Glycyrrhizic Acid Inhibits High-Mobility Group Box-1 and Homocysteine-Induced Vascular Dysfunction
Abstract
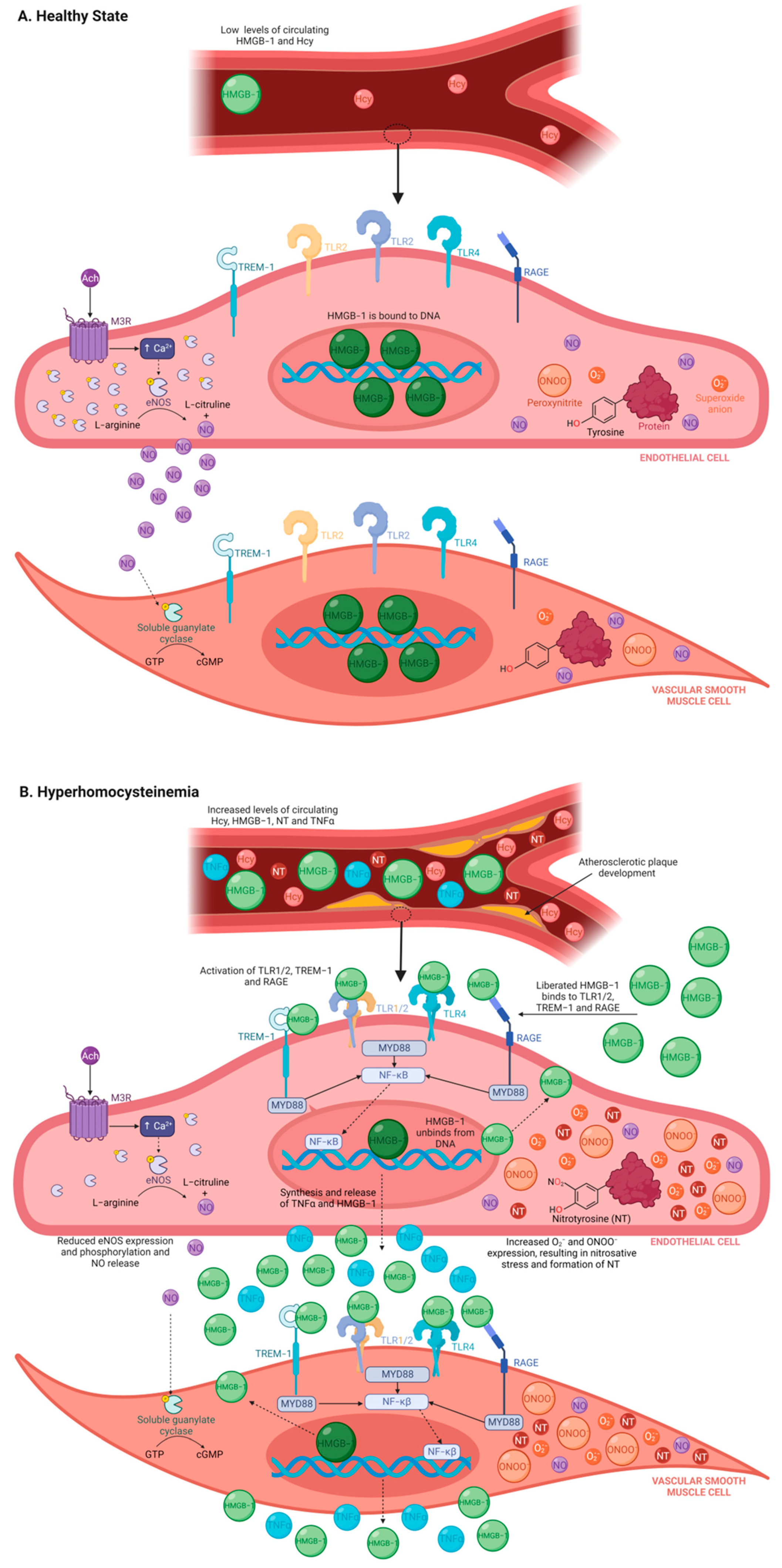
1. Introduction
2. Materials and Methods
2.1. Antibodies, Chemicals, and Kits
2.2. Animal Model and Ethics Approval
2.3. HHcy-Induced Atherogenesis Diet
2.4. Sedation, Anesthesia, and Humane Dispatch
2.5. Serum and Krebs HMGB-1 Quantification
2.6. Incubations and Isometric Tension Analysis
2.7. Immunohistochemistry Technique and Semi-Quantification Analysis
2.8. Statistical Analysis
3. Results
3.1. Serum HMGB-1 Concentration Is Significantly Elevated in Rabbits Fed a 4-Week AD
3.2. HMGB-1 Is Released by AA Rings in Response to Hcy Exposure
3.3. Incubation with rhHMGB-1 or Hcy Cause Similar Reductions in Ach-Mediated Relaxation
3.4. Inhibiting HMGB-1 with Glyz Enhances Ach-Induced Relaxation after Acute or Chronic HHcy
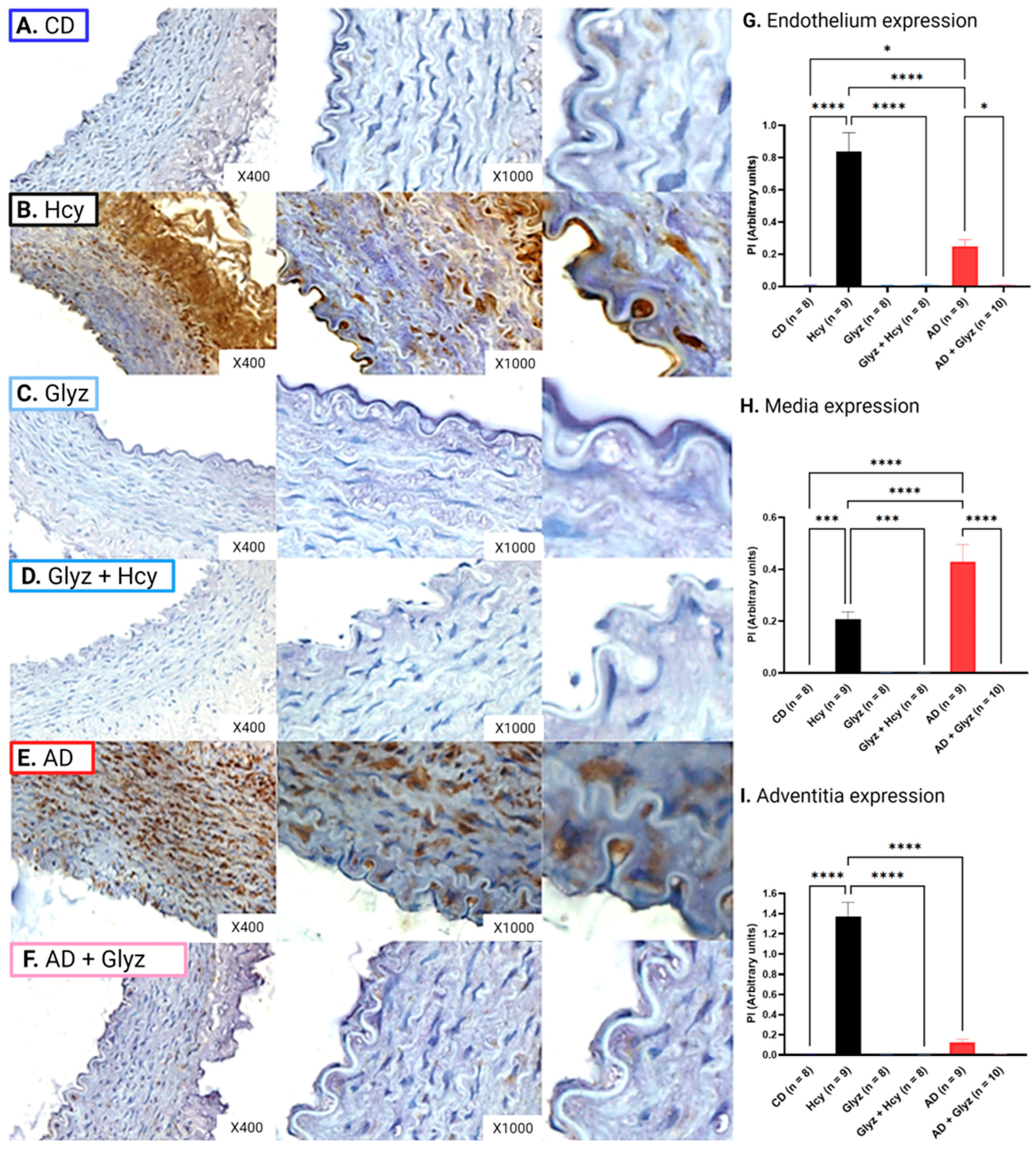
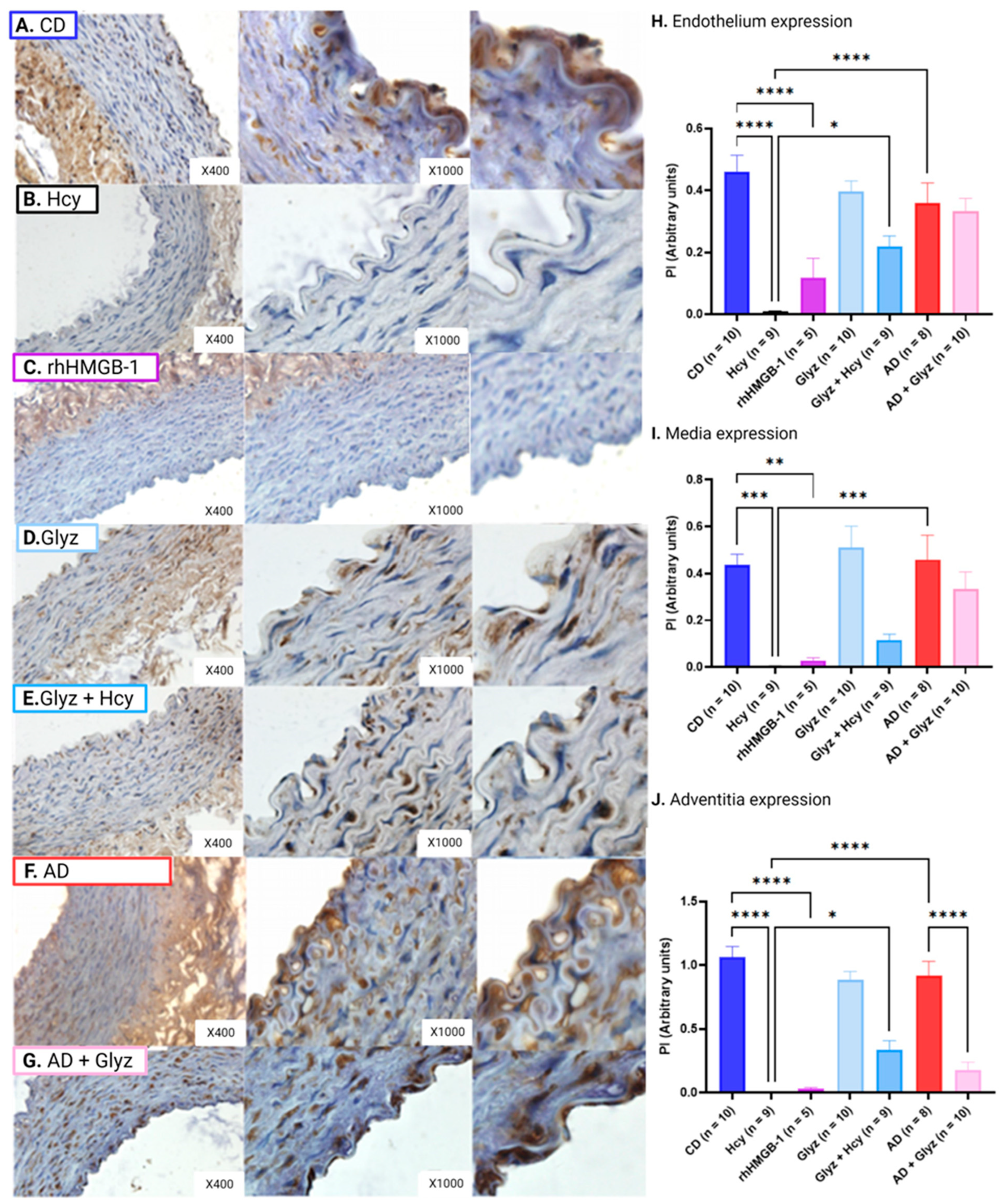
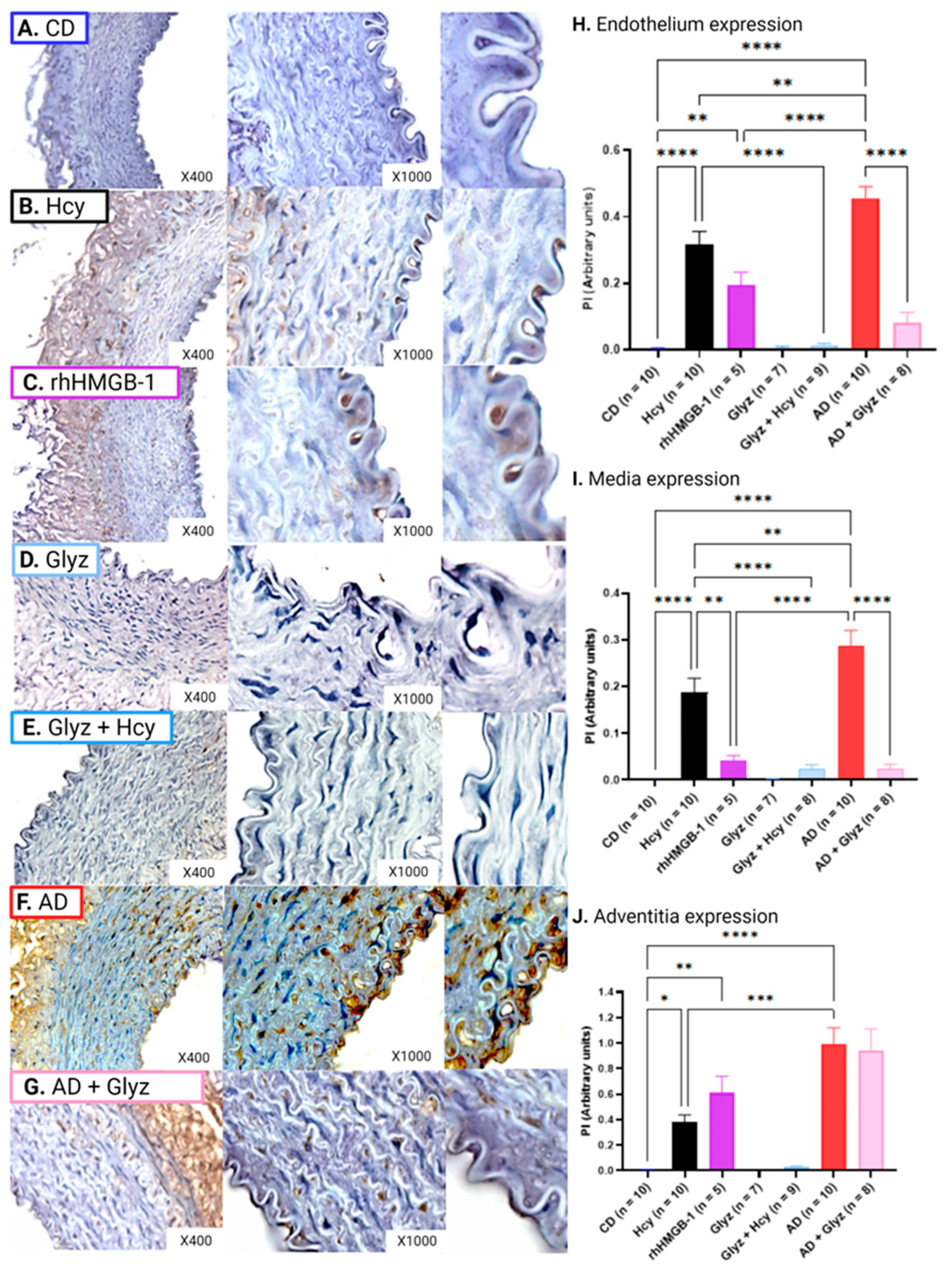
3.5. Glyz Blocks Increased Expression of HMGB-1 after Hcy Exposure or a 4-Week AD
3.6. Inhibiting HMGB-1 Increases eNOS Expression after Hcy Incubation
3.7. Inhibiting HMGB-1 with Glyz Reduces Nitrotyrosine Expression after Hcy Exposure or a 4-Week AD
3.8. Inhibiting HMGB-1 with Glyz Reduces TNFα Expression after Hcy Exposure or a 4-Week AD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mc Namara, K.; Alzubaidi, H.; Jackson, J.K. Cardiovascular disease as a leading cause of death: How are pharmacists getting involved? Integr. Pharm. Res. Pract. 2019, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Gao, C.; Cueto, R.; Liu, L.; Fu, H.; Shao, Y.; Yang, W.Y.; Fang, P.; Choi, E.T.; Wu, Q. Homocysteine-methionine cycle is a metabolic sensor system controlling methylation-regulated pathological signaling. Redox Biol. 2020, 28, 101322. [Google Scholar] [CrossRef] [PubMed]
- Lentz, S. Mechanisms of homocysteine-induced atherothrombosis. J. Thromb. Haemost. 2005, 3, 1646–1654. [Google Scholar] [CrossRef]
- Balint, B.; Jepchumba, V.K.; Guéant, J.-L.; Guéant-Rodriguez, R.-M. Mechanisms of homocysteine-induced damage to the endothelial, medial and adventitial layers of the arterial wall. Biochimie 2020, 173, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Sreckovic, B.; Sreckovic, V.D.; Soldatovic, I.; Colak, E.; Sumarac-Dumanovic, M.; Janeski, H.; Janeski, N.; Gacic, J.; Mrdovic, I. Homocysteine is a marker for metabolic syndrome and atherosclerosis. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, 179–182. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, L.; Miao, Y.; Yang, J.; Wang, X.; Wang, C.-C.; Feng, J.; Wang, L. Homocysteine causes vascular endothelial dysfunction by disrupting endoplasmic reticulum redox homeostasis. Redox Biol. 2019, 20, 46–59. [Google Scholar] [CrossRef]
- Ardestani, S.B.; Eftedal, I.; Pedersen, M.; Jeppesen, P.B.; Nørregaard, R.; Matchkov, V.V. Endothelial dysfunction in small arteries and early signs of atherosclerosis in ApoE knockout rats. Sci. Rep. 2020, 10, 15296. [Google Scholar] [CrossRef]
- Romerio, S.C.; Linder, L.; Nyfeler, J.; Wenk, M.; Litynsky, P.; Asmis, R.; Haefeli, W.E. Acute hyperhomocysteinemia decreases NO bioavailability in healthy adults. Atherosclerosis 2004, 176, 337–344. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, Z.; Wang, X.; Luo, Z.; Lai, B.; Xiao, L.; Wang, N. Stachydrine protects eNOS uncoupling and ameliorates endothelial dysfunction induced by homocysteine. Mol. Med. 2018, 24, 10. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, F.; Tan, H.; Liao, D.; Bryan, R.M., Jr.; Randhawa, J.K.; Rumbaut, R.E.; Durante, W.; Schafer, A.I.; Yang, X. Hyperhomocystinemia impairs endothelial function and eNOS activity via PKC activation. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2515–2521. [Google Scholar] [CrossRef]
- Sood, H.S.; Cox, M.J.; Tyagi, S.C. Generation of nitrotyrosine precedes activation of metalloproteinase in myocardium of hyperhomocysteinemic rats. Antioxid. Redox Signal. 2002, 4, 799–804. [Google Scholar] [CrossRef]
- Dickhout, J.G.; Hossain, G.S.; Pozza, L.M.; Zhou, J.; Lhotάk, S.A.R.; Austin, R.C. Peroxynitrite causes endoplasmic reticulum stress and apoptosis in human vascular endothelium: Implications in atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2623–2629. [Google Scholar] [CrossRef]
- Bhalodia, Y.S.; Sheth, N.R.; Vaghasiya, J.D.; Jivani, N.P. Homocysteine-dependent endothelial dysfunction induced by renal ischemia/reperfusion injury. J. Nephrol. 2011, 24, 631–635. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxidative Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef]
- Wahid, A.; Chen, W.; Wang, X.; Tang, X. High-mobility group box 1 serves as an inflammation driver of cardiovascular disease. Biomed. Pharmacother. 2021, 139, 111555. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Z.; Chen, R.; Shi, R.; Zeng, P.; Chen, R.; Leng, Y.; Chen, A.F. NRP1 regulates HMGB1 in vascular endothelial cells under high homocysteine condition. Am. J. Physiol.-Heart Circ. Physiol. 2019, 316, H1039–H1046. [Google Scholar] [CrossRef]
- Wang, R.; Wu, W.; Li, W.; Huang, S.; Li, Z.; Liu, R.; Shan, Z.; Zhang, C.; Li, W.; Wang, S. Activation of NLRP3 inflammasome promotes foam cell formation in vascular smooth muscle cells and atherogenesis via HMGB1. J. Am. Heart Assoc. 2018, 7, e008596. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Peng, X.; Li, X.; Tu, T.; Yang, H.; Teng, S.; Zhang, W.; Xing, Z.; Tang, J.; Hu, X. HMGB1 impairs endothelium-dependent relaxation in diabetes through TLR4/eNOS pathway. FASEB J. 2020, 34, 8641–8652. [Google Scholar] [CrossRef]
- Yan, X.X.; Lu, L.; Peng, W.H.; Wang, L.J.; Zhang, Q.; Zhang, R.Y.; Chen, Q.J.; Shen, W.F. Increased serum HMGB1 level is associated with coronary artery disease in nondiabetic and type 2 diabetic patients. Atherosclerosis 2009, 205, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Luan, Z.-G.; Zhang, H.; Yang, P.-T.; Ma, X.-C.; Zhang, C.; Guo, R.-X. HMGB1 activates nuclear factor-κB signaling by RAGE and increases the production of TNF-α in human umbilical vein endothelial cells. Immunobiology 2010, 215, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liang, C.; Sun, W.; Chen, J.; Chen, X. Glycyrrhizic acid ameliorates myocardial ischemic injury by the regulation of inflammation and oxidative state. Drug Des. Dev. Ther. 2018, 12, 1311. [Google Scholar] [CrossRef] [PubMed]
- Vergoten, G.; Bailly, C. Analysis of glycyrrhizin binding to protein HMGB1. Med. Drug Discov. 2020, 7, 100058. [Google Scholar] [CrossRef]
- Markina, Y.V.; Kirichenko, T.V.; Markin, A.M.; Yudina, I.Y.; Starodubova, A.V.; Sobenin, I.A.; Orekhov, A.N. Atheroprotective Effects of Glycyrrhiza glabra L. Molecules 2022, 27, 4697. [Google Scholar] [CrossRef] [PubMed]
- Gadanec, L.K.; Qaradakhi, T.; McSweeney, K.R.; Matsoukas, J.M.; Apostolopoulos, V.; Burrell, L.M.; Zulli, A. Diminazene aceturate uses different pathways to induce relaxation in healthy and atherogenic blood vessels. Biochem. Pharmacol. 2023, 208, 115397. [Google Scholar] [CrossRef] [PubMed]
- Tacey, A.; Qaradakhi, T.; Smith, C.; Pittappillil, C.; Hayes, A.; Zulli, A.; Levinger, I. The effect of an atherogenic diet and acute hyperglycaemia on endothelial function in rabbits is artery specific. Nutrients 2020, 12, 2108. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Hare, D.L.; Zulli, A. A physiologically relevant atherogenic diet causes severe endothelial dysfunction within 4 weeks in rabbit. Int. J. Exp. Pathol. 2009, 90, 598–604. [Google Scholar] [CrossRef]
- Zulli, A.; Hare, D.L. High dietary methionine plus cholesterol stimulates early atherosclerosis and late fibrous cap development which is associated with a decrease in GRP78 positive plaque cells. Int. J. Exp. Pathol. 2009, 90, 311–320. [Google Scholar] [CrossRef]
- Zulli, A.; Widdop, R.E.; Hare, D.L.; Buxton, B.F.; Black, M.J. High methionine and cholesterol diet abolishes endothelial relaxation. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1358–1363. [Google Scholar] [CrossRef]
- Setyarani, M.; Zinellu, A.; Carru, C.; Zulli, A. High dietary taurine inhibits myocardial apoptosis during an atherogenic diet: Association with increased myocardial HSP70 and HSF-1 but not caspase 3. Eur. J. Nutr. 2014, 53, 929–937. [Google Scholar] [CrossRef]
- Smith, R.M.; Rai, S.; Kruzliak, P.; Hayes, A.; Zulli, A. Putative Nox2 inhibitors worsen homocysteine-induced impaired acetylcholine-mediated relaxation. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 856–864. [Google Scholar] [CrossRef]
- El-Hawli, A.; Qaradakhi, T.; Hayes, A.; Rybalka, E.; Smith, R.; Caprnda, M.; Opatrilova, R.; Gazdikova, K.; Benckova, M.; Kruzliak, P. IRAP inhibition using HFI419 prevents moderate to severe acetylcholine mediated vasoconstriction in a rabbit model. Biomed. Pharmacother. 2017, 86, 23–26. [Google Scholar] [CrossRef]
- Wang, X.J.; Tian, D.C.; Wang, F.W.; Zhang, M.H.; Fan, C.D.; Chen, W.; Wang, M.H.; Fu, X.Y.; Ma, J.K. Astaxanthin inhibits homocysteine-induced endothelial cell dysfunction via the regulation of the reactive oxygen species-dependent VEGF-VEGFR2-FAK signaling pathway. Mol. Med. Rep. 2019, 19, 4753–4760. [Google Scholar] [CrossRef] [PubMed]
- Borkowska, A.; Ziolkowski, W.; Kaczor, K.; Herman-Antosiewicz, A.; Knap, N.; Wronska, A.; Antosiewicz, J. Homocysteine-induced decrease in HUVEC cells’ resistance to oxidative stress is mediated by Akt-dependent changes in iron metabolism. Eur. J. Nutr. 2021, 60, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Ito, T.; Kibata, K.; Inagaki-Katashiba, N.; Amuro, H.; Nishizawa, T.; Son, Y.; Ozaki, Y.; Nomura, S. Serum high-mobility group box 1 is correlated with interferon-α and may predict disease activity in patients with systemic lupus erythematosus. Lupus 2019, 28, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Marjanac, I.; Lovrić, R.; Barbić, J. Serum levels of the high-mobility group box 1 protein (HMGB1) in children with type 1 diabetes mellitus: Case-control study. Cent.-Eur. J. Immunol. 2019, 44, 33. [Google Scholar] [CrossRef]
- Ishibashi, A.; Ikeda, Y.; Inoue, M.; Takata, H.; Kumon, Y.; Terada, Y. Serum HMGB1 Level in Patients with Type 2 Diabetes. In DIABETES; American Diabetes Association: Arlington, VA, USA, 2010; p. A438. [Google Scholar]
- Hu, X.; Jiang, H.; Bai, Q.; Zhou, X.; Xu, C.; Lu, Z.; Cui, B.; Wen, H. Increased serum HMGB1 is related to the severity of coronary artery stenosis. Clin. Chim. Acta 2009, 406, 139–142. [Google Scholar] [CrossRef]
- Giovannini, S.; Tinelli, G.; Biscetti, F.; Straface, G.; Angelini, F.; Pitocco, D.; Mucci, L.; Landolfi, R.; Flex, A. Serum high mobility group box-1 and osteoprotegerin levels are associated with peripheral arterial disease and critical limb ischemia in type 2 diabetic subjects. Cardiovasc. Diabetol. 2017, 16, 99. [Google Scholar] [CrossRef]
- Kanellakis, P.; Agrotis, A.; Kyaw, T.S.; Koulis, C.; Ahrens, I.; Mori, S.; Takahashi, H.K.; Liu, K.; Peter, K.; Nishibori, M. High-mobility group box protein 1 neutralization reduces development of diet-induced atherosclerosis in apolipoprotein E–deficient mice. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 313–319. [Google Scholar] [CrossRef]
- Ding, J.-W.; Luo, C.-Y.; Wang, X.-A.; Zhou, T.; Zheng, X.-X.; Zhang, Z.-Q.; Yu, B.; Zhang, J.; Tong, X.-H. Glycyrrhizin, a high-mobility group box 1 inhibitor, improves lipid metabolism and suppresses vascular inflammation in apolipoprotein E knockout mice. J. Vasc. Res. 2018, 55, 365–377. [Google Scholar] [CrossRef]
- Pan, Y.; Ke, H.; Yan, Z.; Geng, Y.; Asner, N.; Palani, S.; Munirathinam, G.; Dasari, S.; Nitiss, K.C.; Bliss, S. The western-type diet induces anti-HMGB1 autoimmunity in Apoe−/− mice. Atherosclerosis 2016, 251, 31–38. [Google Scholar] [CrossRef]
- Kake, S.; Kawaguchi, H.; Nagasato, T.; Yamada, T.; Ito, T.; Maruyama, I.; Miura, N.; Tanimoto, A. Association between HMGB1 and thrombogenesis in a hyperlipaemia-induced microminipig model of atherosclerosis. In Vivo 2020, 34, 1871–1874. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Chen, R.; Chen, R.; He, S.; Shi, X.; Zhou, X.; Zhang, Z.; Chen, A.F. HMGB1 mediates homocysteine-induced endothelial cells pyroptosis via cathepsin V-dependent pathway. Biochem. Biophys. Res. Commun. 2020, 532, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Kohno, T.; Anzai, T.; Kaneko, H.; Sugano, Y.; Shimizu, H.; Shimoda, M.; Miyasho, T.; Okamoto, M.; Yokota, H.; Yamada, S. High-mobility group box 1 protein blockade suppresses development of abdominal aortic aneurysm. J. Cardiol. 2012, 59, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, F.; Pokharel, S.; Ma, T.; Wang, X.; Wang, Y.; Wang, W.; Lin, R. Lycium barbarum polysaccharide protects against Homocysteine-induced Vascular smooth muscle cell proliferation and phenotypic transformation via PI3K/Akt pathway. J. Mol. Histol. 2020, 51, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Luo, H.; Zhang, L.; Huang, Y.; Liu, B.; Ma, K.; Feng, J.; Xie, J.; Zheng, J.; Hu, J. Hyperhomocysteinemia Exaggerates Adventitial Inflammation and Angiotensin II− Induced Abdominal Aortic Aneurysm in Mice. Circ. Res. 2012, 111, 1261–1273. [Google Scholar] [CrossRef]
- Degryse, B.; Bonaldi, T.; Scaffidi, P.; Müller, S.; Resnati, M.; Sanvito, F.; Arrigoni, G.; Bianchi, M.E. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J. Cell Biol. 2001, 152, 1197–1206. [Google Scholar] [CrossRef]
- Ming, L.J.; Yin, A.C.Y. Therapeutic effects of glycyrrhizic acid. Nat. Prod. Commun. 2013, 8, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Mollica, L.; De Marchis, F.; Spitaleri, A.; Dallacosta, C.; Pennacchini, D.; Zamai, M.; Agresti, A.; Trisciuoglio, L.; Musco, G.; Bianchi, M.E. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem. Biol. 2007, 14, 431–441. [Google Scholar] [CrossRef]
- Okuma, Y.; Liu, K.; Wake, H.; Liu, R.; Nishimura, Y.; Hui, Z.; Teshigawara, K.; Haruma, J.; Yamamoto, Y.; Yamamoto, H. Glycyrrhizin inhibits traumatic brain injury by reducing HMGB1–RAGE interaction. Neuropharmacology 2014, 85, 18–26. [Google Scholar] [CrossRef]
- Kim, S.-W.; Jin, Y.; Shin, J.-H.; Kim, I.-D.; Lee, H.-K.; Park, S.; Han, P.-L.; Lee, J.-K. Glycyrrhizic acid affords robust neuroprotection in the postischemic brain via anti-inflammatory effect by inhibiting HMGB1 phosphorylation and secretion. Neurobiol. Dis. 2012, 46, 147–156. [Google Scholar] [CrossRef]
- Wu, C.-X.; He, L.-X.; Guo, H.; Tian, X.-X.; Liu, Q.; Sun, H. Inhibition effect of glycyrrhizin in lipopolysaccharide-induced high-mobility group box 1 releasing and expression from RAW264. 7 cells. Shock 2015, 43, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, F.; Jing, Z.; Wang, X.; Hua, X.; Wan, L. Glycyrrhizic acid exerts anti-inflammatory effect to improve cerebral vasospasm secondary to subarachnoid hemorrhage in a rat model. Neurol. Res. 2017, 39, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Haloul, M.; Vinjamuri, S.J.; Naquiallah, D.; Mirza, M.I.; Qureshi, M.; Hassan, C.; Masrur, M.; Bianco, F.M.; Frederick, P.; Cristoforo, G.P. Hyperhomocysteinemia and low folate and vitamin B12 are associated with vascular dysfunction and impaired nitric oxide sensitivity in morbidly obese patients. Nutrients 2020, 12, 2014. [Google Scholar] [CrossRef]
- Kim, S.-M.; Huh, J.-W.; Kim, E.-Y.; Shin, M.-K.; Park, J.-E.; Kim, S.W.; Lee, W.; Choi, B.; Chang, E.-J. Endothelial dysfunction induces atherosclerosis: Increased aggrecan expression promotes apoptosis in vascular smooth muscle cells. BMB Rep. 2019, 52, 145. [Google Scholar] [CrossRef]
- Liu, S.; Hu, R.; Du, J.; Li, Y.; Li, X. Glycyrrhizin ameliorates vascular endothelial cell senescence by inhibiting HMGB1 in HFD/STZ-induced diabetic rats and human umbilical vein endothelial cells. Eur. J. Pharmacol. 2022, 931, 175196. [Google Scholar] [CrossRef]
- Teng, S.; Zhu, Z.; Li, Y.; Hu, X.; Fang, Z.; Liu, Z.; Zhou, S. A novel Glycyrrhizin acid-coated stent reduces neointima formation in a rabbit iliac artery model. Front. Pharmacol. 2023, 14, 1177. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Tu, T.; Tai, S.; Tang, L.; Yang, H.; Zhu, Z. Endothelial specific deletion of HMGB1 increases blood pressure and retards ischemia recovery through eNOS and ROS pathway in mice. Redox Biol. 2021, 41, 101890. [Google Scholar] [CrossRef]
- Quidim, A.V.; Bruno, T.; Leocádio, P.C.L.; Santos, I.S.; Alvarez-Leite, J.I.; dos Reis Menta, P.L.; Lotufo, P.A.; Benseñor, I.M.; Goulart, A.C. The prognostic value of nitrotyrosine levels in coronary heart disease: Long-term evaluation in the Acute Coronary Syndrome Registry Strategy (ERICO study). Clin. Biochem. 2019, 66, 37–43. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Jin, H.; Ebin, Z.; Brodsky, S.; Goligorsky, M.S. Effects of homocysteine on endothelial nitric oxide production. Am. J. Physiol.-Ren. Physiol. 2000, 279, F671–F678. [Google Scholar] [CrossRef]
- Fischer, P.A.; Dominguez, G.N.; Cuniberti, L.A.; Martinez, V.; Werba, J.P.; Ramirez, A.J.; Masnatta, L.D. Hyperhomocysteinemia induces renal hemodynamic dysfunction: Is nitric oxide involved? J. Am. Soc. Nephrol. 2003, 14, 653–660. [Google Scholar] [CrossRef]
- Chen, H.; Guan, B.; Wang, B.; Pu, H.; Bai, X.; Chen, X.; Liu, J.; Li, C.; Qiu, J.; Yang, D. Glycyrrhizin prevents hemorrhagic transformation and improves neurological outcome in ischemic stroke with delayed thrombolysis through targeting peroxynitrite-mediated HMGB1 signaling. Transl. Stroke Res. 2020, 11, 967–982. [Google Scholar] [CrossRef]
- Loukili, N.; Rosenblatt-Velin, N.; Li, J.; Clerc, S.; Pacher, P.; Feihl, F.; Waeber, B.; Liaudet, L. Peroxynitrite induces HMGB1 release by cardiac cells in vitro and HMGB1 upregulation in the infarcted myocardium in vivo. Cardiovasc. Res. 2011, 89, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Genovese, T.; Menegazzi, M.; Mazzon, E.; Crisafulli, C.; Di Paola, R.; Dal Bosco, M.; Zou, Z.; Suzuki, H.; Cuzzocrea, S. Glycyrrhizin reduces secondary inflammatory process after spinal cord compression injury in mice. Shock 2009, 31, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.-L.; Yang, L.-F.; Shi, L.; Li, L.-F.; He, P.; Chen, J.; Zheng, B.-J.; Yang, P.; Wen, A.-D. Post-treatment with glycyrrhizin can attenuate hepatic mitochondrial damage induced by acetaminophen in mice. Exp. Biol. Med. 2021, 246, 1219–1227. [Google Scholar] [CrossRef]
- Menegazzi, M.; Di Paola, R.; Mazzon, E.; Genovese, T.; Crisafulli, C.; Dal Bosco, M.; Zou, Z.; Suzuki, H.; Cuzzocrea, S. Glycyrrhizin attenuates the development of carrageenan-induced lung injury in mice. Pharmacol. Res. 2008, 58, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Han, N.J.; Kim, J.J.; Lee, M.J.; Park, S.-K. TNF-α activates high-mobility group box 1-toll-like receptor 4 signaling pathway in human aortic endothelial cells. Cell. Physiol. Biochem. 2016, 38, 2139–2151. [Google Scholar] [CrossRef]
- Abdel Aziz, M.; Fouad, H.; Mohsen, G.; Mansour, M.; Abdel Ghaffar, S. TNF-alpha and homocysteine levels in type 1 diabetes mellitus. EMHJ-East. Mediterr. Health J. 2001, 7, 679–688. [Google Scholar] [CrossRef]
- Elkind, M.S.; Cheng, J.; Boden-Albala, B.; Rundek, T.; Thomas, J.; Chen, H.; Rabbani, L.E.; Sacco, R.L. Tumor necrosis factor receptor levels are associated with carotid atherosclerosis. Stroke 2002, 33, 31–38. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Heusch, G.; Schulz, R. TNFα in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol. Ther. 2010, 127, 295–314. [Google Scholar] [CrossRef]
- Fiuza, C.; Bustin, M.; Talwar, S.; Tropea, M.; Gerstenberger, E.; Shelhamer, J.H.; Suffredini, A.F. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood J. Am. Soc. Hematol. 2003, 101, 2652–2660. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, W.; Zhang, D. Gycyrrhizic acid alleviates atherosclerotic lesions in rats with diabetes mellitus. Mol. Med. Rep. 2021, 24, 755. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Li, B.; Peng, W.; Xu, Z. Protective effect of glycyrrhizin on coronary microembolization-induced myocardial dysfunction in rats. Pharmacol. Res. Perspect. 2021, 9, e00714. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.C.; Hao, Y.J.; Jiao, Y.; Wang, Y.H.; Xu, L.B.; Mao, C.Y.; Yang, X.L.; Yang, A.N.; Tian, J.; Zhang, M.H. Homocysteine-induced oxidative stress through TLR4/NF-κB/DNMT1-mediated LOX-1 DNA methylation in endothelial cells. Mol. Med. Rep. 2017, 16, 9181–9188. [Google Scholar] [CrossRef] [PubMed]
- Omar, H.R.; Komarova, I.; El-Ghonemi, M.; Fathy, A.; Rashad, R.; Abdelmalak, H.D.; Yerramadha, M.R.; Ali, Y.; Helal, E.; Camporesi, E.M. Licorice abuse: Time to send a warning message. Ther. Adv. Endocrinol. Metab. 2012, 3, 125–138. [Google Scholar] [CrossRef]
- Deutch, M.R.; Grimm, D.; Wehland, M.; Infanger, M.; Krüger, M. Bioactive candy: Effects of licorice on the cardiovascular system. Foods 2019, 8, 495. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lee, S.-W.; Han, M.J. Biotransformation of glycyrrhizin to 18β-glycyrrhetinic acid-3-O-β-D-glucuronide by Streptococcus LJ-22, a human intestinal bacterium. Biol. Pharm. Bull. 1999, 22, 320–322. [Google Scholar] [CrossRef]
- Calὸ, L.A.; Zaghetto, F.; Pagnin, E.; Davis, P.A.; De Mozzi, P.; Sartorato, P.; Martire, G.; Fiore, C.; Armanini, D. Effect of aldosterone and glycyrrhetinic acid on the protein expression of PAI-1 and p22phox in human mononuclear leukocytes. J. Clin. Endocrinol. Metab. 2004, 89, 1973–1976. [Google Scholar] [CrossRef]
- Ferrari, P. The role of 11β-hydroxysteroid dehydrogenase type 2 in human hypertension. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2010, 1802, 1178–1187. [Google Scholar] [CrossRef]
- Hadoke, P.W.; Christy, C.; Kotelevtsev, Y.V.; Williams, B.C.; Kenyon, C.J.; Seckl, J.R.; Mullins, J.J.; Walker, B.R. Endothelial cell dysfunction in mice after transgenic knockout of type 2, but not type 1, 11β-hydroxysteroid dehydrogenase. Circulation 2001, 104, 2832–2837. [Google Scholar] [CrossRef]
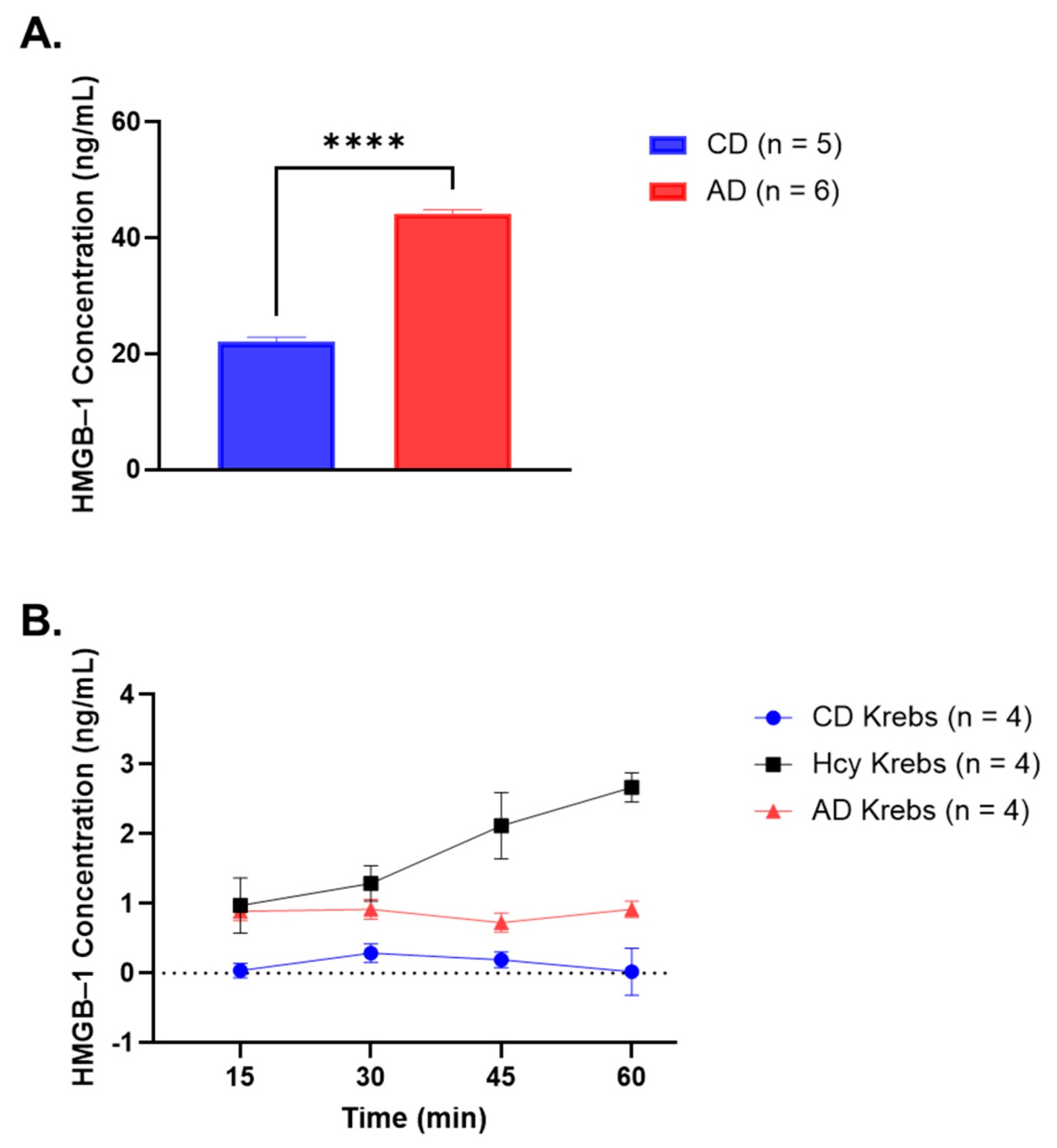



| Time Intervals (min) | CD Krebs vs. AD Krebs (ng/mL) (Mean ± SEM) | CD Krebs vs. Hcy Krebs (ng/mL) (Mean ± SEM) | Hcy Krebs vs. AD Krebs (ng/mL) (Mean ± SEM) |
|---|---|---|---|
| 15 | CD: 0.42 ± 0.10 vs. AD: 0.89 ± 0.13, p = 0.0496 | CD: 0.42 ± 0.10 vs. Hcy: 0.97 ± 0.40, p = 0.0278 | Hcy: 0.97 ± 0.40 vs. AD: 0.89 ± 0.13, p = 0.9705 |
| 30 | CD: 0.29 ± 0.13 vs. AD: 0.92 ± 0.14, p = 0.1791 | CD: 0.29 ± 0.13 vs. Hcy: 1.29 ± 0.25, p = 0.0178 | Hcy: 1.29 ± 0.25 vs. AD: 0.92 ± 0.14, p = 0.411 |
| 45 | CD: 0.19 ± 0.11 vs. AD: 0.73 ± 0.11, p = 0.2808 | CD: 0.19 ± 0.11 vs. Hcy: 2.12 ± 0.48, p < 0.0001 | Hcy: 2.12 ± 0.48 vs. AD: 0.73 ± 0.11, p = 0.0008 |
| 60 | CD: 0.02 ± 0.34 vs. AD: 0.92 ± 0.12, p = 0.0336 | CD: 0.02 ± 0.34 vs. Hcy: 2.67 ± 0.21, p < 0.0001 | Hcy: 2.67 ± 0.21 vs. AD: 0.92 ± 0.12, p < 0.0001 |
| Log [Ach], M | CD vs. Hcy (%) (Mean ± SEM) | CD vs. rhHMGB-1 (%) (Mean ± SEM) | Hcy vs. rhHMGB-1 (%) (Mean ± SEM) |
|---|---|---|---|
| −8.0 | CD: −21.83 ± 2.04 vs. Hcy: −11.43 ± 2.75, p = 0.0319 | CD: −21.83 ± 2.04 vs. rhHMGB-1: −13.20 ± 3.92, p = 0.2989 | Hcy: −11.43 ± 2.75 vs. rhHMGB-1: −13.20 ± 3.92, p = 0.9500 |
| −7.5 | CD: −46.48 ± 3.76 vs. Hcy: −20.58 ± 3.49, p < 0.0001 | CD: −46.48 ± 3.76 vs. rhHMGB-1: −22.53 ± 5.93, p = 0.0020 | Hcy: −20.58 ± 3.49 vs. rhHMGB-1: −22.53 ± 5.93, p = 0.9398 |
| −7.0 | CD: −65.75 ± 3.99 vs. Hcy: −36.37 ± 3.75, p < 0.0001 | CD: −46.48 ± 3.76 vs. rhHMGB-1: −30.17 ± 6.37, p < 0.0001 | Hcy: −36.37 ± 3.75 vs. rhHMGB-1: −30.17 ± 6.37, p = 0.5346 |
| −6.5 | CD: −80.21 ± 2.65 vs. Hcy: −48.06 ± 2.47, p < 0.0001 | CD: −46.48 ± 3.76 vs. rhHMGB-1: −46.53 ± 5.27, p < 0.0001 | Hcy: −48.06 ± 2.47 vs. rhHMGB-1: −46.53 ± 5.27, p = 0.9625 |
| −6.0 | CD: −84.80 ± 2.00 vs. Hcy: −52.87 ± 2.31, p < 0.0001 | CD: −46.48 ± 3.76 vs. rhHMGB-1: −52.04 ± 6.55, p < 0.0001 | Hcy: −52.87 ± 2.31 vs. rhHMGB-1: −52.04 ± 6.55, p = 0.9890 |
| −5.5 | CD: −90.10 ± 1.32 vs. Hcy: −53.30 ± 2.21, p < 0.0001 | CD: −46.48 ± 3.76 vs. rhHMGB-1: −56.18 ± 7.18, p < 0.0001 | Hcy: −53.30 ± 2.21 vs. rhHMGB-1: −56.18 ± 7.18, p = 0.8731 |
| −5.0 | CD: −93.13 ± 1.97 vs. Hcy: −52.29 ± 2.71, p < 0.0001 | CD: −46.48 ± 3.76 vs. rhHMGB-1: −55.84 ± 8.02, p < 0.0001 | Hcy: −52.29 ± 2.71 vs. rhHMGB-1: −55.84 ± 8.02, p = 0.8116 |
| Log [Ach], M | CD vs. Hcy (%) (Mean ± SEM) | CD vs. Glyz (%) (Mean ± SEM) | Hcy vs. Glyz + Hcy (%) (Mean ± SEM) |
|---|---|---|---|
| −8.0 | CD: −21.83 ± 2.04 vs. Hcy: −11.43 ± 2.75, p = 0.0319 | CD: −21.83 ± 2.04 vs. Glyz: −16.20 ± 3.14, p = 0.5321 | Hcy: −11.43 ± 2.75 vs. Glyz + Hcy: −10.28 ± 3.35, p = 0.9928 |
| −7.5 | CD: −46.48 ± 3.76 vs. Hcy: −20.58 ± 3.49, p < 0.0001 | CD: −46.48 ± 3.76 vs. Glyz: −35.51 ± 3.00, p = 0.0444 | Hcy: −20.58 ± 3.49 vs. Glyz + Hcy: −27.45 ± 4.45, p = 0.3550 |
| −7.0 | CD: −65.75 ± 3.99 vs. Hcy: −36.37 ± 3.75, p < 0.0001 | CD: −65.75 ± 3.99 vs. Glyz: −56.79 ± 2.86, p = 0.1403 | Hcy: −36.37 ± 3.75 vs. Glyz + Hcy: −53.56 ± 3.42, p = 0.0003 |
| −6.5 | CD: −80.21 ± 2.65 vs. Hcy: −48.06 ± 2.47, p < 0.0001 | CD: −80.21 ± 2.65 vs. Glyz: −67.96 ± 2.48, p = 0.0186 | Hcy: −48.06 ± 2.47 vs. Glyz + Hcy: −66.87 ± 2.49, p < 0.0001 |
| −6.0 | CD: −84.80 ± 2.00 vs. Hcy: −52.87 ± 2.31, p < 0.0001 | CD: −84.80 ± 2.00 vs. Glyz: −74.32 ± 2.80, p = 0.0602 | Hcy: −52.87 ± 2.31 vs. Glyz + Hcy: −79.26 ± 2.33, p < 0.0001 |
| −5.5 | CD: −90.10 ± 1.32 vs. Hcy: −53.30 ± 2.21, p < 0.0001 | CD: −84.80 ± 2.00 vs. Glyz: −78.64 ± 2.83, p = 0.0322 | Hcy: −53.30 ± 2.21 vs. Glyz + Hcy: −84.26 ± 2.53, p < 0.0001 |
| −5.0 | CD: −93.13 ± 1.97 vs. Hcy: −52.29 ± 2.71, p < 0.0001 | CD: −93.13 ± 1.97 vs. Glyz: −80.01 ± 2.48, p = 0.0105 | Hcy: −52.29 ± 2.71 vs. Glyz + Hcy: −83.20 ± 2.28, p < 0.0001 |
| Log [Ach], M | CD vs. AD (%) (Mean ± SEM) | AD vs. AD + Glyz (%) (Mean ± SEM) |
|---|---|---|
| −8.0 | CD: −21.83 ± 2.04 vs. AD: −4.21 ± 2.48, p < 0.0001 | AD: −4.21 ± 2.48 vs. AD + Glyz: −11.21 ± 1.60, p = 0.1850 |
| −7.5 | CD: −46.48 ± 3.76 vs. AD: −14.15 ± 3.07, p < 0.0001 | AD: −14.15 ± 3.07 vs. AD + Glyz: −29.711 ± 2.66, p = 0.0040 |
| −7.0 | CD: −65.75 ± 3.99 vs. AD: −29.83 ± 4.06, p < 0.0001 | AD: −29.83 ± 4.06 vs. AD + Glyz: −55.72 ± 2.35, p < 0.0001 |
| −6.5 | CD: −80.21 ± 2.65 vs. AD: −42.77 ± 2.78, p < 0.0001 | AD: −42.77 ± 2.78 vs. AD + Glyz: −72.39 ± 1.01, p < 0.0001 |
| −6.0 | CD: −84.80 ± 2.00 vs. AD: −50.95 ± 2.62, p < 0.0001 | AD: −50.95 ± 2.62 vs. AD + Glyz: −79.56 ± 1.13, p < 0.0001 |
| −5.5 | CD: −90.10 ± 1.32 vs. AD: −49.91 ± 2.38, p < 0.0001 | AD: −49.91 ± 2.38 vs. AD + Glyz: −80.85 ± 1.19, p < 0.0001 |
| −5.0 | CD: −93.13 ± 1.97 vs. AD: −52.35 ± 2.23, p < 0.0001 | AD: −52.35 ± 2.23 vs. AD + Glyz: −80.42 ± 1.46, p < 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gadanec, L.K.; Andersson, U.; Apostolopoulos, V.; Zulli, A. Glycyrrhizic Acid Inhibits High-Mobility Group Box-1 and Homocysteine-Induced Vascular Dysfunction. Nutrients 2023, 15, 3186. https://doi.org/10.3390/nu15143186
Gadanec LK, Andersson U, Apostolopoulos V, Zulli A. Glycyrrhizic Acid Inhibits High-Mobility Group Box-1 and Homocysteine-Induced Vascular Dysfunction. Nutrients. 2023; 15(14):3186. https://doi.org/10.3390/nu15143186
Chicago/Turabian StyleGadanec, Laura Kate, Ulf Andersson, Vasso Apostolopoulos, and Anthony Zulli. 2023. "Glycyrrhizic Acid Inhibits High-Mobility Group Box-1 and Homocysteine-Induced Vascular Dysfunction" Nutrients 15, no. 14: 3186. https://doi.org/10.3390/nu15143186
APA StyleGadanec, L. K., Andersson, U., Apostolopoulos, V., & Zulli, A. (2023). Glycyrrhizic Acid Inhibits High-Mobility Group Box-1 and Homocysteine-Induced Vascular Dysfunction. Nutrients, 15(14), 3186. https://doi.org/10.3390/nu15143186







