Acute Cardiometabolic and Exercise Responses to Breakfast Omission Versus Breakfast Consumption in Adolescent Girls: A Randomised Crossover Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Recruitment
2.2. Sample Size Estimation
2.3. Preliminary Measurements
2.4. Experimental Design
2.5. Test Meals
2.6. Blood Sampling and Analysis
2.7. Expired Gas Sampling and Indirect Calorimetry
2.8. Incremental Exercise Protocol and Fatmax Estimation
2.9. Perceived Mood and Appetite
2.10. Statistical Analyses
3. Results
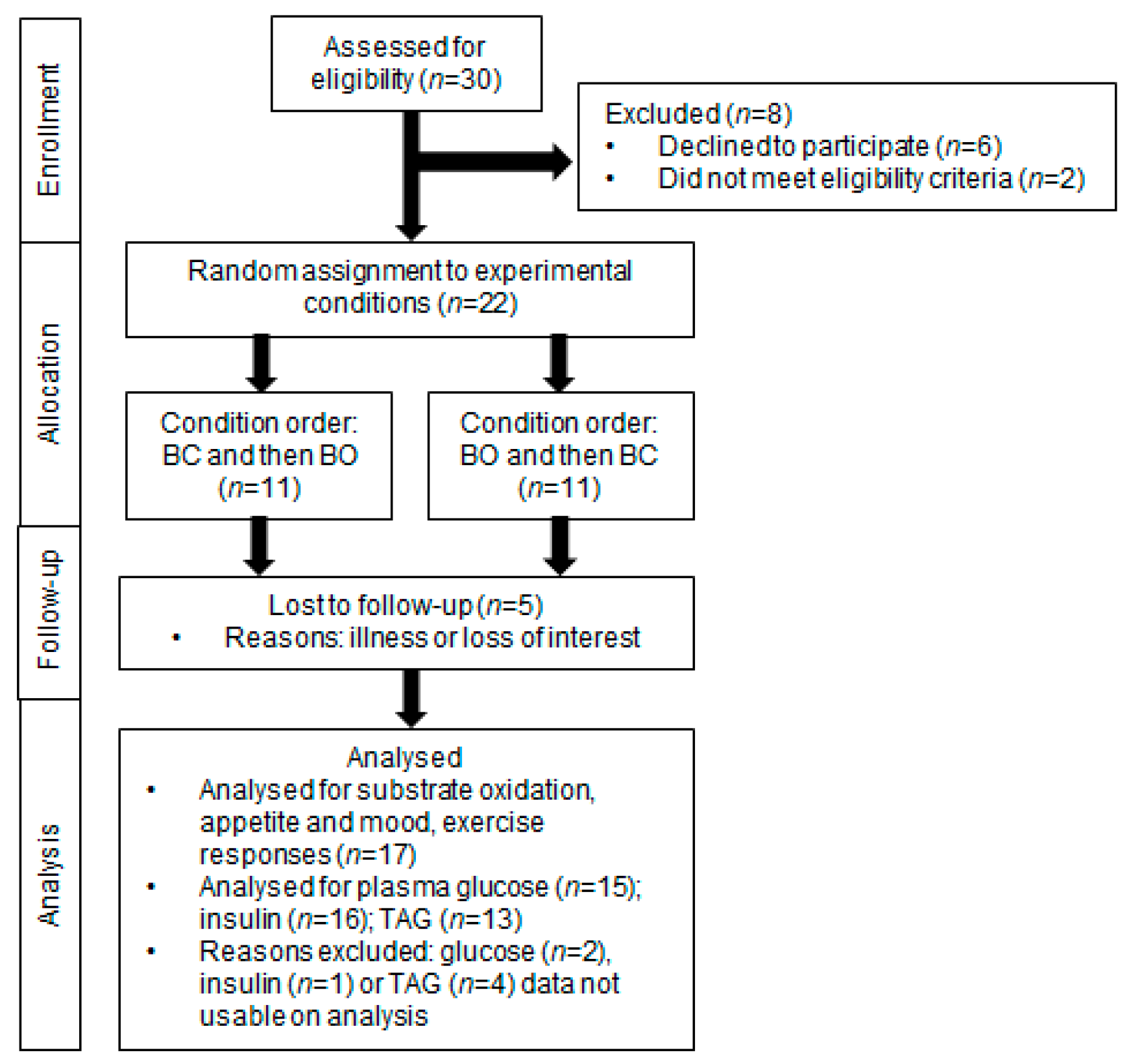
3.1. Participants
3.2. Test Meal Characteristics
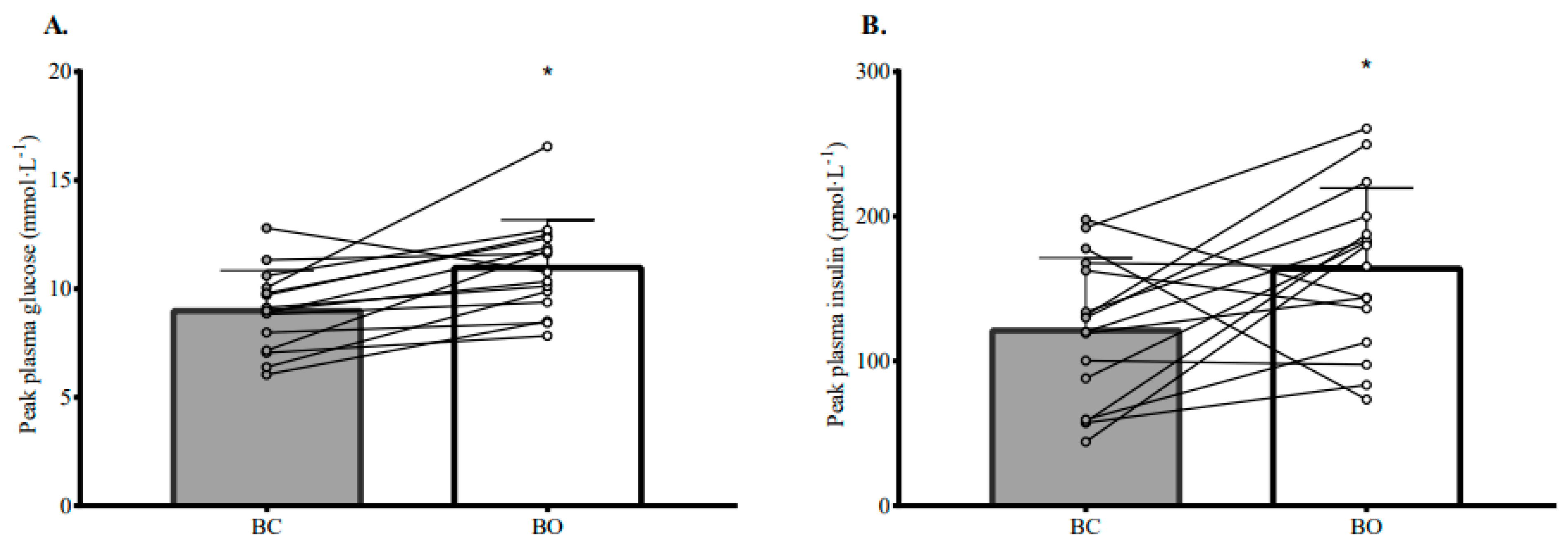
3.3. Plasma Glucose, Insulin, and TAG Responses
3.4. Resting Substrate Oxidation
3.5. Exercise Responses
3.6. Perceptions of Appetite and Mood
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blaak, E.E.; Antoine, J.M.; Benton, D.; Björck, I.; Bozzetto, L.; Brouns, F.; Diamant, M.; Dye, L.; Hulshof, T.; Holst, J.J.; et al. Impact of postprandial glycaemia on health and prevention of disease. Obes. Rev. 2012, 13, 923–984. [Google Scholar] [CrossRef] [PubMed]
- Andes, L.J.; Cheng, Y.J.; Rolka, D.B.; Gregg, E.W.; Imperatore, G. Prevalence of prediabetes among adolescents and young adults in the United States, 2005–2016. JAMA Pediatr. 2020, 174, e194498. [Google Scholar] [CrossRef] [PubMed]
- Candler, T.P.; Mahmoud, O.; Lynn, R.M.; Majbar, A.A.; Barrett, T.G.; Shield, J.P.H. Continuing rise of Type 2 diabetes incidence in children and young people in the UK. Diabet. Med. 2018, 35, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.M.; Divers, J.; Isom, S.; Saydah, S.; Imperatore, G.; Pihoker, C.; Marcovina, S.M.; Mayer-Davis, E.J.; Hamman, R.F.; Dolan, L.; et al. Trends in prevalence of Type 1 and Type 2 diabetes in children and adolescents in the US, 2001–2017. JAMA 2021, 326, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.S.; Day, F.R.; Lakshman, R.; Ong, K.K. Association of puberty timing with type 2 diabetes: A systematic review and meta-analysis. PLoS Med. 2020, 17, e1003017. [Google Scholar] [CrossRef]
- Goran, M.I.; Gower, B.A. Longitudinal study on pubertal insulin resistance. Diabetes 2001, 50, 2444–2450. [Google Scholar] [CrossRef]
- Levy-Marchal, C.; Arslanian, S.; Cutfield, W.; Sinaiko, A.; Druet, C.; Marcovecchio, M.L.; Chiarelli, F. Insulin resistance in children: Consensus, perspective, and future directions. J. Clin. Endocrinol. Metab. 2010, 95, 5189–5198. [Google Scholar] [CrossRef]
- Zakrzewski-Fruer, J.K.; Thackray, A.E. Enhancing cardiometabolic health through physical activity and breakfast manipulations in children and adolescents: Good for humans, good for the planet. Proc. Nutr. Soc. 2022, 11, 1–30. [Google Scholar] [CrossRef]
- Corder, K.; van Sluijs, E.M.; Ridgway, C.L.; Steele, R.M.; Prynne, C.J.; Stephen, A.M.; Bamber, D.J.; Dunn, V.J.; Goodyer, I.M.; Ekelund, U. Breakfast consumption and physical activity in adolescents: Daily associations and hourly patterns. Am. J. Clin. Nutr. 2014, 99, 361–368. [Google Scholar] [CrossRef]
- Timlin, M.T.; Pereira, M.A.; Story, M.; Neumark-Sztainer, D. Breakfast eating and weight change in a 5-year prospective analysis of adolescents: Project EAT (Eating Among Teens). Pediatrics 2008, 121, e638–e645. [Google Scholar] [CrossRef]
- Farooq, A.; Martin, A.; Janssen, X.; Wilson, M.G.; Gibson, A.M.; Hughes, A.; Reilly, J.J. Longitudinal changes in moderate-to-vigorous-intensity physical activity in children and adolescents: A systematic review and meta-analysis. Obes. Rev. 2020, 21, e12953. [Google Scholar] [CrossRef]
- Steene-Johannessen, J.; Hansen, B.H.; Dalene, K.E.; Kolle, E.; Northstone, K.; Møller, N.C.; Grøntved, A.; Wedderkopp, N.; Kriemler, S.; Page, A.S.; et al. Variations in accelerometry measured physical activity and sedentary time across Europe-harmonized analyses of 47,497 children and adolescents. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 38. [Google Scholar] [CrossRef]
- The Health and Social Care Information Centre. Health Survey for England—2016. Trend Tables. 2017. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/health-survey-for-england-2016 (accessed on 13 June 2023).
- Zakrzewski-Fruer, J.K.; Wells, E.K.; Crawford, N.S.G.; Afeef, S.M.O.; Tolfrey, K. Physical activity duration but not energy expenditure differs between daily compared with intermittent breakfast consumption in adolescent girls: A randomized crossover trial. J. Nutr. 2018, 148, 236–244. [Google Scholar] [CrossRef]
- Todd, A.S.; Street, S.J.; Ziviani, J.; Byrne, N.M.; Hills, A.P. Overweight and obese adolescent girls: The importance of promoting sensible eating and activity behaviors from the start of the adolescent period. Int. J. Environ. Res. Public Health 2015, 12, 2306–2329. [Google Scholar] [CrossRef]
- Chowdhury, E.A.; Richardson, J.D.; Tsintzas, K.; Thompson, D.; Betts, J.A. Carbohydrate-rich breakfast attenuates glycaemic, insulinaemic and ghrelin response to ad libitum lunch relative to morning fasting in lean adults. Br. J. Nutr. 2015, 114, 98–107. [Google Scholar] [CrossRef]
- Jovanovic, A.; Gerrard, J.; Taylor, R. The second-meal phenomenon in type 2 diabetes. Diabetes Care 2009, 32, 1199–1201. [Google Scholar] [CrossRef]
- Jovanovic, A.; Leverton, E.; Solanky, B.; Ravikumar, B.; Snaar, J.E.; Morris, P.G.; Taylor, R. The second-meal phenomenon is associated with enhanced muscle glycogen storage in humans. Clin. Sci. 2009, 117, 119–127. [Google Scholar] [CrossRef]
- Donin, A.S.; Nightingale, C.M.; Owen, C.G.; Rudnicka, A.R.; Perkin, M.R.; Jebb, S.A.; Stephen, A.M.; Sattar, N.; Cook, D.G.; Whincup, P.H. Regular breakfast consumption and type 2 diabetes risk markers in 9- to 10-year-old children in the child heart and health study in England (CHASE): A cross-sectional analysis. PLoS Med. 2014, 11, e1001703. [Google Scholar] [CrossRef]
- Hallström, L.; Labayen, I.; Ruiz, J.R.; Patterson, E.; Vereecken, C.A.; Breidenassel, C.; Gottrand, F.; Huybrechts, I.; Manios, Y.; Mistura, L.; et al. Breakfast consumption and CVD risk factors in European adolescents: The HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study. Public Health Nutr. 2013, 16, 1296–1305. [Google Scholar] [CrossRef]
- Smith, K.J.; Gall, S.L.; McNaughton, S.A.; Blizzard, L.; Dwyer, T.; Venn, A.J. Skipping breakfast: Longitudinal associations with cardiometabolic risk factors in the Childhood Determinants of Adult Health Study. Am. J. Clin. Nutr. 2010, 92, 1316–1325. [Google Scholar] [CrossRef]
- Ballon, A.; Neuenschwander, M.; Schlesinger, S. Breakfast skipping is associated with increased risk of type 2 diabetes among adults: A systematic review and meta-analysis of prospective cohort studies. J. Nutr. 2019, 149, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Ofori-Asenso, R.; Owen, A.J.; Liew, D. Skipping breakfast and the risk of cardiovascular disease and death: A systematic review of prospective cohort studies in primary prevention settings. J. Cardiovasc. Dev. Dis. 2019, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.; Dalla Man, C.; Nandy, D.K.; Levine, J.A.; Bharucha, A.E.; Rizza, R.A.; Basu, R.; Carter, R.E.; Cobelli, C.; Kudva, Y.C.; et al. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 2012, 61, 2691–2700. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Almeda-Valdes, P.; Patterson, B.W.; Okunade, A.L.; Imai, S.; Mittendorfer, B.; Klein, S. Diurnal variation in insulin sensitivity of glucose metabolism is associated with diurnal variations in whole-body and cellular fatty acid metabolism in metabolically normal women. J. Clin. Endocrinol. Metab. 2014, 99, e1666–e1670. [Google Scholar] [CrossRef]
- Timmons, B.W.; Bar-Or, O.; Riddell, M.C. Influence of age and pubertal status on substrate utilization during exercise with and without carbohydrate intake in healthy boys. Appl. Physiol. Nutr. Metab. 2007, 32, 416–425. [Google Scholar] [CrossRef]
- Riddell, M.C. The endocrine response and substrate utilization during exercise in children and adolescents. J. Appl. Physiol. 2008, 105, 725–733. [Google Scholar] [CrossRef]
- Timmons, B.W.; Bar-Or, O.; Riddell, M.C. Energy substrate utilization during prolonged exercise with and without carbohydrate intake in preadolescent and adolescent girls. J. Appl. Physiol. 2007, 103, 995–1000. [Google Scholar] [CrossRef]
- Gonzalez, J.T. Paradoxical second-meal phenomenon in the acute postexercise period. Nutrition 2014, 30, 961–967. [Google Scholar] [CrossRef]
- Kovacic, K.; Zhang, L.; Nugent Liegl, M.; Pawela, L.; Simpson, P.; Sood, M.R. Gastric emptying in healthy children using the Spirulina breath test: The impact of gender, body size, and pubertal development. Neurogastroenterol. Motil. 2021, 33, e14063. [Google Scholar] [CrossRef]
- Alwattar, A.Y.; Thyfault, J.P.; Leidy, H.J. The effect of breakfast type and frequency of consumption on glycemic response in overweight/obese late adolescent girls. Eur. J. Clin. Nutr. 2015, 69, 885–890. [Google Scholar] [CrossRef]
- Bauer, L.B.; Reynolds, L.J.; Douglas, S.M.; Kearney, M.L.; Hoertel, H.A.; Shafer, R.S.; Thyfault, J.P.; Leidy, H.J. A pilot study examining the effects of consuming a high-protein vs normal-protein breakfast on free-living glycemic control in overweight/obese ‘breakfast skipping’ adolescents. Int. J. Obes. 2015, 39, 1421–1424. [Google Scholar] [CrossRef]
- Emerson, S.R.; Haub, M.D.; Teeman, C.S.; Kurti, S.P.; Rosenkranz, S.K. Summation of blood glucose and TAG to characterise the ‘metabolic load index’. Br. J. Nutr. 2016, 116, 1553–1563. [Google Scholar] [CrossRef]
- Thomas, E.A.; Higgins, J.; Bessesen, D.H.; McNair, B.; Cornier, M.A. Usual breakfast eating habits affect response to breakfast skipping in overweight women. Obesity 2015, 23, 750–759. [Google Scholar] [CrossRef]
- McCarthy, H.D.; Jarrett, K.V.; Emmett, P.M.; Rogers, I. Trends in waist circumferences in young British children: A comparative study. Int. J. Obes. 2005, 29, 157–162. [Google Scholar] [CrossRef]
- Brooks-Gunn, J.; Warren, M.P.; Rosso, J.; Gargiulo, J. Validity of self-report measures of girls’ pubertal status. Child Dev. 1987, 58, 829–841. [Google Scholar] [CrossRef]
- Tanner, J.M. Growth at Adolescents; Blackwell Scientific: Oxford, UK, 1962. [Google Scholar]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard for child overweight and obesity worldwide: International survey. BMJ 2000, 320, 1240–1243. [Google Scholar] [CrossRef]
- Zakrzewski-Fruer, J.K.; Seall, C.; Tolfrey, K. Breakfast consumption suppresses appetite but does not increase daily energy intake or physical activity energy expenditure when compared with breakfast omission in adolescent girls who habitually skip breakfast: A 7-day randomised crossover trial. Nutrients 2021, 13, 4261. [Google Scholar] [CrossRef]
- Cockcroft, E.J.; Williams, C.A.; Weaver, H.; O’Connor, A.; Jackman, S.R.; Armstrong, N.; Barker, A.R. Acute exercise and insulin sensitivity in boys: A time-course study. Int. J. Sports Med. 2017, 38, 967–974. [Google Scholar] [CrossRef]
- Zakrzewski, J.K.; Tolfrey, K. Acute effect of Fatmax exercise on the metabolism in overweight and nonoverweight girls. Med. Sci. Sports Exerc. 2012, 44, 1698–1705. [Google Scholar] [CrossRef]
- Chu, L.; Riddell, M.C.; Schneiderman, J.E.; McCrindle, B.W.; Hamilton, J.K. The effect of puberty on fat oxidation rates during exercise in overweight and normal-weight girls. J. Appl. Physiol. 2014, 116, 76–82. [Google Scholar] [CrossRef]
- Brown, S.A.; Jiang, B.; McElwee-Malloy, M.; Wakeman, C.; Breton, M.D. Fluctuations of hyperglycemia and insulin sensitivity are linked to menstrual cycle phases in women with T1D. J. Diabetes Sci. Technol. 2015, 9, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Oosthuyse, T.; Bosch, A.N. The effect of the menstrual cycle on exercise metabolism: Implications for exercise performance in eumenorrhoeic women. Sports Med. 2010, 40, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Compher, C.; Frankenfield, D.; Keim, N.; Roth-Yousey, L. Evidence Analysis Working Group. Best practice methods to apply to measurement of resting metabolic rate in adults: A systematic review. J. Am. Diet. Assoc. 2006, 106, 881–903. [Google Scholar] [CrossRef] [PubMed]
- Mellecker, R.R.; McManus, A.M. Measurement of resting energy expenditure in healthy children. J. Parenter. Enteral. Nutr. 2009, 33, 640–645. [Google Scholar] [CrossRef]
- O’Neil, C.E.; Byrd-Bredbenner, C.; Hayes, D.; Jana, L.; Klinger, S.E.; Stephenson-Martin, S. The role of breakfast in health: Definition and criteria for a quality breakfast. J. Acad. Nutr. Diet. 2014, 114 (Suppl. S12), S8–S26. [Google Scholar] [CrossRef]
- Timlin, M.T.; Pereira, M.A. Breakfast frequency and quality in the etiology of adult obesity and chronic diseases. Nutr. Rev. 2007, 65, 268–281. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef]
- Wolever, T.M.; Jenkins, D.J. The use of the glycemic index in predicting the blood glucose response to mixed meals. Am. J. Clin. Nutr. 1986, 43, 167–172. [Google Scholar] [CrossRef]
- Bonuccelli, S.; Muscelli, E.; Gastaldelli, A.; Barsotti, E.; Astiarraga, B.D.; Holst, J.J.; Mari, A.; Ferrannini, E. Improved tolerance to sequential glucose loading (Staub-Traugott effect): Size and mechanisms. Am. J. Physiol. Endocrinol. Metab. 2009, 297, e532–e537. [Google Scholar] [CrossRef]
- Liljeberg, H.; Björck, I. Effects of a low-glycaemic index spaghetti meal on glucose tolerance and lipaemia at a subsequent meal in healthy subjects. Eur. J. Clin. Nutr. 2000, 54, 24–28. [Google Scholar] [CrossRef]
- Nilsson, A.C.; Ostman, E.M.; Granfeldt, Y.; Björck, I.M. Effect of cereal test breakfasts differing in glycemic index and content of indigestible carbohydrates on daylong glucose tolerance in healthy subjects. Am. J. Clin. Nutr. 2008, 87, 645–654. [Google Scholar] [CrossRef]
- Barton, B.A.; Eldridge, A.L.; Thompson, D.; Affenito, S.G.; Striegel-Moore, R.H.; Franko, D.L.; Albertson, A.M.; Crockett, S.J. The relationship of breakfast and cereal consumption to nutrient intake and body mass index: The National Heart, Lung, and Blood Institute Growth and Health Study. J. Am. Diet. Assoc. 2005, 105, 1383–1389. [Google Scholar] [CrossRef]
- Deshmukh-Taskar, P.R.; Nicklas, T.A.; O’Neil, C.E.; Keast, D.R.; Radcliffe, J.D.; Cho, S. The relationship of breakfast skipping and type of breakfast consumption with nutrient intake and weight status in children and adolescents: The National Health and Nutrition Examination Survey 1999–2006. J. Am. Diet. Assoc. 2010, 110, 869–878. [Google Scholar] [CrossRef]
- Tolfrey, K.; Zakrzewski, J.K. Breakfast, glycaemic index and health in young people. J. Sport Health Sci. 2012, 1, 149–159. [Google Scholar] [CrossRef]
- Zakrzewski, J.K.; Stevenson, E.J.; Tolfrey, K. Effect of breakfast glycemic index on metabolic responses during rest and exercise in overweight and non-overweight adolescent girls. Eur. J. Clin. Nutr. 2012, 66, 436–442. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Shashaj, B.; Luciano, R.; Contoli, B.; Morino, G.S.; Spreghini, M.R.; Rustico, C.; Sforza, R.W.; Dallapiccola, B.; Manco, M. Reference ranges of HOMA-IR in normal-weight and obese young Caucasians. Acta Diabetol. 2016, 53, 251–260. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44 (Suppl. S1), S15–S33. [Google Scholar] [CrossRef]
- Frayn, K.N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. 1983, 55, 628–634. [Google Scholar] [CrossRef]
- Arundell, L.; Hinkley, T.; Veitch, J.; Salmon, J. Contribution of the after-school period to children’s daily participation in physical activity and sedentary behaviours. PLoS ONE 2015, 10, e0140132. [Google Scholar] [CrossRef]
- Zakrzewski, J.K.; Tolfrey, K. Exercise protocols to estimate Fatmax and maximal fat oxidation in children. Pediatr. Exerc. Sci. 2011, 23, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Aucouturier, J.; Rance, M.; Meyer, M.; Isacco, L.; Thivel, D.; Fellmann, N.; Duclos, M.; Duché, P. Determination of the maximal fat oxidation point in obese children and adolescents: Validity of methods to assess maximal aerobic power. Eur. J. Appl. Physiol. 2009, 105, 325–331. [Google Scholar] [CrossRef]
- Wasserman, K.; Hansen, J.; Whipp, B.J. Principles of Exercise Testing and Interpretation; Lea & Febiger: Philadelphia, PA, USA, 1986. [Google Scholar]
- Brandou, F.; Savy-Pacaux, A.M.; Marie, J.; Brun, J.F.; Mercier, J. Comparison of the type of substrate oxidation during exercise between pre and post pubertal markedly obese boys. Int. J. Sports Med. 2006, 27, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, K.A.; Pivarnik, J.M.; Womack, C.J.; Reeves, M.J.; Malina, R.M. Reliability and validity of the Borg and OMNI rating of perceived exertion scales in adolescent girls. Med. Sci. Sports Exerc. 2002, 34, 2057–2061. [Google Scholar] [CrossRef] [PubMed]
- Motl, R.W.; Dishman, R.K.; Saunders, R.; Dowda, M.; Felton, G.; Pate, R.R. Measuring enjoyment of physical activity in adolescent girls. Am. J. Prev. Med. 2001, 21, 110–117. [Google Scholar] [CrossRef]
- Cooper, S.B.; Bandelow, S.; Nute, M.; Dring, K.; Stannard, R.L.; Morris, J.; Nevill, M.E. Sprint-based exercise and cognitive function in adolescents. Prev. Med. Rep. 2016, 4, 155–161. [Google Scholar] [CrossRef]
- Senn, S. Cross-Over Trials in Clinical Research; Wiley: Chichester, UK, 1993. [Google Scholar]
- Atkinson, G. Analysis of repeated measurements in physical therapy research: Multiple comparisons amongst level means and multifactorial designs. Phys. Ther. Sport 2002, 3, 191–203. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Achten, J.; Jeukendrup, A.E. The effect of pre-exercise carbohydrate feedings on the intensity that elicits maximal fat oxidation. J. Sports Sci. 2003, 21, 1017–1024. [Google Scholar] [CrossRef]
- Roozbeh, B.; Mohebbi, H. Comparison of different timing effect of carbohydrate intake before exercise on MFO and Fatmax in endurance runners. Sport Physiol. 2017, 9, 147–162. [Google Scholar]
- Clark, C.A.; Gardiner, J.; McBurney, M.I.; Anderson, S.; Weatherspoon, L.J.; Henry, D.N.; Hord, N.G. Effects of breakfast meal composition on second meal metabolic responses in adults with Type 2 diabetes mellitus. Eur. J. Clin. Nutr. 2006, 60, 1122–1129. [Google Scholar] [CrossRef]
- Aucouturier, J.; Duché, P.; Timmons, B.W. Metabolic flexibility and obesity in children and youth. Obes. Rev. 2011, 12, e44–e53. [Google Scholar] [CrossRef]
- Zakrzewski-Fruer, J.K.; Plekhanova, T.; Mandila, D.; Lekatis, Y.; Tolfrey, K. Effect of breakfast omission and consumption on energy intake and physical activity in adolescent girls: A randomised controlled trial. Br. J. Nutr. 2017, 118, 392–400. [Google Scholar] [CrossRef]
- Biddle, S.J.H.; Whitehead, S.H.; O’Donovan, T.M.; Nevill, M.E. Correlates of participation in physical activity for adolescent girls: A systematic review of recent literature. J. Phys. Act. Health 2005, 2, 423–434. [Google Scholar] [CrossRef]
- Dishman, R.K.; McIver, K.L.; Dowda, M.; Pate, R.R. Declining physical activity and motivation from middle school to high school. Med. Sci. Sports Exerc. 2018, 50, 1206–1215. [Google Scholar] [CrossRef]
- Rasberry, C.N.; Lee, S.M.; Robin, L.; Laris, B.A.; Russell, L.A.; Coyle, K.K.; Nihiser, A.J. The association between school-based physical activity, including physical education, and academic performance: A systematic review of the literature. Prev. Med. 2011, 52 (Suppl. S1), S10–S20. [Google Scholar] [CrossRef]
- Ning, F.; Zhang, L.; Dekker, J.M.; Onat, A.; Stehouwer, C.D.; Yudkin, J.S.; Laatikainen, T.; Tuomilehto, J.; Pyörälä, K.; Qiao, Q.; et al. Development of coronary heart disease and ischemic stroke in relation to fasting and 2-h plasma glucose levels in the normal range. Cardiovasc. Diabetol. 2012, 11, 76. [Google Scholar] [CrossRef]
- Fiorentino, T.V.; Marini, M.A.; Andreozzi, F.; Arturi, F.; Succurro, E.; Perticone, M.; Sciacqua, A.; Hribal, M.L.; Perticone, F.; Sesti, G. One-hour postload hyperglycemia is a stronger predictor of type 2 diabetes than impaired fasting glucose. J. Clin. Endocrinol. Metab. 2015, 100, 3744–3751. [Google Scholar] [CrossRef]


| Value ± Variability | |
|---|---|
| Age (years) | 13.2 ± 0.7 |
| Stature (m) | 1.56 ± 0.06 |
| Body mass (kg) | 51.2 ± 13.9 |
| Body fat % | 27.6 ± 7.0 |
| Waist circumference (cm) | 66.8 ± 9.1 |
| Body mass index (kg∙m−2) | 20.9 ± 4.9 |
| Breast development (stage) 2 | 4 (1) |
| Pubic hair (stage) 2 | 4 (1) |
| Resting metabolic rate (kcal/day) | 1566 ± 244 |
| Breakfast | Lunch | |
|---|---|---|
| Energy (kcal) | 588 ± 92 | 768 ± 120 |
| Carbohydrate (g) | 93.9 ± 14.6 | 125.3 ± 19.5 |
| Fat (g) | 8.9 ± 1.4 | 23.0 ± 3.6 |
| Protein (g) | 21.0 ± 3.3 | 11.3 ± 1.8 |
| Fibre (g) | 20.7 ± 3.2 | 4.6 ± 0.7 |
| Pre-Lunch (3 h) | Post-Lunch (2 h) | p | ||||
|---|---|---|---|---|---|---|
| BC | BO | BC | BO | Condition Main Effect | Condition by Time Interaction | |
| Glucose IAUC (mmol·L−1) | 81 (−1, 163) | −55 (−137, 27) | 318 (235, 400) | 559 (477, 641) | 0.210 | <0.0005 |
| Glucose TAUC (mmol·L−1) | 999 (919, 1080) | 879 (799, 960) | 1163 (1083, 1244) | 1347 (1266, 1427) | 0.395 | 0.001 |
| Insulin IAUC (pmol·L−1) | 4203 (2399, 6007) | −284 (−2085, 1516) | 7907 (6103, 9711) | 14,772 (12,972, 16,573) | 0.145 | <0.0005 |
| Insulin TAUC (pmol·L−1) | 5655 (3830, 7479) | 1197 (−624, 3017) | 9889 (8064, 11,713) | 15,621 (13,800, 17,441) | 0.413 | <0.0005 |
| TAG IAUC (mmol·L−1) | 5.2 (−4.8, 15.2) | −0.5 (−10.5, 9.5) | 1.3 (−8.7, 11.3) | −13.0 (−23.0, −3.0) | 0.070 | 0.430 |
| TAG TAUC (mmol·L−1) | 157 (145, 169) | 151 (138, 163) | 143 (130, 155) | 114 (102, 127) | 0.004 | 0.060 |
| Metabolic load index IAUC (mmol·L−1) | 89 (−2, 180) | −53 (−144, 38) | 323 (232, 414) | 568 (476, 659) | 0.264 | <0.0005 |
| Metabolic load index TAUC (mmol·L−1) | 1164 (1076, 1251) | 1036 (949, 1123) | 1317 (1229, 1404) | 1485 (1398, 1572) | 0.608 | 0.001 |
| Fat oxidation IAUC (mg) | −3966 (−5049, −2883) | 1223 (140, 2307) | −853 (−1936, 230) | −3083 (−4167, −1999) | 0.016 | <0.0005 |
| Fat oxidation TAUC (mg) | 5561 (4418, 6704) | 10,209 (9064, 11,355) | 2895 (1752, 4038) | 5014 (3868, 6160) | <0.0005 | 0.015 |
| Carbohydrate oxidation IAUC (mg) | 19,293 (16,177, 22,409) | −299 (−3418, 2820) | 6774 (3658, 9890) | 11,179 (8060, 14,297) | <0.0005 | <0.0005 |
| Carbohydrate oxidation TAUC (mg) | 50,129 (47,062, 53,196) | 31,300 (28,230, 34,369) | 39,864 (36,798, 42,931) | 31,135 (28,065, 34,205) | <0.0005 | <0.0005 |
| Energy expenditure IAUC (kcal) | 41.6 (27.6, 55.5) | 9.8 (−4.2, 23.7) |
3.3 (−10.7, 17.3) |
1.9 (−12.0, 15.9) | 0.003 | 0.006 |
| Energy expenditure TAUC (kcal) | 251 (235, 266) | 217 (202, 233) | 183 (167, 198) | 168 (153, 184) | <0.0005 | 0.067 |
| BC | BO | p | |
|---|---|---|---|
| Fatmax (heart rate, beats·min−1) | 125 (122, 137) | 124 (112, 137) | 0.944 |
| Peak fat oxidation rate (mg·min−1) | 49 (36, 62) | 59 (46, 72) | 0.095 |
| Perceived exercise exertion | 4 (3, 4) | 4 (3, 5) | 0.306 |
| Physical activity enjoyment | 34 (30, 37) | 31 (27, 34) | 0.055 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakrzewski-Fruer, J.K.; Morari, V.; Champion, R.B.; Bailey, D.P.; Ferrandino, L.E.; Jones, R.L. Acute Cardiometabolic and Exercise Responses to Breakfast Omission Versus Breakfast Consumption in Adolescent Girls: A Randomised Crossover Trial. Nutrients 2023, 15, 3210. https://doi.org/10.3390/nu15143210
Zakrzewski-Fruer JK, Morari V, Champion RB, Bailey DP, Ferrandino LE, Jones RL. Acute Cardiometabolic and Exercise Responses to Breakfast Omission Versus Breakfast Consumption in Adolescent Girls: A Randomised Crossover Trial. Nutrients. 2023; 15(14):3210. https://doi.org/10.3390/nu15143210
Chicago/Turabian StyleZakrzewski-Fruer, Julia K., Victoria Morari, Rachael B. Champion, Daniel P. Bailey, Louise E. Ferrandino, and Rebecca L. Jones. 2023. "Acute Cardiometabolic and Exercise Responses to Breakfast Omission Versus Breakfast Consumption in Adolescent Girls: A Randomised Crossover Trial" Nutrients 15, no. 14: 3210. https://doi.org/10.3390/nu15143210
APA StyleZakrzewski-Fruer, J. K., Morari, V., Champion, R. B., Bailey, D. P., Ferrandino, L. E., & Jones, R. L. (2023). Acute Cardiometabolic and Exercise Responses to Breakfast Omission Versus Breakfast Consumption in Adolescent Girls: A Randomised Crossover Trial. Nutrients, 15(14), 3210. https://doi.org/10.3390/nu15143210






