Sulforaphane Inhibits Exhaustive Exercise-Induced Liver Injury and Transcriptome-Based Mechanism Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
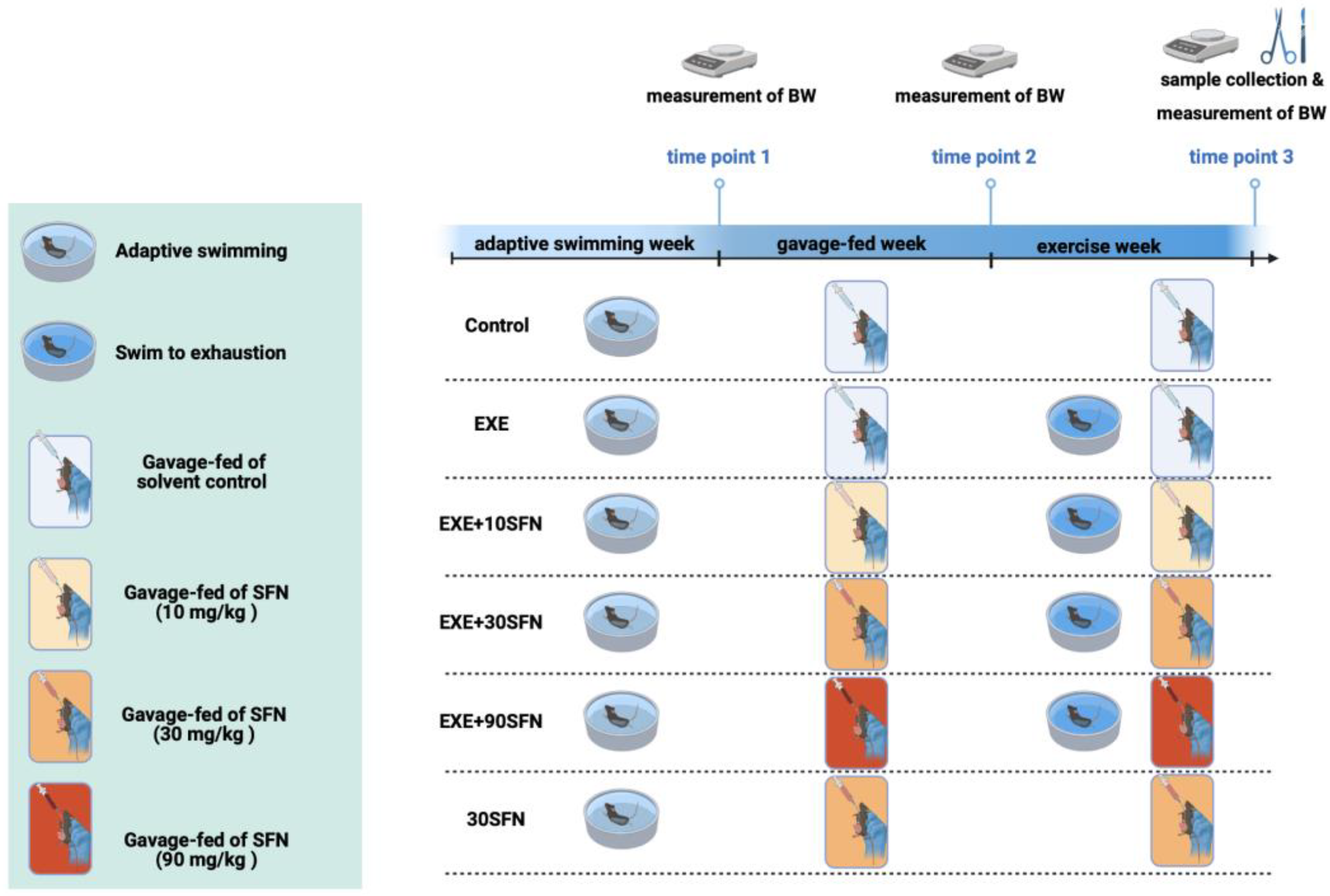
2.2. Animals, Intervention Protocol, and Sample Collection
2.3. Serum Biochemical Parameters
2.4. H&E Staining
2.5. Detection of Oxidative Stress
2.6. RNA Sequencing and Bioinformatic Analysis
2.7. RNA Isolation and qRT-PCR
2.8. Statistical Analysis
3. Results
3.1. Sulforaphane Intervention Combined with EE Slowed down the Age-Dependent Body Weight Gain in Mice
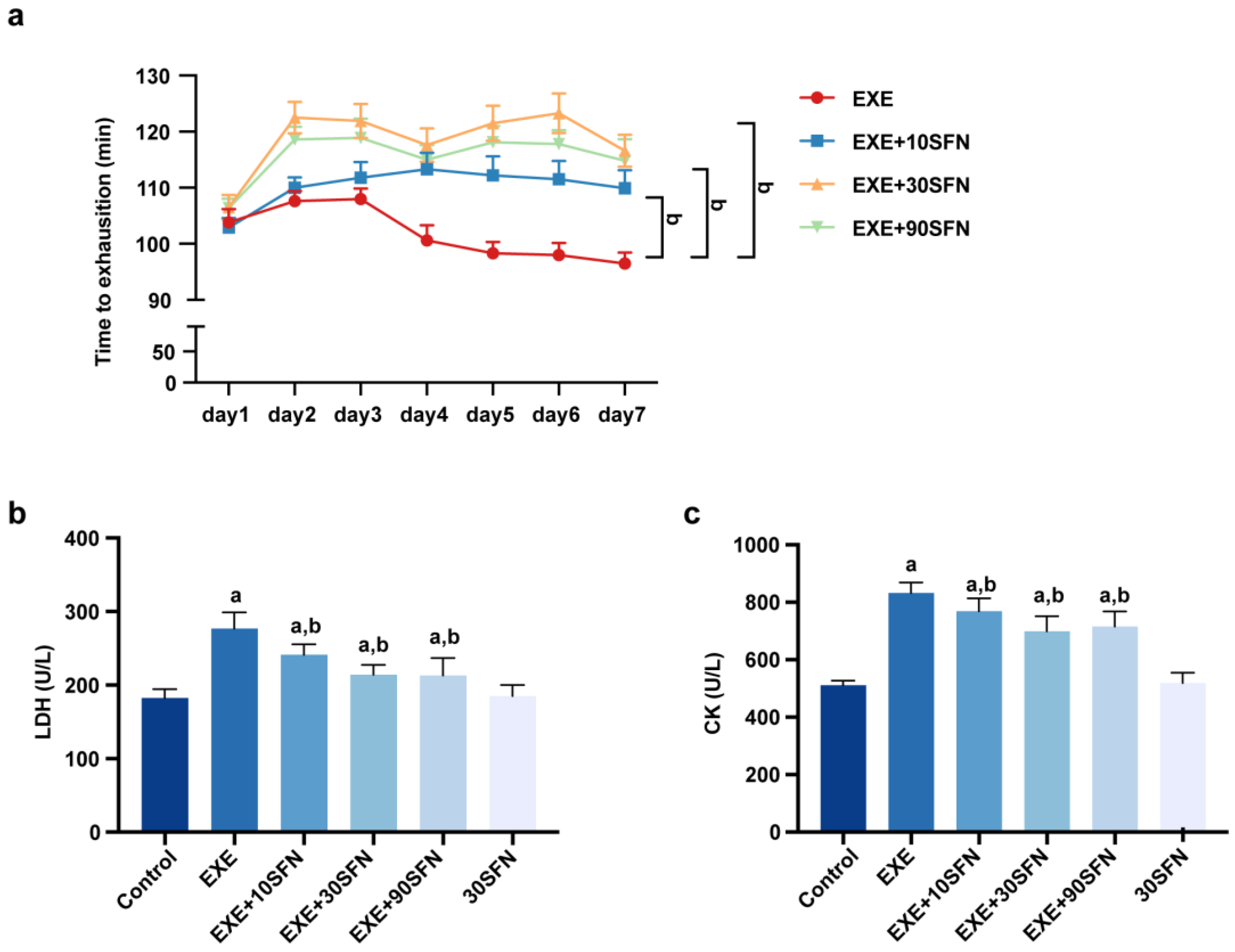
3.2. Sulforaphane Improved Exercise Performance and Fatigue Biochemical Parameters in Mice Subjected to EE
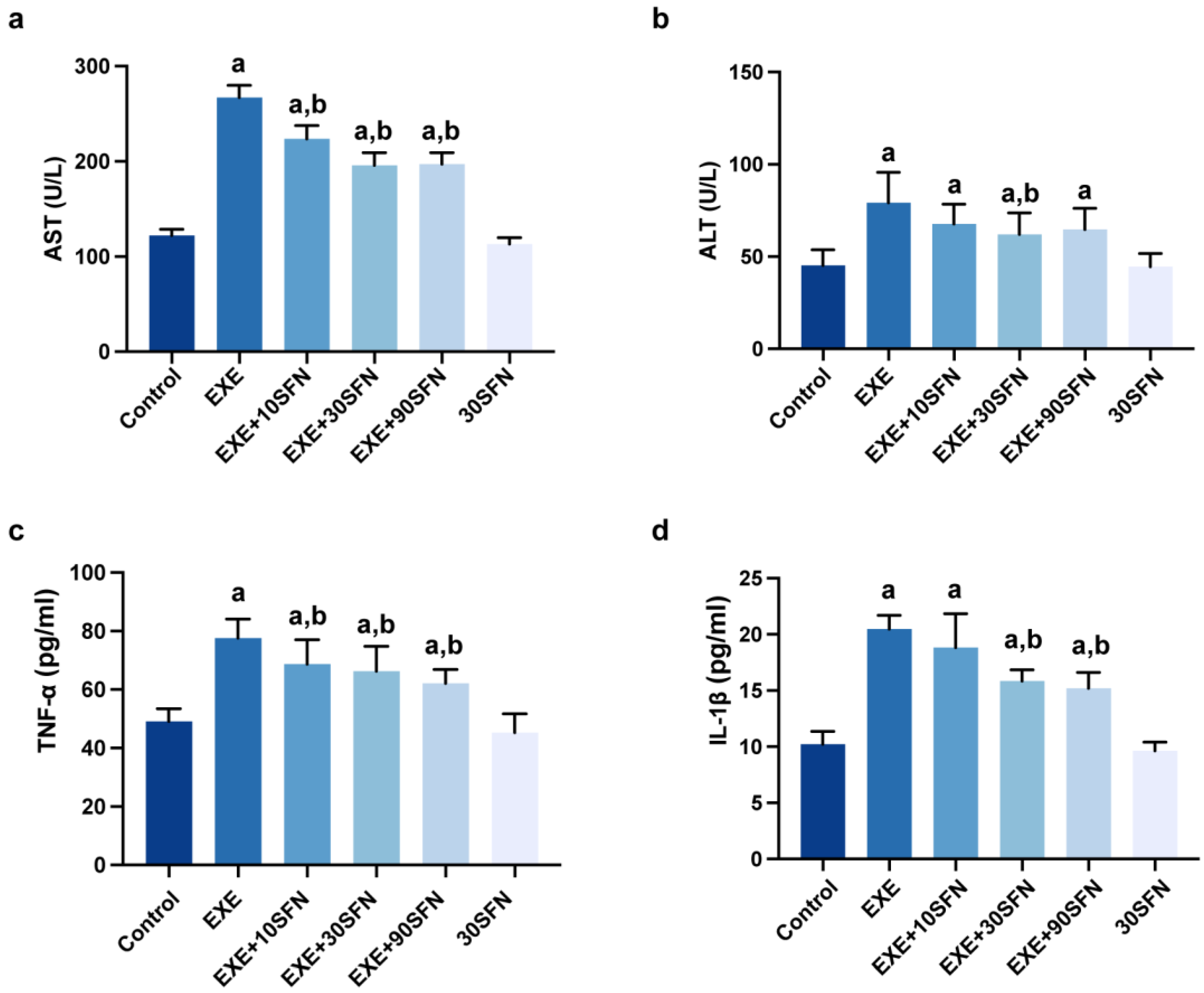
3.3. Sulforaphane Attenuated EE-Induced Inflammation and Liver Enzyme Elevation
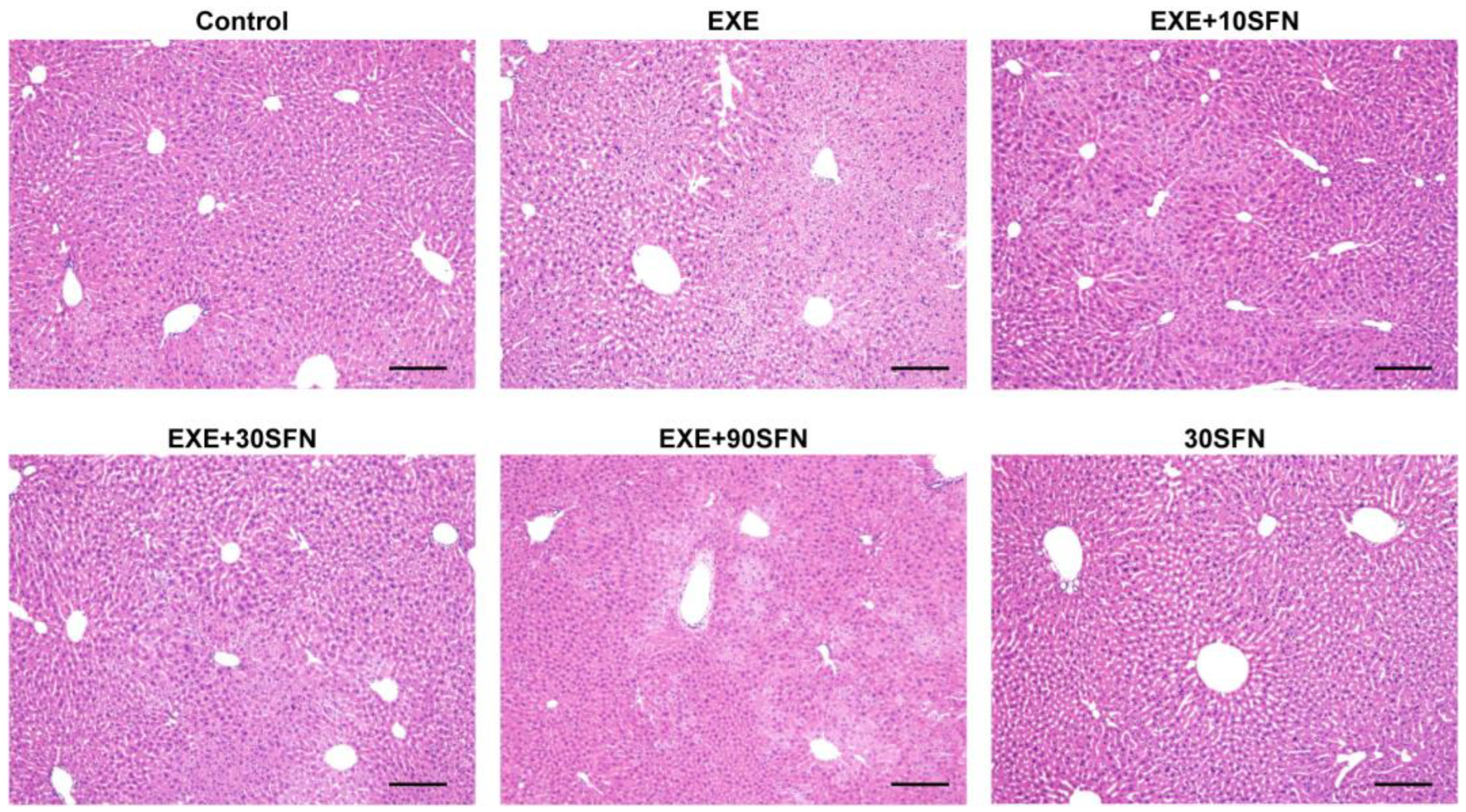
3.4. Sulforaphane Improved EE-Induced Liver Pathological Morphology Abnormality
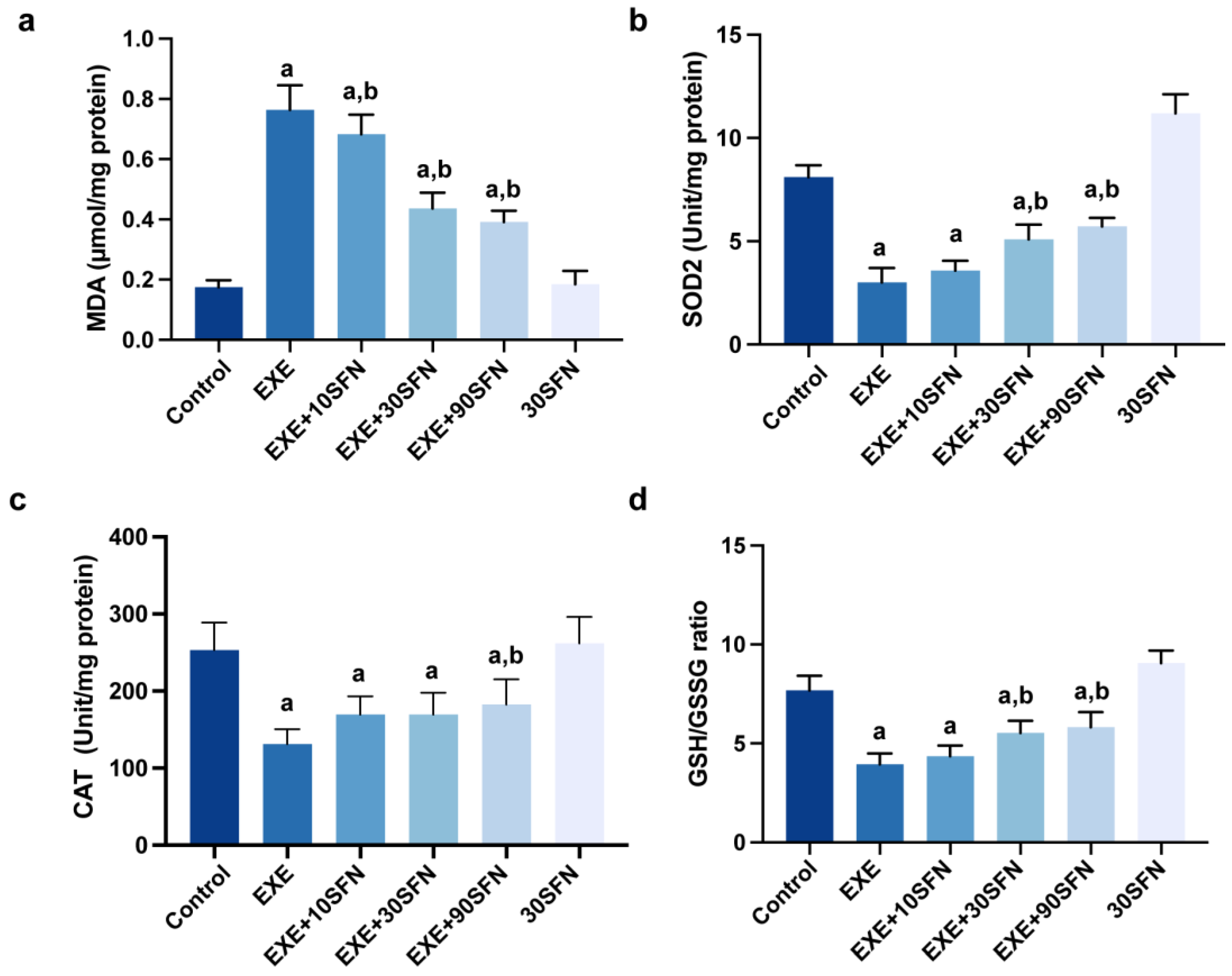
3.5. Sulforaphane Attenuated EE-Induced Liver Oxidative Stress
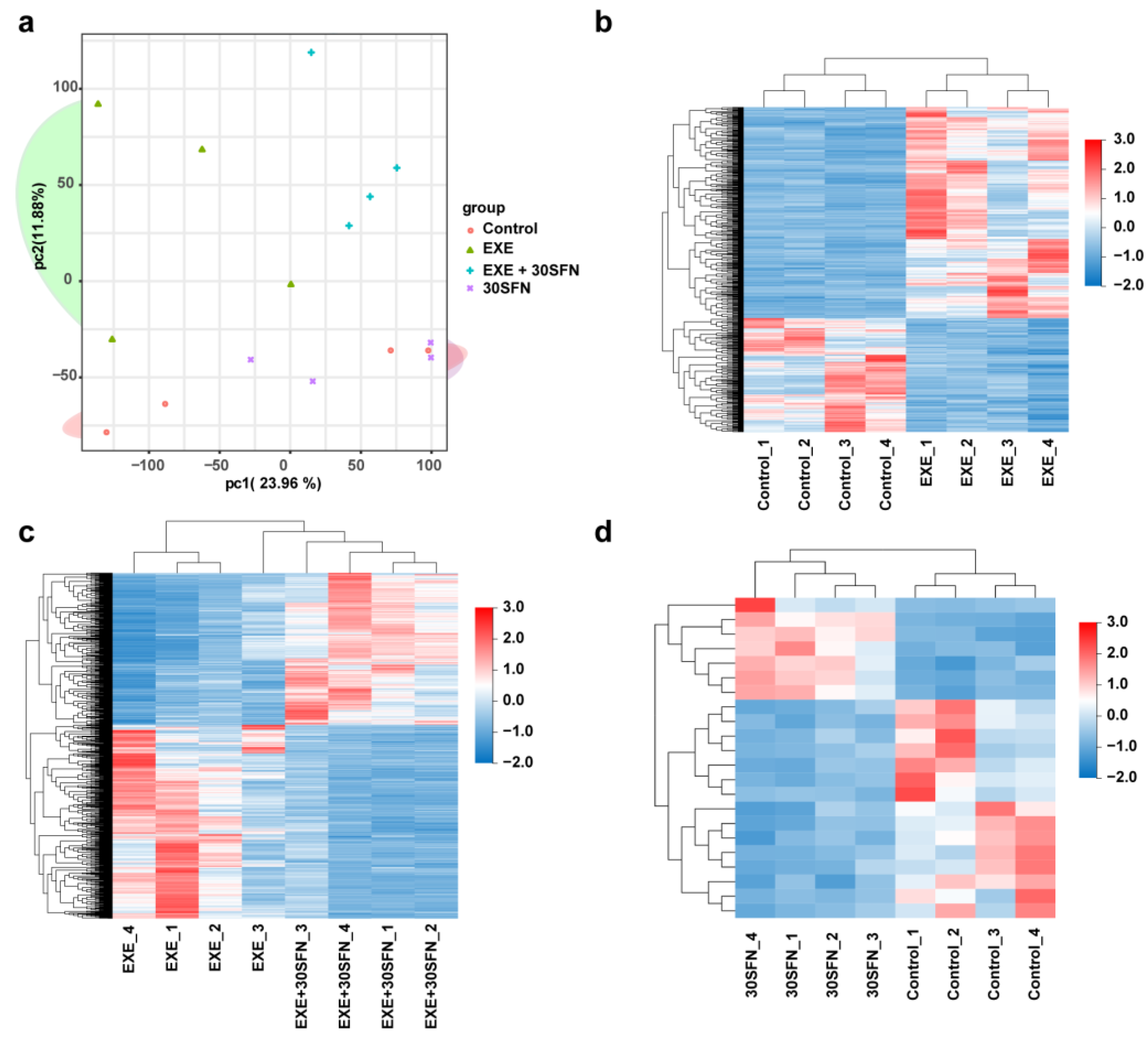
3.6. Liver Transcriptome Alterations in the SFN Intervened EE Mice Model
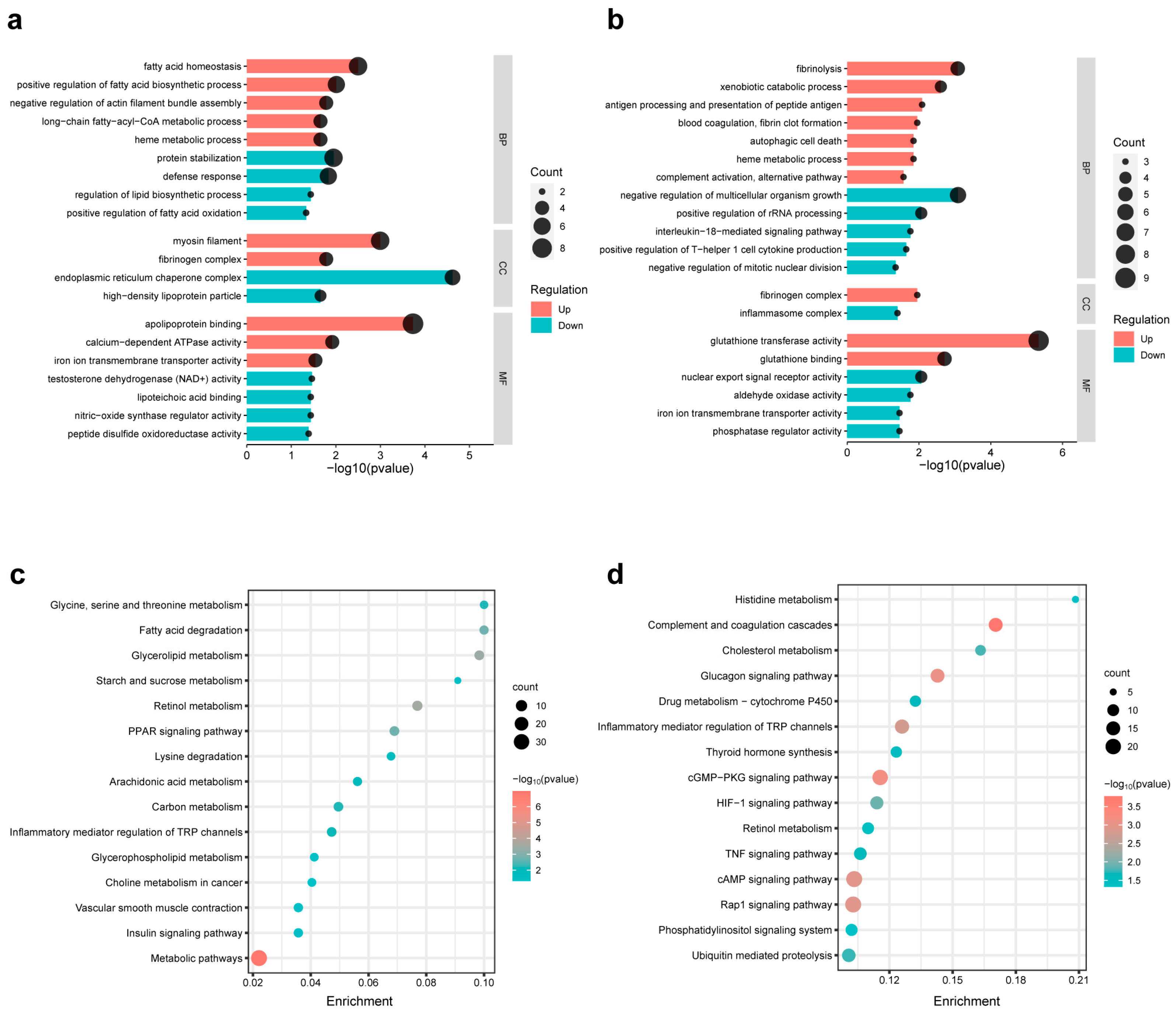
3.7. Pathway Enrichment Analysis of the DEGs of Different Comparison Groups
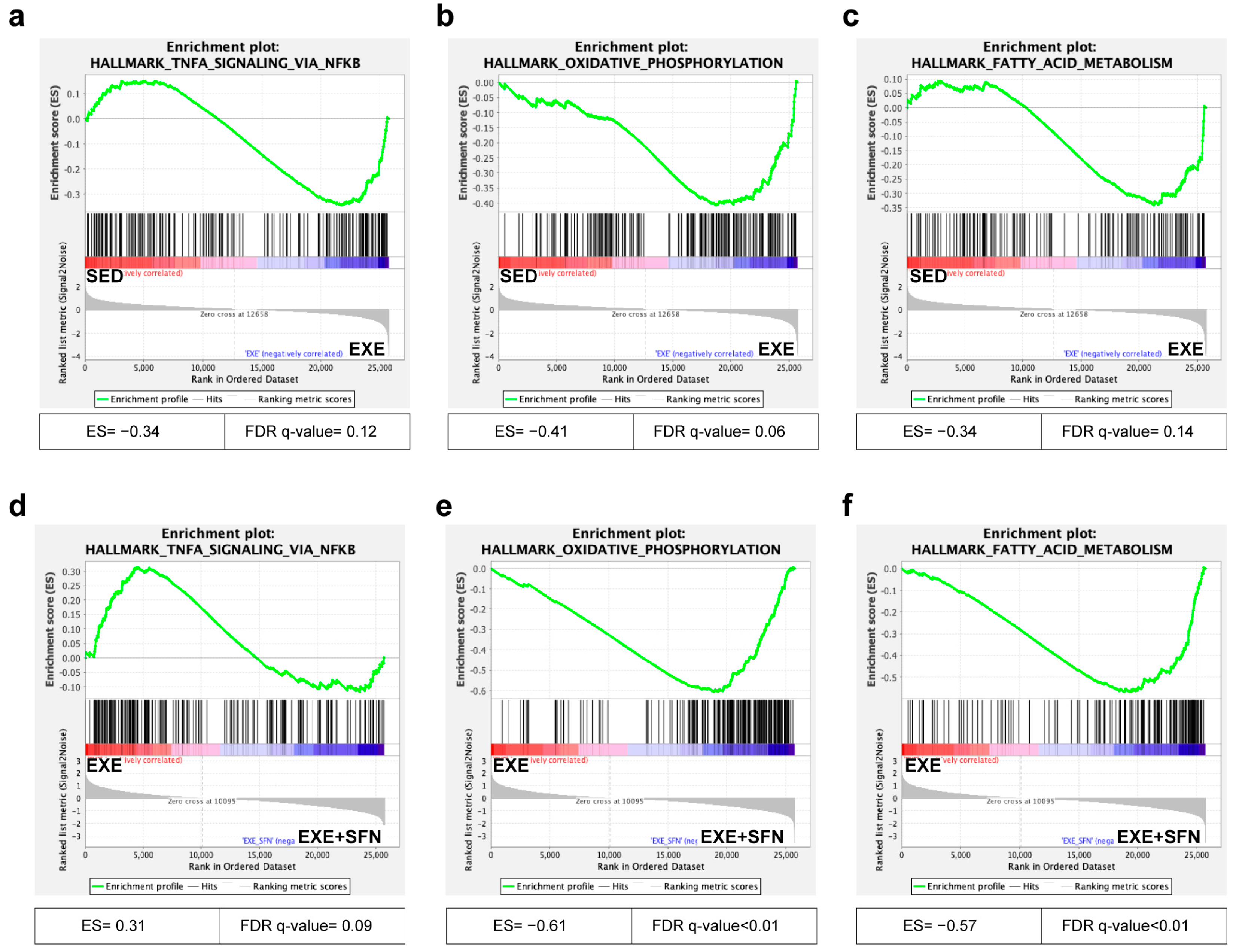
3.8. Gene Set Enrichment Analysis (GSEA) of the Liver Transcriptomes
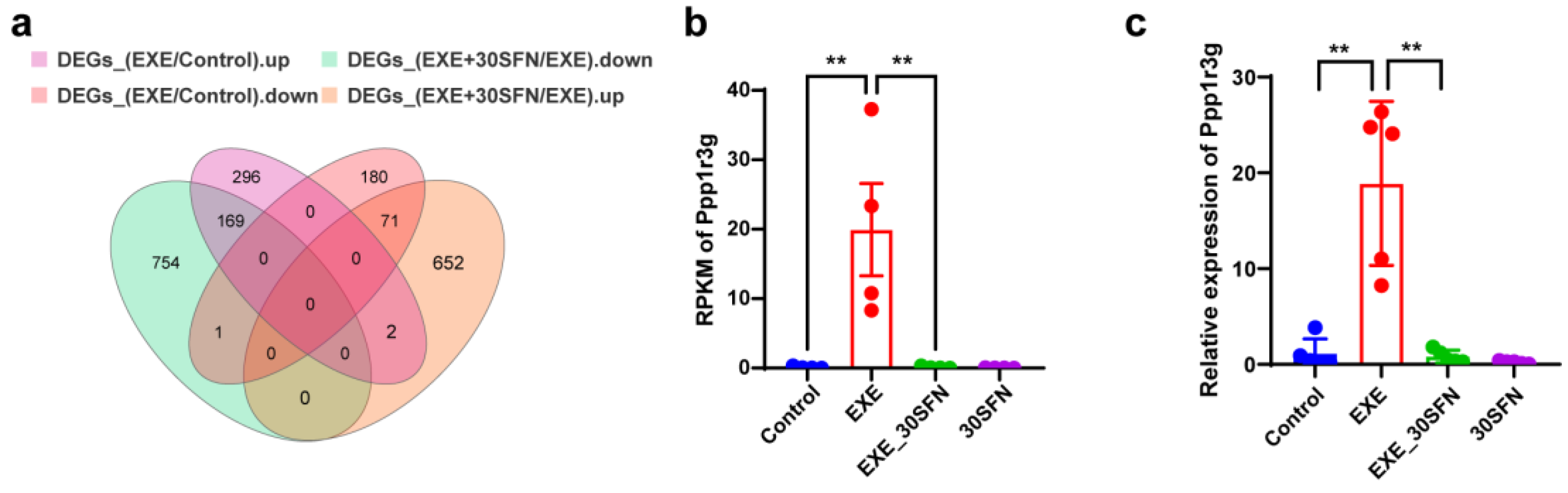
3.9. Ppp1r3g Is a Candidate Gene for the Protective Effect of Sulforaphane on EE-Induced Liver Injury
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Chen, Z.; Pan, L.; Ma, Z.M.; Zhang, H.; Li, X.J.; Li, X. Effect of Moderate and Vigorous Aerobic Exercise on Incident Diabetes in Adults with Obesity: A 10-Year Follow-up of a Randomized Clinical Trial. JAMA Intern. Med. 2023, 183, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Wang, H.; Lin, K.; Wang, R.; Guo, S.; Chen, P.; Wu, H.; Liu, T.; Wang, R. Exercise-induced microbial changes in preventing type 2 diabetes. Sci. China Life Sci. 2023, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Liu, Z.; Yu, X.; Huang, T.; Chen, J.; Wang, J.; Wilhelm, J.; Li, S.; Song, J.; Li, W.; et al. Lactate increases stemness of CD8 + T cells to augment anti-tumor immunity. Nat. Commun. 2022, 13, 4981. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Rezende, L.F.; Joh, H.-K.; Keum, N.; Ferrari, G.; Rey-Lopez, J.P.; Rimm, E.B.; Tabung, F.K.; Giovannucci, E.L. Long-Term Leisure-Time Physical Activity Intensity and All-Cause and Cause-Specific Mortality: A Prospective Cohort of US Adults. Circulation 2022, 146, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.T.; Fuchs, C.J.; Betts, J.A.; van Loon, L.J. Liver glycogen metabolism during and after prolonged endurance-type exercise. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E543–E553. [Google Scholar] [CrossRef]
- Khatri, P.; Neupane, A.; Sapkota, S.R.; Bashyal, B.; Sharma, D.; Chhetri, A.; Chirag, K.C.; Banjade, A.; Sapkota, P.; Bhandari, S. Strenuous Exercise-Induced Tremendously Elevated Transaminases Levels in a Healthy Adult: A Diagnostic Dilemma. Case Rep. Hepatol. 2021, 2021, 6653266. [Google Scholar] [CrossRef]
- Gao, C.; Liu, Y.; Jiang, C.; Liu, L.; Li, J.; Li, D.; Guo, X.; Wang, Z.; Yang, Y.; Tang, Y.; et al. Intensive Running Enhances NF-kappa B Activity in the Mice Liver and the Intervention Effects of Quercetin. Nutrients 2020, 12, 2770. [Google Scholar] [CrossRef]
- Sestili, P.; Fimognari, C. Cytotoxic and Antitumor Activity of Sulforaphane: The Role of Reactive Oxygen Species. BioMed Res. Int. 2015, 2015, 402386. [Google Scholar] [CrossRef]
- Sato, K.; Kihara, H.; Kumazawa, Y.; Tatara, K. Oral chronic sulforaphane effects on heavy resistance exercise: Implications for inflammatory and muscle damage parameters in young practitioners. Nutrition 2021, 90, 111266. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Suzuki, K. The Integrative Role of Sulforaphane in Preventing Inflammation, Oxidative Stress and Fatigue: A Review of a Potential Protective Phytochemical. Antioxidants 2020, 9, 521. [Google Scholar] [CrossRef]
- Sivapalan, T.; Melchini, A.; Saha, S.; Needs, P.W.; Traka, M.; Tapp, H.; Dainty, J.R.; Mithen, R.F. Bioavailability of Glucoraphanin and Sulforaphane from High-Glucoraphanin Broccoli. Mol. Nutr. Food Res. 2017, 62, e1700911. [Google Scholar] [CrossRef]
- Russo, M.; Spagnuolo, C.; Russo, G.L.; Skalicka-Woźniak, K.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.M. Nrf2 targeting by sulforaphane: A potential therapy for cancer treatment. Crit. Rev. Food Sci. Nutr. 2017, 58, 1391–1405. [Google Scholar] [CrossRef] [PubMed]
- Liebman, S.E.; Le, T.H. Eat Your Broccoli: Oxidative Stress, NRF2, and Sulforaphane in Chronic Kidney Disease. Nutrients 2021, 13, 266. [Google Scholar] [CrossRef] [PubMed]
- Ruhee, R.T.; Ma, S.; Suzuki, K. Protective Effects of Sulforaphane on Exercise-Induced Organ Damage via Inducing Antioxidant Defense Responses. Antioxidants 2020, 9, 136. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.C.; Copp, S.W.; Colburn, T.D.; Craig, J.C.; Allen, D.L.; Sturek, M.; O’Leary, D.S.; Zucker, I.H.; Musch, T.I. Guidelines for animal exercise and training protocols for cardiovascular studies. Am. J. Physiol. Circ. Physiol. 2020, 318, H1100–H1138. [Google Scholar] [CrossRef] [PubMed]
- Bei, Y.; Wang, L.; Ding, R.; Che, L.; Fan, Z.; Gao, W.; Liang, Q.; Lin, S.; Liu, S.; Xiao, J.; et al. Animal exercise studies in cardiovascular research: Current knowledge and optimal design—A position paper of the Committee on Cardiac Rehabilitation, Chinese Medical Doctors’ Association. J. Sport Health Sci. 2021, 10, 660–674. [Google Scholar] [CrossRef]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and Eosin Staining of Tissue and Cell Sections. Cold Spring Harb. Protoc. 2008, 2008, pdb.prot4986. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. feature Counts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Perry, M. Heatmaps: Flexible Heatmaps for Functional Genomics and Sequence Features, version 1.8.0. R Package. R Foundation for Statistical Computing: Vienna, Austria, 2019.
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef]
- Ginestet, C. ggplot2: Elegant graphics for data analysis. J. R. Stat. Soc. Ser. A 2011, 174, 245–246. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.-F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C.(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liu, R.; Wu, L.; Du, Q.; Ren, J.-W.; Chen, Q.-H.; Li, D.; Mao, R.-X.; Liu, X.-R.; Li, Y. Small Molecule Oligopeptides Isolated from Walnut (Juglans regia L.) and Their Anti-Fatigue Effects in Mice. Molecules 2018, 24, 45. [Google Scholar] [CrossRef]
- Xu, X.; Ding, Y.; Yang, Y.; Gao, Y.; Sun, Q.; Liu, J.; Yang, X.; Wang, J.; Zhang, J. β-glucan Salecan Improves Exercise Performance and Displays Anti-Fatigue Effects through Regulating Energy Metabolism and Oxidative Stress in Mice. Nutrients 2018, 10, 858. [Google Scholar] [CrossRef]
- Wolf, P.L. Biochemical diagnosis of liver disease. Indian J. Clin. Biochem. 1999, 14, 59–90. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef]
- Munro, S.; Ceulemans, H.; Bollen, M.; Diplexcito, J.; Cohen, P.T. A novel glycogen-targeting subunit of protein phosphatase 1 that is regulated by insulin and shows differential tissue distribution in humans and rodents. FEBS J. 2005, 272, 1478–1489. [Google Scholar] [CrossRef]
- Guo, S.; Huang, Y.; Zhang, Y.; Huang, H.; Hong, S.; Liu, T. Impacts of exercise interventions on different diseases and organ functions in mice. J. Sport Health Sci. 2019, 9, 53–73. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise—A Systematic Review. Front. Physiol. 2020, 10, 1550. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.S.; Dighe, P.A.; Mezera, V.; Monternier, P.A.; Brand, M.D. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J. Biol. Chem. 2017, 292, 16804–16809. [Google Scholar] [CrossRef] [PubMed]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative eustress: On constant alert for redox homeostasis. Redox Biol. 2021, 41, 101867. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, E.; Mündel, T.; Barnes, M.J. Nutritional Compounds to Improve Post-Exercise Recovery. Nutrients 2022, 14, 5069. [Google Scholar] [CrossRef]
- Socała, K.; Nieoczym, D.; Kowalczuk-Vasilev, E.; Wyska, E.; Wlaź, P. Increased seizure susceptibility and other toxicity symptoms following acute sulforaphane treatment in mice. Toxicol. Appl. Pharmacol. 2017, 326, 43–53. [Google Scholar] [CrossRef]
- Saeidi, A.; Soltani, M.; Daraei, A.; Nohbaradar, H.; Haghighi, M.M.; Khosravi, N.; Johnson, K.E.; Laher, I.; Hackney, A.C.; VanDusseldorp, T.A.; et al. The Effects of Aerobic-Resistance Training and Broccoli Supplementation on Plasma Dectin-1 and Insulin Resistance in Males with Type 2 Diabetes. Nutrients 2021, 13, 3144. [Google Scholar] [CrossRef]
- Çakır, I.; Lining Pan, P.; Hadley, C.K.; El-Gamal, A.; Fadel, A.; Elsayegh, D.; Mohamed, O.; Rizk, N.M.; Ghamari-Langroudi, M. Sulforaphane reduces obesity by reversing leptin resistance. eLife 2022, 11, e67368. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, X.; Chen, Z.; Luo, T.; Zhu, C.; Ji, Y.; Bian, Z. The Protective Effects of Sulforaphane on High-Fat Diet-Induced Obesity in Mice Through Browning of White Fat. Front. Pharmacol. 2021, 12, 5069. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Chen, S.Y.; Wang, A.S.; Yao, A.J.; Fu, J.F.; Zhao, J.S.; Chen, F.; Zou, Z.Q.; Zhang, X.H.; Shan, Y.J.; et al. Sulforaphane induces adipocyte browning and promotes glucose and lipid utilization. Mol. Nutr. Food Res. 2016, 60, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Shiroguchi, K.; Jia, T.Z.; Sims, P.A.; Xie, X.S. Digital RNA sequencing minimizes sequence-dependent bias and amplification noise with optimized single-molecule barcodes. Proc. Natl. Acad. Sci. USA 2012, 109, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.C.; Ellefsen, S.; Baar, K. Adaptations to Endurance and Strength Training. Cold Spring Harb. Perspect. Med. 2017, 8, a029769. [Google Scholar] [CrossRef] [PubMed]
- Aggen, J.B.; Nairn, A.C.; Chamberlin, R. Regulation of protein phosphatase-1. Chem. Biol. 2000, 7, R13–R23. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.S.; Choy, M.S.; Koveal, D.M.; Lorinsky, M.K.; Lyons, S.P.; Kettenbach, A.N.; Page, R.; Peti, W. Identification of the substrate recruitment mechanism of the muscle glycogen protein phosphatase 1 holoenzyme. Sci. Adv. 2018, 4, eaau6044. [Google Scholar] [CrossRef]
- Gasa, R.; Jensen, P.B.; Berman, H.K.; Brady, M.J.; DePaoli-Roach, A.A.; Newgard, C.B. Distinctive Regulatory and Metabolic Properties of Glycogen-targeting Subunits of Protein Phosphatase-1 (PTG, GL, GM/RGl) Expressed in Hepatocytes. J. Biol. Chem. 2000, 275, 26396–26403. [Google Scholar] [CrossRef]
- Newgard, C.B.; Brady, M.J.; O’Doherty, R.M.; Saltiel, A.R. Organizing glucose disposal: Emerging roles of the glycogen targeting subunits of protein phosphatase-1. Diabetes 2000, 49, 1967–1977. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, Y.; Ruan, X.; Jiang, X.; Zhu, L.; Wang, X.; Ding, Q.; Liu, W.; Pan, Y.; Wang, Z.; et al. Fasting-Induced Protein Phosphatase 1 Regulatory Subunit Contributes to Postprandial Blood Glucose Homeostasis via Regulation of Hepatic Glycogenesis. Diabetes 2011, 60, 1435–1445. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Q.; Zhang, J.; Zhou, L.; Zhang, W.; Chua, B.; Chen, Y.; Xu, L.; Li, P. The Protein Phosphatase 1 Complex Is a Direct Target of AKT that Links Insulin Signaling to Hepatic Glycogen Deposition. Cell Rep. 2019, 28, 3406–3422.e7. [Google Scholar] [CrossRef]
| Group | Before Gavage Fed (g) | Before the EE (g) | After the EE (g) |
|---|---|---|---|
| Control | 25.35 ± 0.63 | 25.70 ± 1.18 | 27.46 ± 1.87 *,# |
| EXE | 25.61 ± 0.80 | 26.55 ± 0.71 | 27.41 ± 1.05 # |
| EXE + 10SFN | 25.97 ± 0.82 | 26.88 ± 0.82 | 27.58 ± 0.98 # |
| EXE + 30SFN | 25.65 ± 0.95 | 26.19 ± 1.58 | 27.16 ± 1.53 |
| EXE + 90SFN | 25.78 ± 1.42 | 26.98 ± 1.47 | 27.04 ± 1.43 |
| 30SFN | 25.66 ± 1.00 | 26.11 ± 0.97 | 27.50 ± 1.18 *,# |
| Gene ID | Gene Symbol | FDR of Control vs. EXE | Log2FC of Control vs. EXE |
|---|---|---|---|
| 76487 | Ppp1r3g | 0.00755186 | 7.4671905 |
| 54698 | Crtam | 0.00794266 | 6.65883683 |
| 83770 | Tas1r2 | 0.00638426 | 6.35433732 |
| 73435 | Tex35 | 0.01319879 | 5.87021818 |
| 22420 | Wnt6 | 0.03151093 | 5.42157778 |
| 14120 | Fbp2 | 0.00638426 | 5.36280401 |
| 18227 | Nr4a2 | 0.03290719 | 5.29711301 |
| 100502635 | Gm11525 | 0.03715784 | 5.21183416 |
| 15186 | Hdc | 0.01275262 | 4.77473087 |
| 29870 | Gtse1 | 0.01580759 | 4.62222956 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Guo, X.; Li, T.; Xie, Y.; Wang, D.; Yi, L.; Mi, M. Sulforaphane Inhibits Exhaustive Exercise-Induced Liver Injury and Transcriptome-Based Mechanism Analysis. Nutrients 2023, 15, 3220. https://doi.org/10.3390/nu15143220
Yang J, Guo X, Li T, Xie Y, Wang D, Yi L, Mi M. Sulforaphane Inhibits Exhaustive Exercise-Induced Liver Injury and Transcriptome-Based Mechanism Analysis. Nutrients. 2023; 15(14):3220. https://doi.org/10.3390/nu15143220
Chicago/Turabian StyleYang, Jining, Xinxin Guo, Tianyou Li, Yingquan Xie, Dawei Wang, Long Yi, and Mantian Mi. 2023. "Sulforaphane Inhibits Exhaustive Exercise-Induced Liver Injury and Transcriptome-Based Mechanism Analysis" Nutrients 15, no. 14: 3220. https://doi.org/10.3390/nu15143220
APA StyleYang, J., Guo, X., Li, T., Xie, Y., Wang, D., Yi, L., & Mi, M. (2023). Sulforaphane Inhibits Exhaustive Exercise-Induced Liver Injury and Transcriptome-Based Mechanism Analysis. Nutrients, 15(14), 3220. https://doi.org/10.3390/nu15143220






