Neuro-Vulnerability in Energy Metabolism Regulation: A Comprehensive Narrative Review
Abstract
1. Introduction
1.1. Regulation of Brain Metabolism
1.2. Methodology of Search
2. Neurobiological Basis of Energy Metabolism
2.1. Metabolic Localization of Glycogen in the Brain
2.2. Regulatory Mechanisms of Glycogen in the Brain
2.3. Lactate as a Metabolite?
3. Neuro-Vulnerability and Metabolic Dysregulation
4. Neuroendocrine Interactions in Energy Metabolism
5. Neural Regulation of Food Intake and Satiety
6. Neural Control of Energy Expenditure
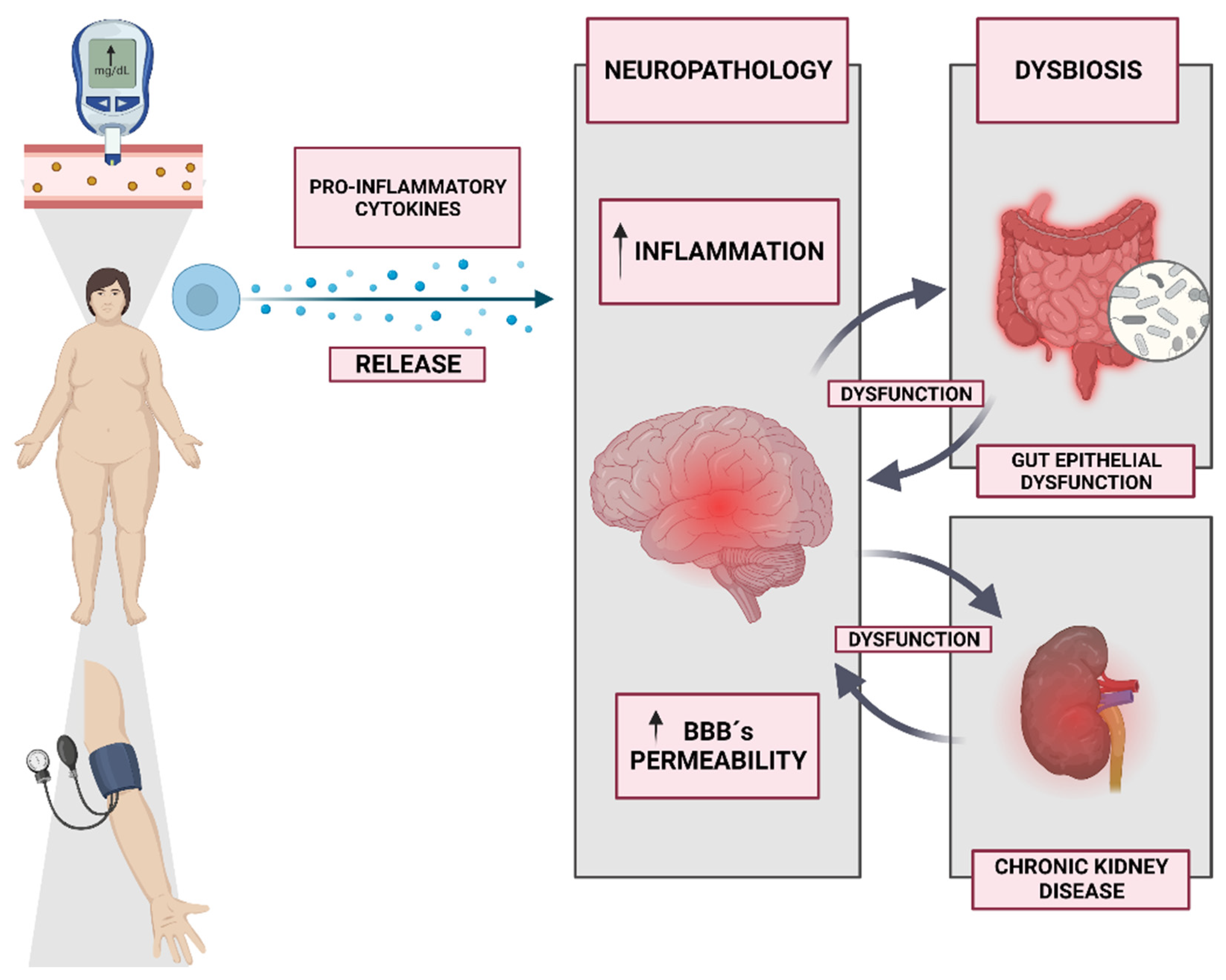
7. Neuroinflammation and Metabolic Health
8. Neuroimaging Techniques in Studying Neuro-Vulnerability
9. Neuro-Vulnerability and Eating Disorders
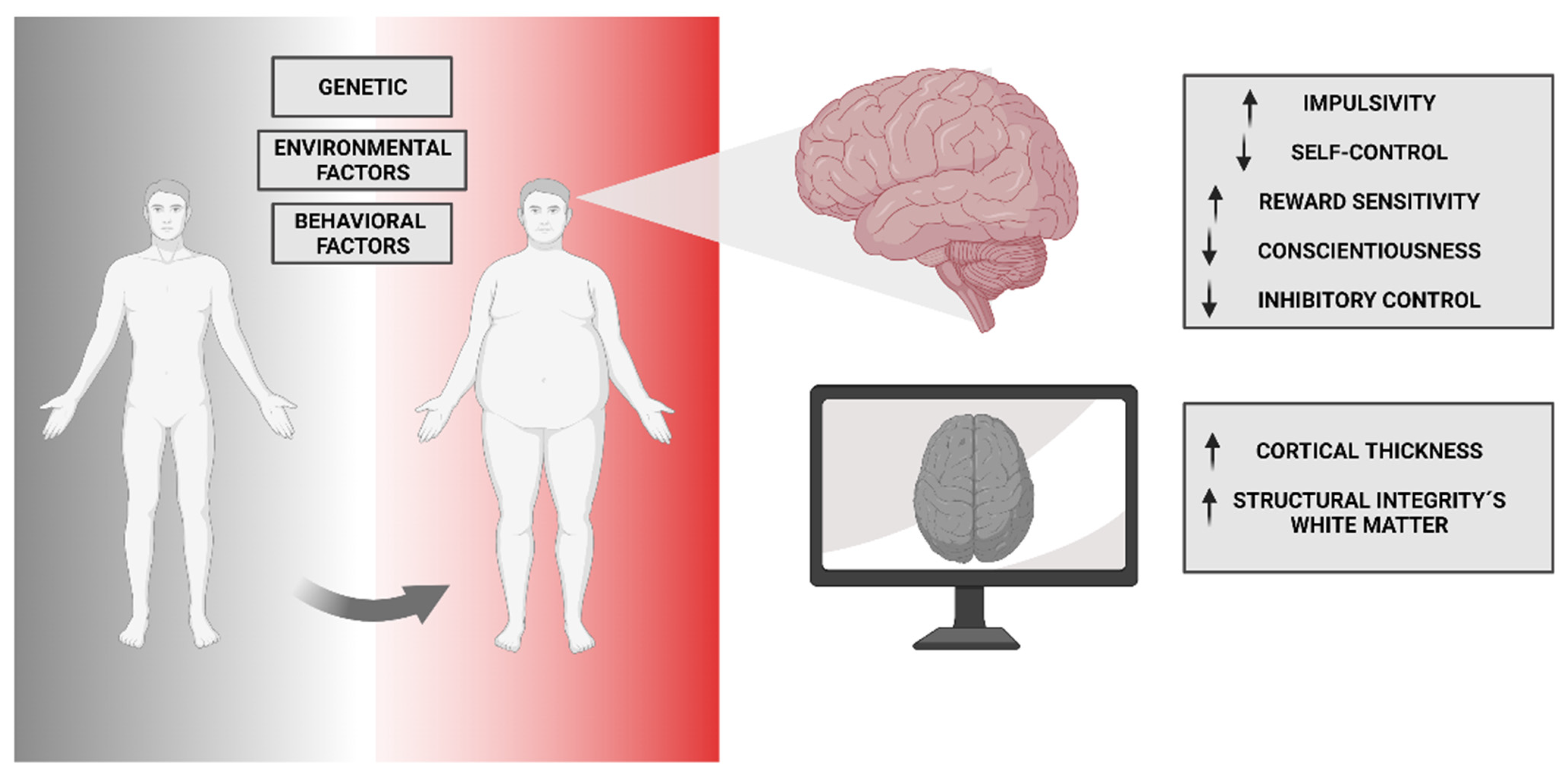
10. Neuro-Vulnerability and Obesity
11. Therapeutic Strategies for Modulating Neuro-Vulnerability
12. Future Directions and Research Gaps
- Longitudinal Studies: Conducting longitudinal studies that track individuals over time can provide valuable insights into the temporal dynamics of neuro-vulnerability and its association with metabolic dysregulation. This approach can help identify early markers or predictors of neuro-vulnerability and inform targeted interventions.
- Genetic and Epigenetic Influences: Investigating the genetic and epigenetic factors underlying neuro-vulnerability can enhance our understanding of individual differences in energy metabolism regulation. Identifying specific genetic variants or epigenetic modifications associated with neuro-vulnerability may open avenues for personalized interventions and precision medicine approaches.
- Gut–Brain Axis: A further exploration of the bidirectional communication between the gut and the brain, particularly through the gut–brain axis, is crucial for unraveling the mechanisms involved in energy metabolism regulation. Understanding how gut-derived signals modulate the neural circuits involved in metabolic control can pave the way for novel therapeutic strategies.
- Sex Differences: Examining potential sex differences in neuro-vulnerability and metabolic dysregulation is important, as sex hormones and other sex-specific factors may contribute to variations in energy metabolism regulation. Investigating whether neuro-vulnerability manifests differently between males and females could guide the development of sex-specific interventions.
- Neuroinflammation and Metabolism: Elucidating the intricate relationship between neuroinflammation and metabolic health is an area of ongoing investigation. Identifying the underlying mechanisms linking neuroinflammation, neuro-vulnerability, and metabolic dysregulation can provide insights into novel therapeutic targets for managing metabolic disorders.
- Advanced Neuroimaging Techniques: Utilizing advanced neuroimaging techniques, such as functional magnetic resonance imaging (fMRI), positron emission tomography (PET), and magnetic resonance spectroscopy (MRS), can provide more detailed information about neuro-vulnerability and its impact on energy metabolism regulation. These techniques can help elucidate the neural circuits and metabolic pathways involved, allowing for targeted interventions.
- Neuro-Vulnerability and Aging: Investigating the impact of neuro-vulnerability on energy metabolism regulation in the context of aging is an important area for future research. Understanding how age-related changes in the brain and neuroendocrine systems influence metabolic dysregulation can inform strategies for healthy aging and prevent age-related metabolic disorders.
- Novel Therapeutic Approaches: Developing innovative therapeutic approaches that specifically target neuro-vulnerability holds promise for improving metabolic health. Exploring neuroprotective agents, neuromodulation techniques, and precision medicine strategies tailored to individuals with neuro-vulnerability can pave the way for more effective interventions.
- Translational Research: Bridging the gap between basic research and clinical applications is crucial for translating the findings into meaningful interventions. Conducting translational studies that integrate preclinical models, human research, and clinical trials can accelerate the development of targeted therapies for individuals with neuro-vulnerability.
- Socioeconomic Factors: Considering the influence of socioeconomic factors on neuro-vulnerability and metabolic dysregulation is important for addressing health disparities. Investigating how socioeconomic status, access to healthy food, and environmental factors interact with neuro-vulnerability can inform public health policies and interventions aimed at promoting metabolic health.
- Comorbidities and Multifactorial Approaches: Given the complex nature of metabolic disorders, exploring the interplay between neuro-vulnerability and comorbid conditions (e.g., cardiovascular disease, diabetes) is necessary. Adopting a multifactorial approach that considers the contributions of both neuro-vulnerability and other systemic factors can enhance our understanding of metabolic dysregulation.
- Lifestyle Interventions: Investigating the effects of lifestyle interventions, such as dietary modifications, physical activity, and behavioral interventions, on neuro-vulnerability and metabolic health is crucial. Understanding how lifestyle factors interact with neuro-vulnerability can inform evidence-based recommendations for preventing and managing metabolic disorders.
- Data Sharing and Collaboration: Encouraging data sharing and collaboration among researchers is vital for advancing our knowledge of neuro-vulnerability in energy metabolism regulation. Promoting open science practices and establishing collaborative networks can facilitate the pooling of resources, data, and expertise, leading to more comprehensive and impactful research outcomes.
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Traxler, L.; Lagerwall, J.; Eichhorner, S.; Stefanoni, D.; D’Alessandro, A.; Mertens, J. Metabolism Navigates Neural Cell Fate in Development, Aging and Neurodegeneration. Dis. Model. Mech. 2021, 14, dmm048993. [Google Scholar] [CrossRef] [PubMed]
- Mergenthaler, P.; Lindauer, U.; Dienel, G.A.; Meisel, A. Sugar for the Brain: The Role of Glucose in Physiological and Pathological Brain Function. Trends Neurosci. 2013, 36, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S. Metabolic Contribution and Cerebral Blood Flow Regulation by Astrocytes in the Neurovascular Unit. Cells 2022, 11, 813. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.A.; Farooqui, T.; Panza, F.; Frisardi, V. Metabolic Syndrome as a Risk Factor for Neurological Disorders. Cell. Mol. Life Sci. 2012, 69, 741–762. [Google Scholar] [CrossRef]
- Boguszewski, C.L.; Paz-Filho, G.; Velloso, L.A. Neuroendocrine Body Weight Regulation: Integration between Fat Tissue, Gastrointestinal Tract, and the Brain. Endokrynol. Pol. 2010, 61, 194–206. [Google Scholar]
- Skowron, K.; Kurnik-Łucka, M.; Dadański, E.; Bętkowska-Korpała, B.; Gil, K. Backstage of Eating Disorder-About the Biological Mechanisms behind the Symptoms of Anorexia Nervosa. Nutrients 2020, 12, 2604. [Google Scholar] [CrossRef]
- Kaye, W. Neurobiology of Anorexia and Bulimia Nervosa. Physiol. Behav. 2008, 94, 121–135. [Google Scholar] [CrossRef]
- Kaye, W.H.; Bailer, U.F. Understanding the Neural Circuitry of Appetitive Regulation in Eating Disorders. Biol. Psychiatry 2011, 70, 704–705. [Google Scholar] [CrossRef]
- Narayanaswami, V.; Dwoskin, L.P. Obesity: Current and Potential Pharmacotherapeutics and Targets. Pharmacol. Ther. 2017, 170, 116–147. [Google Scholar] [CrossRef]
- Cunnane, S.C.; Trushina, E.; Morland, C.; Prigione, A.; Casadesus, G.; Andrews, Z.B.; Beal, M.F.; Bergersen, L.H.; Brinton, R.D.; de la Monte, S.; et al. Brain Energy Rescue: An Emerging Therapeutic Concept for Neurodegenerative Disorders of Ageing. Nat. Rev. Drug Discov. 2020, 19, 609–633. [Google Scholar] [CrossRef]
- Bélanger, M.; Allaman, I.; Magistretti, P.J. Brain Energy Metabolism: Focus on Astrocyte-Neuron Metabolic Cooperation. Cell Metab. 2011, 14, 724–738. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef] [PubMed]
- Obel, L.F.; Müller, M.S.; Walls, A.B.; Sickmann, H.M.; Bak, L.K.; Waagepetersen, H.S.; Schousboe, A. Brain Glycogen-New Perspectives on Its Metabolic Function and Regulation at the Subcellular Level. Front. Neuroenergetics 2012, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.M. Brain Glycogen Re-Awakened. J. Neurochem. 2004, 89, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.A.; Carrasco, M.; Godoy, A.; Reinicke, K.; Montecinos, V.P.; Aguayo, L.G.; Tapia, J.C.; Vera, J.C.; Nualart, F. Elevated Expression of Glucose Transporter-1 in Hypothalamic Ependymal Cells Not Involved in the Formation of the Brain-Cerebrospinal Fluid Barrier. J. Cell. Biochem. 2001, 80, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.M.; Ransom, B.R. Astrocyte Glycogen and Brain Energy Metabolism. Glia 2007, 55, 1263–1271. [Google Scholar] [CrossRef]
- Wu, L. Perturbed Brain Energy Metabolism in Alzheimer’s Disease and Diabetes. Ph.D. Thesis, University of Kansas, Lawrence, KS, USA, 2017. [Google Scholar]
- Pellerin, L.; Bouzier-Sore, A.-K.; Aubert, A.; Serres, S.; Merle, M.; Costalat, R.; Magistretti, P.J. Activity-Dependent Regulation of Energy Metabolism by Astrocytes: An Update. Glia 2007, 55, 1251–1262. [Google Scholar] [CrossRef]
- Gentric, G.; Mieulet, V.; Mechta-Grigoriou, F. Heterogeneity in Cancer Metabolism: New Concepts in an Old Field. Antioxid. Redox Signal. 2017, 26, 462–485. [Google Scholar] [CrossRef] [PubMed]
- Wikstrom, J.D.; Twig, G.; Shirihai, O.S. What Can Mitochondrial Heterogeneity Tell Us about Mitochondrial Dynamics and Autophagy? Int. J. Biochem. Cell Biol. 2009, 41, 1914–1927. [Google Scholar] [CrossRef]
- Murali Mahadevan, H.; Hashemiaghdam, A.; Ashrafi, G.; Harbauer, A.B. Mitochondria in Neuronal Health: From Energy Metabolism to Parkinson’s Disease. Adv. Biol. 2021, 5, e2100663. [Google Scholar] [CrossRef]
- Vannucci, S.J.; Maher, F.; Simpson, I.A. Glucose Transporter Proteins in Brain: Delivery of Glucose to Neurons and Glia. Glia 1997, 21, 2–21. [Google Scholar] [CrossRef]
- Lalo, U.; Pankratov, Y.; Parpura, V.; Verkhratsky, A. Ionotropic Receptors in Neuronal-Astroglial Signalling: What Is the Role of “Excitable” Molecules in Non-Excitable Cells. Biochim. Biophys. Acta 2011, 1813, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Falkowska, A.; Gutowska, I.; Goschorska, M.; Nowacki, P.; Chlubek, D.; Baranowska-Bosiacka, I. Energy Metabolism of the Brain, Including the Cooperation between Astrocytes and Neurons, Especially in the Context of Glycogen Metabolism. Int. J. Mol. Sci. 2015, 16, 25959–25981. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.-C.; Huang, C.-J.; Chang, J.C.-H.; Teng, W.-Y.; Baba, O.; Fann, M.-J.; Hwang, P.-P. Glycogen Phosphorylase in Glycogen-Rich Cells Is Involved in the Energy Supply for Ion Regulation in Fish Gill Epithelia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R482–R491. [Google Scholar] [CrossRef] [PubMed]
- Zois, C.E.; Harris, A.L. Glycogen Metabolism Has a Key Role in the Cancer Microenvironment and Provides New Targets for Cancer Therapy. J. Mol. Med. 2016, 94, 137–154. [Google Scholar] [CrossRef]
- Hardie, D.G.; Carling, D.; Gamblin, S.J. AMP-Activated Protein Kinase: Also Regulated by ADP? Trends Biochem. Sci. 2011, 36, 470–477. [Google Scholar] [CrossRef]
- Oernbo, E.K.; Steffensen, A.B.; Razzaghi Khamesi, P.; Toft-Bertelsen, T.L.; Barbuskaite, D.; Vilhardt, F.; Gerkau, N.J.; Tritsaris, K.; Simonsen, A.H.; Lolansen, S.D.; et al. Membrane Transporters Control Cerebrospinal Fluid Formation Independently of Conventional Osmosis to Modulate Intracranial Pressure. Fluids Barriers CNS 2022, 19, 65. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, N.; Gupta, R.; Gupta, N. Pentose Phosphate Pathway. In Fundamentals of Bacterial Physiology and Metabolism; Springer: Singapore, 2021; pp. 289–305. [Google Scholar]
- Castro, M.A.; Angulo, C.; Brauchi, S.; Nualart, F.; Concha, I.I. Ascorbic Acid Participates in a General Mechanism for Concerted Glucose Transport Inhibition and Lactate Transport Stimulation. Pflugers Arch. 2008, 457, 519–528. [Google Scholar] [CrossRef]
- Castro, M.A.; Beltrán, F.A.; Brauchi, S.; Concha, I.I. A Metabolic Switch in Brain: Glucose and Lactate Metabolism Modulation by Ascorbic Acid. J. Neurochem. 2009, 110, 423–440. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Allaman, I. Lactate in the Brain: From Metabolic End-Product to Signalling Molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef]
- Duatti, A. Lactate-Induced COL1A1/DDR1 Axis Promotes Prostate Cancer Aggressiveness and Enhances Metastatic Colonization. Ph.D. Thesis, Università di Siena, Siena, Italy, 2023. [Google Scholar]
- Wu, C.; Khan, S.A.; Peng, L.-J.; Lange, A.J. Roles for Fructose-2,6-Bisphosphate in the Control of Fuel Metabolism: Beyond Its Allosteric Effects on Glycolytic and Gluconeogenic Enzymes. Adv. Enzyme Regul. 2006, 46, 72–88. [Google Scholar] [CrossRef]
- Allaman, I.; Bélanger, M.; Magistretti, P.J. Methylglyoxal, the Dark Side of Glycolysis. Front. Neurosci. 2015, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Pestana, F.; Edwards-Faret, G.; Belgard, T.G.; Martirosyan, A.; Holt, M.G. No Longer Underappreciated: The Emerging Concept of Astrocyte Heterogeneity in Neuroscience. Brain Sci. 2020, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.C.; Rothstein, J.D.; Sontheimer, H. Compromised Glutamate Transport in Human Glioma Cells: Reduction-Mislocalization of Sodium-Dependent Glutamate Transporters and Enhanced Activity of Cystine-Glutamate Exchange. J. Neurosci. 1999, 19, 10767–10777. [Google Scholar] [CrossRef]
- Pellerin, L. How Astrocytes Feed Hungry Neurons. Mol. Neurobiol. 2005, 32, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Díaz-García, C.M.; Mongeon, R.; Lahmann, C.; Koveal, D.; Zucker, H.; Yellen, G. Neuronal Stimulation Triggers Neuronal Glycolysis and Not Lactate Uptake. Cell Metab. 2017, 26, 361–374.e4. [Google Scholar] [CrossRef] [PubMed]
- Bolaños, J.P.; Almeida, A. The Pentose-Phosphate Pathway in Neuronal Survival against Nitrosative Stress. IUBMB Life 2010, 62, 14–18. [Google Scholar] [CrossRef]
- Martín, E.D.; Fernández, M.; Perea, G.; Pascual, O.; Haydon, P.G.; Araque, A.; Ceña, V. Adenosine Released by Astrocytes Contributes to Hypoxia-Induced Modulation of Synaptic Transmission. Glia 2007, 55, 36–45. [Google Scholar] [CrossRef]
- Kambe, Y. Recent Behavioral Findings of Pathophysiological Involvement of Lactate in the Central Nervous System. Biochim. Biophys. Acta Gen. Subj. 2022, 1866, 130137. [Google Scholar] [CrossRef]
- Maurer, M.H.; Canis, M.; Kuschinsky, W.; Duelli, R. Correlation between Local Monocarboxylate Transporter 1 (MCT1) and Glucose Transporter 1 (GLUT1) Densities in the Adult Rat Brain. Neurosci. Lett. 2004, 355, 105–108. [Google Scholar] [CrossRef]
- Hertz, L.; Dienel, G.A. Lactate Transport and Transporters: General Principles and Functional Roles in Brain Cells. J. Neurosci. Res. 2005, 79, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Arevalo, J.A.; Osmond, A.D.; Leija, R.G.; Curl, C.C.; Tovar, A.P. Lactate in Contemporary Biology: A Phoenix Risen. J. Physiol. 2022, 600, 1229–1251. [Google Scholar] [CrossRef]
- Vijay, N.; Morris, M.E. Role of Monocarboxylate Transporters in Drug Delivery to the Brain. Curr. Pharm. Des. 2014, 20, 1487–1498. [Google Scholar] [CrossRef]
- Sugino, K.; Clark, E.; Schulmann, A.; Shima, Y.; Wang, L.; Hunt, D.L.; Hooks, B.M.; Tränkner, D.; Chandrashekar, J.; Picard, S.; et al. Mapping the Transcriptional Diversity of Genetically and Anatomically Defined Cell Populations in the Mouse Brain. Elife 2019, 8, e38619. [Google Scholar] [CrossRef]
- Frishberg, A.; Peshes-Yaloz, N.; Cohn, O.; Rosentul, D.; Steuerman, Y.; Valadarsky, L.; Yankovitz, G.; Mandelboim, M.; Iraqi, F.A.; Amit, I.; et al. Cell Composition Analysis of Bulk Genomics Using Single-Cell Data. Nat. Methods 2019, 16, 327–332. [Google Scholar] [CrossRef]
- Uhlen, M.; Karlsson, M.J.; Zhong, W.; Tebani, A.; Pou, C.; Mikes, J.; Lakshmikanth, T.; Forsström, B.; Edfors, F.; Odeberg, J.; et al. A Genome-Wide Transcriptomic Analysis of Protein-Coding Genes in Human Blood Cells. Science 2019, 366, eaax9198. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, J.A.; Kavanagh, E.; Engskog-Vlachos, P.; Engskog, M.K.R.; Herrera, A.J.; Espinosa-Oliva, A.M.; Joseph, B.; Hajji, N.; Venero, J.L.; Burguillos, M.A. Microglia: Agents of the CNS Pro-Inflammatory Response. Cells 2020, 9, 1717. [Google Scholar] [CrossRef]
- Marschallinger, J.; Iram, T.; Zardeneta, M.; Lee, S.E.; Lehallier, B.; Haney, M.S.; Pluvinage, J.V.; Mathur, V.; Hahn, O.; Morgens, D.W.; et al. Lipid-Droplet-Accumulating Microglia Represent a Dysfunctional and Proinflammatory State in the Aging Brain. Nat. Neurosci. 2020, 23, 194–208. [Google Scholar] [CrossRef]
- Fan, H.; Tang, H.-B.; Chen, Z.; Wang, H.-Q.; Zhang, L.; Jiang, Y.; Li, T.; Yang, C.-F.; Wang, X.-Y.; Li, X.; et al. Inhibiting HMGB1-RAGE Axis Prevents pro-Inflammatory Macrophages/Microglia Polarization and Affords Neuroprotection after Spinal Cord Injury. J. Neuroinflamm. 2020, 17, 295. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Xue, M.; Yong, V.W. Microglia and Macrophage Phenotypes in Intracerebral Haemorrhage Injury: Therapeutic Opportunities. Brain 2020, 143, 1297–1314. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, Y.; Wang, C.; Han, T.; Liu, H.; Sun, L.; Hong, J.; Hashimoto, M.; Wei, J. The Reciprocal Interactions between Microglia and T Cells in Parkinson’s Disease: A Double-Edged Sword. J. Neuroinflammation 2023, 20, 33. [Google Scholar] [CrossRef]
- Masuda, T.; Sankowski, R.; Staszewski, O.; Prinz, M. Microglia Heterogeneity in the Single-Cell Era. Cell Rep. 2020, 30, 1271–1281. [Google Scholar] [CrossRef]
- Borst, K.; Dumas, A.A.; Prinz, M. Microglia: Immune and Non-Immune Functions. Immunity 2021, 54, 2194–2208. [Google Scholar] [CrossRef] [PubMed]
- Olah, M.; Menon, V.; Habib, N.; Taga, M.F.; Ma, Y.; Yung, C.J.; Cimpean, M.; Khairallah, A.; Coronas-Samano, G.; Sankowski, R.; et al. Single Cell RNA Sequencing of Human Microglia Uncovers a Subset Associated with Alzheimer’s Disease. Nat. Commun. 2020, 11, 6129. [Google Scholar] [CrossRef]
- Chen, X.; Firulyova, M.; Manis, M.; Herz, J.; Smirnov, I.; Aladyeva, E.; Wang, C.; Bao, X.; Finn, M.B.; Hu, H.; et al. Microglia-Mediated T Cell Infiltration Drives Neurodegeneration in Tauopathy. Nature 2023, 615, 668–677. [Google Scholar] [CrossRef]
- Burmeister, A.R.; Johnson, M.B.; Marriott, I. Murine Astrocytes Are Responsive to the Pro-Inflammatory Effects of IL-20. Neurosci. Lett. 2019, 708, 134334. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Yao, L. The Role of Inflammation in Epileptogenesis. Acta Epileptol. 2020, 2, 15. [Google Scholar] [CrossRef]
- Araki, T.; Ikegaya, Y.; Koyama, R. The Effects of Microglia- and Astrocyte-Derived Factors on Neurogenesis in Health and Disease. Eur. J. Neurosci. 2021, 54, 5880–5901. [Google Scholar] [CrossRef] [PubMed]
- MacMahon Copas, A.N.; McComish, S.F.; Fletcher, J.M.; Caldwell, M.A. The Pathogenesis of Parkinson’s Disease: A Complex Interplay Between Astrocytes, Microglia, and T Lymphocytes? Front. Neurol. 2021, 12, 666737. [Google Scholar] [CrossRef]
- Gharagozloo, M.; Smith, M.D.; Jin, J.; Garton, T.; Taylor, M.; Chao, A.; Meyers, K.; Kornberg, M.D.; Zack, D.J.; Ohayon, J.; et al. Complement Component 3 from Astrocytes Mediates Retinal Ganglion Cell Loss during Neuroinflammation. Acta Neuropathol. 2021, 142, 899–915. [Google Scholar] [CrossRef]
- Raphael, I.; Gomez-Rivera, F.; Raphael, R.A.; Robinson, R.R.; Nalawade, S.; Forsthuber, T.G. TNFR2 Limits Proinflammatory Astrocyte Functions during EAE Induced by Pathogenic DR2b-Restricted T Cells. JCI Insight 2019, 4, e132527. [Google Scholar] [CrossRef]
- Giovannoni, F.; Quintana, F.J. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020, 41, 805–819. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Hamblin, M.R.; Abrahamse, H. Differentiation of Mesenchymal Stem Cells to Neuroglia: In the Context of Cell Signalling. Stem Cell Rev. Rep. 2019, 15, 814–826. [Google Scholar] [CrossRef]
- Bakaeva, Z.; Goncharov, M.; Krasilnikova, I.; Zgodova, A.; Frolov, D.; Grebenik, E.; Timashev, P.; Pinelis, V.; Surin, A. Acute and Delayed Effects of Mechanical Injury on Calcium Homeostasis and Mitochondrial Potential of Primary Neuroglial Cell Culture: Potential Causal Contributions to Post-Traumatic Syndrome. Int. J. Mol. Sci. 2022, 23, 3858. [Google Scholar] [CrossRef]
- Wu, Q.; Kong, D.; Peng, W.; Zong, R.; Yu, X.; Feng, S. Deciphering the Biochemical Similarities and Differences Among Human Neuroglial Cells and Glioma Cells Using Fourier Transform Infrared Spectroscopy. World Neurosurg. 2022, 168, e562–e569. [Google Scholar] [CrossRef]
- van Beek, A.A.; Van den Bossche, J.; Mastroberardino, P.G.; de Winther, M.P.J.; Leenen, P.J.M. Metabolic Alterations in Aging Macrophages: Ingredients for Inflammaging? Trends Immunol. 2019, 40, 113–127. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H.M. Redox Metabolism: ROS as Specific Molecular Regulators of Cell Signaling and Function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef] [PubMed]
- Greco, E.A.; Lenzi, A.; Migliaccio, S.; Gessani, S. Epigenetic Modifications Induced by Nutrients in Early Life Phases: Gender Differences in Metabolic Alteration in Adulthood. Front. Genet. 2019, 10, 795. [Google Scholar] [CrossRef] [PubMed]
- Kazemian, N.; Mahmoudi, M.; Halperin, F.; Wu, J.C.; Pakpour, S. Gut Microbiota and Cardiovascular Disease: Opportunities and Challenges. Microbiome 2020, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Carbajal, A.; Nirmalkar, K.; Pérez-Lizaur, A.; Hernández-Quiroz, F.; Ramírez-Del-Alto, S.; García-Mena, J.; Hernández-Guerrero, C. Gut Microbiota and Predicted Metabolic Pathways in a Sample of Mexican Women Affected by Obesity and Obesity Plus Metabolic Syndrome. Int. J. Mol. Sci. 2019, 20, 438. [Google Scholar] [CrossRef]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of Oxidative Stress in Metabolic Syndrome and Associated Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 8267234. [Google Scholar] [CrossRef] [PubMed]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Son, D.-H.; Lee, H.S.; Lee, Y.-J.; Lee, J.-H.; Han, J.-H. Comparison of Triglyceride-Glucose Index and HOMA-IR for Predicting Prevalence and Incidence of Metabolic Syndrome. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 596–604. [Google Scholar] [CrossRef]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Ruga, S.; Zito, M.C.; et al. From Metabolic Syndrome to Neurological Diseases: Role of Autophagy. Front. Cell Dev. Biol. 2021, 9, 651021. [Google Scholar] [CrossRef]
- Barber, T.M.; Kyrou, I.; Randeva, H.S.; Weickert, M.O. Mechanisms of Insulin Resistance at the Crossroad of Obesity with Associated Metabolic Abnormalities and Cognitive Dysfunction. Int. J. Mol. Sci. 2021, 22, 546. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Ryan, P.M.; Wiley, N.; Carafa, I.; Sherwin, E.; Moloney, G.; Franciosi, E.; Mandal, R.; Wishart, D.S.; Tuohy, K.; et al. Gamma-Aminobutyric Acid-Producing Lactobacilli Positively Affect Metabolism and Depressive-like Behaviour in a Mouse Model of Metabolic Syndrome. Sci. Rep. 2019, 9, 16323. [Google Scholar] [CrossRef]
- Polis, B.; Samson, A.O. Role of the Metabolism of Branched-Chain Amino Acids in the Development of Alzheimer’s Disease and Other Metabolic Disorders. Neural Regen. Res. 2020, 15, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Cang, H.; Guo, B. Crosstalk between Cancer and Immune Cells: Role of Tumor-Associated Macrophages in the Tumor Microenvironment. Cancer Med. 2019, 8, 4709–4721. [Google Scholar] [CrossRef]
- Schwager, S.; Detmar, M. Inflammation and Lymphatic Function. Front. Immunol. 2019, 10, 308. [Google Scholar] [CrossRef]
- Barker, P.A.; Mantyh, P.; Arendt-Nielsen, L.; Viktrup, L.; Tive, L. Nerve Growth Factor Signaling and Its Contribution to Pain. J. Pain Res. 2020, 13, 1223–1241. [Google Scholar] [CrossRef]
- Delzenne, N.M.; Knudsen, C.; Beaumont, M.; Rodriguez, J.; Neyrinck, A.M.; Bindels, L.B. Contribution of the Gut Microbiota to the Regulation of Host Metabolism and Energy Balance: A Focus on the Gut-Liver Axis. Proc. Nutr. Soc. 2019, 78, 319–328. [Google Scholar] [CrossRef]
- Coll, A.P.; Chen, M.; Taskar, P.; Rimmington, D.; Patel, S.; Tadross, J.A.; Cimino, I.; Yang, M.; Welsh, P.; Virtue, S.; et al. GDF15 Mediates the Effects of Metformin on Body Weight and Energy Balance. Nature 2020, 578, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Farooqi, I.S.; Friedman, J.M.; Klein, S.; Loos, R.J.F.; Mangelsdorf, D.J.; O’Rahilly, S.; Ravussin, E.; Redman, L.M.; Ryan, D.H.; et al. The Energy Balance Model of Obesity: Beyond Calories in, Calories Out. Am. J. Clin. Nutr. 2022, 115, 1243–1254. [Google Scholar] [CrossRef]
- Bitzer-Quintero, O.K.; Ortiz, G.G.; Jaramillo-Bueno, S.; Ramos-González, E.J.; Márquez-Rosales, M.G.; Delgado-Lara, D.L.C.; Torres-Sánchez, E.D.; Tejeda-Martínez, A.R.; Ramirez-Jirano, J. Psycho-Neuro-Endocrine-Immunology: A Role for Melatonin in This New Paradigm. Molecules 2022, 27, 4888. [Google Scholar] [CrossRef]
- Jänig, W. The Integrative Action of the Autonomic Nervous System: Neurobiology of Homeostasis; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Dinarello, C.A. Proinflammatory Cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Helle, K.B. The Granin Family of Uniquely Acidic Proteins of the Diffuse Neuroendocrine System: Comparative and Functional Aspects. Biol. Rev. Camb. Philos. Soc. 2004, 79, 769–794. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.R. Proinflammatory Cytokines and Osteoporosis. Curr. Osteoporos. Rep. 2009, 7, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Strober, W.; Fuss, I.J. Proinflammatory Cytokines in the Pathogenesis of Inflammatory Bowel Diseases. Gastroenterology 2011, 140, 1756–1767. [Google Scholar] [CrossRef]
- Wieseler-Frank, J.; Maier, S.F.; Watkins, L.R. Central Proinflammatory Cytokines and Pain Enhancement. Neurosignals 2005, 14, 166–174. [Google Scholar] [CrossRef]
- Maley, B.E. Immunohistochemical Localization of Neuropeptides and Neurotransmitters in the Nucleus Solitarius. Chem. Senses 1996, 21, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Shi, X.; Li, X.; Zou, J.; Zhou, C.; Liu, W.; Shao, H.; Chen, H.; Shi, L. Neurotransmitter and Neuropeptide Regulation of Mast Cell Function: A Systematic Review. J. Neuroinflamm. 2020, 17, 356. [Google Scholar] [CrossRef] [PubMed]
- Benomar, Y.; Taouis, M. Molecular Mechanisms Underlying Obesity-Induced Hypothalamic Inflammation and Insulin Resistance: Pivotal Role of Resistin/TLR4 Pathways. Front. Endocrinol. 2019, 10, 140. [Google Scholar] [CrossRef]
- Ono, H. Molecular Mechanisms of Hypothalamic Insulin Resistance. Int. J. Mol. Sci. 2019, 20, 1317. [Google Scholar] [CrossRef]
- Abuzzahab, M.J.; Roth, C.L.; Shoemaker, A.H. Hypothalamic Obesity: Prologue and Promise. Horm. Res. Paediatr. 2019, 91, 128–136. [Google Scholar] [CrossRef]
- Friedman, J.M. Leptin and the Endocrine Control of Energy Balance. Nat. Metab. 2019, 1, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Poetsch, M.S.; Strano, A.; Guan, K. Role of Leptin in Cardiovascular Diseases. Front. Endocrinol. 2020, 11, 354. [Google Scholar] [CrossRef]
- Varkaneh Kord, H.; Tinsley, G.; Santos, H.O.; Zand, H.; Nazary, A.; Fatahi, S.; Mokhtari, Z.; Salehi-Sahlabadi, A.; Tan, S.C.; Rahmani, J.; et al. The Influence of Fasting and Energy-Restricted Diets on Leptin and Adiponectin Levels in Humans: A Systematic Review and Meta-Analysis. Clin. Nutr. 2021, 40, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Dallner, O.S.; Marinis, J.M.; Lu, Y.-H.; Birsoy, K.; Werner, E.; Fayzikhodjaeva, G.; Dill, B.D.; Molina, H.; Moscati, A.; Kutalik, Z.; et al. Dysregulation of a Long Noncoding RNA Reduces Leptin Leading to a Leptin-Responsive Form of Obesity. Nat. Med. 2019, 25, 507–516. [Google Scholar] [CrossRef]
- Butiaeva, L.I.; Slutzki, T.; Swick, H.E.; Bourguignon, C.; Robins, S.C.; Liu, X.; Storch, K.-F.; Kokoeva, M.V. Leptin Receptor-Expressing Pericytes Mediate Access of Hypothalamic Feeding Centers to Circulating Leptin. Cell Metab. 2021, 33, 1433–1448.e5. [Google Scholar] [CrossRef]
- Trinh, T.; Broxmeyer, H.E. Role for Leptin and Leptin Receptors in Stem Cells During Health and Diseases. Stem Cell Rev. Rep. 2021, 17, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Bertucci, J.I.; Blanco, A.M.; Sundarrajan, L.; Rajeswari, J.J.; Velasco, C.; Unniappan, S. Nutrient Regulation of Endocrine Factors Influencing Feeding and Growth in Fish. Front. Endocrinol. 2019, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Régnier, M.; Van Hul, M.; Knauf, C.; Cani, P.D. Gut Microbiome, Endocrine Control of Gut Barrier Function and Metabolic Diseases. J. Endocrinol. 2021, 248, R67–R82. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.M.; Qualls-Creekmore, E.; Berthoud, H.-R.; Soto, P.; Yu, S.; McDougal, D.H.; Münzberg, H.; Morrison, C.D. FGF21 and the Physiological Regulation of Macronutrient Preference. Endocrinology 2020, 161, bqaa019. [Google Scholar] [CrossRef]
- Becattini, B.; Molinaro, A.; Henricsson, M.; Boren, J.; Solinas, G. Adipocyte PI3K Links Adipostasis with Insulin Secretion through an Adipoincretin Effect. bioRxiv 2023, 2004–2023. [Google Scholar] [CrossRef]
- Ghadge, A.A.; Khaire, A.A. Leptin as a Predictive Marker for Metabolic Syndrome. Cytokine 2019, 121, 154735. [Google Scholar] [CrossRef]
- Spezani, R.; da Silva, R.R.; Martins, F.F.; de Souza Marinho, T.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Intermittent Fasting, Adipokines, Insulin Sensitivity, and Hypothalamic Neuropeptides in a Dietary Overload with High-Fat or High-Fructose Diet in Mice. J. Nutr. Biochem. 2020, 83, 108419. [Google Scholar] [CrossRef]
- García-Carrizo, F.; Picó, C.; Rodríguez, A.M.; Palou, A. High-Esterified Pectin Reverses Metabolic Malprogramming, Improving Sensitivity to Adipostatic/Adipokine Hormones. J. Agric. Food Chem. 2019, 67, 3633–3642. [Google Scholar] [CrossRef]
- Deloose, E.; Janssen, P.; Depoortere, I.; Tack, J. The Migrating Motor Complex: Control Mechanisms and Its Role in Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 271–285. [Google Scholar] [CrossRef]
- Sakahara, S.; Xie, Z.; Koike, K.; Hoshino, S.; Sakata, I.; Oda, S.; Takahashi, T.; Sakai, T. Physiological Characteristics of Gastric Contractions and Circadian Gastric Motility in the Free-Moving Conscious House Musk Shrew (Suncus murinus). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1106–R1113. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Kawamoto, Y.; Yanaka, T.; Tsutsui, C.; Sakata, I.; Oda, S.-I.; Tanaka, T.; Sakai, T. Myenteric Neural Network Activated by Motilin in the Stomach of Suncus murinus (House Musk Shrew). Neurogastroenterol. Motil. 2011, 23, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Xie, Z.; Miyano, Y.; Tsutsui, C.; Sakata, I.; Kawamoto, Y.; Aizawa, S.; Tanaka, T.; Oda, S.; Sakai, T. Coordination of Motilin and Ghrelin Regulates the Migrating Motor Complex of Gastrointestinal Motility in Suncus murinus. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1207–G1215. [Google Scholar] [CrossRef] [PubMed]
- Peeters, T.L.; Vantrappen, G.; Janssens, J. Fasting Plasma Motilin Levels Are Related to the Interdigestive Motility Complex. Gastroenterology 1980, 79, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Janssens, J.; Vantrappen, G.; Peeters, T.L. The Activity Front of the Migrating Motor Complex of the Human Stomach but Not of the Small Intestine Is Motilin-Dependent. Regul. Pept. 1983, 6, 363–369. [Google Scholar] [CrossRef]
- Tack, J.; Depoortere, I.; Bisschops, R.; Delporte, C.; Coulie, B.; Meulemans, A.; Janssens, J.; Peeters, T. Influence of Ghrelin on Interdigestive Gastrointestinal Motility in Humans. Gut 2006, 55, 327–333. [Google Scholar] [CrossRef]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin Is a Growth-Hormone-Releasing Acylated Peptide from Stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- Wren, A.M.; Small, C.J.; Ward, H.L.; Murphy, K.G.; Dakin, C.L.; Taheri, S.; Kennedy, A.R.; Roberts, G.H.; Morgan, D.G.; Ghatei, M.A.; et al. The Novel Hypothalamic Peptide Ghrelin Stimulates Food Intake and Growth Hormone Secretion. Endocrinology 2000, 141, 4325–4328. [Google Scholar] [CrossRef]
- Tschöp, M.; Smiley, D.L.; Heiman, M.L. Ghrelin Induces Adiposity in Rodents. Nature 2000, 407, 908–913. [Google Scholar] [CrossRef]
- Date, Y.; Shimbara, T.; Koda, S.; Toshinai, K.; Ida, T.; Murakami, N.; Miyazato, M.; Kokame, K.; Ishizuka, Y.; Ishida, Y.; et al. Peripheral Ghrelin Transmits Orexigenic Signals through the Noradrenergic Pathway from the Hindbrain to the Hypothalamus. Cell Metab. 2006, 4, 323–331. [Google Scholar] [CrossRef]
- Kojima, M.; Hosoda, H.; Kangawa, K. Purification and Distribution of Ghrelin: The Natural Endogenous Ligand for the Growth Hormone Secretagogue Receptor. Horm. Res. 2001, 56 (Suppl. S1), 93–97. [Google Scholar] [CrossRef]
- Deloose, E.; Vos, R.; Corsetti, M.; Depoortere, I.; Tack, J. Endogenous Motilin, but Not Ghrelin Plasma Levels Fluctuate in Accordance with Gastric Phase III Activity of the Migrating Motor Complex in Man. Neurogastroenterol. Motil. 2015, 27, 63–71. [Google Scholar] [CrossRef]
- Tack, J.; Deloose, E.; Ang, D.; Scarpellini, E.; Vanuytsel, T.; Van Oudenhove, L.; Depoortere, I. Motilin-Induced Gastric Contractions Signal Hunger in Man. Gut 2016, 65, 214–224. [Google Scholar] [CrossRef]
- Deloose, E.; Vos, R.; Janssen, P.; Van den Bergh, O.; Van Oudenhove, L.; Depoortere, I.; Tack, J. The Motilin Receptor Agonist Erythromycin Stimulates Hunger and Food Intake through a Cholinergic Pathway. Am. J. Clin. Nutr. 2016, 103, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Hequing, H.; Mondal, A.; Yoshimura, M.; Ito, K.; Mikami, T.; Takemi, S.; Jogahara, T.; Sakata, I.; Sakai, T. Ghrelin Is an Essential Factor for Motilin-Induced Gastric Contraction in Suncus murinus. Endocrinology 2015, 156, 4437–4447. [Google Scholar] [CrossRef] [PubMed]
- Tschöp, M.; Weyer, C.; Tataranni, P.A.; Devanarayan, V.; Ravussin, E.; Heiman, M.L. Circulating Ghrelin Levels Are Decreased in Human Obesity. Diabetes 2001, 50, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.K.; Dall, R.; Hosoda, H.; Kojima, M.; Kangawa, K.; Christiansen, J.S.; Jørgensen, J.O.L. Weight Loss Increases Circulating Levels of Ghrelin in Human Obesity. Clin. Endocrinol. 2002, 56, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Otto, B.; Cuntz, U.; Fruehauf, E.; Wawarta, R.; Folwaczny, C.; Riepl, R.L.; Heiman, M.L.; Lehnert, P.; Fichter, M.; Tschöp, M. Weight Gain Decreases Elevated Plasma Ghrelin Concentrations of Patients with Anorexia Nervosa. Eur. J. Endocrinol. 2001, 145, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J. The Physiology of Glucagon-like Peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef]
- Drucker, D.J.; Habener, J.F.; Holst, J.J. Discovery, Characterization, and Clinical Development of the Glucagon-like Peptides. J. Clin. Investig. 2017, 127, 4217–4227. [Google Scholar] [CrossRef]
- Nogueiras, R.; Pérez-Tilve, D.; Veyrat-Durebex, C.; Morgan, D.A.; Varela, L.; Haynes, W.G.; Patterson, J.T.; Disse, E.; Pfluger, P.T.; López, M.; et al. Direct Control of Peripheral Lipid Deposition by CNS GLP-1 Receptor Signaling Is Mediated by the Sympathetic Nervous System and Blunted in Diet-Induced Obesity. J. Neurosci. 2009, 29, 5916–5925. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Glucagon-like Peptide-1 Receptors in the Brain: Controlling Food Intake and Body Weight. J. Clin. Investig. 2014, 124, 4223–4226. [Google Scholar] [CrossRef]
- Gaykema, R.P.; Newmyer, B.A.; Ottolini, M.; Raje, V.; Warthen, D.M.; Lambeth, P.S.; Niccum, M.; Yao, T.; Huang, Y.; Schulman, I.G.; et al. Activation of Murine Pre-Proglucagon-Producing Neurons Reduces Food Intake and Body Weight. J. Clin. Investig. 2017, 127, 1031–1045. [Google Scholar] [CrossRef]
- Wang, X.-F.; Liu, J.-J.; Xia, J.; Liu, J.; Mirabella, V.; Pang, Z.P. Endogenous Glucagon-like Peptide-1 Suppresses High-Fat Food Intake by Reducing Synaptic Drive onto Mesolimbic Dopamine Neurons. Cell Rep. 2015, 12, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Reiner, D.J.; Leon, R.M.; McGrath, L.E.; Koch-Laskowski, K.; Hahn, J.D.; Kanoski, S.E.; Mietlicki-Baase, E.G.; Hayes, M.R. Glucagon-like Peptide-1 Receptor Signaling in the Lateral Dorsal Tegmental Nucleus Regulates Energy Balance. Neuropsychopharmacology 2018, 43, 627–637. [Google Scholar] [CrossRef]
- Terrill, S.J.; Jackson, C.M.; Greene, H.E.; Lilly, N.; Maske, C.B.; Vallejo, S.; Williams, D.L. Role of Lateral Septum Glucagon-like Peptide 1 Receptors in Food Intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R124–R132. [Google Scholar] [CrossRef] [PubMed]
- Ong, Z.Y.; Liu, J.-J.; Pang, Z.P.; Grill, H.J. Paraventricular Thalamic Control of Food Intake and Reward: Role of Glucagon-Like Peptide-1 Receptor Signaling. Neuropsychopharmacology 2017, 42, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, J.F.; Friis-Hansen, L.; Goetze, J.P.; Hansen, T.V.O. The Biology of Cholecystokinin and Gastrin Peptides. Curr. Top. Med. Chem. 2007, 7, 1154–1165. [Google Scholar] [CrossRef]
- Chaudhri, O.; Small, C.; Bloom, S. Gastrointestinal Hormones Regulating Appetite. Philos. Trans. R. Soc. B 2006, 361, 1187–1209. [Google Scholar] [CrossRef]
- Crawley, J.N.; Corwin, R.L. Biological Actions of Cholecystokinin. Peptides 1994, 15, 731–755. [Google Scholar] [CrossRef]
- Asin, K.E.; Bednarz, L.; Nikkel, A.L.; Gore, P.A.J.; Nadzan, A.M. A-71623, a Selective CCK-A Receptor Agonist, Suppresses Food Intake in the Mouse, Dog, and Monkey. Pharmacol. Biochem. Behav. 1992, 42, 699–704. [Google Scholar] [CrossRef]
- D’Agostino, G.; Lyons, D.J.; Cristiano, C.; Burke, L.K.; Madara, J.C.; Campbell, J.N.; Garcia, A.P.; Land, B.B.; Lowell, B.B.; Dileone, R.J.; et al. Appetite Controlled by a Cholecystokinin Nucleus of the Solitary Tract to Hypothalamus Neurocircuit. Elife 2016, 5, e12225. [Google Scholar] [CrossRef] [PubMed]
- Peikin, S.R. Role of Cholecystokinin in the Control of Food Intake. Gastroenterol. Clin. N. Am. 1989, 18, 757–775. [Google Scholar] [CrossRef]
- Boden, G.; Chen, X.; Mozzoli, M.; Ryan, I. Effect of Fasting on Serum Leptin in Normal Human Subjects. J. Clin. Endocrinol. Metab. 1996, 81, 3419–3423. [Google Scholar] [CrossRef] [PubMed]
- Bjørbaek, C.; Elmquist, J.K.; Michl, P.; Ahima, R.S.; van Bueren, A.; McCall, A.L.; Flier, J.S. Expression of Leptin Receptor Isoforms in Rat Brain Microvessels. Endocrinology 1998, 139, 3485–3491. [Google Scholar] [CrossRef]
- Elmquist, J.K.; Bjørbaek, C.; Ahima, R.S.; Flier, J.S.; Saper, C.B. Distributions of Leptin Receptor MRNA Isoforms in the Rat Brain. J. Comp. Neurol. 1998, 395, 535–547. [Google Scholar] [CrossRef]
- Bates, S.H.; Stearns, W.H.; Dundon, T.A.; Schubert, M.; Tso, A.W.K.; Wang, Y.; Banks, A.S.; Lavery, H.J.; Haq, A.K.; Maratos-Flier, E.; et al. STAT3 Signalling Is Required for Leptin Regulation of Energy Balance but Not Reproduction. Nature 2003, 421, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, I.S.; Bullmore, E.; Keogh, J.; Gillard, J.; O’Rahilly, S.; Fletcher, P.C. Leptin Regulates Striatal Regions and Human Eating Behavior. Science 2007, 317, 1355. [Google Scholar] [CrossRef]
- Münzberg, H.; Qualls-Creekmore, E.; Berthoud, H.-R.; Morrison, C.D.; Yu, S. Neural Control of Energy Expenditure. Handb. Exp. Pharmacol. 2016, 233, 173–194. [Google Scholar] [CrossRef]
- Krashes, M.J.; Shah, B.P.; Madara, J.C.; Olson, D.P.; Strochlic, D.E.; Garfield, A.S.; Vong, L.; Pei, H.; Watabe-Uchida, M.; Uchida, N.; et al. An Excitatory Paraventricular Nucleus to AgRP Neuron Circuit That Drives Hunger. Nature 2014, 507, 238–242. [Google Scholar] [CrossRef]
- Tucker, J.A.L.; Bornath, D.P.D.; McCarthy, S.F.; Hazell, T.J. Leptin and Energy Balance: Exploring Leptin’s Role in the Regulation of Energy Intake and Energy Expenditure. Nutr. Neurosci. 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kullmann, S.; Kleinridders, A.; Small, D.M.; Fritsche, A.; Häring, H.-U.; Preissl, H.; Heni, M. Central Nervous Pathways of Insulin Action in the Control of Metabolism and Food Intake. Lancet Diabetes Endocrinol. 2020, 8, 524–534. [Google Scholar] [CrossRef]
- Walczak, K.; Sieminska, L. Obesity and Thyroid Axis. Int. J. Environ. Res. Public Health 2021, 18, 9434. [Google Scholar] [CrossRef]
- Hewagalamulage, S.D.; Lee, T.K.; Clarke, I.J.; Henry, B.A. Stress, Cortisol, and Obesity: A Role for Cortisol Responsiveness in Identifying Individuals Prone to Obesity. Domest. Anim. Endocrinol. 2016, 56, S112–S120. [Google Scholar] [CrossRef]
- Coppari, R.; Bjørbæk, C. Leptin Revisited: Its Mechanism of Action and Potential for Treating Diabetes. Nat. Rev. Drug Discov. 2012, 11, 692–708. [Google Scholar] [CrossRef]
- Myers, M.G.; Cowley, M.A.; Münzberg, H. Mechanisms of Leptin Action and Leptin Resistance. Annu. Rev. Physiol. 2008, 70, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Elmquist, J.K.; Elias, C.F.; Saper, C.B. From Lesions to Leptin: Hypothalamic Control of Food Intake and Body Weight. Neuron 1999, 22, 221–232. [Google Scholar] [CrossRef]
- Liu, L.; Karkanias, G.B.; Morales, J.C.; Hawkins, M.; Barzilai, N.; Wang, J.; Rossetti, L. Intracerebroventricular Leptin Regulates Hepatic but Not Peripheral Glucose Fluxes. J. Biol. Chem. 1998, 273, 31160–31167. [Google Scholar] [CrossRef]
- Heilig, M.; Widerlöv, E. Neuropeptide Y: An Overview of Central Distribution, Functional Aspects, and Possible Involvement in Neuropsychiatric Illnesses. Acta Psychiatr. Scand. 1990, 82, 95–114. [Google Scholar] [CrossRef]
- Kuo, L.E.; Kitlinska, J.B.; Tilan, J.U.; Li, L.; Baker, S.B.; Johnson, M.D.; Lee, E.W.; Burnett, M.S.; Fricke, S.T.; Kvetnansky, R.; et al. Neuropeptide Y Acts Directly in the Periphery on Fat Tissue and Mediates Stress-Induced Obesity and Metabolic Syndrome. Nat. Med. 2007, 13, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Desai, D. Physiological and Therapeutic Roles of Neuropeptide Y on Biological Functions. Adv. Exp. Med. Biol. 2020, 1237, 37–47. [Google Scholar] [CrossRef]
- Mani, B.K.; Shankar, K.; Zigman, J.M. Ghrelin’s Relationship to Blood Glucose. Endocrinology 2019, 160, 1247–1261. [Google Scholar] [CrossRef]
- Müller, T.D.; Nogueiras, R.; Andermann, M.L.; Andrews, Z.B.; Anker, S.D.; Argente, J.; Batterham, R.L.; Benoit, S.C.; Bowers, C.Y.; Broglio, F. Ghrelin. Mol. Metab. 2015, 4, 437–460. [Google Scholar] [CrossRef]
- Cummings, D.E.; Purnell, J.Q.; Frayo, R.S.; Schmidova, K.; Wisse, B.E.; Weigle, D.S. A Preprandial Rise in Plasma Ghrelin Levels Suggests a Role in Meal Initiation in Humans. Diabetes 2001, 50, 1714–1719. [Google Scholar] [CrossRef]
- Perelló, M.; Zigman, J.M. The Role of Ghrelin in Reward-Based Eating. Biol. Psychiatry 2012, 72, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Madden, C.J.; Tupone, D.; Morrison, S.F. Orexin Modulates Brown Adipose Tissue Thermogenesis. Biomol. Concepts 2012, 3, 381–386. [Google Scholar] [CrossRef]
- Lin, L.; Lee, J.H.; Wang, R.; Wang, R.; Sheikh-Hamad, D.; Zang, Q.S.; Sun, Y. AP2-Cre Mediated Ablation of GHS-R Attenuates Adiposity and Improves Insulin Sensitivity during Aging. Int. J. Mol. Sci. 2018, 19, 3002. [Google Scholar] [CrossRef] [PubMed]
- Shabalina, I.G.; Nedergaard, J. Mitochondrial (‘mild’) Uncoupling and ROS Production: Physiologically Relevant or Not? Biochem. Soc. Trans. 2011, 39, 1305–1309. [Google Scholar] [CrossRef]
- Iwen, K.A.; Schröder, E.; Brabant, G. Thyroid Hormones and the Metabolic Syndrome. Eur. Thyroid J. 2013, 2, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Yes, Even Human Brown Fat Is on Fire! J. Clin. Investig. 2012, 122, 486–489. [Google Scholar] [CrossRef]
- Dallman, M.F.; Pecoraro, N.C.; la Fleur, S.E. Chronic Stress and Comfort Foods: Self-Medication and Abdominal Obesity. Brain. Behav. Immun. 2005, 19, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, N.; Reyes, F.; Gomez, F.; Bhargava, A.; Dallman, M.F. Chronic Stress Promotes Palatable Feeding, Which Reduces Signs of Stress: Feedforward and Feedback Effects of Chronic Stress. Endocrinology 2004, 145, 3754–3762. [Google Scholar] [CrossRef]
- Dallman, M.F.; la Fleur, S.E.; Pecoraro, N.C.; Gomez, F.; Houshyar, H.; Akana, S.F. Minireview: Glucocorticoids--Food Intake, Abdominal Obesity, and Wealthy Nations in 2004. Endocrinology 2004, 145, 2633–2638. [Google Scholar] [CrossRef]
- la Fleur, S.E.; Houshyar, H.; Roy, M.; Dallman, M.F. Choice of Lard, but Not Total Lard Calories, Damps Adrenocorticotropin Responses to Restraint. Endocrinology 2005, 146, 2193–2199. [Google Scholar] [CrossRef]
- Jéquier, E. Leptin Signaling, Adiposity, and Energy Balance. Ann. N. Y. Acad. Sci. 2002, 967, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Jastreboff, A.M. Stress as a Common Risk Factor for Obesity and Addiction. Biol. Psychiatry 2013, 73, 827–835. [Google Scholar] [CrossRef]
- Mitchell, D.; Shireman, J.; Sierra Potchanant, E.A.; Lara-Velazquez, M.; Dey, M. Neuroinflammation in Autoimmune Disease and Primary Brain Tumors: The Quest for Striking the Right Balance. Front. Cell. Neurosci. 2021, 15, 716947. [Google Scholar] [CrossRef] [PubMed]
- Han, V.X.; Patel, S.; Jones, H.F.; Dale, R.C. Maternal Immune Activation and Neuroinflammation in Human Neurodevelopmental Disorders. Nat. Rev. Neurol. 2021, 17, 564–579. [Google Scholar] [CrossRef]
- Kölliker-Frers, R.; Udovin, L.; Otero-Losada, M.; Kobiec, T.; Herrera, M.I.; Palacios, J.; Razzitte, G.; Capani, F. Neuroinflammation: An Integrating Overview of Reactive-Neuroimmune Cell Interactions in Health and Disease. Mediators Inflamm. 2021, 2021, 9999146. [Google Scholar] [CrossRef]
- Yong, H.Y.F.; Rawji, K.S.; Ghorbani, S.; Xue, M.; Yong, V.W. The Benefits of Neuroinflammation for the Repair of the Injured Central Nervous System. Cell. Mol. Immunol. 2019, 16, 540–546. [Google Scholar] [CrossRef]
- Zhao, J.; Bi, W.; Xiao, S.; Lan, X.; Cheng, X.; Zhang, J.; Lu, D.; Wei, W.; Wang, Y.; Li, H.; et al. Neuroinflammation Induced by Lipopolysaccharide Causes Cognitive Impairment in Mice. Sci. Rep. 2019, 9, 5790. [Google Scholar] [CrossRef]
- Zindler, E.; Zipp, F. Neuronal Injury in Chronic CNS Inflammation. Best Pract. Res. Clin. Anaesthesiol. 2010, 24, 551–562. [Google Scholar] [CrossRef]
- Bertram, L.; Tanzi, R.E. The Genetic Epidemiology of Neurodegenerative Disease. J. Clin. Investig. 2005, 115, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Abramov, A.Y. Mechanism of Oxidative Stress in Neurodegeneration. Oxid. Med. Cell. Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef] [PubMed]
- Lezi, E.; Swerdlow, R.H. Mitochondria in Neurodegeneration. Adv. Exp. Med. Biol. 2012, 942, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Ahumada-Castro, U.; Sanhueza, M.; Gonzalez-Billault, C.; Court, F.A.; Cárdenas, C. Mitochondria and Calcium Regulation as Basis of Neurodegeneration Associated with Aging. Front. Neurosci. 2018, 12, 470. [Google Scholar] [CrossRef] [PubMed]
- Matus, S.; Glimcher, L.H.; Hetz, C. Protein Folding Stress in Neurodegenerative Diseases: A Glimpse into the ER. Curr. Opin. Cell Biol. 2011, 23, 239–252. [Google Scholar] [CrossRef]
- de Araújo Boleti, A.P.; de Oliveira Flores, T.M.; Moreno, S.E.; dos Anjos, L.; Mortari, M.R.; Migliolo, L. Neuroinflammation: An Overview of Neurodegenerative and Metabolic Diseases and of Biotechnological Studies. Neurochem. Int. 2020, 136, 104714. [Google Scholar] [CrossRef]
- Bellia, C.; Lombardo, M.; Meloni, M.; Della-Morte, D.; Bellia, A.; Lauro, D. Diabetes and Cognitive Decline. Adv. Clin. Chem. 2022, 108, 37–71. [Google Scholar] [CrossRef]
- Ehtewish, H.; Arredouani, A.; El-Agnaf, O. Diagnostic, Prognostic, and Mechanistic Biomarkers of Diabetes Mellitus-Associated Cognitive Decline. Int. J. Mol. Sci. 2022, 23, 6144. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-P.; Yang, S.-L.; Zhao, S.; Zheng, C.-H.; Li, H.-H.; Zhang, Y.; Huang, R.-X.; Li, M.-Z.; Gao, Y.; Zhang, S.-J.; et al. Biomarkers for Early Diagnostic of Mild Cognitive Impairment in Type-2 Diabetes Patients: A Multicentre, Retrospective, Nested Case-Control Study. eBioMedicine 2016, 5, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Liu, T. Hypothalamic Inflammation: A Double-Edged Sword to Nutritional Diseases. Ann. N. Y. Acad. Sci. 2011, 1243, E1–E39. [Google Scholar] [CrossRef]
- Miller, A.A.; Spencer, S.J. Obesity and Neuroinflammation: A Pathway to Cognitive Impairment. Brain. Behav. Immun. 2014, 42, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Martín-Rodríguez, A.; Tornero-Aguilera, J.F.; López-Pérez, P.J.; Clemente-Suárez, V.J. Overweight and Executive Functions, Psychological and Behavioral Profile of Spanish Adolescents. Physiol. Behav. 2022, 254, 113901. [Google Scholar] [CrossRef] [PubMed]
- Lara, V.P.; Caramelli, P.; Teixeira, A.L.; Barbosa, M.T.; Carmona, K.C.; Carvalho, M.G.; Fernandes, A.P.; Gomes, K.B. High Cortisol Levels Are Associated with Cognitive Impairment No-Dementia (CIND) and Dementia. Clin. Chim. Acta. 2013, 423, 18–22. [Google Scholar] [CrossRef]
- Rhebergen, D.; Korten, N.C.M.; Penninx, B.W.J.H.; Stek, M.L.; van der Mast, R.C.; Oude Voshaar, R.; Comijs, H.C. Hypothalamic-Pituitary-Adrenal Axis Activity in Older Persons with and without a Depressive Disorder. Psychoneuroendocrinology 2015, 51, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Cope, E.C.; LaMarca, E.A.; Monari, P.K.; Olson, L.B.; Martinez, S.; Zych, A.D.; Katchur, N.J.; Gould, E. Microglia Play an Active Role in Obesity-Associated Cognitive Decline. J. Neurosci. 2018, 38, 8889–8904. [Google Scholar] [CrossRef]
- Adlercreutz, H. Western Diet and Western Diseases: Some Hormonal and Biochemical Mechanisms and Associations. Scand. J. Clin. Lab. Investig. 1990, 201, 3–23. [Google Scholar] [CrossRef]
- Alhabeeb, H.; AlFaiz, A.; Kutbi, E.; AlShahrani, D.; Alsuhail, A.; AlRajhi, S.; Alotaibi, N.; Alotaibi, K.; AlAmri, S.; Alghamdi, S.; et al. Gut Hormones in Health and Obesity: The Upcoming Role of Short Chain Fatty Acids. Nutrients 2021, 13, 481. [Google Scholar] [CrossRef]
- Astrup, A.; Magkos, F.; Bier, D.M.; Brenna, J.T.; de Oliveira Otto, M.C.; Hill, J.O.; King, J.C.; Mente, A.; Ordovas, J.M.; Volek, J.S.; et al. Saturated fats and health: A reassessment of evidence and proposal for food-based recommendations. J. Am. Coll. Cardiol. 2020, 76, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Clifton, P. Metabolic Syndrome-Role of Dietary Fat Type and Quantity. Nutrients 2019, 11, 1438. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Guo, D.-H.; Hernandez, C.M.; Stranahan, A.M. Endothelial Adora2a Activation Promotes Blood-Brain Barrier Breakdown and Cognitive Impairment in Mice with Diet-Induced Insulin Resistance. J. Neurosci. 2019, 39, 4179–4192. [Google Scholar] [CrossRef]
- Mauro, C.; De Rosa, V.; Marelli-Berg, F.; Solito, E. Metabolic Syndrome and the Immunological Affair with the Blood-Brain Barrier. Front. Immunol. 2014, 5, 677. [Google Scholar] [CrossRef] [PubMed]
- Van Dyken, P.; Lacoste, B. Impact of Metabolic Syndrome on Neuroinflammation and the Blood-Brain Barrier. Front. Neurosci. 2018, 12, 930. [Google Scholar] [CrossRef]
- Kwon, Y.N.; Kim, Y.-J. Gut-Brain-Microbiota Axis and Hypertension: A Literature Review. Curr. Pharm. Des. 2021, 27, 3939–3946. [Google Scholar] [CrossRef]
- Sharma, R.K.; Yang, T.; Oliveira, A.C.; Lobaton, G.O.; Aquino, V.; Kim, S.; Richards, E.M.; Pepine, C.J.; Sumners, C.; Raizada, M.K. Microglial Cells Impact Gut Microbiota and Gut Pathology in Angiotensin II-Induced Hypertension. Circ. Res. 2019, 124, 727–736. [Google Scholar] [CrossRef]
- Richards, E.M.; Li, J.; Stevens, B.R.; Pepine, C.J.; Raizada, M.K. Gut Microbiome and Neuroinflammation in Hypertension. Circ. Res. 2022, 130, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.A. Neurologic Conditions and Disorders of Uremic Syndrome of Chronic Kidney Disease: Presentations, Causes, and Treatment Strategies. Expert Rev. Clin. Pharmacol. 2019, 12, 61–90. [Google Scholar] [CrossRef]
- Miranda, A.S.; Cordeiro, T.M.; Dos Santos Lacerda Soares, T.M.; Ferreira, R.N.; Simões e Silva, A.C. Kidney-Brain Axis Inflammatory Cross-Talk: From Bench to Bedside. Clin. Sci. 2017, 131, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Corona, R.; Ordaz, B.; Robles-Osorio, L.; Sabath, E.; Morales, T. Neuroimmunoendocrine Link Between Chronic Kidney Disease and Olfactory Deficits. Front. Integr. Neurosci. 2022, 16, 763986. [Google Scholar] [CrossRef]
- Moalem, G.; Leibowitz-Amit, R.; Yoles, E.; Mor, F.; Cohen, I.R.; Schwartz, M. Autoimmune T Cells Protect Neurons from Secondary Degeneration after Central Nervous System Axotomy. Nat. Med. 1999, 5, 49–55. [Google Scholar] [CrossRef]
- Ceriello, A. The Emerging Challenge in Diabetes: The “Metabolic Memory”. Vascul. Pharmacol. 2012, 57, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.C.; Luk, K.; Purtell, K.; Burke Nanni, S.; Stoessl, A.J.; Trudeau, L.-E.; Yue, Z.; Krainc, D.; Oertel, W.; Obeso, J.A.; et al. Neuronal Vulnerability in Parkinson Disease: Should the Focus Be on Axons and Synaptic Terminals? Mov. Disord. 2019, 34, 1406–1422. [Google Scholar] [CrossRef] [PubMed]
- Jordan, L.C.; DeBaun, M.R.; Donahue, M.J. Advances in Neuroimaging to Improve Care in Sickle Cell Disease. Lancet. Neurol. 2021, 20, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Hirose, D.; Hatanaka, H.; Takenoshita, N.; Kaneko, Y.; Ogawa, Y.; Sakurai, H.; Hanyu, H. Role of Neuroimaging as a Biomarker for Neurodegenerative Diseases. Front. Neurol. 2018, 9, 265. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.J.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The Diagnosis of Dementia Due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimers. Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhao, X.-M. Deep Learning of Brain Magnetic Resonance Images: A Brief Review. Methods 2021, 192, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Ambler, G.; Lee, K.-J.; Lim, J.-S.; Shiozawa, M.; Koga, M.; Li, L.; Lovelock, C.; Chabriat, H.; Hennerici, M. Cerebral Microbleeds and Stroke Risk after Ischaemic Stroke or Transient Ischaemic Attack: A Pooled Analysis of Individual Patient Data from Cohort Studies. Lancet Neurol. 2019, 18, 653–665. [Google Scholar] [CrossRef]
- Mei, L.; Wang, Y.; Liu, C.; Mou, J.; Yuan, Y.; Qiu, L.; Gong, Q. Study of Sex Differences in Unmedicated Patients with Major Depressive Disorder by Using Resting State Brain Functional Magnetic Resonance Imaging. Front. Neurosci. 2022, 16, 814410. [Google Scholar] [CrossRef]
- He, N.; Chen, Y.; LeWitt, P.A.; Yan, F.; Haacke, E.M. Application of Neuromelanin MR Imaging in Parkinson Disease. J. Magn. Reson. Imaging 2023, 57, 337–352. [Google Scholar] [CrossRef]
- Li, T.-R.; Wu, Y.; Jiang, J.-J.; Lin, H.; Han, C.-L.; Jiang, J.-H.; Han, Y. Radiomics Analysis of Magnetic Resonance Imaging Facilitates the Identification of Preclinical Alzheimer’s Disease: An Exploratory Study. Front. Cell Dev. Biol. 2020, 8, 605734. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-Y.; Yu, J.-T.; Liu, Y.; Yin, R.-H.; Wang, H.-F.; Wang, J.; Tan, L.; Radua, J.; Tan, L. Voxel-Based Meta-Analysis of Grey Matter Changes in Alzheimer’s Disease. Transl. Neurodegener. 2015, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.-T.; Zheng, Q.; Xu, Z.-Q.; Yin, J.-H.; Lu, L.-G.; Zuo, Q.; Yang, S.; Zhang, C.-L.; Jiao, L. Positron Emission Computed Tomography/Single Photon Emission Computed Tomography in Parkinson Disease. Chin. Med. J. 2020, 133, 1448–1455. [Google Scholar] [CrossRef]
- Bhat, S.; Acharya, U.R.; Hagiwara, Y.; Dadmehr, N.; Adeli, H. Parkinson’s Disease: Cause Factors, Measurable Indicators, and Early Diagnosis. Comput. Biol. Med. 2018, 102, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Kägi, G.; Bhatia, K.P.; Tolosa, E. The Role of DAT-SPECT in Movement Disorders. J. Neurol. Neurosurg. Psychiatry 2010, 81, 5–12. [Google Scholar] [CrossRef]
- Shao, W.; Rowe, S.P.; Du, Y. Artificial Intelligence in Single Photon Emission Computed Tomography (SPECT) Imaging: A Narrative Review. Ann. Transl. Med. 2021, 9, 820. [Google Scholar] [CrossRef] [PubMed]
- Shiiba, T.; Takano, K.; Takaki, A.; Suwazono, S. Dopamine Transporter Single-Photon Emission Computed Tomography-Derived Radiomics Signature for Detecting Parkinson’s Disease. EJNMMI Res. 2022, 12, 39. [Google Scholar] [CrossRef]
- Höller, Y.; Bathke, A.C.; Uhl, A.; Strobl, N.; Lang, A.; Bergmann, J.; Nardone, R.; Rossini, F.; Zauner, H.; Kirschner, M.; et al. Combining SPECT and Quantitative EEG Analysis for the Automated Differential Diagnosis of Disorders with Amnestic Symptoms. Front. Aging Neurosci. 2017, 9, 290. [Google Scholar] [CrossRef]
- Lilenfeld, L.R.R.; Wonderlich, S.; Riso, L.P.; Crosby, R.; Mitchell, J. Eating Disorders and Personality: A Methodological and Empirical Review. Clin. Psychol. Rev. 2006, 26, 299–320. [Google Scholar] [CrossRef]
- Monteleone, A.M.; Castellini, G.; Volpe, U.; Ricca, V.; Lelli, L.; Monteleone, P.; Maj, M. Neuroendocrinology and Brain Imaging of Reward in Eating Disorders: A Possible Key to the Treatment of Anorexia Nervosa and Bulimia Nervosa. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 80 Pt B, 132–142. [Google Scholar] [CrossRef]
- Frank, G.K.; Bailer, U.F.; Henry, S.; Wagner, A.; Kaye, W.H. Neuroimaging Studies in Eating Disorders. CNS Spectr. 2004, 9, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Frank, G.K.W. Advances from Neuroimaging Studies in Eating Disorders. CNS Spectr. 2015, 20, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Greer, P.; Bailer, U.F.; Frank, G.K.; Henry, S.E.; Putnam, K.; Meltzer, C.C.; Ziolko, S.K.; Hoge, J.; McConaha, C.; et al. Normal Brain Tissue Volumes after Long-Term Recovery in Anorexia and Bulimia Nervosa. Biol. Psychiatry 2006, 59, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Stice, E.; Spoor, S.; Ng, J.; Zald, D.H. Relation of Obesity to Consummatory and Anticipatory Food Reward. Physiol. Behav. 2009, 97, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Uher, R.; Murphy, T.; Brammer, M.J.; Dalgleish, T.; Phillips, M.L.; Ng, V.W.; Andrew, C.M.; Williams, S.C.R.; Campbell, I.C.; Treasure, J. Medial Prefrontal Cortex Activity Associated with Symptom Provocation in Eating Disorders. Am. J. Psychiatry 2004, 161, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Fladung, A.-K.; Grön, G.; Grammer, K.; Herrnberger, B.; Schilly, E.; Grasteit, S.; Wolf, R.C.; Walter, H.; von Wietersheim, J. A Neural Signature of Anorexia Nervosa in the Ventral Striatal Reward System. Am. J. Psychiatry 2010, 167, 206–212. [Google Scholar] [CrossRef]
- Keating, C.; Tilbrook, A.J.; Rossell, S.L.; Enticott, P.G.; Fitzgerald, P.B. Reward Processing in Anorexia Nervosa. Neuropsychologia 2012, 50, 567–575. [Google Scholar] [CrossRef]
- Kullmann, S.; Pape, A.-A.; Heni, M.; Ketterer, C.; Schick, F.; Häring, H.-U.; Fritsche, A.; Preissl, H.; Veit, R. Functional Network Connectivity Underlying Food Processing: Disturbed Salience and Visual Processing in Overweight and Obese Adults. Cereb. Cortex 2013, 23, 1247–1256. [Google Scholar] [CrossRef]
- Culbert, K.M.; Sisk, C.L.; Klump, K.L. A Narrative Review of Sex Differences in Eating Disorders: Is There a Biological Basis? Clin. Ther. 2021, 43, 95–111. [Google Scholar] [CrossRef]
- Asarian, L.; Geary, N. Sex Differences in the Physiology of Eating. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1215-67. [Google Scholar] [CrossRef]
- Pursey, K.M.; Stanwell, P.; Callister, R.J.; Brain, K.; Collins, C.E.; Burrows, T.L. Neural Responses to Visual Food Cues According to Weight Status: A Systematic Review of Functional Magnetic Resonance Imaging Studies. Front. Nutr. 2014, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Vainik, U.; Baker, T.E.; Dadar, M.; Zeighami, Y.; Michaud, A.; Zhang, Y.; García Alanis, J.C.; Misic, B.; Collins, D.L.; Dagher, A. Neurobehavioral Correlates of Obesity Are Largely Heritable. Proc. Natl. Acad. Sci. USA 2018, 115, 9312–9317. [Google Scholar] [CrossRef] [PubMed]
- Fotuhi, M.; Lubinski, B. The Effects of Obesity on Brain Structure and Size. Pract. Neurol. 2013. Available online: https://neurogrow.com/wp-content/uploads/2020/07/2013_-_fotuhi_-_practical_neurology.pdf (accessed on 20 June 2023).
- Stice, E.; Yokum, S. Neural Vulnerability Factors That Increase Risk for Future Weight Gain. Psychol. Bull. 2016, 142, 447–471. [Google Scholar] [CrossRef] [PubMed]
- Ronan, L.; Alexander-Bloch, A.F.; Wagstyl, K.; Farooqi, S.; Brayne, C.; Tyler, L.K.; Fletcher, P.C. Obesity Associated with Increased Brain Age from Midlife. Neurobiol. Aging 2016, 47, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kullmann, S.; Schweizer, F.; Veit, R.; Fritsche, A.; Preissl, H. Compromised White Matter Integrity in Obesity. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2015, 16, 273–281. [Google Scholar] [CrossRef]
- Morys, F.; García-García, I.; Dagher, A. Is Obesity Related to Enhanced Neural Reactivity to Visual Food Cues? A Review and Meta-Analysis. Soc. Cogn. Affect. Neurosci. 2020, 18, 1438. [Google Scholar] [CrossRef]
- Sharma, L.; Markon, K.E.; Clark, L.A. Toward a Theory of Distinct Types of “Impulsive” Behaviors: A Meta-Analysis of Self-Report and Behavioral Measures. Psychol. Bull. 2014, 140, 374–408. [Google Scholar] [CrossRef]
- Michaud, A.; Vainik, U.; Garcia-Garcia, I.; Dagher, A. Overlapping Neural Endophenotypes in Addiction and Obesity. Front. Endocrinol. 2017, 8, 127. [Google Scholar] [CrossRef]
- Bryant, E.J.; King, N.A.; Blundell, J.E. Disinhibition: Its Effects on Appetite and Weight Regulation. Obes. Rev. 2008, 9, 409–419. [Google Scholar] [CrossRef]
- Davis, C.; Curtis, C.; Levitan, R.D.; Carter, J.C.; Kaplan, A.S.; Kennedy, J.L. Evidence That “food Addiction” Is a Valid Phenotype of Obesity. Appetite 2011, 57, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Catena, A.; Megías, A.; Maldonado, A.; Cándido, A.; Verdejo-García, A.; Perales, J.C. Emotional and Non-Emotional Pathways to Impulsive Behavior and Addiction. Front. Hum. Neurosci. 2013, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, G.; Herpertz, S.; Loeber, S. Personality Traits and Obesity: A Systematic Review. Obes. Rev. 2015, 16, 32–63. [Google Scholar] [CrossRef] [PubMed]
- Meule, A.; Blechert, J. Trait Impulsivity and Body Mass Index: A Cross-Sectional Investigation in 3073 Individuals Reveals Positive, but Very Small Relationships. Health Psychol. Open 2016, 3, 2055102916659164. [Google Scholar] [CrossRef]
- Hays, N.P.; Roberts, S.B. Aspects of Eating Behaviors “Disinhibition” and “Restraint” Are Related to Weight Gain and BMI in Women. Obesity 2008, 16, 52–58. [Google Scholar] [CrossRef]
- Bryant, E.J.; Rehman, J.; Pepper, L.B.; Walters, E.R. Obesity and Eating Disturbance: The Role of TFEQ Restraint and Disinhibition. Curr. Obes. Rep. 2019, 8, 363–372. [Google Scholar] [CrossRef]
- Emery, R.L.; King, K.M.; Fischer, S.F.; Davis, K.R. The Moderating Role of Negative Urgency on the Prospective Association between Dietary Restraint and Binge Eating. Appetite 2013, 71, 113–119. [Google Scholar] [CrossRef]
- Platt, M.L.; Watson, K.K.; Hayden, B.Y.; Shepherd, S.V.; Klein, J.T. Neuroeconomics: Implications for Understanding the Neurobiology of Addiction; Kuhn, C.M., Koob, G.F., Eds.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Brogan, A.; Hevey, D.; O’Callaghan, G.; Yoder, R.; O’Shea, D. Impaired Decision Making among Morbidly Obese Adults. J. Psychosom. Res. 2011, 70, 189–196. [Google Scholar] [CrossRef]
- Bartholdy, S.; Dalton, B.; O’Daly, O.G.; Campbell, I.C.; Schmidt, U. A Systematic Review of the Relationship between Eating, Weight and Inhibitory Control Using the Stop Signal Task. Neurosci. Biobehav. Rev. 2016, 64, 35–62. [Google Scholar] [CrossRef]
- Hall, P.A.; Lowe, C.; Vincent, C. Executive Control Resources and Snack Food Consumption in the Presence of Restraining versus Facilitating Cues. J. Behav. Med. 2014, 37, 587–594. [Google Scholar] [CrossRef]
- Wu, X.; Nussbaum, M.A.; Madigan, M.L. Executive Function and Measures of Fall Risk Among People with Obesity. Percept. Mot. Skills 2016, 122, 825–839. [Google Scholar] [CrossRef]
- Chase, H.W.; Eickhoff, S.B.; Laird, A.R.; Hogarth, L. The Neural Basis of Drug Stimulus Processing and Craving: An Activation Likelihood Estimation Meta-Analysis. Biol. Psychiatry 2011, 70, 785–793. [Google Scholar] [CrossRef] [PubMed]
- García-García, I.; Horstmann, A.; Jurado, M.A.; Garolera, M.; Chaudhry, S.J.; Margulies, D.S.; Villringer, A.; Neumann, J. Reward Processing in Obesity, Substance Addiction and Non-Substance Addiction. Obes. Rev. 2014, 15, 853–869. [Google Scholar] [CrossRef]
- Barry, D.; Clarke, M.; Petry, N.M. Obesity and Its Relationship to Addictions: Is Overeating a Form of Addictive Behavior? Am. J. Addict. 2009, 18, 439–451. [Google Scholar] [CrossRef]
- Barry, D.; Petry, N.M. Associations between Body Mass Index and Substance Use Disorders Differ by Gender: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Addict. Behav. 2009, 34, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Sansone, R.A.; Sansone, L.A. Obesity and Substance Misuse: Is There a Relationship? Innov. Clin. Neurosci. 2013, 10, 30–35. [Google Scholar]
- Tanaka, H.; Gourley, D.D.; Dekhtyar, M.; Haley, A.P. Cognition, Brain Structure, and Brain Function in Individuals with Obesity and Related Disorders. Curr. Obes. Rep. 2020, 9, 544–549. [Google Scholar] [CrossRef]
- Karasu, S.R. Psychotherapy-Lite: Obesity and the Role of the Mental Health Practitioner. Am. J. Psychother. 2013, 67, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Brownell, K.D.; Walsh, B.T. Eating Disorders and Obesity: A Comprehensive Handbook; Guilford Publications: New York, NY, USA, 2017. [Google Scholar]
- Daubenmier, J.; Moran, P.J.; Kristeller, J.; Acree, M.; Bacchetti, P.; Kemeny, M.E.; Dallman, M.; Lustig, R.H.; Grunfeld, C.; Nixon, D.F.; et al. Effects of a Mindfulness-Based Weight Loss Intervention in Adults with Obesity: A Randomized Clinical Trial. Obesity 2016, 24, 794–804. [Google Scholar] [CrossRef]
- Kober, H.; Boswell, R.G. Potential Psychological & Neural Mechanisms in Binge Eating Disorder: Implications for Treatment. Clin. Psychol. Rev. 2018, 60, 32–44. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wise, R.A. How Can Drug Addiction Help Us Understand Obesity? Nat. Neurosci. 2005, 8, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Formolo, D.A.; Gaspar, J.M.; Melo, H.M.; Eichwald, T.; Zepeda, R.J.; Latini, A.; Okun, M.S.; Walz, R. Deep Brain Stimulation for Obesity: A Review and Future Directions. Front. Neurosci. 2019, 13, 323. [Google Scholar] [CrossRef]
- Orrù, G.; Cesari, V.; Malloggi, E.; Conversano, C.; Menicucci, D.; Rotondo, A.; Scarpazza, C.; Marchi, L.; Gemignani, A. The Effects of Transcranial Direct Current Stimulation on Food Craving and Food Intake in Individuals Affected by Obesity and Overweight: A Mini Review of the Magnitude of the Effects. AIMS Neurosci. 2022, 9, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Figlewski, K.; Blicher, J.U.; Mortensen, J.; Severinsen, K.E.; Nielsen, J.F.; Andersen, H. Transcranial Direct Current Stimulation Potentiates Improvements in Functional Ability in Patients with Chronic Stroke Receiving Constraint-Induced Movement Therapy. Stroke 2017, 48, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Geda, E.; Codella, R.; Faelli, E.; Panascì, M.; Ranieri, L.E.; Pollastri, L.; Brighenti, S.; Molino, L.; Riba, U.; et al. Effects of Bilateral Dorsolateral Prefrontal Cortex High-Definition Transcranial Direct-Current Stimulation on Physiological and Performance Responses at Severe-Intensity Exercise Domain in Elite Road Cyclists. Int. J. Sports Physiol. Perform. 2022, 17, 1085–1093. [Google Scholar] [CrossRef]
- Gadde, K.M.; Martin, C.K.; Berthoud, H.-R.; Heymsfield, S.B. Obesity: Pathophysiology and Management. J. Am. Coll. Cardiol. 2018, 71, 69–84. [Google Scholar] [CrossRef] [PubMed]
- de Ceglia, M.; Decara, J.; Gaetani, S.; Rodríguez de Fonseca, F. Obesity as a Condition Determined by Food Addiction: Should Brain Endocannabinoid System Alterations Be the Cause and Its Modulation the Solution? Pharmaceuticals 2021, 14, 1002. [Google Scholar] [CrossRef]
- Epstein, L.H.; Salvy, S.J.; Carr, K.A.; Dearing, K.K.; Bickel, W.K. Food Reinforcement, Delay Discounting and Obesity. Physiol. Behav. 2010, 100, 438–445. [Google Scholar] [CrossRef]
- Carr, K.A.; Daniel, T.O.; Lin, H.; Epstein, L.H. Reinforcement Pathology and Obesity. Curr. Drug Abuse Rev. 2011, 4, 190–196. [Google Scholar] [CrossRef]
- Rollins, B.Y.; Dearing, K.K.; Epstein, L.H. Delay Discounting Moderates the Effect of Food Reinforcement on Energy Intake among Non-Obese Women. Appetite 2010, 55, 420–425. [Google Scholar] [CrossRef]
- Carr, K.A.; Lin, H.; Fletcher, K.D.; Epstein, L.H. Food Reinforcement, Dietary Disinhibition and Weight Gain in Nonobese Adults. Obesity 2014, 22, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J. Multidisciplinary intervention in the treatment of mixed anxiety and depression disorder. Physiol. Behav. 2020, 219, 112858. [Google Scholar] [CrossRef] [PubMed]
- Carreira-Míguez, M.; Navarro-Jiménez, E.; Clemente-Suárez, V.J. Behavioral Patterns of Depression Patients and Control Population. Int. J. Environ. Res. Public Health 2022, 19, 9506. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Bustamante-Sanchez, Á.; Tornero-Aguilera, J.F.; Ruisoto, P.; Mielgo-Ayuso, J. Inflammation in COVID-19 and the effects of non-pharmacological interventions during the pandemic: A review. Int. J. Mol. Sci. 2022, 23, 15584. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Ramírez-Goerke, M.I.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; Ramos-Campo, D.J.; Navarro-Jiménez, E.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. The Impact of Anorexia Nervosa and the Basis for Non-Pharmacological Interventions. Nutrients 2023, 15, 2594. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Redondo-Flórez, L.; López-Mora, C.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. New Insights and Potential Therapeutic Interventions in Metabolic Diseases. Int. J. Mol. Sci. 2023, 24, 10672. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Redondo-Flórez, L.; Ruisoto, P.; Navarro-Jiménez, E.; Ramos-Campo, D.J.; Tornero-Aguilera, J.F. Metabolic health, mitochondrial fitness, physical activity, and cancer. Cancers 2023, 15, 814. [Google Scholar] [CrossRef]
- Carreira-Míguez, M.; Belinchón-deMiguel, P.P.; Clemente-Suárez, V.J. Behavioural, odontological and physical activity patterns of hypertense and control population. Physiol. Behav. 2022, 252, 113841. [Google Scholar] [CrossRef]
- Bustamante-Sanchez, A.; Villegas-Mora, B.E.; Martínez-Guardado, I.; Tornero-Aguilera, J.F.; Ardigò, L.P.; Nobari, H.; Clemente-Suárez, V.J. Physical activity and nutritional pattern related to maturation and development. Sustainability 2022, 14, 16958. [Google Scholar] [CrossRef]
- Ciccozzi, M.; Lai, A.; Zehender, G.; Borsetti, A.; Cella, E.; Ciotti, M.; Sagnelli, E.; Sagnelli, C.; Angeletti, S. The phylogenetic approach for viral infectious disease evolution and epidemiology: An updating review. J. Med. Virol. 2019, 91, 1707–1724. [Google Scholar] [CrossRef] [PubMed]
- Carreira Míguez, M.; Clemente Suárez, V.J. Physical activity levels affect mental health and behavior in men. J. Men’s Health 2023, 1, 12. [Google Scholar] [CrossRef]
- Carreira-Míguez, M.; Ramos-Campo, D.J.; Clemente-Suárez, V.J. Differences in Nutritional and Psychological Habits in Hypertension Patients. BioMed Res. Int. 2022, 2022, 1920996. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Navarro-Jiménez, E.; Jimenez, M.; Hormeño-Holgado, A.; Martinez-Gonzalez, M.B.; Benitez-Agudelo, J.C.; Perez-Palencia, N.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. Impact of COVID-19 pandemic in public mental health: An extensive narrative review. Sustainability 2021, 13, 3221. [Google Scholar] [CrossRef]
- Martín-Rodríguez, A.; Tornero-Aguilera, J.F.; López-Pérez, P.J.; Clemente-Suárez, V.J. The effect of loneliness in psychological and behavioral profile among high school students in Spain. Sustainability 2021, 14, 168. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. Neuro-Vulnerability in Energy Metabolism Regulation: A Comprehensive Narrative Review. Nutrients 2023, 15, 3106. https://doi.org/10.3390/nu15143106
Clemente-Suárez VJ, Beltrán-Velasco AI, Redondo-Flórez L, Martín-Rodríguez A, Yáñez-Sepúlveda R, Tornero-Aguilera JF. Neuro-Vulnerability in Energy Metabolism Regulation: A Comprehensive Narrative Review. Nutrients. 2023; 15(14):3106. https://doi.org/10.3390/nu15143106
Chicago/Turabian StyleClemente-Suárez, Vicente Javier, Ana Isabel Beltrán-Velasco, Laura Redondo-Flórez, Alexandra Martín-Rodríguez, Rodrigo Yáñez-Sepúlveda, and José Francisco Tornero-Aguilera. 2023. "Neuro-Vulnerability in Energy Metabolism Regulation: A Comprehensive Narrative Review" Nutrients 15, no. 14: 3106. https://doi.org/10.3390/nu15143106
APA StyleClemente-Suárez, V. J., Beltrán-Velasco, A. I., Redondo-Flórez, L., Martín-Rodríguez, A., Yáñez-Sepúlveda, R., & Tornero-Aguilera, J. F. (2023). Neuro-Vulnerability in Energy Metabolism Regulation: A Comprehensive Narrative Review. Nutrients, 15(14), 3106. https://doi.org/10.3390/nu15143106








