Comparison of the Effects of Monounsaturated Fatty Acids and Polyunsaturated Fatty Acids on Liver Lipid Disorders in Obese Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Fatty Acid Solutions
2.2. Cell Culture
2.3. Cell Viability Detection
2.4. Cell Apoptosis Detection
2.5. ROS Detection
2.6. Western Blot Analysis
2.7. Real-Time PCR
2.8. Animals
2.9. Calculation of Liver Index
2.10. Biochemical Analysis
2.11. Serum Insulin Detection
2.12. Fasting Blood Glucose (FBG), Glucose Tolerance Test (GTT), and Insulin Tolerance Test (ITT)
2.13. Electron Microscopy
2.14. Pathological Staining
2.15. Oxidoreductases Detection
2.16. Triglyceride (TG) and Total Cholesterol (TC) Contents Detection
2.17. Targeted Lipidomics
2.18. Statistical Analysis
3. Results
3.1. UFAs Improve SFA-Induced Cell Viability Damage and Apoptosis
3.2. UFAs Attenuate PA-Induced ER Stress and Inflammatory Gene Expression
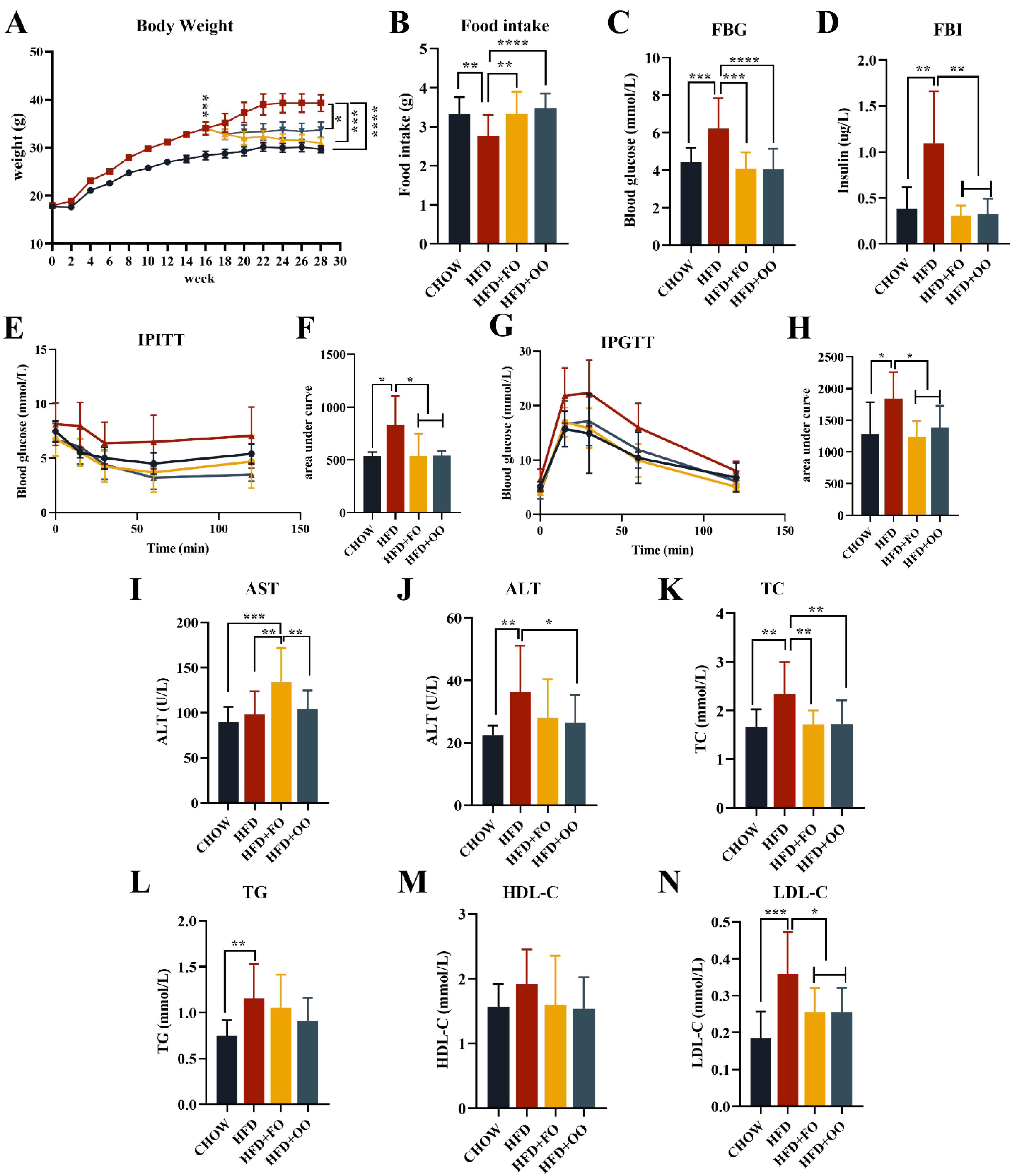
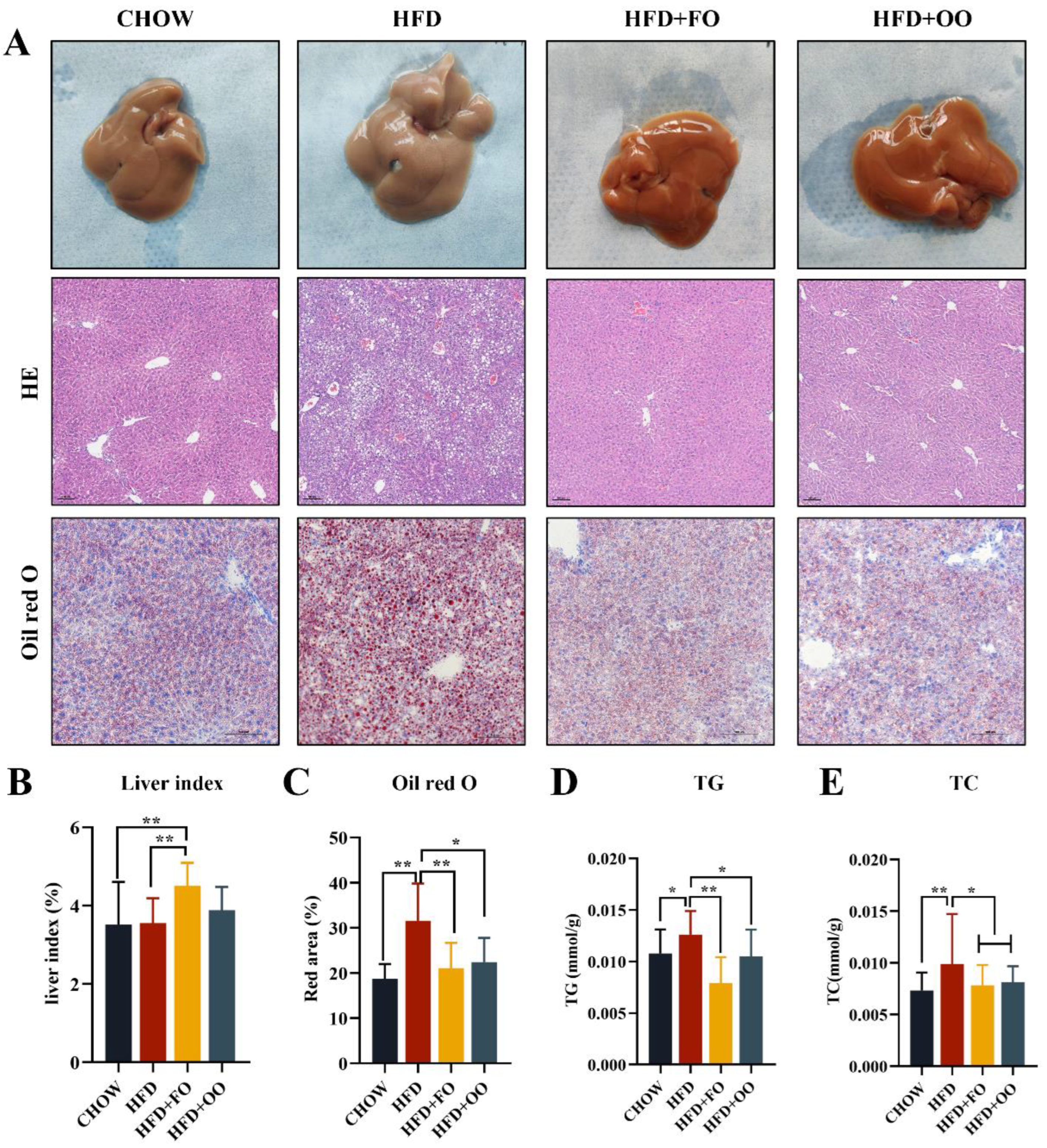
3.3. Fish Oil/Olive Oil Supplementation Improves IR and Hepatic Steatosis in Obese Mice
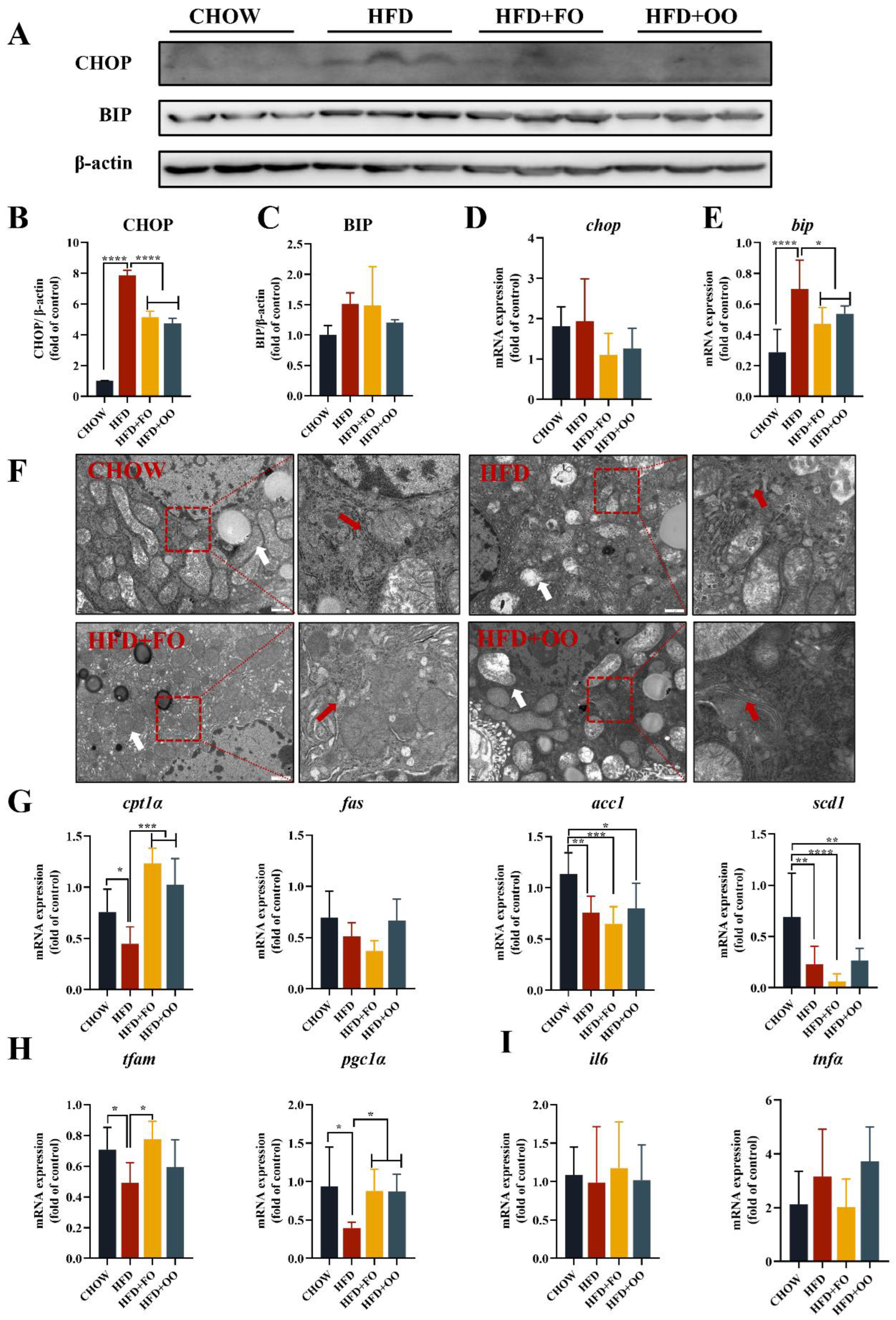
3.4. Fish Oil/Olive Oil Ameliorates HFD-Induced ER Stress in the Livers of Mice
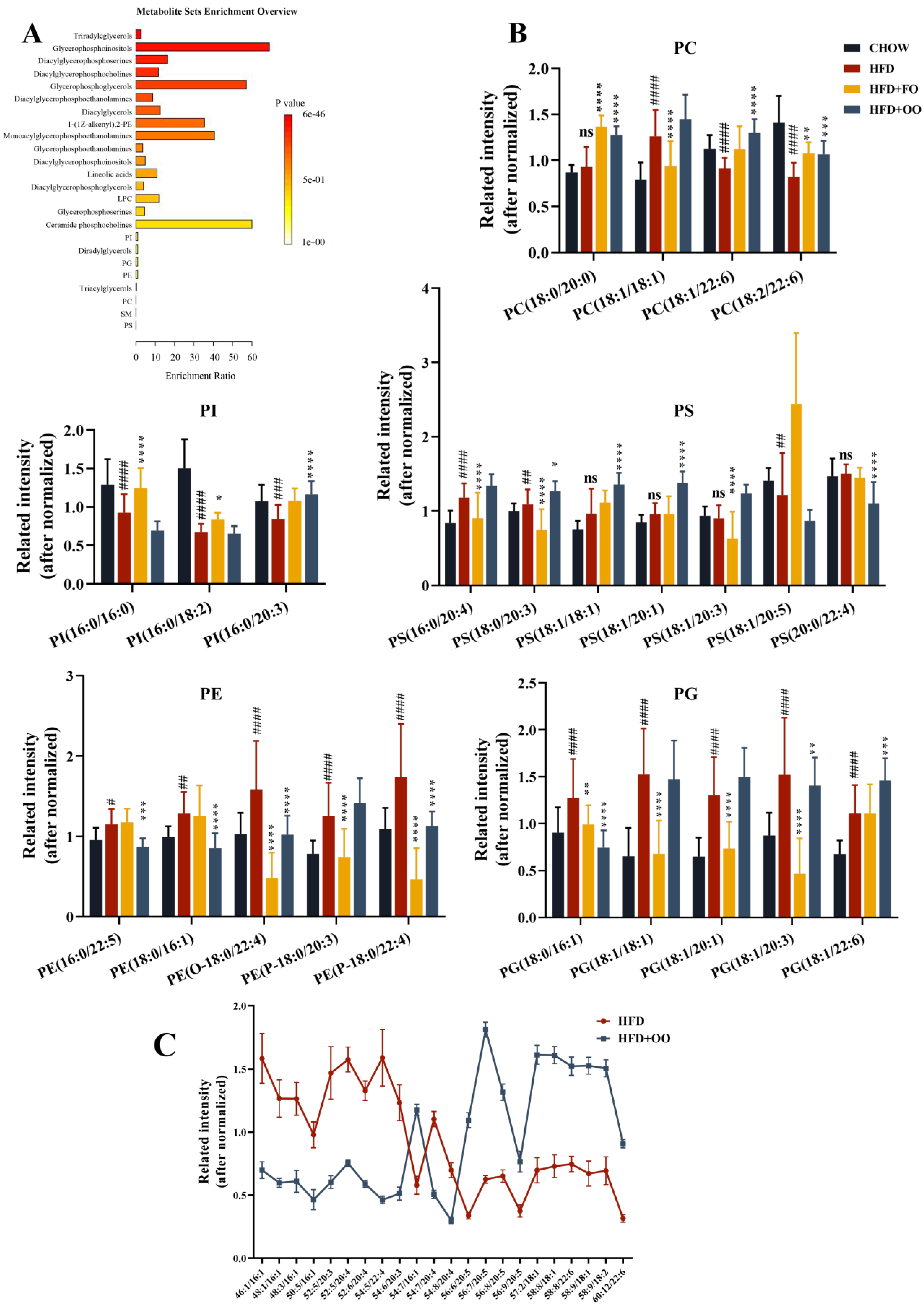
3.5. UFA Diets Regulated the Disturbed Lipid Metabolism Induced in Obese Mice
4. Discussion
4.1. UFAs Ameliorate SFA-Induced Lipotoxicity
4.2. UFA Diet Improves IRand Hepatic Steatosis in Obese Mice
4.3. Targeted Lipidomics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| T2D | Type 2 Diabetes |
| NASH | Non-alcoholic steatohepatitis |
| NAFLD | Non-alcoholic fatty liver disease |
| EPA | Eicosapentaenoic acid |
| DHA | Docosahexaenoic acid |
| ALA | α-linoleic acid |
| AA | Arachidonic acid |
| PA | Palmitic acid |
| SA | Stearic acid |
| OA | Oleic acid |
| POA | Palmitoleic acid |
| LA | Linoleic acid |
| MUFAs | Monounsaturated fatty acids |
| PUFAs | Polyunsaturated fatty acids |
| SFAs | Saturated fatty acids |
| CCK8 | Cell-Counting Kit-8 |
| QC | Quality control |
| HFD | High-fat diet |
| IPGTT | Intraperitoneal glucose tolerance test |
| IPITT | Intraperitoneal insulin tolerance test |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| LDH | Lactate dehydrogenase |
| LDL-C | Low-density Lipoprotein cholesterol |
| HDL-C | High-density lipoprotein cholesterol |
| FBI | Fasting blood insulin |
| FBG | Fasting blood glucose |
| IL-6 | Interleukin-6 |
| TNFα | Tumor Necrosis Factor α |
| ROS | Reactive oxygen species |
| MDA | Malondialdehyde |
| DAG | Diglycerides |
| TC | Total cholesterol |
| TG | Triglyceride |
| FO | Fish oil |
| OO | Olive Oil |
| PCA | Principal component analysis |
| OPLS-DA | Orthogonal Partial Least Squares Discriminant Analysis |
References
- Grander, C.; Grabherr, F.; Moschen, A.R.; Tilg, H. Non-Alcoholic Fatty Liver Disease: Cause or Effect of Metabolic Syndrome. Viszeralmedizin 2016, 32, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Kusminski, C.M.; Shetty, S.; Orci, L.; Unger, R.H.; Scherer, P.E. Diabetes and apoptosis: Lipotoxicity. Apoptosis 2009, 14, 1484–1495. [Google Scholar] [CrossRef] [PubMed]
- Trufelli, H.; Famiglini, G.; Termopoli, V.; Cappiello, A. Profiling of non-esterified fatty acids in human plasma using liquid chromatography-electron ionization mass spectrometry. Anal. Bioanal. Chem. 2011, 400, 2933–2941. [Google Scholar] [CrossRef] [PubMed]
- Song, M.J.; Kim, K.H.; Yoon, J.M.; Kim, J.B. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem. Biophys. Res. Commun. 2006, 346, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, L.; Liu, X.; Luo, R.; Liao, G.; Li, L.; Chen, Y. Oleic acid protects saturated fatty acid mediated lipotoxicity in hepatocytes and rat of non-alcoholic steatohepatitis. Life Sci. 2018, 203, 291–304. [Google Scholar] [CrossRef]
- Furstova, V.; Kopska, T.; James, R.F.; Kovar, J. Comparison of the effect of individual saturated and unsaturated fatty acids on cell growth and death induction in the human pancreatic beta-cell line NES2Y. Life Sci. 2008, 82, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, M.Y.; Bae, U.J.; Lim, J.M.; Kwon, K.S.; Park, B.H. n-3 Polyunsaturated fatty acids protect against pancreatic beta-cell damage due to ER stress and prevent diabetes development. Mol. Nutr. Food Res. 2015, 59, 1791–1802. [Google Scholar] [CrossRef]
- Abenavoli, L.; Milanovic, M.; Milic, N.; Luzza, F.; Giuffre, A.M. Olive oil antioxidants and non-alcoholic fatty liver disease. Expert. Rev. Gastroenterol. Hepatol. 2019, 13, 739–749. [Google Scholar] [CrossRef]
- Eid, S.; Sas, K.M.; Abcouwer, S.F.; Feldman, E.L.; Gardner, T.W.; Pennathur, S.; Fort, P.E. New insights into the mechanisms of diabetic complications: Role of lipids and lipid metabolism. Diabetologia 2019, 62, 1539–1549. [Google Scholar] [CrossRef]
- Albracht-Schulte, K.; Kalupahana, N.S.; Ramalingam, L.; Wang, S.; Rahman, S.M.; Robert-McComb, J.; Moustaid-Moussa, N. Omega-3 fatty acids in obesity and metabolic syndrome: A mechanistic update. J. Nutr. Biochem. 2018, 58, 1–16. [Google Scholar] [CrossRef]
- Imamura, F.; Micha, R.; Wu, J.H.; de Oliveira Otto, M.C.; Otite, F.O.; Abioye, A.I.; Mozaffarian, D. Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials. PLoS Med. 2016, 13, e1002087. [Google Scholar] [CrossRef]
- Harari, A.; Frenkel, A.L.; Barshack, I.; Sagee, A.; Cohen, H.; Kamari, Y.; Harats, D.; Kandel Kfir, M.; Shaish, A. Addition of fish oil to atherogenic high fat diet inhibited atherogenesis while olive oil did not, in LDL receptor KO mice. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, X.; Su, S.; Yuan, Y.; Liu, W.; Zhu, M.; Chen, Y. Oleic acid improves hepatic lipotoxicity injury by alleviating autophagy dysfunction. Exp. Cell Res. 2023, 429, 113655. [Google Scholar] [CrossRef]
- Astarita, G.; McKenzie, J.H.; Wang, B.; Strassburg, K.; Doneanu, A.; Johnson, J.; Kang, J.X. A protective lipidomic biosignature associated with a balanced omega-6/omega-3 ratio in fat-1 transgenic mice. PLoS ONE 2014, 9, e96221. [Google Scholar] [CrossRef] [PubMed]
- Lamaziere, A.; Richard, D.; Bausero, P.; Barbe, U.; Kefi, K.; Wolf, C.; Visioli, F. Comparison of docosahexaenoic acid uptake in murine cardiomyocyte culture and tissue: Significance to physiologically relevant studies. Prostaglandins Leukot. Essent. Fat. Acids 2015, 94, 49–54. [Google Scholar] [CrossRef]
- Zeng, X.; Zhu, M.; Liu, X.; Chen, X.; Yuan, Y.; Li, L.; Chen, Y. Oleic acid ameliorates palmitic acid induced hepatocellular lipotoxicity by inhibition of ER stress and pyroptosis. Nutr. Metab. 2020, 17, 11. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, X.; Chen, X.; Luo, R.; Li, L.; Wang, C.; Chen, Y. Oleic acid protects insulin-secreting INS-1E cells against palmitic acid-induced lipotoxicity along with an amelioration of ER stress. Endocrine 2019, 64, 512–524. [Google Scholar] [CrossRef]
- Luo, R.; Li, L.; Liu, X.; Yuan, Y.; Zhu, W.; Li, L.; Chen, Y. Mesenchymal stem cells alleviate palmitic acid-induced endothelial-to-mesenchymal transition by suppressing endoplasmic reticulum stress. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E961–E980. [Google Scholar] [CrossRef]
- Chen, J.; Ruan, X.; Sun, Y.; Li, X.; Yuan, S.; Larsson, S.C. Plasma phospholipid arachidonic acid in relation to non-alcoholic fatty liver disease: Mendelian randomization study. Nutrition 2023, 106, 111910. [Google Scholar] [CrossRef]
- Sztolsztener, K.; Chabowski, A.; Harasim-Symbor, E.; Bielawiec, P.; Konstantynowicz-Nowicka, K. Arachidonic Acid as an Early Indicator of Inflammation during Non-Alcoholic Fatty Liver Disease Development. Biomolecules 2020, 10, 1133. [Google Scholar] [CrossRef]
- Fu, S.; Yang, L.; Li, P.; Hofmann, O.; Dicker, L.; Hide, W.; Hotamisligil, G.S. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 2011, 473, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ge, M.; Ciani, L.; Kuriakose, G.; Westover, E.J.; Dura, M.; Tabas, I. Enrichment of endoplasmic reticulum with cholesterol inhibits sarcoplasmic-endoplasmic reticulum calcium ATPase-2b activity in parallel with increased order of membrane lipids: Implications for depletion of endoplasmic reticulum calcium stores and apoptosis in cholesterol-loaded macrophages. J. Biol. Chem. 2004, 279, 37030–37039. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.L.; Urano, F. Endoplasmic reticulum stress in beta cells and autoimmune diabetes. Curr. Opin. Immunol. 2016, 43, 60–66. [Google Scholar] [CrossRef]
- Sriburi, R.; Jackowski, S.; Mori, K.; Brewer, J.W. XBP1: A link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J. Cell Biol. 2004, 167, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Danino, H.; Ben-Dror, K.; Birk, R. Exocrine pancreas ER stress is differentially induced by different fatty acids. Exp. Cell Res. 2015, 339, 397–406. [Google Scholar] [CrossRef]
- Man, W.C.; Miyazaki, M.; Chu, K.; Ntambi, J. Colocalization of SCD1 and DGAT2: Implying preference for endogenous monounsaturated fatty acids in triglyceride synthesis. J. Lipid Res. 2006, 47, 1928–1939. [Google Scholar] [CrossRef]
- Capel, F.; Acquaviva, C.; Pitois, E.; Laillet, B.; Rigaudière, J.P.; Jouve, C.; Morio, B. DHA at nutritional doses restores insulin sensitivity in skeletal muscle by preventing lipotoxicity and inflammation. J. Nutr. Biochem. 2015, 26, 949–959. [Google Scholar] [CrossRef]
- Tang, D.G.; Guan, K.L.; Li, L.; Honn, K.V.; Chen, Y.Q.; Rice, R.L.; Taylor, J.D.; Porter, A.T. Suppression of W256 carcinosarcoma cell apoptosis by arachidonic acid and other polyunsaturated fatty acids. Int. J. Cancer 1997, 72, 1078–1087. [Google Scholar] [CrossRef]
- Roy, S.; Ripon, M.A.R.; Begum, R.; Bhowmik, D.R.; Amin, M.T.; Islam, M.A.; Ahmed, F.; Hossain, M.S. Arachidonic acid supplementation attenuates adipocyte inflammation but not adiposity in high fat diet induced obese mice. Biochem. Biophys. Res. Commun. 2022, 608, 90–95. [Google Scholar] [CrossRef]
- Lee, C.H.; Fu, Y.; Yang, S.J.; Chi, C.C. Effects of Omega-3 Polyunsaturated Fatty Acid Supplementation on Non-Alcoholic Fatty Liver: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2769. [Google Scholar] [CrossRef]
- Kuschner, C.E.; Choi, J.; Yin, T.; Shinozaki, K.; Becker, L.B.; Lampe, J.W.; Kim, J. Comparing phospholipid profiles of mitochondria and whole tissue: Higher PUFA content in mitochondria is driven by increased phosphatidylcholine unsaturation. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1093, 147–157. [Google Scholar] [CrossRef]
- Bargut, T.C.; Frantz, E.D.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Effects of a diet rich in n-3 polyunsaturated fatty acids on hepatic lipogenesis and beta-oxidation in mice. Lipids 2014, 49, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Okada, L.; Oliveira, C.P.; Stefano, J.T.; Nogueira, M.A.; da Silva ID, C.G.; Cordeiro, F.B.; Waitzberg, D.L. Omega-3 PUFA modulate lipogenesis, ER stress, and mitochondrial dysfunction markers in NASH-Proteomic and lipidomic insight. Clin. Nutr. 2018, 37, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Chapkin, R.S.; Wang, N.; Fan, Y.Y.; Lupton, J.R.; Prior, I.A. Docosahexaenoic acid alters the size and distribution of cell surface microdomains. Biochim. Biophys. Acta 2008, 1778, 466–471. [Google Scholar] [CrossRef]
- Zhu, C.H.; Sawrey-Kubicek, L.; Beals, E.; Hughes, R.L.; Rhodes, C.H.; Sacchi, R.; Zivkovic, A.M. The HDL lipidome is widely remodeled by fast food versus Mediterranean diet in 4 days. Metabolomics 2019, 15, 114. [Google Scholar] [CrossRef]
- Fernández-Castillejo, S.; Pedret, A.; Catalán, Ú.; Valls, R.M.; Farràs, M.; Rubió, L.; Solà, R. Virgin Olive Oil Phenolic Compounds Modulate the HDL Lipidome in Hypercholesterolaemic Subjects: A Lipidomic Analysis of the VOHF Study. Mol. Nutr. Food Res. 2021, 65, e2001192. [Google Scholar] [CrossRef]


| Gene | Sequence |
|---|---|
| H-CHOP-F | CACTCTCCAGATTCCAGTCAG |
| H-CHOP-R | AGCCGTTCATTCTCTTCAGC |
| H-BIP-F | AAGAACCAGCTCCAATTGCA |
| H-BIP-R | CACCTTGAACGGCACGAACT |
| H-IL6-F | GAAAGCAGCAAAGAGGCA |
| H-IL6-R | CACCAAGTTGAGGGAATGA |
| H-TNFα-F | ACCTCCTCTCTGCCATCAAG |
| H-TNFα-R | GAGTCGATCACCCTTCTCCA |
| H-SCD1-F | CCTGGTTTCACTTGGAGCTGTG |
| H-SCD1-R | TGTGGTGAAGTTGATGTGCCAGC |
| H-ACTIN-F | CCACGAAACTACCTTCAACTCC |
| H-ACTIN-R | GTGATCTCCTTCTGCATCCTGT |
| H-FAS-F | GGACCCAGAATACCAAGTGCAG |
| H-FAS-R | GGACCCAGAATACCAAGTGCAG |
| H-DGAT1-F | CACAGAGGCCACAGAAGTGA |
| H-DGAT1-R | AGGGCAGATACCTCCAGACA |
| H-CPT1α-F | GATCCTGGACAATACCTCGGAG |
| H- CPT1α-R | CTCCACAGCATCAAGAGACTGC |
| H-ACC1-F | TTCACTCCACCTTGTCAGCGGA |
| H-ACC1-R | GTCAGAGAAGCAGCCCATCACT |
| M-bip-F | A TGA TGAAGTTCACTGTGGTGG |
| M-bip-R | CTGATCGTTGGCTATGATCTCC |
| M-actin-F | GTGGGAATGGGTCAGAAGGA |
| M-actin-R | CTTCTCCATGTCGTCCCAGT |
| M-chop-F | CGGGTGGCAGCGACAGAG |
| M-chop-R | CAGGTGTGGTGGTGTATGAAGATG |
| M-scd1-F | AACATTCAATCCCGGGAGAATA |
| M-scd1-R | GAAACTTTCTTCCGGTCGTAAG |
| M-fas-F | TAAAGCATGACCTCGTGATGAA |
| M-fas-R | GAAGTTCAGTGAGGCGTAGTAG |
| M-dgat1-F | CCGATTCTTCCAAGGGAACTAT |
| M-dgat1-R | ATCGTAGTTGAGCACGTAGTAG |
| M-cpt1α-F | ACCGCCACCTCTTCTGCCT |
| M-cpt1α-R | AGTTCCACCTGCTGCTGAG |
| M-acc1-F | CCCAGAGATGTTTCGGCAGTCAC |
| M-acc1-R | GTCAGGATGTCGGAAGGCAAAGG |
| M-tfam-F | GTGAGCAAGTATAAAGAGCAGC |
| M-tfam -R | CTGAACGAGGTCTTTTTGGTTT |
| M-pgc1α-F | GGATATACTTTACGCAGGTCGA |
| M-pgc1α-R | CGTCTGAGTTGGTATCTAGGTC |
| M-tnfα-F | GCCACCACGCTCTTCTGTCTAC |
| M-tnfα-R | GGTTTGTGAGTGTGAGGGTCTGG |
| M-il6-F | ACAAGTCGGAGGCTTAATTACACAT |
| M-il6-R | TTGCCATTGCACAACTCTTTTC |
| 60%HFD | HFD+ FO | HFD + OO | |||||
|---|---|---|---|---|---|---|---|
| Mass Ratio (%) | Energy Ratio (%) | Mass Ratio (%) | Energy Ratio (%) | Mass Ratio (%) | Energy Ratio (%) | ||
| protein | 23.25 | 18.14 | 23.25 | 18.14 | 23.25 | 18.14 | |
| fat | Lard oil | 34.55 | 60.64 | 23.15 | 40.64 | 23.15 | 40.64 |
| Fish oil | 0 | 11.4 | 20 | 0 | 20 | ||
| Olive oil | 0 | 0 | 0 | 11.4 | 0 | ||
| carbs | 27.2 | 21.22 | 27.2 | 21.22 | 27.2 | 21.22 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Zhu, M.; Gong, M.; Zheng, W.; Zeng, X.; Zheng, Q.; Li, X.; Fu, F.; Chen, Y.; Cheng, J.; et al. Comparison of the Effects of Monounsaturated Fatty Acids and Polyunsaturated Fatty Acids on Liver Lipid Disorders in Obese Mice. Nutrients 2023, 15, 3200. https://doi.org/10.3390/nu15143200
Liu W, Zhu M, Gong M, Zheng W, Zeng X, Zheng Q, Li X, Fu F, Chen Y, Cheng J, et al. Comparison of the Effects of Monounsaturated Fatty Acids and Polyunsaturated Fatty Acids on Liver Lipid Disorders in Obese Mice. Nutrients. 2023; 15(14):3200. https://doi.org/10.3390/nu15143200
Chicago/Turabian StyleLiu, Wen, Min Zhu, Meng Gong, Wen Zheng, Xin Zeng, Qing Zheng, Xiaoyu Li, Fudong Fu, Yingyi Chen, Jingqiu Cheng, and et al. 2023. "Comparison of the Effects of Monounsaturated Fatty Acids and Polyunsaturated Fatty Acids on Liver Lipid Disorders in Obese Mice" Nutrients 15, no. 14: 3200. https://doi.org/10.3390/nu15143200
APA StyleLiu, W., Zhu, M., Gong, M., Zheng, W., Zeng, X., Zheng, Q., Li, X., Fu, F., Chen, Y., Cheng, J., Rao, Z., Lu, Y., & Chen, Y. (2023). Comparison of the Effects of Monounsaturated Fatty Acids and Polyunsaturated Fatty Acids on Liver Lipid Disorders in Obese Mice. Nutrients, 15(14), 3200. https://doi.org/10.3390/nu15143200








