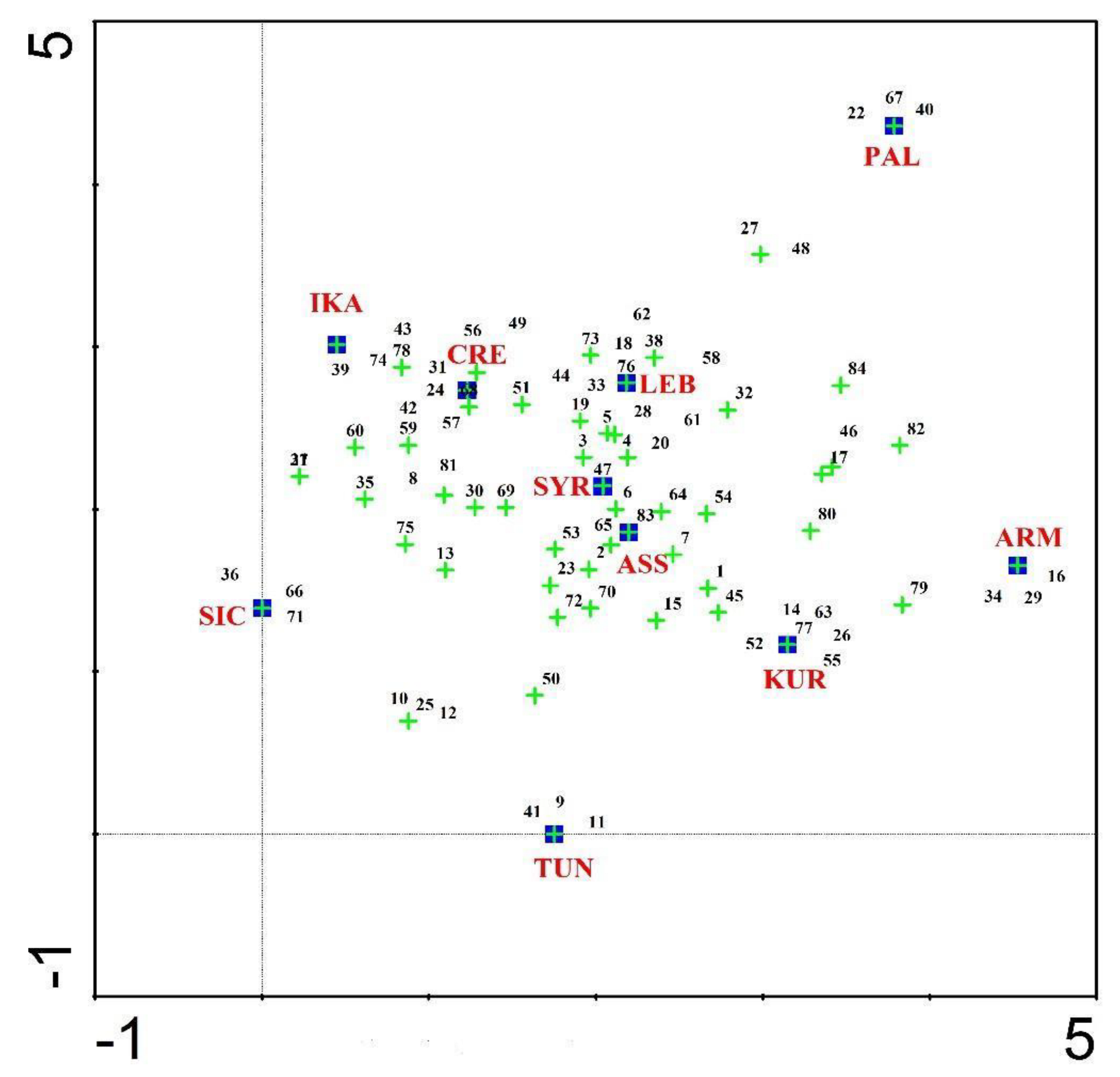
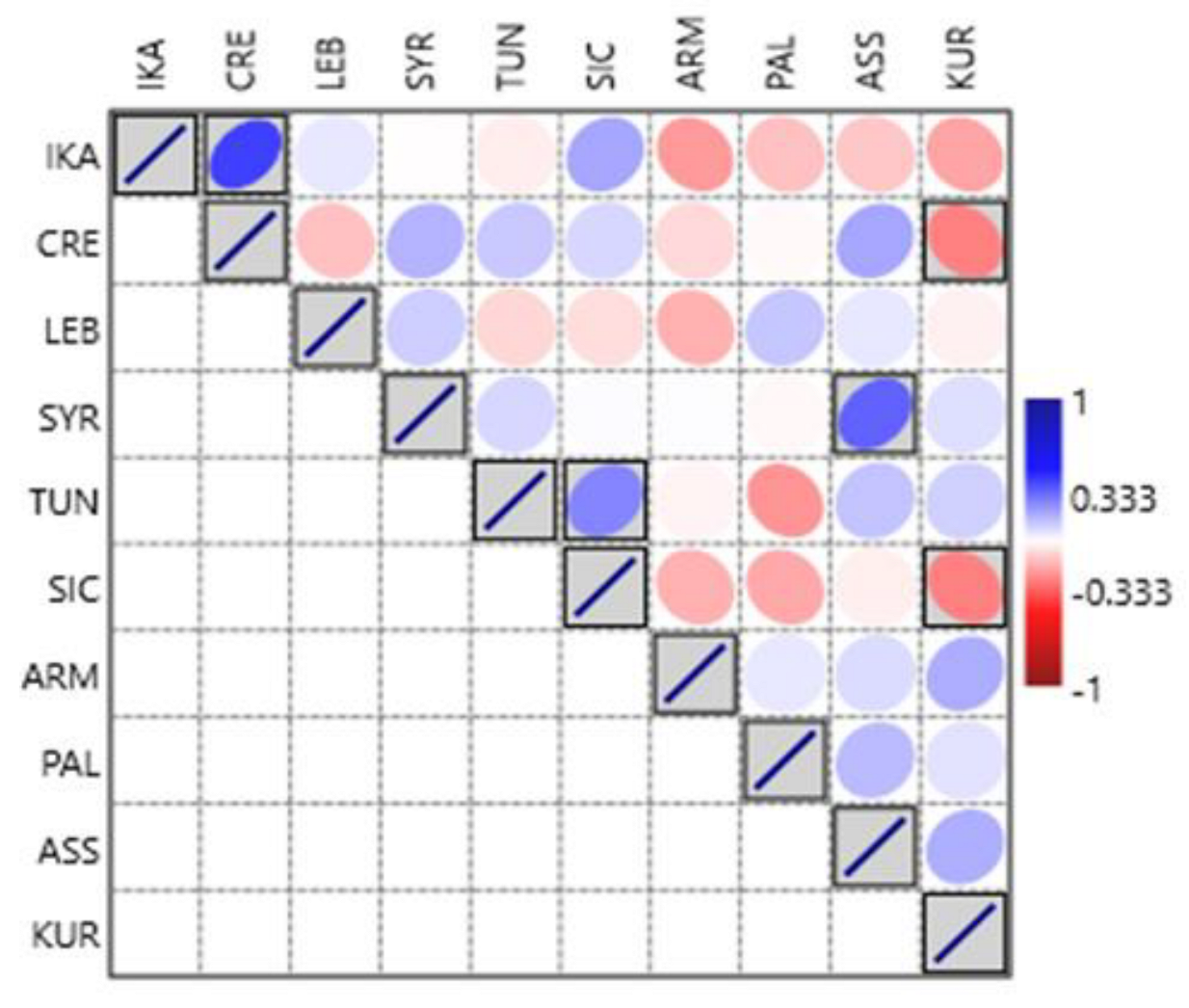
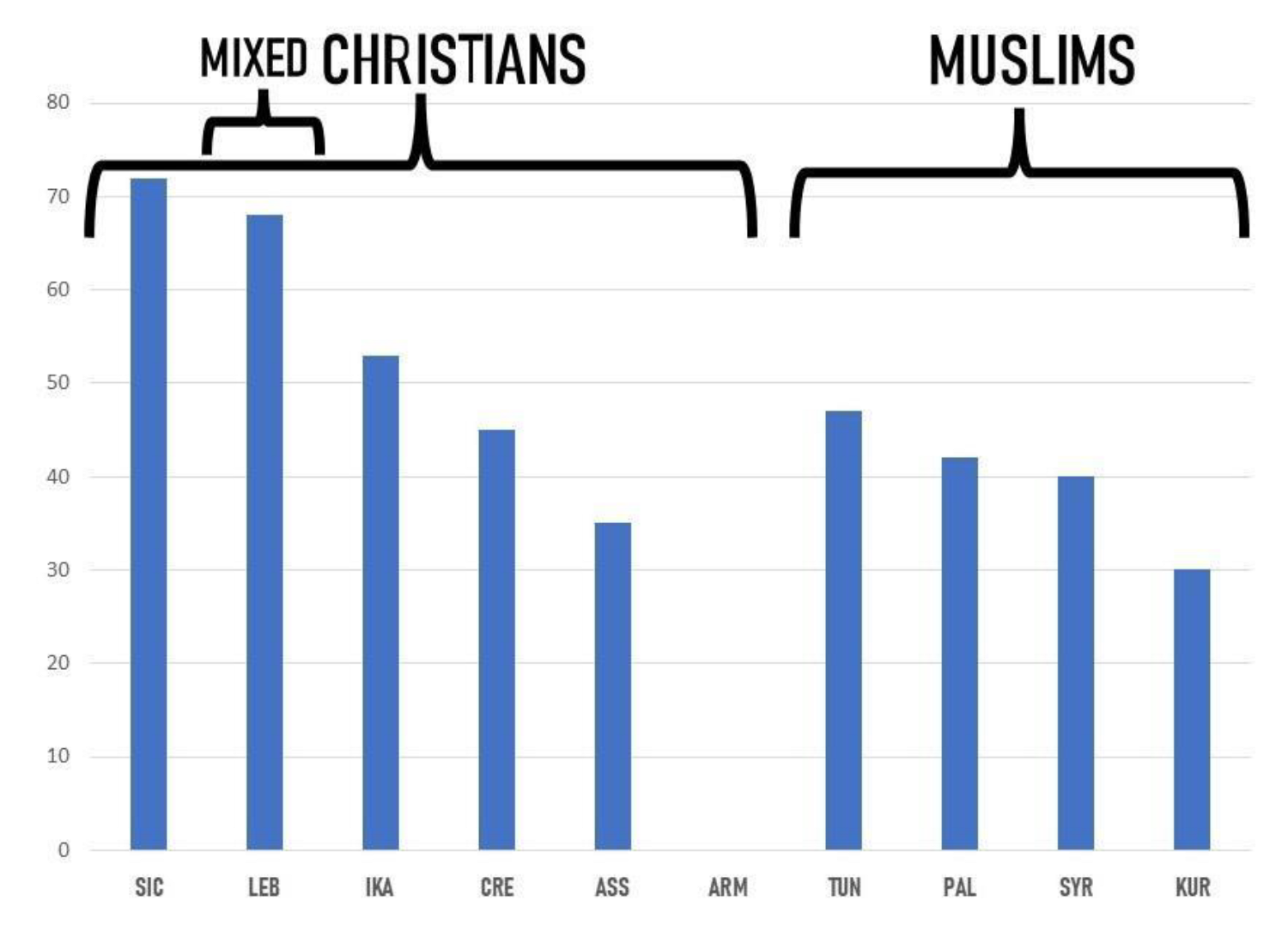
3.2. Cross-Cultural Comparisons
3.3. Bitter Is Better
3.4. How to Make Food Heritage of Wild Greens Trendy Again
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keys, A. Seven Countries: A Multivariate Analysis of Death and Coronary Heart Disease; Harvard University Press: Cambridge, MA, USA, 1980. [Google Scholar]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet: A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health benefits of the Mediterranean diet: Metabolic and molecular mechanisms. J. Gerontol. Ser. A 2018, 73, 318–326. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean diet: A review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean diet and cardiovascular health: A critical review. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Martín-Peláez, S.; Fito, M.; Castaner, O. Mediterranean diet effects on type 2 diabetes prevention, disease progression, and related mechanisms. A review. Nutrients 2020, 12, 2236. [Google Scholar] [CrossRef] [PubMed]
- UNESCO. Mediterranean Diet. Available online: https://ich.unesco.org/en/RL/mediterranean-diet-00884 (accessed on 18 March 2023).
- Serra-Majem, L.; Medina, F.X. The Mediterranean Diet as an Intangible and Sustainable Food Culture. In The Mediterranean Diet: An Evidence-Based Approach; Preedy, V.R., Watson, R.R., Eds.; University of Arizona: Tucson, AZ, USA, 2015; pp. 37–46. [Google Scholar]
- Naska, A.; Trichopoulou, A. Back to the future: The Mediterranean diet paradigm. Nutr. Metab. Cardiovasc. Dis. 2020, 24, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Diolintzi, A.; Panagiotakos, D.B.; Sidossis, L.S. From Mediterranean diet to Mediterranean lifestyle: A narrative review. Public Health Nutr. 2019, 22, 2703–2713. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef]
- Obeid, C.A.; Gubbels, J.S.; Jaalouk, D.; Kremers, S.P.; Oenema, A. Adherence to the Mediterranean diet among adults in Mediterranean countries: A systematic literature review. Eur. J. Nutr. 2022, 61, 3327–3344. [Google Scholar] [CrossRef]
- D’Innocenzo, S.; Biagi, C.; Lanari, M. Obesity and the Mediterranean Diet: A Review of Evidence of the Role and Sustainability of the Mediterranean Diet. Nutrients 2019, 11, 1306. [Google Scholar] [CrossRef]
- Buettner, D.; Skemp, S. Blue Zones: Lessons from the World’s Longest Lived. Am. J. Lifestyle Med. 2016, 10, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Nieddu, A.; Vindas, L.; Errigo, A.; Vindas, J.; Pes, G.M.; Dore, M.P. Dietary Habits, Anthropometric Features and Daily Performance in Two Independent Long-Lived Populations from Nicoya peninsula (Costa Rica) and Ogliastra (Sardinia). Nutrients 2020, 12, 1621. [Google Scholar] [CrossRef] [PubMed]
- Pes, G.M.; Dore, M.P.; Tsofliou, F.; Poulain, M. Diet and longevity in the Blue Zones: A set-and-forget issue? Maturitas 2022, 164, 31–37. [Google Scholar] [CrossRef]
- Biscotti, N.; Pieroni, A. The hidden Mediterranean diet: Wild greens traditionally gathered and consumed in the Gargano area, Apulia, SE Italy. Acta Soc. Bot. Pol. 2015, 84, 327–338. [Google Scholar] [CrossRef]
- Łuczaj, Ł.; Zovkokoncic, M.; Milicevic, T.; Dolina, K.; Pandza, M. Wild vegetable mixes sold in the markets of Dalmatia (southern Croatia). J. Ethnobiol. Ethnomed. 2013, 9, 2. [Google Scholar] [CrossRef]
- Łuczaj, Ł.; Dolina, K. A hundred years of change in wild vegetable use in southern Herzegovina. J. Ethnopharmacol. 2015, 166, 297–304. [Google Scholar] [CrossRef]
- Dolina, K.; Łuczaj, Ł. Wild food plants used on the Dubrovnik coast (south-eastern Croatia). Acta Soc. Bot. Pol. 2014, 83, 175–181. [Google Scholar] [CrossRef]
- Dogan, Y. Traditionally used wild edible greens in the Aegean Region of Turkey. Acta Soc. Bot. Pol. 2012, 81, 329–341. [Google Scholar] [CrossRef]
- Dogan, Y.; Ugulu, I.; Durkan, N. Wild edible plants sold in the local markets of Izmir, Turkey. Pak. J. Bot. 2013, 45, 177–184. [Google Scholar]
- Ertuğ, F. Wild edible plants of the Bodrum area (Mula, Turkey). Turk. J. Bot. 2004, 28, 161–174. [Google Scholar]
- Della, A.; Paraskeva-Hadjichambi, D.; Hadjichambis, A. An ethnobotanical survey of wild edible plants of Paphos and Larnaca countryside of Cyprus. J. Ethnobiol. Ethnomed. 2006, 2, 34. [Google Scholar] [CrossRef] [PubMed]
- Hadjichambis, A.; Paraskeva-Hadjichambi, D.; Della, A.; Giusti, M.E.; de Pasquale, C.; Lenzarini, C.; Censorii, E.; Reyes Gonzales-Tejero, M.; Patricia Sanchez-Rojas, C.; Ramiro-Gutierrez, J.M.; et al. Wild and semi-domesticated food plant consumption in seven circum-Mediterranean areas. Int. J. Food Sci. Nutr. 2008, 59, 383–414. [Google Scholar] [CrossRef] [PubMed]
- Leonti, M.; Nebel, S.; Rivera, D.; Heinrich, M. Wild gathered food plants in the European Mediterranean: A comparative analysis. Econ. Bot. 2006, 60, 130–142. [Google Scholar] [CrossRef]
- Pieroni, A.; Nebel, S.; Santoro, R.F.; Heinrich, M. Food for two seasons: Culinary uses of non-cultivated local vegetables and mushrooms in a south Italian village. Int. J. Food Sci. Nutr. 2005, 56, 245–272. [Google Scholar] [CrossRef] [PubMed]
- Nebel, S.; Pieroni, A.; Heinrich, M. Ta chorta: Wild edible greens used in the Graecanic area in Calabria, southern Italy. Appetite 2006, 47, 333–342. [Google Scholar] [CrossRef]
- Geraci, A.; Amato, F.; Di Noto, G.; Bazan, G.; Schicchi, R. The wild taxa utilized as vegetables in Sicily (Italy): A traditional component of the Mediterranean diet. J. Ethnobiol. Ethnomed. 2018, 14, 14. [Google Scholar] [CrossRef]
- Pieroni, A.; Cattero, V. Wild greens do not lie: Comparative gastronomic ethnobotany and ethnolinguistics on the Greek traces of the Mediterranean Diet of southeastern Italy. Acta Bot. Bras. 2019, 33, 198–211. [Google Scholar] [CrossRef]
- Motti, R.; Bonanomi, G.; Lanzotti, V.; Sacchi, R. The contribution of wild edible plants to the Mediterranean Diet: An ethnobotanical case study along the coast of Campania (Southern Italy). Econ. Bot. 2020, 74, 249–272. [Google Scholar] [CrossRef]
- Pieroni, A.; Nebel, S.; Quave, C.L.; Münz, H.; Heinrich, M. Ethnopharmacology of liakra, traditional weedy vegetables of the Arbëreshë of the Vulture area in southern Italy. J. Ethnopharmacol. 2002, 81, 165–185. [Google Scholar] [CrossRef]
- Cucinotta, F.; Pieroni, A. “If you want to get married, you have to collect virdura”: The vanishing custom of gathering and cooking wild food plants on Vulcano, Aeolian Islands, Sicily. Food Cult. Soc. 2018, 31, 539–567. [Google Scholar] [CrossRef]
- Local Food-Nutraceuticals Consortium. Local Food-Nutraceuticals Consortium. Understanding local Mediterranean diets: A multidisciplinary pharmacological and ethnobotanical approach. Pharmacol. Res. 2005, 52, 353–366. [Google Scholar] [CrossRef]
- Chatzopoulou, E.; Carocho, M.; Di Gioia, F.; Petropoulos, S.A. The beneficial health effects of vegetables and wild edible greens: The case of the Mediterranean Diet and its sustainability. Appl. Sci. 2020, 10, 24. [Google Scholar] [CrossRef]
- García-Herrera, P.; Morales, P.; Cámara, M.; Fernández-Ruiz, V.; Tardío, J.; Sánchez-Mata, M.C. Nutritional and phytochemical composition of Mediterranean wild greens after culinary treatment. Foods 2020, 9, 1761. [Google Scholar] [CrossRef]
- Leonti, M. The co-evolutionary perspective of the food-medicine continuum and wild gathered and cultivated vegetables. Gen. Res. Crop Evol. 2012, 59, 1295–1302. [Google Scholar] [CrossRef]
- Stepp, R.J.; Moerman, D.E. The importance of weeds in Ethnopharmacology. J. Ethnopharmacol. 2001, 75, 19–23. [Google Scholar] [CrossRef]
- Finger, T.E.; Kinnamon, S.C. Taste isn’t just for taste buds anymore. F1000 Biol. Rep. 2011, 3, 20. [Google Scholar] [CrossRef]
- Harmon, C.P.; Deng, D.; Breslin, P.A. Bitter taste receptors (T2Rs) are sentinels that coordinate metabolic and immunological defense responses. Curr. Opin. Physiol. 2021, 20, 70–76. [Google Scholar] [CrossRef]
- Cui, M.; Chen, B.; Xu, K.; Rigakou, A.; Diamantakos, P.; Melliou, E.; Logothetis, D.E.; Magiatis, P. Activation of specific bitter taste receptors by olive oil phenolics and secoiridoids. Sci. Rep. 2021, 11, 22340. [Google Scholar] [CrossRef]
- Des Gachons, C.P.; Uchida, K.; Bryant, B.; Shima, A.; Sperry, J.B.; Dankulich-Nagrudny, L.; Tominaga, M.; Smith, A.B.; Beauchamp, G.K.; Breslin, P.A. Unusual pungency from extra-virgin olive oil is attributable to restricted spatial expression of the receptor of oleocanthal. J. Neurosci. 2011, 31, 999–1009. [Google Scholar] [CrossRef]
- Crespo, M.C.; Tomé-Carneiro, J.; Dávalos, A.; Visioli, F. Pharma-nutritional properties of olive oil phenols. Transfer of new findings to human nutrition. Foods 2018, 7, 90. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Keast, R.S.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.; Smith, A.B.; Breslin, P.A. Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef]
- Torri, L.; Bondioli, P.; Folegatti, L.; Rovellini, P.; Piochi, M.; Morini, G. Development of Perilla seed oil and extra virgin olive oil blends for nutritional, oxidative stability and consumer acceptance improvements. Food Chem. 2019, 286, 584–591. [Google Scholar] [CrossRef]
- Tuorila, H.; Recchia, A. Sensory perception and other factors affecting consumer choice of olive oil. In Olive Oil Sensory Science; Monteleone, E., Langstaff, S., Eds.; Wiley: Hoboken, NJ, USA, 2014; pp. 55–80. [Google Scholar]
- Roura, E.; Aldayyani, A.; Thavaraj, P.; Prakash, S.; Greenway, D.; Thomas, W.G.; Meyerhof, W.; Roudnitzky, N.; Foster, S.R. Variability in human bitter taste sensitivity to chemically diverse compounds can be accounted for by differential TAS2R activation. Chem. Senses 2015, 40, 427–435. [Google Scholar] [CrossRef]
- von Heldreich, T. Die Nutzpflanzen Griechenlands mit Besonderer Berücksichtigung der Neugriechischen und Pelasgischen Vulgarnamen; Karl Vilberg: Athens, Greece, 1862. [Google Scholar]
- Sordinas, A. Wild plant for subsistence on the Island of Corfu, Greece. In Proceedings of the 70th Annual Meeting of the American Anthropological Association, New York, NY, USA, 30 November 1971. [Google Scholar]
- Psaroudaki, A.; Dimitropoulakis, P.; Constantinidis, T.; Katsiotis, A.; Skaracis, G.N. Ten indigenous edible plants: Contemporary use in Eastern Crete, Greece. Cult. Agric. Food Env. 2012, 34, 172–177. [Google Scholar] [CrossRef]
- Stavridakis, K.G. Wild Edible Plants of Crete; K.G. Stavridakis: Rethymno, Greece, 2006. [Google Scholar]
- Kochilas, D. Ikaria. Lessons on Food, Life, and Longevity from the Greek Island Where Peoples Forget to Die; Rodale: New York, NY, USA, 2014. [Google Scholar]
- Rechinger, K.H. Flora Aegaea: Flora d. Inseln u. Halbinseln d. aegaeischen Meeres; Springer: Vienna, Austria, 1944. [Google Scholar]
- Strid, A. Atlas of the Aegean Flora; Botanic Garden and Botanical Museum Berlin, Freie Universität Berlin: Berlin, Germany, 2016. [Google Scholar] [CrossRef]
- Turland, N.J.; Chilton, L.; Press, J.R. Flora of the Cretan Area. Annotated Checklist & Atlas; HMSO: London, UK, 1995. [Google Scholar]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular Plants of Greece: An Annotated Checklist (and Its Supplement); Botanic Garden and Botanical Museum Berlin-Dahlem: Berlin, Germany; The Hellenic Botanical Society: Athens, Greece, 2013. [Google Scholar]
- Internationals Society of Ethnobiology. Code of Ethics. 2006. Available online: https://www.ethnobiology.net/what-we-do/core-programs/ise-ethics-program/code-of-ethics/ (accessed on 10 May 2023).
- Pieroni, A.; Sulaiman, N.; Sõukand, R. Chorta (Wild Greens) in Central Crete: The Bio-Cultural Heritage of a Hidden and Resilient Ingredient of the Mediterranean Diet. Biology 2022, 11, 673. [Google Scholar] [CrossRef]
- The World Flora Online. Available online: http://www.worldfloraonline.org/ (accessed on 18 March 2023).
- Stevens, P.F.; Angiosperm Phylogeny Website. Version 14. Available online: http://www.mobot.org/MOBOT/research/APweb/ (accessed on 18 March 2023).
- Baydoun, S.; Hani, N.; Nasser, H.; Ulian, T.; Arnold-Apostolides, N. Wild leafy vegetables: A potential source for a traditional Mediterranean food from Lebanon. Front. Sustain. Food Syst. 2023, 6, 991979. [Google Scholar] [CrossRef]
- Pieroni, A.; Sõukand, R.; Amin, H.I.M.; Zahir, H.; Kukk, T. Celebrating multi-religious co-existence in central Kurdistan: The bio-culturally diverse traditional gathering of wild greens among Yazidis, Assyrians, and Muslim Kurds. Hum. Ecol. 2018, 46, 217–227. [Google Scholar] [CrossRef]
- Abdalla, M. Wild growing plants in the cuisine of modern Assyrians in the Eastern Syrian-Turkish borderland. J. Assyrian Acad. Stu. 2004, 18, 50–58. [Google Scholar]
- Frahm, E. (Ed.) A Companion to Assyria; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Berlin, B. Ethnobiological Classification: Principles of Categorization of Plants and Animals in Traditional Societies; Princeton University Press: Princeton, NJ, USA, 2014; Volume 185. [Google Scholar]
- Dop, M.C.; Kefi, F.; Karous, O.; Verger, E.O.; Bahrini, A.; Ghrabi, Z.; El Ati, J.; Kennedy, G.; Termote, C. MEDINA Study Group. Identification and frequency of consumption of wild edible plants over a year in central Tunisia: A mixed-methods approach. Public Health Nutr. 2020, 23, 782–794. [Google Scholar] [CrossRef]
- Sulaiman, N.; Pieroni, A.; Sõukand, R.; Polesny, Z. Food behavior in emergency time: Wild plant use for human nutrition during the conflict in Syria. Foods 2022, 11, 177. [Google Scholar] [CrossRef]
- Ali-Shtayeh, M.S.; Jamous, R.M.; Al-Shafie’, J.H.; Elgharabah, W.A.; Kherfan, F.A.; Qarariah, K.H.; Khdair, I.S.; Soos, I.M.; Musleh, A.A.; Isa, B.A.; et al. Traditional knowledge of wild edible plants used in Palestine (Northern West Bank): A comparative study. J. Ethnobiol. Ethnomed. 2008, 4, 13. [Google Scholar] [CrossRef]
- Pieroni, A.; Hovsepyan, R.; Manduzai, A.K.; Sõukand, R. Wild food plants traditionally gathered in central Armenia: Archaic ingredients or future sustainable foods? Environ. Dev. Sustain. 2021, 23, 2358–2381. [Google Scholar] [CrossRef]
- Ter Braak, C.J. Canonical correspondence analysis: A new eigenvector technique for multivariate direct gradient analysis. Ecology 1986, 67, 1167–1179. [Google Scholar] [CrossRef]
- Izenman, A.J. Modern Multivariate Statistical Techniques. Regression, Classification, and Manifold Learning; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Abdi, H.; Lynne, J.W. Principal Component Analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 28 May 2023).
- Sebastien, L.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Alboukadel, K.; Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 20 May 2023).
- Yanagisawa, T.; Misaka, T. Characterization of the Human Bitter Taste Receptor Response to Sesquiterpene Lactones from Edible Asteraceae Species and Suppression of Bitterness through pH Control. ACS Omega 2021, 6, 4401–4407. [Google Scholar] [CrossRef]
- Zidorn, C. Sesquiterpene lactones and their precursors as chemosystematic markers in the tribe Cichorieae of the Asteraceae. Phytochemistry 2008, 69, 2270–2296. [Google Scholar] [CrossRef]
- Bell, L.; Oloyede, O.O.; Lignou, S.; Wagstaff, C.; Methven, L. Taste and flavor perceptions of glucosinolates, isothiocyanates, and related compounds. Mol. Nutr. Food Res. 2018, 62, 1700990. [Google Scholar] [CrossRef]
- Beauchamp, G.K. Basic Taste: A Perceptual Concept. J. Agric. Food Chem. 2019, 67, 13860–13869. [Google Scholar] [CrossRef]
- Behrens, M.; Meyerhof, W. Vertebrate bitter taste receptors: Keys for survival in changing environments. J. Agric. Food Chem. 2016, 66, 2204–2213. [Google Scholar] [CrossRef]
- Green, B.G. Chemesthesis: Pungency as a component of flavor. Trends Food Sci. Technol. 1996, 71, 415–420. [Google Scholar] [CrossRef]
- Blankenship, M.L.; Grigorova, M.; Katz, D.B.; Maier, J.X. Retronasal Odor Perception Requires Taste Cortex, but Orthonasal Does Not. Curr. Biol. 2019, 29, 62–69.e3. [Google Scholar] [CrossRef]
- Lawless, H. The sense of smell in food quality and sensory evaluation. J. Food Qual. 1991, 14, 33–60. [Google Scholar] [CrossRef]
- Shepherd, G.M. Smell images and the flavour system in the human brain. Nature 2006, 444, 316–321. [Google Scholar] [CrossRef]
- Robino, A.; Mezzavilla, M.; Pirastu, N.; Dognini, M.; Tepper, B.J.; Gasparini, P. A population-based approach to study the impact of PROP perception on food liking in populations along the Silk Road. PLoS ONE 2014, 9, e91716. [Google Scholar] [CrossRef]
- Risso, D.S.; Mezzavilla, M.; Pagani, L.; Robino, A.; Morini, G.; Tofanelli, S.; Carrai, M.; Campa, D.; Barale, R.; Caradonna, F.; et al. Global diversity in the TAS2R38 bitter taste receptor: Revisiting a classic evolutionary PROPosal. Sci. Rep. 2016, 6, 25506. [Google Scholar] [CrossRef]
- Lipchock, S.V.; Mennella, J.A.; Spielman, A.I.; Reed, D.R. Human bitter perception correlates with bitter receptor messenger RNA expression in taste cells. Am. J. Clin. Nutr. 2013, 98, 1136–1143. [Google Scholar] [CrossRef]
- Sandell, M.A.; Breslin, P.A.S. Variability in a gene determines whether we taste toxins in food. Curr. Biol. 2006, 16, 792–794. [Google Scholar] [CrossRef]
- Baranowski, T.; Baranowski, J.C.; Watson, K.B.; Jago, R.; Islam, N.; Beltran, A.; Martin, S.J.; Nguyen, N.; Tepper, B.J. 6-n-Propylthiouracil taster status not related to reported cruciferous vegetable intake among ethnically diverse children. Nutr. Res. 2011, 31, 594–600. [Google Scholar] [CrossRef]
- Bayer, S.; Mayer, A.I.; Borgonovo, G.; Morini, G.; Di Pizio, A.; Bassoli, A. Chemoinformatics View on Bitter Taste Receptor Agonists in Food. J. Agric. Food Chem. 2021, 69, 13916–13924. [Google Scholar] [CrossRef]
- Sherman, P.W.; Billing, J. Darwinian gastronomy: Why we use spices: Spices taste good because they are good for us. BioScience 1999, 49, 453–463. [Google Scholar] [CrossRef]
- Nilius, B.; Appendino, G. Tasty and healthy TR (i) Ps: The human quest for culinary pungency. EMBO Rep. 2011, 12, 1094–1101. [Google Scholar] [CrossRef]
- Vitaglione, P.; Savarese, M.; Paduano, A.; Scalfi, L.; Fogliano, V.; Sacchi, R. Healthy virgin olive oil: A matter of bitterness. Crit. Rev. Food Sci. Nutr. 2015, 55, 1808–1818. [Google Scholar] [CrossRef] [PubMed]
- Dragoș, D.; Petran, M.; Gradinaru, T.-C.; Gilca, M. Phytochemicals and Inflammation: Is Bitter Better? Plants 2022, 11, 2991. [Google Scholar] [CrossRef] [PubMed]
- Morini, G.; Luiselli, D.; Risso, D.S.; Tofanelli, S. Il gusto degli Italiani. In La Cultura del Cibo; UTET Grandi Opere: Torino, Italy, 2015; pp. 365–375. [Google Scholar]
- Saleh, H.M. The Civilization of Egypt in the Shadow of Shiite Islam; Dar Al-Juman: Beirut, Lebanon, 2003. [Google Scholar]
- Walker, B. This Kind Only Comes Out by Prayer (and Fasting): Fasting, Ritual Efficacy and Magical Thinking in Early Christianity. J. Ritual Stud. 2017, 31, 43–52. [Google Scholar]
- Mentzer, R.A. Fasting, Piety, and Political Anxiety among French Reformed Protestants. Church Hist. 2007, 76, 330–362. [Google Scholar] [CrossRef]
- Musurillo, H. The Problem of Ascetical Fasting in the Greek Patristic Writers. Traditio 1956, 12, 1–64. [Google Scholar] [CrossRef]
- Arbesmann, R. Fasting and Prophecy in Pagan and Christian Antiquity. Traditio 1951, 7, 1–71. [Google Scholar] [CrossRef]
- Barnett, E. Reforming Food and Eating in Protestant England, c. 1560–c. 1640. Hist. J. 2020, 63, 507–527. [Google Scholar] [CrossRef]
- Watts, S. Enlightened Fasting: Religious Conviction, Scientific Inquiry, and Medical Knowledge in Early Modern France. In Food and Faith in Christian Culture; Albala, K., Eden, T., Eds.; Columbia University Press: New York, NY, USA, 2011; pp. 105–124. [Google Scholar]
- Dumanowski, J. Old Polish Fasting: Discourse and Dietary Practices in the 16th–18th Century. In Gruppenidentitäten in Ostmitteleuropa; Dybaś, B., Bojarski, J., Eds.; V & R Unipress: Göttingen, Germany, 2021; pp. 93–116. [Google Scholar]
- Klymenko, I. The Fasting of Others: Constructing Interreligious Boundaries Through Bodily Practices in Christian Discourses Around 1600. Hist. Stud. Cent. Eur. 2022, 2, 60–75. [Google Scholar] [CrossRef]
- Heyberger, B. Fasting: The Limits of Catholic Confessionalization in Eastern Christianity in the Eighteenth Century, in Scholarship between Europe and the Levant; Loop, J., Kraye, J., Eds.; Brill: Leiden, The Netherlands, 2020; pp. 217–235. [Google Scholar] [CrossRef]
- Ivanov, A.V. A Spiritual Revolution: The Impact of Reformation and Enlightenment in Orthodox Russia, 1700–1825; University of Wisconsin Press: Madison, WI, USA, 2020. [Google Scholar]
- Sarri, K.; Linardakis, M.; Bervanaki, F.; Tzanakis, N.; Kafatos, A. Greek Orthodox fasting rituals: A hidden characteristic of the Mediterranean diet of Crete. Br. J. Nutr. 2004, 92, 277–284. [Google Scholar] [CrossRef]
- Pfeilstetter, R. Heritage entrepreneurship. Agency-driven promotion of the Mediterranean diet in Spain. Int. J. Herit. Stud. 2015, 21, 215–231. [Google Scholar] [CrossRef]
- Legrand, R.; Nuemi, G.; Poulain, M.; Manckoundia, P. Description of Lifestyle, Including Social Life, Diet and Physical Activity, of People ≥90 years Living in Ikaria, a Longevity Blue Zone. Int. J. Environ. Res. Public Health 2021, 18, 6602. [Google Scholar] [CrossRef]
- Zouras, E. Belonging as a concept in placemaking: Exploring perceptions in Ikaria, Greece: A study of belonging in the elderly in the Greek Blue Zone of Ikaria. Master’s Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2020. Available online: https://www.diva-portal.org/smash/get/diva2:1592906/FULLTEXT01.pdf (accessed on 28 May 2023).
- Hartmann, C.; Dohle, S.; Siegrist, M. Importance of cooking skills for balanced food choices. Appetite 2013, 65, 125–131. [Google Scholar] [CrossRef]
- Goldfield, H. The Sweet Rewards of Bitter Food. The New York Times Magazine, 22 November 2017. Available online: https://www.nytimes.com/2017/11/22/t-magazine/bitter-food.html (accessed on 12 June 2023).
- Caremoli, F.; Bassoli, A.; Borgonovo, G.; Morini, G.; Torri, L. Do you like it bitter? A preliminary study on food preferences for bitter taste in a young population. In World Food Trends and the Future of Food; Nobile, M., Ed.; Ledizioni: Milano, Italy, 2015; pp. 75–94. [Google Scholar]
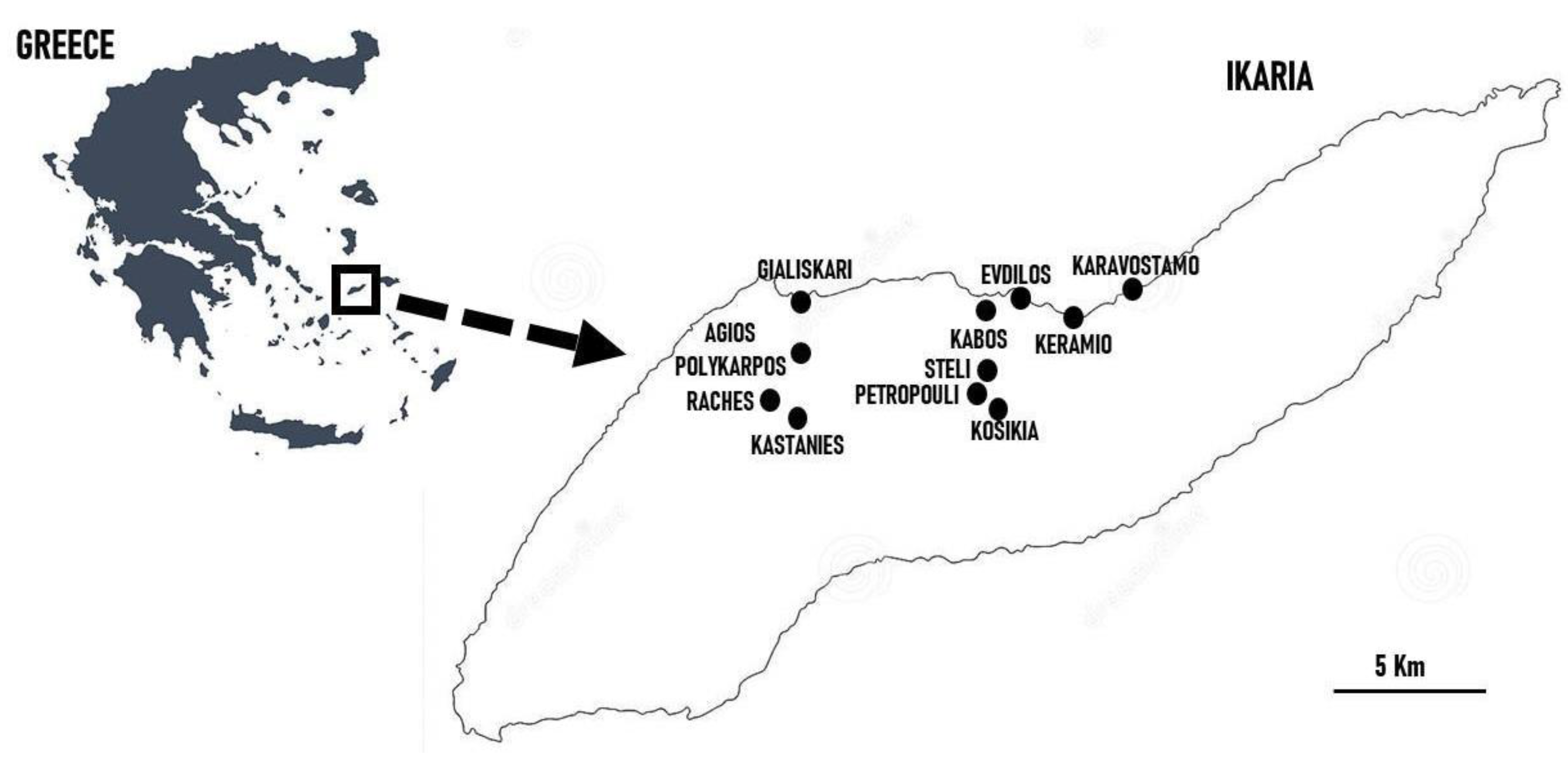


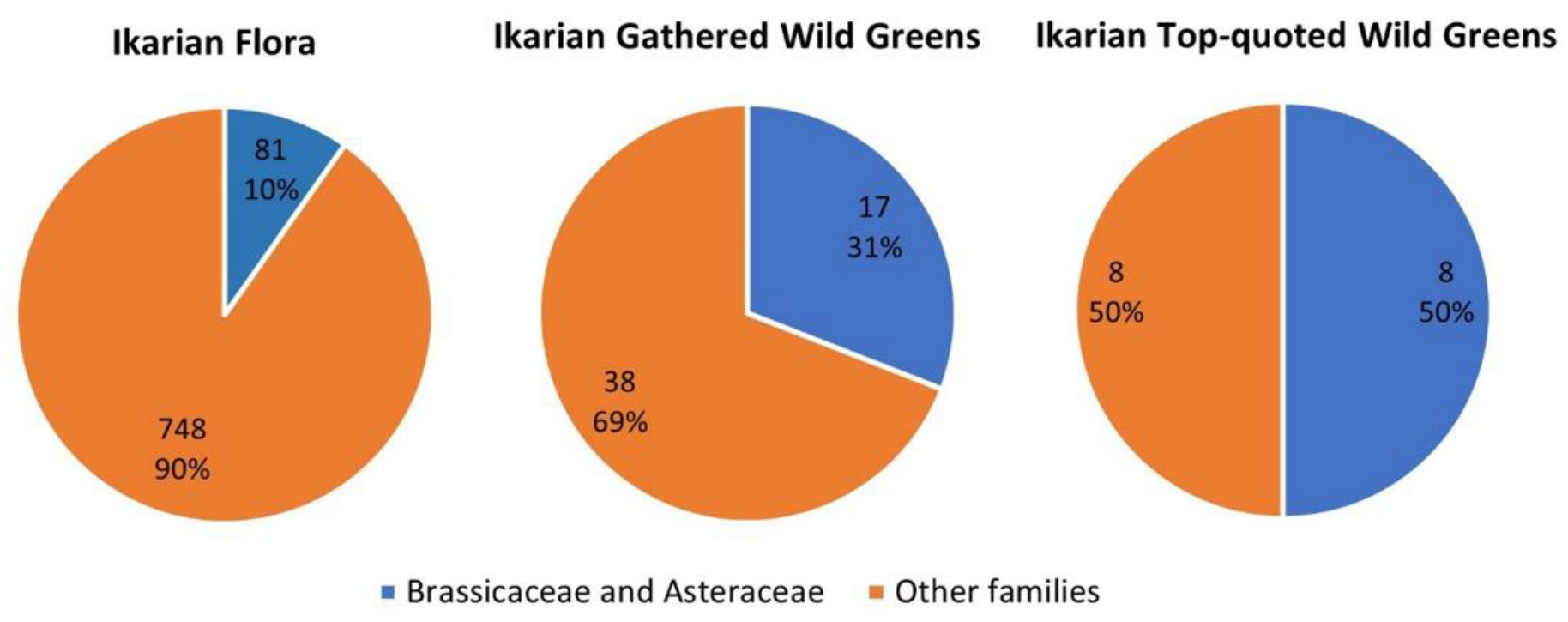
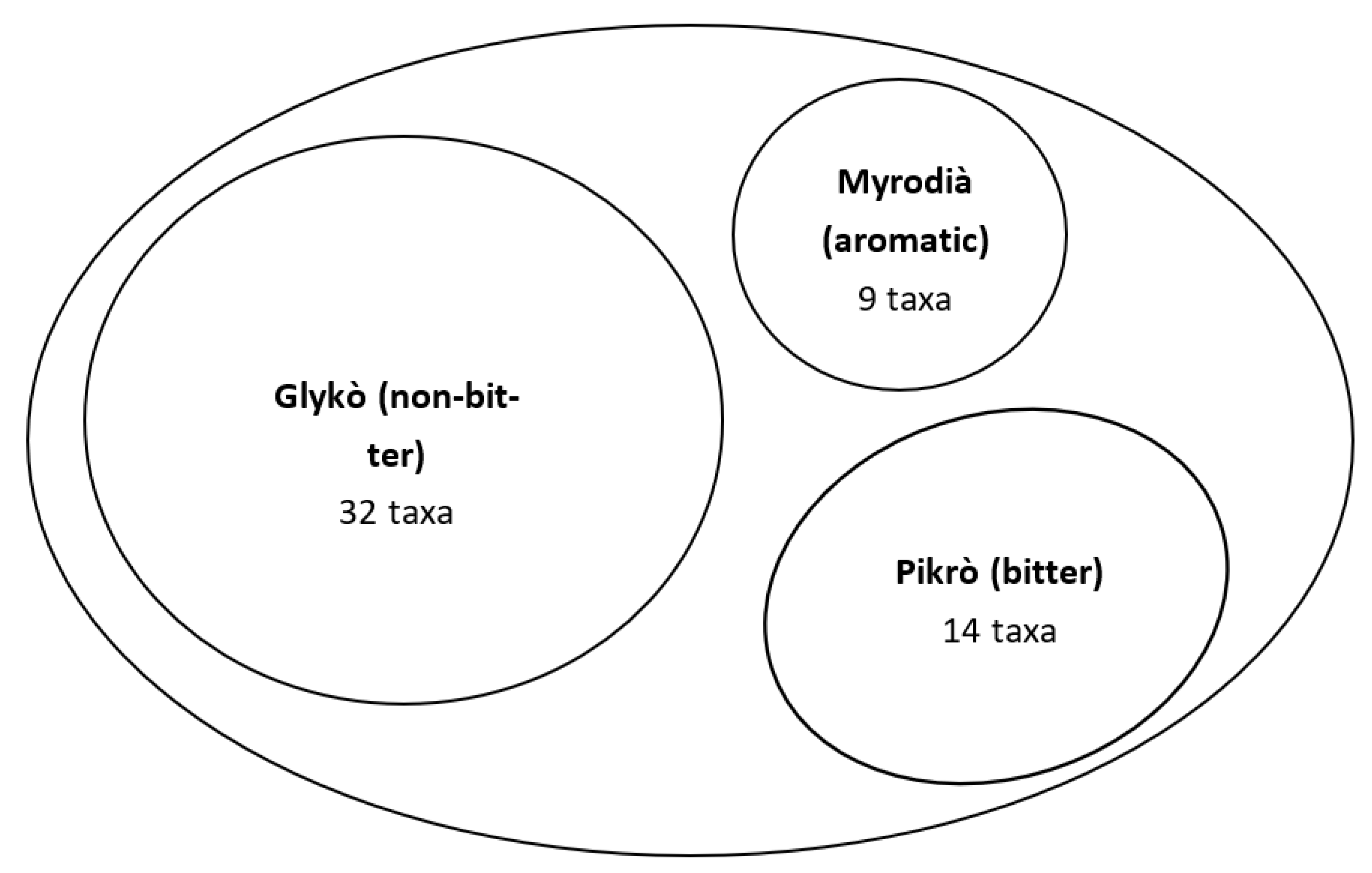



| Site Code | Area and Country | Main Ethnicity of the Considered Sample | Religion of the Considered Sample | Reference |
|---|---|---|---|---|
| IKA | Central Ikaria, Greece | Greeks | Orthodox Christians | (Present study) |
| CRE | Central Crete, Greece | Greeks | Orthodox Christians | [58] |
| SIC | (entire) Sicily, Italy | Italians | Catholic Christians | [29] |
| TUN | Sidi Bouzid area, Tunisia | Arabs | Sunni Muslims | [66] |
| SYR | Tartus area, Syria | Arabs | (mainly) Alawite Muslims | [67] |
| LEB | (entire) Lebanon | Arabs | (Various denominations of) Christians and Muslims | [61] |
| PAL | Northern West Bank, Palestine | Arabs | (mainly) Sunni Muslims | [68] |
| ASS | Syrian-Turkish borderland and NW Kurdistan region, Northern Iraq | Assyrians | Eastern Christians | [62,63] |
| KUR | Kurdistan region, Northern Iraq | Kurds | Sunni Muslims | [62] |
| ARM | Central Armenia | Armenians | Orthodox Christians | [69] |
| Botanical Taxon or Taxa; Botanical Family (Botanical Voucher Specimen Code) | Recorded Local Name(s) (in Singular or Plural) | Used Parts | Local Sensory Classification | Local Culinary Uses | Quotation Index |
|---|---|---|---|---|---|
| Alcea rosea L.; Malvaceae (IK17) | Molocha | Leaves | Gly | Boiled | R |
| Allium ampeloprasum L.; Amaryllidaceae (IK28) | Agriopraso | Whole plant | Myr | Pies cooked with rice | C |
| Allium vineale L.; Amaryllidaceae | Agrioskordo | Whole plant | Myr | Seasoning | R |
| Amaranthus blitum L. and possibly other Amaranthus species; Amaranthaceae | Vlito | Leaves | Gly | Boiled, stewed with tomatoes | VC |
| Anchusa hybrida Ten.; Boraginaceae (IK36) | Shipita | Young aerial parts | Gly | Salads, boiled | C |
| Asparagus acutifolius L.; Asparagaceae (IK27) | Agriasfaragia | Shoots | Pik | Cooked with eggs | C |
| Calendula arvensis L.; Asteraceae (IK01) | Not recorded | Leaves | Gly | Boiled | R |
| Carduus pycnocephalus L.; Asteraceae (IK10) | Gaidoroga, Agathi | Young stems | Pik | Boiled | C |
| Crithmum maritimum L.; Apiaceae (IK29) | Kritamo | Young aerial part | Gly | Pickled, salads | C |
| Cichorium spinosum L.; Asteraceae | Stamnagathi | Rosettes | Pik | Salads, boiled | C |
| Cirsium vulgare (Savi) Ten.; Asteraceae (IK11) | Gaidoraga, Agathi | Young leaves | Pik | Boiled | C |
| Colocasia esculenta (L.) Schott; Araceae * | Glikopatates | Young leaves | Gly | Diverse wrapping material in cooking | R |
| Crepis fraasii Sch.Bip. (IK33), C. sancta (L.) Bornm. (IK15), and possibly other Crepis spp.; Asteraceae | Radiki | Rosettes | Pik | Boiled | VC |
| Cucurbita maxima Duchesne; Cucurbitaceae * | Kolokithi | Shoots | Gly | Boiled | R |
| Cynara cardunculus subsp. cardunculus; Asteraceae | Agriaginara | Flower receptacles | Gly | Salads, boiled | C |
| Daucus carota L.; Apiaceae | Agriokaroto | Young aerial parts | Myr | Pies | C |
| Dioscorea communis (L.) Caddick and Wilkin; Dioscoreaceae | Ovries | Shoots | Pik | Cooked with eggs | C |
| Echium plantagineum L.; Boraginaceae | Voidoglossa | Leaves | Gly | Boiled | R |
| Eruca vesicaria (L.) Cav.; Brassicaceae (IK05) | Roka | Young aerial parts | Pik | Salads | C |
| Ficus carica L.; Moraceae | Agriasikia | Unripe fruits | Gly | Preserves | C |
| Foeniculum vulgare Mill.; Apiaceae (IK22) | Maratho | Young aerial parts | Myr | Boiled and pies, dolmade filling | VC |
| Galium rotundifolium L.; Rubiaceae (IK26) | Kolitzida | aerial parts | Gly | Boiled | R |
| Hyoseris scabra L.; Asteraceae (IK07) | Radiki | Rosettes | Pik | Boiled | VC |
| Hypochaeris glabra L.; Asteraceae (IK32) | Glikosiridi, Radiki | Rosettes | Gly | Boiled | C |
| Ipomoea batatas (L.) Lam.; Convolvulaceae * | Glikopatates | Shoots | Gly | Boiled | R |
| Lapsana communis L.; Asteraceae (IK02) | Petrouchia, Petrukki | Rosettes | Gly | Boiled and pies | C |
| Leontodon tuberosus L.; Asteraceae (IK14, IK25) | Radiki, Pikrosiridia | Rosettes | Pik | Boiled | VC |
| Lupinus albus L.; Fabaceae (IK30) | Loumbina | Shoots | Gly | Salads | R |
| Malva neglecta Wallr. (IK18), and M. parviflora (IK37); Malvaceae | Molocha | Leaves | Gly | Boiled | R |
| Mentha longifolia (L.) L.; Lamiaceae | Diosmos, Iosmos | Leaves | Myr | Seasoning | C |
| Muscari comosum (L.) Mill.; Asparagaceae | Vorvos | Bulbs | Pik | Boiled and pickled | VC |
| -- | Aerial parts | Gly | Boiled | R | |
| Origanum onites L.; Lamiaceae (IK31) | Rigani | Flowering tops | Myr | Seasoning | VC |
| Papaver rhoeas L.; Papaveraceae | Paparouna | Rosettes | Gly | Boiled and pies | VC |
| Portulaca oleracea L.; Portulacaceae | Glistrida | Aerial parts | Gly | Salads | VC |
| Raphanus raphanistrum L.; Brassicaceae (IK06) | Vrouves | Young aerial parts | Pik | Boiled and pies | VC |
| Reichardia picroides (L.) Roth; Asteraceae (IK21) | Galatsida | Rosettes | Gly | Boiled and pies | VC |
| Rumex conglomeratus Murray; Polygonaceae (IK20) | Lahanes | Leaves | Gly | Boiled, cooked with eggs, pies, dolmades | C |
| Rumex pulcher L.; Polygonaceae (IK35) | Lapatho | Leaves | Gly | Boiled | R |
| Satureja thymbra L.; Lamiaceae | Thimbra | Aerial parts | Myr | Seasoning | R |
| Scolymus hispanicus L.; Asteraceae | Askolimbros | Young aerial parts | Gly | Boiled | C |
| Silene vulgaris (Moench) Garcke; Caryophyllaceae (IK19) | Strifulia | Young aerial parts | Gly | Pies | C |
| Sinapis alba L.; Brassicaceae (IK13) | Vrouves | Young aerial parts | Pik | Boiled and pies | VC |
| Solanum nigrum L.; Solanaceae (IK09) | Stifno | Leaves | Pik | Boiled | C |
| fruits | Fruits | Gly | Chutney | R | |
| Sonchus oleraceus L. (IK12), and possibly other Sonchus spp.; Asteraceae | Tzochos | Young aerial parts | Gly | Boiled and pies | VC |
| Taraxacum minimum (V.Brig.) N.Terracc. (IK16), and possibly other Taraxacum spp.; Asteraceae | Radiki, Pikrosiridia | Rosettes | Pik | Boiled | VC |
| Tordylium apulum L.; Apiaceae | Kaftkalithra | Rosettes | Myr | Salads, boiled | VC |
| Scandix pecten-veneris L.; Apiaceae (IK34) | Kaftkalithra | Rosettes | Myr | Salads, boiled | VC |
| Tragopogon porrifolius L.; Asteraceae | Tragopogon | Young aerial parts | Gly | Pies | R |
| Urospermum picroides (L.) Scop. ex F.W.Schmidt; Asteraceae | Agnosiridio, Glikochorto | Rosettes | Gly | Boiled | C |
| Urtica membranacea Poir. ex Savigny; Urticaceae (IK23) | Tzouknides | Young aerial parts | Gly | Boiled and pies | C |
| Vicia faba L.; Fabaceae * | Kukkia | Shoots | Gly | Boiled | R |
| Vitis vinifera L.; Vitaceae * | Ampeli | Shoots | Gly | Boiled | R |
| Unidentified taxon | Drosseridia | Rosettes | Gly | Boiled | R |
| Wild Greens | Sensory Classification | IKA | CRE | LEB | SYR | TUN | SIC | ARM | PAL | ASS | KUR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcea | nb | 1 | 1 | ||||||||
| Allium | ar | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Amaranthus | b | 1 | 1 | 1 | |||||||
| Anchusa | nb | 1 | 1 | 1 | |||||||
| Anthriscus | ar | 1 | 1 | ||||||||
| Apium | ar | 1 | 1 | ||||||||
| Arum | nb | 1 | 1 | 1 | |||||||
| Asparagus | b | 1 | 1 | 1 | |||||||
| Asphodelus | nb | 1 | |||||||||
| Atriplex | nb | 1 | 1 | ||||||||
| Beta | nb | 1 | |||||||||
| Brassica | b | 1 | 1 | ||||||||
| Capparis | b | 1 | 1 | ||||||||
| Capsella | b | 1 | |||||||||
| Centaurea | b | 1 | 1 | 1 | |||||||
| Chaerophyllum | nb | 1 | |||||||||
| Chenopodium | nb | 1 | 1 | ||||||||
| Chondrilla | b | 1 | |||||||||
| Cichorium | b | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Cirsium | b | 1 | |||||||||
| Crepis | b | 1 | 1 | ||||||||
| Cyclamen | nb | 1 | 1 | ||||||||
| Cynara | b | 1 | 1 | 1 | |||||||
| Daucus | ar | 1 | |||||||||
| Diplotaxis | b | 1 | 1 | ||||||||
| Eremurus | nb | 1 | |||||||||
| Eruca | b | 1 | 1 | ||||||||
| Eryngium | b | 1 | 1 | ||||||||
| Falcaria | ar | 1 | |||||||||
| Foeniculum | ar | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Glebionis | b | 1 | |||||||||
| Gundelia | b | 1 | 1 | 1 | 1 | ||||||
| Helminthotheca | b | 1 | |||||||||
| Heracleum | ar | 1 | |||||||||
| Hirschfeldia | b | 1 | 1 | ||||||||
| Hyoseris | nb | 1 | |||||||||
| Hypochoeris | b | 1 | 1 | ||||||||
| Lactuca | b | 1 | 1 | 1 | 1 | ||||||
| Lapsana | nb | 1 | |||||||||
| Lathyrus | nb | 1 | |||||||||
| Launea | b | 1 | |||||||||
| Leontodon | b | 1 | 1 | 1 | |||||||
| Leopoldia | b | 1 | 1 | ||||||||
| Lepidium | b | 1 | 1 | ||||||||
| Malva | nb | 1 | 1 | 1 | 1 | 1 | |||||
| Mentha | ar | 1 | 1 | 1 | 1 | ||||||
| Micromeria | ar | 1 | |||||||||
| Notobasis | b | 1 | 1 | ||||||||
| Oenanthe | ar | 1 | |||||||||
| Onopordum | b | 1 | 1 | 1 | |||||||
| Origanum | ar | 1 | 1 | 1 | |||||||
| Ornithogalum | nb | 1 | |||||||||
| Papaver | nb | 1 | 1 | 1 | 1 | 1 | |||||
| Plantago | nb | 1 | 1 | ||||||||
| Polygonum | nb | 1 | |||||||||
| Portulaca | nb | 1 | 1 | 1 | |||||||
| Prasium | ar | 1 | |||||||||
| Pseudospermum | b | 1 | |||||||||
| Raphanus | b | 1 | 1 | 1 | |||||||
| Reichardia | nb | 1 | 1 | 1 | |||||||
| Rhagadiolus | b | 1 | |||||||||
| Rhamphospermum ? | nb | 1 | |||||||||
| Rheum | nb | 1 | |||||||||
| Rorippa | b | 1 | 1 | 1 | 1 | ||||||
| Rumex | nb | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Ruscus | b | 1 | |||||||||
| Salvia | ar | 1 | |||||||||
| Scandix | ar | 1 | 1 | 1 | |||||||
| Scolymus | b | 1 | 1 | 1 | |||||||
| Scorzonera | nb | 1 | 1 | ||||||||
| Silene | nb | 1 | |||||||||
| Silybum | b | 1 | 1 | 1 | 1 | ||||||
| Sinapis | b | 1 | 1 | 1 | 1 | 1 | |||||
| Solanum | b | 1 | 1 | ||||||||
| Sonchus | b | 1 | 1 | 1 | 1 | ||||||
| Taraxacum | b | 1 | |||||||||
| Thymus | ar | 1 | 1 | ||||||||
| Tordylium | ar | 1 | 1 | ||||||||
| Trifolium | nb | 1 | 1 | ||||||||
| Tragopogon | nb | 1 | 1 | 1 | |||||||
| Urospermum | b | 1 | 1 | ||||||||
| Urtica | nb | 1 | 1 | 1 | |||||||
| Veronica | nb | 1 | |||||||||
| Vicia | nb | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieroni, A.; Morini, G.; Piochi, M.; Sulaiman, N.; Kalle, R.; Haq, S.M.; Devecchi, A.; Franceschini, C.; Zocchi, D.M.; Migliavada, R.; et al. Bitter Is Better: Wild Greens Used in the Blue Zone of Ikaria, Greece. Nutrients 2023, 15, 3242. https://doi.org/10.3390/nu15143242
Pieroni A, Morini G, Piochi M, Sulaiman N, Kalle R, Haq SM, Devecchi A, Franceschini C, Zocchi DM, Migliavada R, et al. Bitter Is Better: Wild Greens Used in the Blue Zone of Ikaria, Greece. Nutrients. 2023; 15(14):3242. https://doi.org/10.3390/nu15143242
Chicago/Turabian StylePieroni, Andrea, Gabriella Morini, Maria Piochi, Naji Sulaiman, Raivo Kalle, Shiekh Marifatul Haq, Andrea Devecchi, Cinzia Franceschini, Dauro M. Zocchi, Riccardo Migliavada, and et al. 2023. "Bitter Is Better: Wild Greens Used in the Blue Zone of Ikaria, Greece" Nutrients 15, no. 14: 3242. https://doi.org/10.3390/nu15143242
APA StylePieroni, A., Morini, G., Piochi, M., Sulaiman, N., Kalle, R., Haq, S. M., Devecchi, A., Franceschini, C., Zocchi, D. M., Migliavada, R., Prakofjewa, J., Sartori, M., Krigas, N., Ahmad, M., Torri, L., & Sõukand, R. (2023). Bitter Is Better: Wild Greens Used in the Blue Zone of Ikaria, Greece. Nutrients, 15(14), 3242. https://doi.org/10.3390/nu15143242















