The Effectiveness of Early Enteral Nutrition on Clinical Outcomes in Critically Ill Sepsis Patients: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Selection and Databases
2.3. Data Extraction
2.4. Risk of Bias
2.5. Synthesis of Results
3. Results
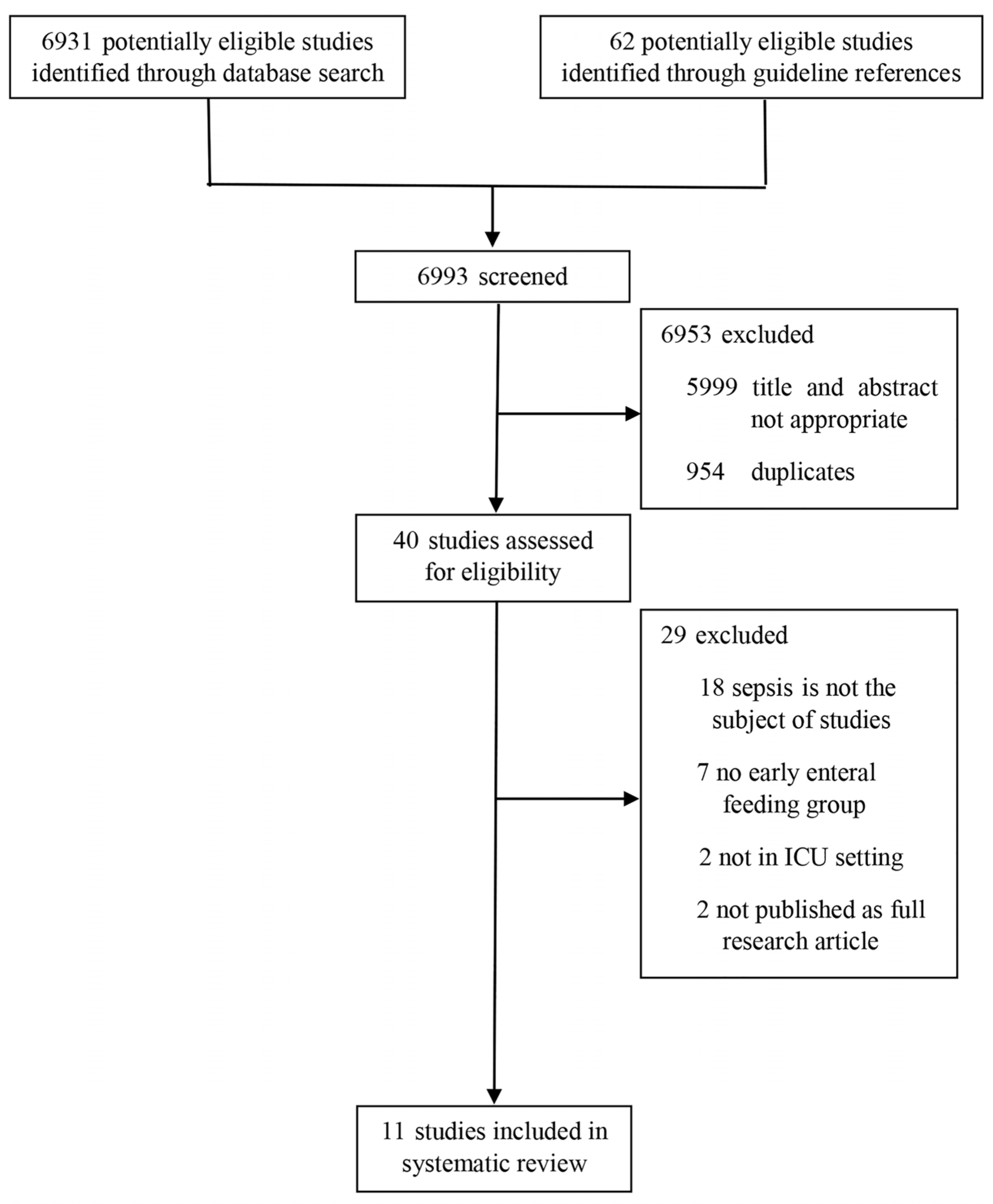
3.1. Flow of Studies
3.2. Clinical Characteristics of Included Studies
3.3. Nutrition Characteristics of Included Studies
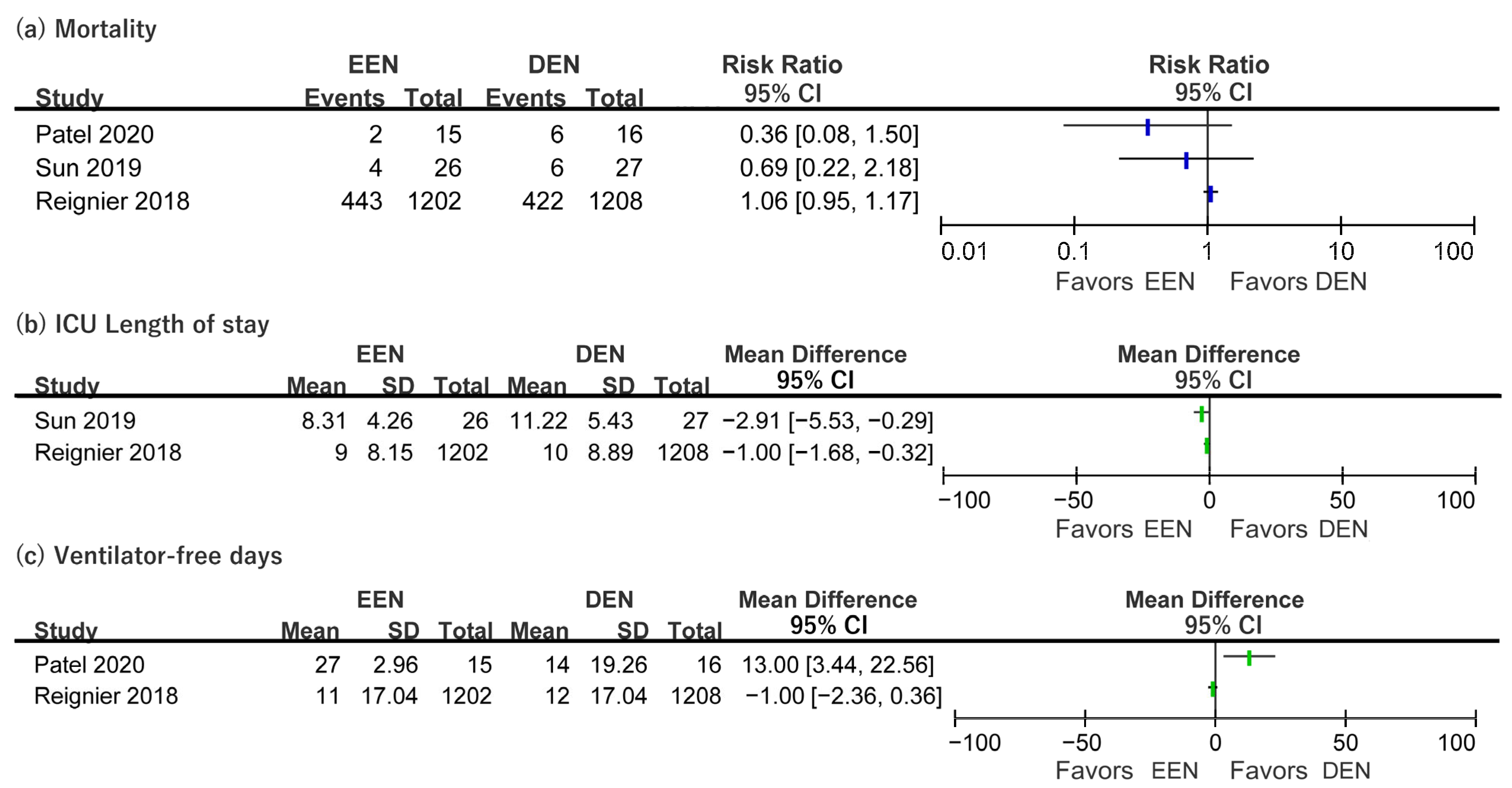
3.4. Clinical Outcomes of Included Patients
3.5. Risk of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Van Niekerk, G.; Engelbrecht, A.M. Inflammation-induced metabolic derangements or adaptation: An immunometabolic perspective. Cytokine Growth Factor Rev. 2018, 43, 47–53. [Google Scholar] [CrossRef]
- Reid, C.L.; Campbell, I.T.; Little, R.A. Muscle wasting and energy balance in critical illness. Clin. Nutr. 2004, 23, 273–280. [Google Scholar] [CrossRef]
- Ochala, J.; Gustafson, A.M.; Diez, M.L.; Renaud, G.; Li, M.; Aare, S.; Qaisar, R.; Banduseela, V.C.; Hedstrom, Y.; Tang, X.; et al. Preferential skeletal muscle myosin loss in response to mechanical silencing in a novel rat intensive care unit model: Underlying mechanisms. J. Physiol. 2011, 589, 2007–2026. [Google Scholar] [CrossRef] [PubMed]
- Sharshar, T.; Bastuji-Garin, S.; Stevens, R.D.; Durand, M.C.; Malissin, I.; Rodriguez, P.; Cerf, C.; Outin, H.; De Jonghe, B.; Groupe de Réflexion et d’Etude des Neuromyopathies En Réanimation. Presence and severity of intensive care unit-acquired paresis at time of awakening are associated with increased intensive care unit and hospital mortality. Crit. Care Med. 2009, 37, 3047–3053. [Google Scholar] [CrossRef] [PubMed]
- Ohbe, H.; Jo, T.; Matsui, H.; Fushimi, K.; Yasunaga, H. Differences in effect of early enteral nutrition on mortality among ventilated adults with shock requiring low-, medium-, and high-dose noradrenaline: A propensity-matched analysis. Clin. Nutr. 2020, 39, 460–467. [Google Scholar] [CrossRef]
- Piton, G.; Le Gouge, A.; Boisrame-Helms, J.; Anguel, N.; Argaud, L.; Asfar, P.; Botoc, V.; Bretagnol, A.; Brisard, L.; Bui, H.N.; et al. Factors associated with acute mesenteric ischemia among critically ill ventilated patients with shock: A post hoc analysis of the NUTRIREA2 trial. Intensive Care Med. 2022, 48, 458–466. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013, 41, 580–637. [Google Scholar] [CrossRef]
- Taylor, B.E.; McClave, S.A.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). Crit. Care Med. 2016, 44, 390–438. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Egi, M.; Ogura, H.; Yatabe, T.; Atagi, K.; Inoue, S.; Iba, T.; Kakihana, Y.; Kawasaki, T.; Kushimoto, S.; Kuroda, Y.; et al. The Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock 2020 (J-SSCG 2020). J. Intensive Care 2021, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Carlet, J.M.; Masur, H.; Gerlach, H.; Calandra, T.; Cohen, J.; Gea-Banacloche, J.; Keh, D.; Marshall, J.C.; Parker, M.M.; et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit. Care Med. 2004, 32, 858–873. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P.; Levy, M.M.; Carlet, J.M. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit. Care Med. 2008, 36, 296–327, Erratum in Crit. Care Med. 2008, 36, 1394–1396. [Google Scholar] [CrossRef] [PubMed]
- Boullata, J.I.; Carrera, A.L.; Harvey, L.; Escuro, A.A.; Hudson, L.; Mays, A.; McGinnis, C.; Wessel, J.J.; Bajpai, S.; Beebe, M.L.; et al. ASPEN Safe Practices for Enteral Nutrition Therapy. J. Parenter. Enter. Nutr. 2017, 41, 15–103. [Google Scholar] [CrossRef] [PubMed]
- Reintam Blaser, A.; Starkopf, J.; Alhazzani, W.; Berger, M.M.; Casaer, M.P.; Deane, A.M.; Fruhwald, S.; Hiesmayr, M.; Ichai, C.; Jakob, S.M.; et al. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. 2017, 43, 380–398. [Google Scholar] [CrossRef]
- Taverny, G.; Lescot, T.; Pardo, E.; Thonon, F.; Maarouf, M.; Alberti, C. Outcomes used in randomised controlled trials of nutrition in the critically ill: A systematic review. Crit. Care 2019, 23, 12. [Google Scholar] [CrossRef]
- Chapple, L.-a.S.; Summers, M.J.; Weinel, L.M.; Deane, A.M. Outcome Measures in Critical Care Nutrition Interventional Trials: A Systematic Review. Nutr. Clin. Pract. 2020, 35, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Li, T.; Deeks, J.J. Chapter 6: Choosing effect measures and computing estimates of effect. In Cochrane Handbook for Systematic Reviews of Interventions, version 6.3 (updated February 2022); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2022. [Google Scholar]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Haac, B.; Henry, S.; Diaz, J.; Scalea, T.; Stein, D. Early enteral nutrition is associated with reduced morbidity in critically Ill soft tissue patients. Am. Surg. 2018, 84, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hu, B.; Zhang, S.; Cai, M.; Chu, X.; Zheng, D.; Lou, M.; Cui, K.; Zhang, M.; Sun, R.; et al. Effects of early enteral nutrition on the prognosis of patients with sepsis: Secondary analysis of acute gastrointestinal injury study. Ann. Palliat. Med. 2020, 9, 3793–3801. [Google Scholar] [CrossRef]
- Koga, Y.; Fujita, M.; Yagi, T.; Todani, M.; Nakahara, T.; Kawamura, Y.; Kaneda, K.; Oda, Y.; Tsuruta, R. Early enteral nutrition is associated with reduced in-hospital mortality from sepsis in patients with sarcopenia. J. Crit. Care 2018, 47, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, W.; Chen, W.; Shen, X.; Fu, R.; Zhao, Y.; Liu, H. Effects of Early Enteral Nutrition on Immune Function and Prognosis of Patients with Sepsis on Mechanical Ventilation. J. Intensive Care Med. 2020, 35, 1053–1061. [Google Scholar] [CrossRef]
- Ortiz-Reyes, L.; Patel, J.J.; Jiang, X.; Coz Yataco, A.; Day, A.G.; Shah, F.; Zelten, J.; Tamae-Kakazu, M.; Rice, T.; Heyland, D.K. Early versus delayed enteral nutrition in mechanically ventilated patients with circulatory shock: A nested cohort analysis of an international multicenter, pragmatic clinical trial. Crit. Care 2022, 26, 173. [Google Scholar] [CrossRef]
- Patel, J.J.; Kozeniecki, M.; Biesboer, A.; Peppard, W.; Ray, A.S.; Thomas, S.; Jacobs, E.R.; Nanchal, R.; Kumar, G. Early Trophic Enteral Nutrition Is Associated with Improved Outcomes in Mechanically Ventilated Patients with Septic Shock: A Retrospective Review. J. Intensive Care Med. 2016, 31, 471–477. [Google Scholar] [CrossRef]
- Patel, J.J.; Kozeniecki, M.; Peppard, W.J.; Peppard, S.R.; Zellner-Jones, S.; Graf, J.; Szabo, A.; Heyland, D.K. Phase 3 Pilot Randomized Controlled Trial Comparing Early Trophic Enteral Nutrition With “No Enteral Nutrition” in Mechanically Ventilated Patients with Septic Shock. J. Parenter. Enter. Nutr. 2020, 44, 866–873. [Google Scholar] [CrossRef]
- Reignier, J.; Boisramé-Helms, J.; Brisard, L.; Lascarrou, J.B.; Ait Hssain, A.; Anguel, N.; Argaud, L.; Asehnoune, K.; Asfar, P.; Bellec, F.; et al. Enteral versus parenteral early nutrition in ventilated adults with shock: A randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2). Lancet 2018, 391, 133–143. [Google Scholar] [CrossRef]
- Sun, J.K.; Yuan, S.T.; Mu, X.W.; Zhang, W.H.; Liu, Y.; Zou, L.; Wang, X.; Zheng, S.Y. Effects of early enteral nutrition on T helper lymphocytes of surgical septic patients. Medicine 2017, 96, e7702. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.K.; Zhang, W.H.; Chen, W.X.; Wang, X.; Mu, X.W. Effects of early enteral nutrition on Th17/Treg cells and IL-23/IL-17 in septic patients. World J. Gastroenterol. 2019, 25, 2799–2808. [Google Scholar] [CrossRef]
- Yuan, Y.; Ren, J.; Gu, G.; Chen, J.; Li, J. Early enteral nutrition improves outcomes of open abdomen in gastrointestinal fistula patients complicated with severe sepsis. Nutr. Clin. Pract. 2011, 26, 688–694. [Google Scholar] [CrossRef]
- Chuntrasakul, C.; Siltharm, S.; Chinswangwatanakul, V.; Pongprasobchai, T.; Chockvivatanavanit, S.; Bunnak, A. Early nutritional support in severe traumatic patients. J. Med. Assoc. Thai. 1996, 79, 21–26. [Google Scholar] [PubMed]
- Van Haren, F.M.; Sleigh, J.W.; Pickkers, P.; Van der Hoeven, J.G. Gastrointestinal perfusion in septic shock. Anaesth. Intensive Care 2007, 35, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Panchal, A.K.; Manzi, J.; Connolly, S.; Christensen, M.; Wakeham, M.; Goday, P.S.; Mikhailov, T.A. Safety of Enteral Feedings in Critically Ill Children Receiving Vasoactive Agents. J. Parenter. Enter. Nutr. 2016, 40, 236–241. [Google Scholar] [CrossRef]
- Ong, C.S.; Brown, P.M.; Yesantharao, P.; Zhou, X.; Young, A.; Canner, J.K.; Quinlan, M.; Brown, E.F.; Sussman, M.S.; Whitman, G.J.R. Vasoactive and Inotropic Support, Tube Feeding, and Ischemic Gut Complications After Cardiac Surgery. J. Parenter. Enter. Nutr. 2020, 44, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Preiser, J.C.; Arabi, Y.M.; Berger, M.M.; Casaer, M.; McClave, S.; Montejo-Gonzalez, J.C.; Peake, S.; Reintam Blaser, A.; Van den Berghe, G.; van Zanten, A.; et al. A guide to enteral nutrition in intensive care units: 10 expert tips for the daily practice. Crit. Care 2021, 25, 424. [Google Scholar] [CrossRef] [PubMed]
| Study Design | Country | Sample Size | Mean Age (Years) | Mean BMI (m2/kg) | Main Sepsis Etiology | Mean SOFA Score | Vasopressor/MV Support | |
|---|---|---|---|---|---|---|---|---|
| Ortiz-Reyes et al., 2022 [27] | Prospective cohort | USA | 626 | 57.70 | 28.20 | Pneumonia | 9.40 | +/+ * |
| Patel et al., 2020 [29] | RCT | USA | 31 | 59.87 | 32.71 | Pneumonia | 10.52 | +/not reported |
| Liu et al., 2020 [26] | Case–control | China | 63 | 47.84 | Not reported | Pneumonia | 8.97 | +/+ |
| Jiang et al., 2020 [24] | Prospective cohort | China | 163 | 70.09 | 20.20 | Abdominal infection | 9.85 | +/+ |
| Sun et al., 2019 [32] | RCT | China | 53 | 58.06 | 24.74 | Abdominal infection | 9.26 | All not reported |
| Reignier et al., 2018 [30] | RCT | France | 2410 | 66.00 | 27.85 | Not reported | 11.00 | +/+ |
| Koga et al., 2018 [25] | Retro-prospective cohort | Japan | 173 | 44.39 | 21.59 | Pneumonia (EEN) and abdominal infection (DEN) | 8.96 | +/not reported |
| Haac et al., 2018 [23] | Case–control | USA | 85 | 59.72 | 37.60 | Necrotizing soft-tissue infection | 7.70 | +/not reported |
| Sun et al., 2017 [31] | Case–control | China | 82 | 71.66 | 22.91 | Abdominal infection | 8.00 | All not reported |
| Patel et al., 2016 [28] | Case–control | USA | 52 | 58.29 | 28.00 (trophic EEN), 26.55 (full EEN) | Pneumonia | Not reported | +/+ |
| Yuan et al., 2011 [33] | Case–control | China | 82 | 44.33 | 20.70 | Trauma | Not reported | Not reported/+ |
| Timing of EEN/DEN Delivery (Range Hours after ICU Admission) | Actual Delivered Energy of EEN/DEN Group (Mean kcal/Day or kcal/kg/Day) | PN Support of EEN/DEN Group (+ or −) | |
|---|---|---|---|
| RCT | |||
| Patel et al., 2020 [29] | 24 to 48 h/after 48 h | 252/307 kcal/day (7 days) | −/− * |
| Sun et al., 2019 [32] | 24 to 48 h/after 96 h | Not reported | −/− |
| Reignier et al., 2018 [30] | 24 to 96 h/after 96 h | 1413/1552 kcal/day (7 days) | −/+ |
| Cohort | |||
| Ortiz-Reyes et al., 2022 [27] | 0 to 48 h/after 48 h | 993/772 kcal/day (12 days) | +/+ |
| Jiang et al., 2020 [24] | 0 to 24 h/after 24 h | Not reported | Not reported |
| Koga et al., 2018 [25] | 0 to 48 h/after 48 h | 10.4/1.4 kcal/kg/day (7 days) | +/+ |
| Case–control | |||
| Liu et al., 2020 [26] | 0 to 48 h/after 48 h | Not reported | −/− |
| Haac et al., 2018 [23] | 0 to 48 h/after 48 h | Not reported | −/− |
| Sun et al., 2017 [31] | 48 to 72 h/after 96 h | Not reported | −/− |
| Patel et al., 2016 [28] | 0 to 48 h/after 48 h | 329 and 778/307 kcal/day (7 days) (trophic EEN and full EEN/DEN) | −/− |
| Yuan et al., 2011 [33] | 0 to 336 h/after 336 h | Not reported | +/+ |
| Definition of Mortality | EEN Group % (Events/Total) | DEN Group % (Events/Total) | Reported Significance, p | |
|---|---|---|---|---|
| RCT | ||||
| Reignier et al., 2018 [30] | Day 90 mortality | 45% (530/1185) | 43% (507/1192) | 0.28 |
| ICU mortality | 33% (429/1202) | 31% (405/1208) | 0.17 | |
| Cohort | ||||
| Ortiz-Reyes et al., 2022 [27] | Day 60 mortality | 40% (211/526) | 45% (45/100) | 0.36 |
| ICU mortality | 31% (161/526) | 33% (33/100) | 0.55 | |
| Jiang et al., 2020 [24] | Day 60 mortality | 37% (31/85) | 53% (41/78) | 0.039 * |
| Koga et al., 2018 [25] | ICU mortality | 12% (9/78) | 20% (23/113) | 0.11 |
| GI Complications (EEN/DEN Group) | Tolerance of EN (EEN/DEN Group) | |
|---|---|---|
| RCT | ||
| Patel et al., 2020 [29] | Vomiting within 72 h: 13% (EEN), 50% (DEN) Vomiting within seven days: 20% (EEN), 56% (DEN) * Ileus within seven days: 0% (EEN), 0% (DEN) Intestinal ischemia within 30 days: 0% (EEN), 0% (DEN) Small bowel obstruction within 30 days: 0% (EEN), 0% (DEN) | Episode of GRV more than 500 mL: 0% (EEN), 0% (DEN) |
| Reignier et al., 2018 [30] | Vomiting within 28 days: 34% (EEN), 24% (DEN) * Diarrhea within 28 days: 36% (EEN), 33% (DEN) * Intestinal ischemia within 28 days: 2% (EEN), less than 1% (DEN) * Acute colonic pseudo-obstruction within 28 days: 1% (EEN), 1% (DEN) * | Not reported (GRV were not monitored) |
| Cohort | ||
| Ortiz-Reyes et al., 2022 [27] | Vomiting within 28 days: 10.5% (EEN), 13.0% (DEN) Diarrhea within 28 days: 2.3% (EEN), 3.0% (DEN) Subjective discomfort within 28 days: 0.8% (EEN), 0% (DEN) Intestinal ischemia (necrotic) within 28 days: 0.3% (EEN), 0% (DEN) (significance not reported) | High GRV: 7.2% (EEN), 7.4% (DEN) |
| Jiang et al., 2020 [24] | Mean global AGI grade: 1.3 (EEN), 1.6 (DEN) | Mean GRV: 98.6 mL (EEN), 77.7 mL (DEN) |
| Case-control | ||
| Patel et al., 2016 [28] | Ileus: 2.7% (trophic EEN), 7.1% (full-calorie EEN), and 6.7% (DEN) (significance not reported) Nonocclusive intestinal ischemia or necrosis: 0% (EEN), 0% (DEN) | Feeding tolerance: 97.3% (trophic EEN), 85.7% (full-calorie EEN), and 86.6% (DEN) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, S.J.; Ko, R.-E.; Park, C.-M.; Suh, G.Y.; Hwang, J.; Chung, C.R. The Effectiveness of Early Enteral Nutrition on Clinical Outcomes in Critically Ill Sepsis Patients: A Systematic Review. Nutrients 2023, 15, 3201. https://doi.org/10.3390/nu15143201
Moon SJ, Ko R-E, Park C-M, Suh GY, Hwang J, Chung CR. The Effectiveness of Early Enteral Nutrition on Clinical Outcomes in Critically Ill Sepsis Patients: A Systematic Review. Nutrients. 2023; 15(14):3201. https://doi.org/10.3390/nu15143201
Chicago/Turabian StyleMoon, Sun Jae, Ryoung-Eun Ko, Chi-Min Park, Gee Young Suh, Jinseub Hwang, and Chi Ryang Chung. 2023. "The Effectiveness of Early Enteral Nutrition on Clinical Outcomes in Critically Ill Sepsis Patients: A Systematic Review" Nutrients 15, no. 14: 3201. https://doi.org/10.3390/nu15143201
APA StyleMoon, S. J., Ko, R.-E., Park, C.-M., Suh, G. Y., Hwang, J., & Chung, C. R. (2023). The Effectiveness of Early Enteral Nutrition on Clinical Outcomes in Critically Ill Sepsis Patients: A Systematic Review. Nutrients, 15(14), 3201. https://doi.org/10.3390/nu15143201






