Adherence to a Mediterranean Diet, Body Composition and Energy Expenditure in Outpatients Adolescents Diagnosed with Anorexia Nervosa: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Dietary Intervention
2.3. Dietary and MD Adherence Assessment
2.4. Anthropometrics
2.5. Body Composition
2.6. Indirect Calorimetry
2.7. Handgrip Strength
2.8. Statistical Analysis
3. Results
3.1. Subjects
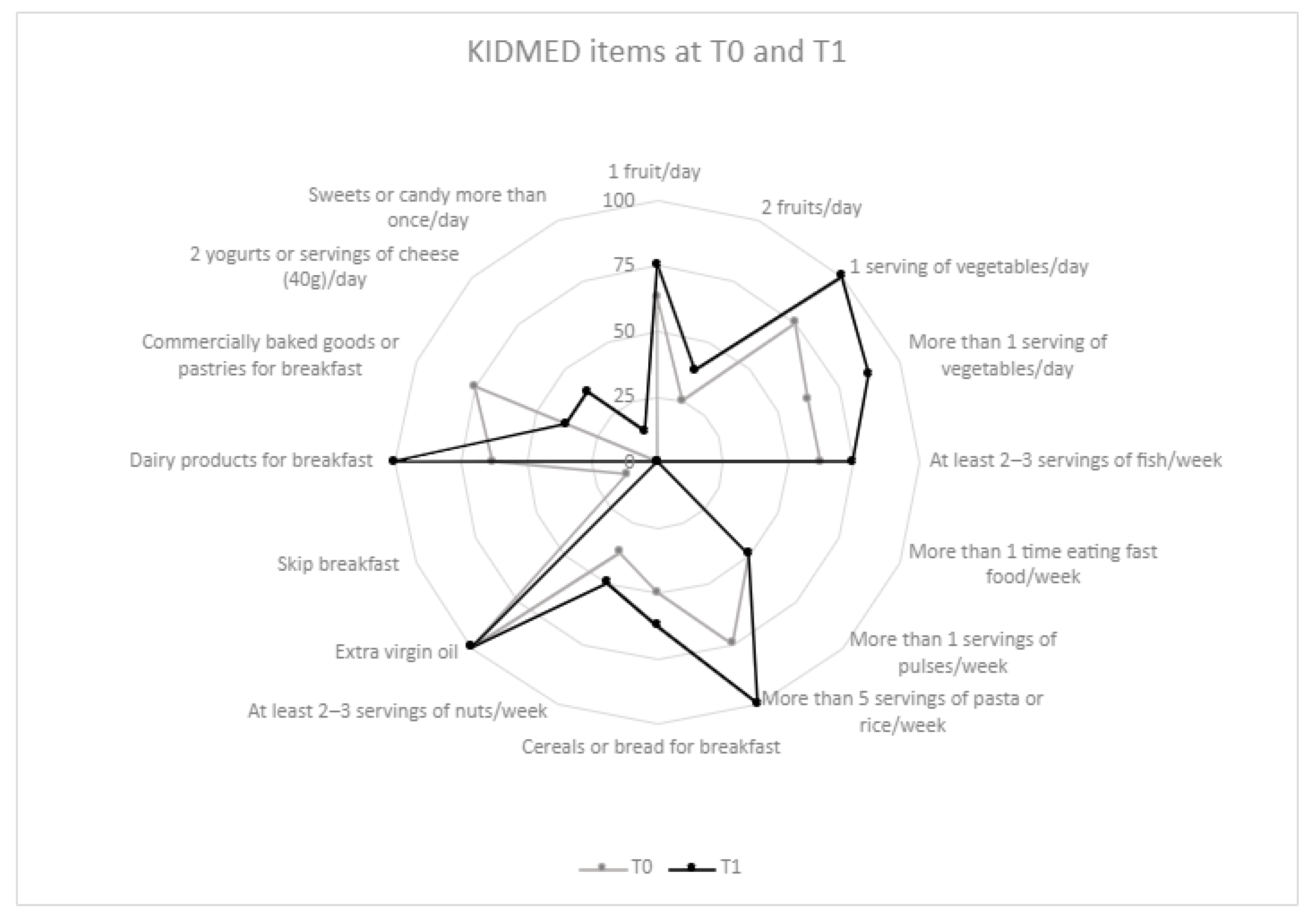
3.2. Dietary Components
3.3. Anthropometry and Body Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Eeden, A.E.; van Hoeken, D.; Hoek, H.W. Incidence, Prevalence and Mortality of Anorexia Nervosa and Bulimia Nervosa. Curr. Opin. Psychiatry 2021, 34, 515. [Google Scholar] [CrossRef]
- Kerruish, K.P.; O’Connor, J.; Humphries, I.R.J.; Kohn, M.R.; Clarke, S.D.; Briody, J.N.; Thomson, E.J.; Wright, K.A.; Gaskin, K.J.; Baur, L.A. Body Composition in Adolescents with Anorexia Nervosa. Am. J. Clin. Nutr. 2002, 75, 31–37. [Google Scholar] [CrossRef] [PubMed]
- NICE. NICE Guideline 69. Eating Disorders: Recognition and Treatment; NICE: London, UK, 2017. [Google Scholar]
- CREA; Centro di Ricerca Alimenti e Nutrizione. Linee Guida per Una Sana Alimentazione—Revisione 2018; CREA: Roma, Italy, 2019. [Google Scholar]
- Hubel, C.; Yilmaz, Z.; Schaumberg, K.E.; Breithaupt, L.; Hunjan, A.; Horne, E.; Garcia-Gonzalez, J.; O’Reilly, P.F.; Bulik, C.M.; Breen, G. Body Composition in Anorexia Nervosa: Meta-Analysis and Meta-Regression of Cross-Sectional and Longitudinal Studies. Int. J. Eat. Disord. 2019, 52, 1205–1223. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, E.; Ciccarese, F.; Di Pietro, E.; Facchini, G.; Moscano, F.; Iero, L.; Monaldi, A.; Battista, G.; Bazzocchi, A. Follow-up of Bone Mineral Density and Body Composition in Adolescents with Restrictive Anorexia Nervosa: Role of Dual-Energy X-ray Absorptiometry. Eur. J. Clin. Nutr. 2014, 68, 247–252. [Google Scholar] [CrossRef] [PubMed]
- El Ghoch, M.; Calugi, S.; Lamburghini, S.; Dalle Grave, R. Anorexia Nervosa and Body Fat Distribution: A Systematic Review. Nutrients 2014, 6, 3895–3912. [Google Scholar] [CrossRef]
- El Ghoch, M.; Milanese, C.; Calugi, S.; Müller, M.J.; Pourhassan, M.; Ruocco, A.; Dalle Grave, R. Regional Fat Distribution in Adolescent and Adult Females with Anorexia Nervosa: A Longitudinal Study. Clin. Nutr. 2015, 34, 1224–1232. [Google Scholar] [CrossRef]
- Ilyas, A.; Hübel, C.; Stahl, D.; Stadler, M.; Ismail, K.; Breen, G.; Treasure, J.; Kan, C. The Metabolic Underpinning of Eating Disorders: A Systematic Review and Meta-Analysis of Insulin Sensitivity. Mol. Cell. Endocrinol. 2019, 497, 110307. [Google Scholar] [CrossRef]
- Prioletta, A.; Muscogiuri, G.; Sorice, G.P.; Lassandro, A.P.; Mezza, T.; Policola, C.; Salomone, E.; Cipolla, C.; Della Casa, S.; Pontecorvi, A.; et al. In Anorexia Nervosa, Even a Small Increase in Abdominal Fat Is Responsible for the Appearance of Insulin Resistance. Clin. Endocrinol. 2011, 75, 202–206. [Google Scholar] [CrossRef]
- Karountzos, V.; Lambrinoudaki, I.; Tsitsika, A.; Deligeoroglou, E. The Role of Total Body Fat Mass and Trunk Fat Mass, Combined with Other Endocrine Factors, in Menstrual Recovery and Psychopathology of Adolescents with Anorexia Nervosa. Gynecol. Endocrinol. 2017, 33, 757–762. [Google Scholar] [CrossRef]
- Eddy, K.T.; Dorer, D.J.; Franko, D.L.; Tahilani, K.; Thompson-Brenner, H.; Herzog, D.B. Diagnostic Crossover in Anorexia Nervosa and Bulimia Nervosa: Implications for DSM-V. Am. J. Psychiatry 2008, 165, 245–250. [Google Scholar] [CrossRef]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean Diet Pyramid: A Cultural Model for Healthy Eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef]
- Di Daniele, N.; Petramala, L.; Di Renzo, L.; Sarlo, F.; Della Rocca, D.G.; Rizzo, M.; Fondacaro, V.; Iacopino, L.; Pepine, C.J.; De Lorenzo, A. Body Composition Changes and Cardiometabolic Benefits of a Balanced Italian Mediterranean Diet in Obese Patients with Metabolic Syndrome. Acta Diabetol. 2013, 50, 409–416. [Google Scholar] [CrossRef]
- Di Daniele, N.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A. Impact of Mediterranean Diet on Metabolic Syndrome, Cancer and Longevity. Oncotarget 2016, 8, 8947–8979. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; De Lorenzo, A. Diet, Nutrition and Chronic Degenerative Diseases. Nutrients 2021, 13, 1372. [Google Scholar] [CrossRef]
- Funtikova, A.N.; Benítez-Arciniega, A.A.; Gomez, S.F.; Fitó, M.; Elosua, R.; Schröder, H. Mediterranean Diet Impact on Changes in Abdominal Fat and 10-Year Incidence of Abdominal Obesity in a Spanish Population. Br. J. Nutr. 2014, 111, 1481–1487. [Google Scholar] [CrossRef]
- Bertoli, S.; Leone, A.; Vignati, L.; Bedogni, G.; Martínez-González, M.Á.; Bes-Rastrollo, M.; Spadafranca, A.; Vanzulli, A.; Battezzati, A. Adherence to the Mediterranean Diet Is Inversely Associated with Visceral Abdominal Tissue in Caucasian Subjects. Clin. Nutr. 2015, 34, 1266–1272. [Google Scholar] [CrossRef]
- Romaguera, D.; Norat, T.; Mouw, T.; May, A.M.; Bamia, C.; Slimani, N.; Travier, N.; Besson, H.; Luan, J.; Wareham, N.; et al. Adherence to the Mediterranean Diet Is Associated with Lower Abdominal Adiposity in European Men and Women. J. Nutr. 2009, 139, 1728–1737. [Google Scholar] [CrossRef]
- Leone, A.; Martínez-González, M.Á.; Lahortiga-Ramos, F.; Molero Santos, P.; Bertoli, S.; Battezzati, A.; Bes-Rastrollo, M. Adherence to the Mediterranean Dietary Pattern and Incidence of Anorexia and Bulimia Nervosa in Women: The SUN Cohort. Nutrition 2018, 54, 19–25. [Google Scholar] [CrossRef]
- Ministero della Salute. Linee di Indirizzo Nazionali per la Riabilitazione Nutrizionale Nei Disturbi dell’Alimentazione; Ministero della Salut: Rome, Italy, 2017.
- Società Italiana di Nutrizione Umana (SINU). LARN—Livelli di Assunzione di Riferimento per la Popolazione Italiana: ENERGIA. Fabbisogno Energetico Medio (AR) Nell’intervallo D’età 1–17 Anni. Available online: https://sinu.it/2019/07/09/fabbisogno-energetico-medio-ar-nellintervallo-deta-1-17-anni/ (accessed on 2 June 2021).
- Buscemi, S.; Rosafio, G.; Vasto, S.; Massenti, F.M.; Grosso, G.; Galvano, F.; Rini, N.; Barile, A.M.; Maniaci, V.; Cosentino, L.; et al. Validation of a Food Frequency Questionnaire for Use in Italian Adults Living in Sicily. Int. J. Food Sci. Nutr. 2015, 66, 426–438. [Google Scholar] [CrossRef]
- Atlante Fotografico delle Porzioni degli Alimenti per Adulti. Available online: https://www.scottibassani.it/atlante-fotografico-delle-porzioni-degli-alimenti/ (accessed on 15 June 2023).
- Serra-Majem, L.; Ribas, L.; Ngo, J.; Ortega, R.M.; García, A.; Pérez-Rodrigo, C.; Aranceta, J. Food, Youth and the Mediterranean Diet in Spain. Development of KIDMED, Mediterranean Diet Quality Index in Children and Adolescents. Public Health Nutr. 2004, 7, 931–935. [Google Scholar] [CrossRef]
- WHO. WHO Anthro Survey Analyser and Other Tools. Available online: https://www.who.int/tools/child-growth-standards/software (accessed on 15 June 2023).
- Cole, T.J.; Flegal, K.M.; Nicholls, D.; Jackson, A.A. Body Mass Index Cut Offs to Define Thinness in Children and Adolescents: International Survey. BMJ 2007, 335, 194. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Lobstein, T. Extended International (IOTF) Body Mass Index Cut-Offs for Thinness, Overweight and Obesity. Pediatr. Obes. 2012, 7, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.R.; Moore, R.H.; Leonard, M.B.; Zemel, B.S. Fat and Lean BMI Reference Curves in Children and Adolescents and Their Utility in Identifying Excess Adiposity Compared with BMI and Percentage Body Fat1234. Am. J. Clin. Nutr. 2013, 98, 49–56. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, A.; Di Renzo, L.; Morini, P.; de Miranda, R.C.; Romano, L.; Colica, C. New Equations to Estimate Resting Energy Expenditure in Obese Adults from Body Composition. Acta Diabetol. 2018, 55, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Weir, J.B.D.B. New Methods for Calculating Metabolic Rate with Special Reference to Protein Metabolism. J. Physiol. 1949, 109, 1–9. [Google Scholar] [CrossRef]
- Pizzigalli, L.; Micheletti Cremasco, M.; LA Torre, A.; Rainoldi, A.; Benis, R. Hand Grip Strength and Anthropometric Characteristics in Italian Female National Basketball Teams. J. Sports Med. Phys. Fit. 2017, 57, 521–528. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Romano, L.; Marrone, G.; Noce, A.; Pujia, A.; Perrone, M.A.; Aiello, V.; Colica, C.; De Lorenzo, A. Role of Personalized Nutrition in Chronic-Degenerative Diseases. Nutrients 2019, 11, 1707. [Google Scholar] [CrossRef]
- Gouveri, E.T.; Tzavara, C.; Drakopanagiotakis, F.; Tsaoussoglou, M.; Marakomichelakis, G.E.; Tountas, Y.; Diamantopoulos, E.J. Mediterranean Diet and Metabolic Syndrome in an Urban Population: The Athens Study. Nutr. Clin. Pract. 2011, 26, 598–606. [Google Scholar] [CrossRef]
- Misra, M.; Soyka, L.A.; Miller, K.K.; Grinspoon, S.; Levitsky, L.L.; Klibanski, A. Regional Body Composition in Adolescents with Anorexia Nervosa and Changes with Weight Recovery. Am. J. Clin. Nutr. 2003, 77, 1361–1367. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical Impedance Analysis—Part I: Review of Principles and Methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Rosa-Caldwell, M.E.; Eddy, K.T.; Rutkove, S.B.; Breithaupt, L. Anorexia Nervosa and Muscle Health: A Systematic Review of Our Current Understanding and Future Recommendations for Study. Int. J. Eat. Disord. 2023, 56, 483–500. [Google Scholar] [CrossRef]
- Bou Khalil, R.; Sultan, A.; Seneque, M.; Richa, S.; Lefebvre, P.; Renard, E.; Courtet, P.; Maimoun, L.; Guillaume, S. Clinical Correlates of Measured and Predicted Resting Energy Expenditure in Patients with Anorexia Nervosa: A Retrospective Cohort Study. Nutrients 2022, 14, 2727. [Google Scholar] [CrossRef]
- Moreira, C.; Santos, R.; Vale, S.; Santos, P.C.; Abreu, S.; Marques, A.I.; Soares-Miranda, L.; Mota, J. Ability of Different Measures of Adiposity to Identify High Metabolic Risk in Adolescents. J. Obes. 2011, 2011, 578106. [Google Scholar] [CrossRef]
- Ayton, A. The Importance of Restoring Body Fat Mass in the Treatment of Anorexia Nervosa: An Expert Commentary. J. Popul. Ther. Clin. Pharmacol. 2019, 26, e9–e13. [Google Scholar] [CrossRef]

| T0 | T1 | p | |
|---|---|---|---|
| Energy (Kcal) | 1219.1 (587.9–1536.2) | 1854.7 (1689.4–2051.6) | 0.025 |
| Carbohydrates (g) | 154.7 (64.5–190.7) | 220.2 (210.0–245.4) | 0.012 |
| Carbohydrates (%) | 45.2 (37.4–48.5) | 48.1 (44.1–49.1) | 0.069 |
| Sugars (g) | 47.7 (22.8–62.0) | 66.4 (52.8–69.6) | 0.012 |
| Sugars (%) | 13.9 (13.4–16.0) | 13.2 (12.0–15.4) | 0.161 |
| Proteins (g) | 59.6 (35.6–84.8) | 87.6 (79.4–95.6) | 0.036 |
| Proteins (%) | 22.8 (21.8–23.8) | 19.1 (17.6–20.0) | 0.025 |
| Animal/plant-based proteins * | 2.4 (1.8–3.8) | 2.0 (1.6–2.3) | 0.124 |
| Lipids (g) | 37.6 (24.7–56.4) | 70.6 (57.5–83.9) | 0.036 |
| Lipids (%) | 33.5 (30.2–38.4) | 32.5 (30.6–36.8) | 1.000 |
| Saturated fatty acids (g) | 9.6 (6.7–11.8) | 21.7 (15.5–25.7) | 0.050 |
| Saturated fatty acids (%) | 8.2 (7.0–12.4) | 10.4 (8.4–11.8) | 0.327 |
| Mono-unsaturated fatty acids (g) | 21.5 (13.4–25.4) | 37.5 (27.5–43.0) | 0.036 |
| Mono-unsaturated fatty acids (%) | 17.3 (15.0–19.8) | 16.6 (15.9–19.4) | 0.575 |
| Poli-unsaturated fatty acids (g) | 4.7 (2.3–7.0) | 7.3 (6.1–8.0) | 0.161 |
| Poli-unsaturated fatty acids (%) | 3.5 (3.1–5.2) | 3.5 (3.2–3.9) | 0.401 |
| Fiber (g) | 14.9 (7.2–22.2) | 22.6 (20.5–26.5) | 0.025 |
| T0 | T1 | p | |
|---|---|---|---|
| Height (cm) | 157.3 (153.1–163.9) | 158.4 (154.2–165.7) | 0.014 |
| Weight (kg) | 38.1 (36.3–43.0) | 46.7 (43.1–49.0) | 0.012 |
| BMI (kg/m2) | 15.8 (15.1–16.5) | 17.8 (16.9–19.5) | 0.012 |
| BMI percentile | 2.5 (1.2–3.9) | 16.8 (5.2–37.8) | 0.012 |
| Waist circumference (cm) | 57.9 (55.2–58.3) | 61.5 (59.5–63.2) | 0.025 |
| Waist-to-height ratio | 0.36 (0.35–0.37) | 0.38 (0.37–0.40) | 0.036 |
| Abdomen circumference (cm) | 68.9 (63.0–73.1) | 71.9 (69.3–75.9) | 0.036 |
| Hip circumference (cm) | 79.3 (76.3–82.0) | 87.2 (83.6–89.7) | 0.017 |
| Mid Harm circumference (cm) | 20.4 (19.9–21.0) | 23.0 (22.2–24.6) | 0.017 |
| Mid-thigh circumference (cm) | 39.6 (38.6–41.8) | 43.0 (42.1–46.7) | 0.017 |
| T0 | T1 | p | ||
|---|---|---|---|---|
| BIA | Rz Resistance | 749.5 (711.5–767) | 701.5 (682.5–755.5) | 0.124 |
| Xc Reactance | 70.0 (66.0–74.5) | 69.5 (66.0–78.5) | 0.779 | |
| TBW (L) | 25.4 (24.1–26.2) | 27.0 (25.9–28.1) | 0.012 | |
| ECW (L) | 11.4 (10.7–13.2) | 12.0 (11.5–12.9) | 0.161 | |
| BCM (kg) | 16.3 (15.0–17.2) | 17.6 (16.7–21.1) | 0.042 | |
| BCMI (kg/m2) | 6.5 (6.1–6.9) | 7.4 (6.7–8.0) | 0.107 | |
| PA (°) | 5.3 (5.1–5.9) | 5.8 (5.2–6.3) | 0.622 | |
| DXA | FFM (kg) | 31.59 (29.24–33.07) | 34.41 (32.59–37.71) | 0.012 |
| FM (kg) | 8.57 (6.84–9.63) | 10.09 (7.18–12.41) | 0.161 | |
| LBM (kg) | 31.40 (28.50–34.51) | 33.28 (32.00–35.73) | 0.036 | |
| FMpct (%) | 21.2 (17.4–23.8) | 21.5 (17.6–26.6) | 0.575 | |
| FMI (kg/m2) | 3.3 (2.9–3.7) | 4.0 (2.9–4.9) | 0.208 | |
| LBMI (kg/m2) | 13.0 (11.8–13.0) | 13.9 (12.8–14.2) | 0.050 | |
| Calorimetry | VO2 (mL/min) | 137 (112.5–150.5) | 161.5 (148.5–169) | 0.012 |
| VCO2 (mL/min) | 122 (96.5–136) | 146.5 (137–157) | 0.035 | |
| RER | 0.88 (0.87–0.96) | 0.90 (0.87–0.97) | 0.575 | |
| REE (kcal) | 921 (781.5–1036.5) | 1124.5 (1036–1154.5) | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cinelli, G.; Croci, I.; De Santis, G.L.; Chianello, I.; Miller, K.P.; Gualtieri, P.; Di Renzo, L.; De Lorenzo, A.; Tozzi, A.E.; Zanna, V. Adherence to a Mediterranean Diet, Body Composition and Energy Expenditure in Outpatients Adolescents Diagnosed with Anorexia Nervosa: A Pilot Study. Nutrients 2023, 15, 3223. https://doi.org/10.3390/nu15143223
Cinelli G, Croci I, De Santis GL, Chianello I, Miller KP, Gualtieri P, Di Renzo L, De Lorenzo A, Tozzi AE, Zanna V. Adherence to a Mediterranean Diet, Body Composition and Energy Expenditure in Outpatients Adolescents Diagnosed with Anorexia Nervosa: A Pilot Study. Nutrients. 2023; 15(14):3223. https://doi.org/10.3390/nu15143223
Chicago/Turabian StyleCinelli, Giulia, Ileana Croci, Gemma Lou De Santis, Ilenia Chianello, Kiersten Pilar Miller, Paola Gualtieri, Laura Di Renzo, Antonino De Lorenzo, Alberto Eugenio Tozzi, and Valeria Zanna. 2023. "Adherence to a Mediterranean Diet, Body Composition and Energy Expenditure in Outpatients Adolescents Diagnosed with Anorexia Nervosa: A Pilot Study" Nutrients 15, no. 14: 3223. https://doi.org/10.3390/nu15143223
APA StyleCinelli, G., Croci, I., De Santis, G. L., Chianello, I., Miller, K. P., Gualtieri, P., Di Renzo, L., De Lorenzo, A., Tozzi, A. E., & Zanna, V. (2023). Adherence to a Mediterranean Diet, Body Composition and Energy Expenditure in Outpatients Adolescents Diagnosed with Anorexia Nervosa: A Pilot Study. Nutrients, 15(14), 3223. https://doi.org/10.3390/nu15143223








