Abstract
Sleep is fundamental for adolescents’ healthy development but undergoes dramatic changes in quantity and quality due to the conflict between biological and social rhythms. Insufficient sleep has been associated with worse physical health status and irregular eating behaviors in adolescents. This review aims to systematically synthesize the longitudinal associations between adolescents’ sleep dimensions (i.e., duration, timing, quality, and insomnia symptoms) and physical health indicators (i.e., anthropometric indices, fat percentage, and risk of obesity), eating behaviors, and nutritional aspects (i.e., type of diet related to the intake of specific foods and nutrients, amount and timing of food consumption, energy expenditure). A total of 28 longitudinal studies were included. The meta-analytic results showed that longer sleep duration, better sleep quality, and lower insomnia symptoms were associated with lower BMI and fat percentage and that shorter sleep duration (<7 h) and lower sleep quality were associated with a higher risk of obesity. Conversely, anthropometric indices were not related to sleep over time. Limited literature examined the bidirectional association between adolescents’ sleep and their eating behaviors and nutritional aspects. Such knowledge sheds new light on the role of sleep for adolescents’ health, highlighting the need to examine further the interplay between these variables.
1. Introduction
Sleep is a fundamental human psychophysiological function, and good sleep quality is essential for adolescents’ healthy development []. Sleep quality can be conceived as a multi-faceted phenomenon referring to satisfaction with sleep, adequate sleep duration, regular sleep timing, initiation and maintenance of sleep, and the ability to maintain alertness during waking hours [] (see Figure 1 for a summary of the main dimensions of sleep considered). According to the National Sleep Foundation [], adolescents (aged 14–17 years) are recommended to sleep around 8–10 h and to maintain a regular sleep timing (keeping almost the same bedtimes and wake-up times amongst weekdays and weekends). These recommendations are considered central assets for promoting adolescents’ physical health (e.g., weight control, hormones regulation) [,,]. However, over the last decades, adolescents’ sleep duration has been significantly reduced, becoming a source of public health concern [,]. This dramatic decline could be due to the interplay between biological (e.g., changes in melatonin secretion; for a review, []) and psychosocial changes (e.g., irregular sleep patterns and changes in physical activity and digital use; for reviews, [,]) that happen during adolescence.
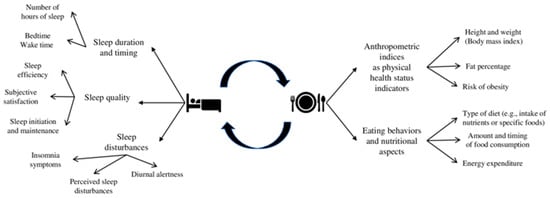
Figure 1.
Overview of the main dimensions.
Importantly, poor sleep quality can be associated with adolescents’ main indicators of physical health status (i.e., weight status, risk of developing obesity), as well as with their eating behaviors and nutritional aspects (i.e., type of diet related to the intake of nutrients or specific foods, amount and timing of food consumption and energy expenditure) [,,] (see Figure 1 for a summary of the main dimensions considered). Nowadays, there is a trend of increasing obesity rates among adolescents [] and that could lead to a greater risk of non-communicable diseases such as cardiovascular diseases and type 2 diabetes [], as well as psychosocial problems later in life, such as depression [] and bullying []. Moreover, eating behaviors and nutritional aspects become more irregular during adolescence in terms of type of diet, amount and timing of food consumption (e.g., skipping breakfast and decline in fruit and vegetable consumption), and energy expenditure [,,]. Moving from this knowledge of an interplay between sleep, physical health status indicators, eating behaviors, and nutritional aspects [,,], this systematic review with meta-analysis aims to shed light on the available longitudinal findings of how adolescents’ sleep, anthropometric indices, eating behaviors, and nutritional aspects are bidirectionally related over time.
1.1. The Interplay between Adolescents’ Sleep and Anthropometric Indices
Anthropometric indices are crucial indicators of the health status of individuals. For example, waist circumference, body fat percentage, and Body Mass Index (BMI, weight/height2; kg/m2) are some of the most used measures of body fatness in children and adolescents []. Moreover, these indicators are helpful in identifying several pathologies. Indeed, based on the World Health Organization, adolescents’ overweight and obesity are defined as a gender- and age-specific BMI at or above the 95th percentile on the growth references []. Over the past decades, adolescents’ obesity has become a global public health issue since its association with cardiometabolic and psychosocial comorbidity [,].
Previous results highlighted an association between short sleep duration (less than the amount of sleep recommended for the considered age group), increased BMI, and obesity among adolescents (for reviews, [,,,,]). One of the mechanisms that was hypothesized to be involved in this relation is the biological alteration in appetite-regulating hormones ghrelin (which stimulates appetite) and leptin (which restrains appetite). Specifically, short sleep duration has been shown to increase ghrelin [] and decrease leptin []. However, results about the effects of sleep on the secretion of leptin and ghrelin are still mixed (for a review []), also suggesting that longer sleep duration can be associated with lower levels of leptin [] or higher levels of ghrelin []. Most of these results are based on cross-sectional studies that included both children and adolescents, so the inconsistency may be partially explained by participants’ heterogeneity (e.g., age, sex, and weight status) and studies’ methodological differences (e.g., measurement tools and participant selection) [].
Another possible explanation of the link between poor sleep quality, short sleep duration, and higher BMI and consequent risk of obesity could be attributed to the trend of adolescents to prefer a pattern of behavior referred to as eveningness, characterized by later bedtime and wake-up times and an increase in activity levels later on the day [,,,,]. In addition, adolescents’ preference for eveningness contrasts with their social schedules (i.e., school entrance time) and often results in short sleep duration [,,]. This can also have diurnal consequences such as daytime sleepiness and higher sedentary time or fatigue, leading to reduced energy expenditure and, consequently, a predisposition to obesity [], creating a vicious circle.
These findings highlight the potential role adolescents’ poor sleep can play in negatively affecting their weight status, predisposing them to developing obesity. Conversely, less is known about the opposite direction of this relation. Thus, among the adult population, only a few studies showed that obesity could be an important risk factor for daytime sleepiness and nocturnal sleep disturbances (for a review []), while among adolescents, evidence is even rarer. In conclusion, both poor sleep and obesity are increasing among adolescents, with major consequences for their health. Nevertheless, there is a lack of knowledge on the mechanism and sleep related aspects involved in this reciprocal association.
1.2. The Interplay between Adolescents’ Sleep, Eating Behaviors, and Nutritional Aspects
In adolescence, youth tend to modify their eating habits, becoming more irregular in timing and diet pattern. Specifically, they tend to drink more energy-dense foods and sweetened beverages, eat away from home more often (e.g., in fast food restaurants), and increase portion sizes [,,]. Dietary patterns formed during adolescence tend to be maintained even in adulthood [,,]. Among the adult population, studies found a bidirectional link between sleeping and eating: on the one hand, sleep duration could influence hunger (e.g., []) and emotional eating (e.g., eating in response to aroused emotional states, []) and, on the other hand, some kind of food such as ones that contain melatonin and tryptophan (e.g., fruits and vegetables) and high-carbohydrate diets could be associated with sleep quality and quantity [,].
Compared to the adult population, less attention has been paid to the interplay between adolescents’ eating behavior and sleep over time. Previous cross-sectional and laboratory studies focused on the role of sleep duration, showing that adolescents’ sleep deprivation (assessed with both objective and subjective measures) led to higher caloric intake and changes in food choices (e.g., greater consumption of high-sugar snacks and dessert) [,,]. Moreover, sleep timing was found to be related to eating patterns (e.g., later bedtimes were associated with higher intake of energy-dense food [,,]). Therefore, sleep timing could be associated with meal timing and, doing so, weight status (i.e., individuals with later bedtimes who wake up later in the morning may skip breakfast and engage in sedentary activity, such as using digital media, during the evening [,,].
Considering the reverse relation between eating pattern and sleep, previous studies found that adolescents with a tendency to consume greater amounts of highly processed food (e.g., sweets and candy, baked goods, pastries), skip breakfast, and have irregular eating schedules tend to show poorer sleep quality, higher sleep-related problems (e.g., insomnia), and less restorative sleep [,,,,]. Additionally, dietary patterns considered healthy, such as the Mediterranean diet (e.g., high consumption of plant-based foods; moderate intake of dairy products, seafood, eggs, and poultry; olive oil as the primary fat source; and low consumption of red meat [,]) has been shown to be associated with longer adolescents’ sleep duration and lower odds of reporting sleep-related problems [,]. Nevertheless, studies on this direction of the association are limited and provide only a scattered picture of this phenomenon.
1.3. The Present Study
Considering the literature previously examined, on the one hand, shorter sleep duration could be related to higher BMI or more irregular eating behaviors (in terms of timing and type of food consumption) (e.g., [,]). On the other hand, eating behaviors characterized by regular timing and healthy nutritional patterns (e.g., high consumption of plant-based food) could be associated with longer sleep duration and less sleep-related problems among adolescents [,]. However, some knowledge gaps need to be addressed in the available literature.
First, most of the studies used cross-sectional designs, making it impossible to examine the different directions of the relation (e.g., []); second, they included different age groups without differentiating between children and adolescents (e.g., []); third, they mainly focused on the effect of sleep on physical health variables and mostly considered a single aspect of sleep (e.g., sleep duration []), not providing a comprehensive understanding of the phenomenon. Similarly, concerning eating behaviors variables, previous studies mainly focused on the negative side of dietary patterns, disregarding how the type of diet or the ordinary amount of food intake are associated with sleep over time. Because of these criticisms, prior systematic reviews and meta-analyses (e.g., []) could provide only a partial picture of the complex longitudinal interplay between adolescents’ sleep, eating behaviors, and nutritional aspects.
The current systematic review with meta-analysis sought to address these gaps by reviewing longitudinal studies focusing on adolescents’ samples and considering how different aspects of sleep and adolescents’ physical health status indicators, potentially related eating behaviors and nutritional aspects, are bidirectionally linked. This can provide a more comprehensive picture of how this reciprocal relation develops over time, clarifying its direction and the different implications of the variables considered. In this vein, this study aims to systematically summarize previous longitudinal evidence on the bidirectional link between sleep variables (i.e., sleep duration, timing, quality, and insomnia symptoms) and (a) anthropometric indices and obesity risk and (b) eating behaviors and nutritional aspects (i.e., type of diet, amount and timing of food consumption, and energy expenditure) (an overview of the factors examined is presented in Figure 1).
2. Materials and Method
This review conformed to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [] (see Document S1 for the PRISMA checklist, PROSPERO preregistration ID: CRD42021281002). The current study is part of a larger project aimed to review available longitudinal research evaluating the interplay between sleep and different aspects of psychosocial development in adolescents, e.g., [,,].
2.1. Eligibility Criteria
Specific eligibility criteria were defined regarding the studies and publications’ characteristics. To meet eligibility criteria, studies needed to (a) include adolescents from the general population as a sample (attending junior or secondary high schools and aged between 10 and 19 years old); (b) use a longitudinal study design (with at least two assessments); (c) examine at least one aspect of sleep measured with either objective (e.g., actigraphy, polysomnography) and subjective measures (e.g., questionnaires, sleep diaries, clinical interviews); (d) examine at least one variable related to anthropometric indices as physical health status indicators (i.e., BMI, fat percentage, and risk of obesity) and/or related eating behaviors and nutritional aspects (i.e., type of diet related to the intake of specific foods and nutrients, amount and timing of food consumption, and energy expenditure) (for details, see Figure 1). Moreover, concerning the publication’s characteristics, both peer-reviewed journal articles and grey literature that can be retrieved through database searches (e.g., doctoral dissertations) were included to avoid selection biases and reinforce the methodological rigor []. Finally, no restrictions were applied based on the year and the language of publication (when articles/dissertations were published in a language other than English, professional translators were contacted).
2.2. Literature Search
The literature search was conducted on the 28th of January 2023 to detect research published in peer-reviewed journal articles or available as grey literature. The full list of databases searched with full query strings used for each are reported in the Supplemental materials, Documents S2. Moreover, using the statistics of the previous search conducted in Web of Science, the websites of the fifteen journals that published most on the topic were screened (for the full list, see Document S3). Afterward, sleep-related conference proceedings (i.e., Journal of Sleep Research and Sleep Medicine) and the reference lists of the most relevant published systematic reviews and meta-analyses (e.g., []) (see Document S4 for the full list) were screened. The last step consisted of screening the reference lists of included studies. The searches and the screening were run and managed on Citavi 6 software.
2.3. Selection of Studies
The results of the search strategies are reported in the PRISMA diagram (Figure 2). The searches yielded 43,538 abstracts, and after removing duplicates, two raters independently screened the remaining records (N = 24,145) with a substantial percentage of agreement (Cohen’s Kappa = 0.77), and disagreements were discussed with a third rater (taking the final decisions with the achievement of the agreement among all raters). Next, the full texts were screened following the same procedure used for the abstract screening (the agreement was high; Cohen’s Kappa = 0.63). In total, 28 studies were included in the systematic review, with 21 also included in the meta-analytic calculation.
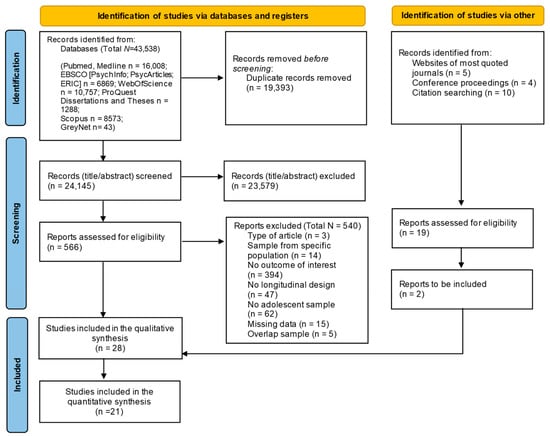
Figure 2.
PRISMA diagram search flow.
2.4. Coding of Primary Studies
All the included studies were coded independently and simultaneously by two independent raters (V.B. and M.G.) (the percentage of agreement was 91.8%) following a coding protocol. The disagreements were discussed with a third rater and solved among the three evaluators. In the first part of the protocol, the publications’ characteristics were coded: type of publication (i.e., journal article or grey literature), year of publication, and the publication language. Next, studies’ characteristics were grouped: funding sources; the number of waves of the longitudinal design; the interval between waves; the dimensions of each study; and the assessment method categorized as subjective (e.g., questionnaires, sleep diaries, clinical interviews) and objective assessment (e.g., actigraphs, polysomnography). Third, participants’ characteristics were coded: sample size, gender composition of the sample (% females), mean age, geographical location, and ethnic composition of the sample.
Finally, data necessary for effect size computations were coded. Due to the high heterogeneity of the studies included, different effect sizes were extracted (i.e., odds ratios, risk ratios, cross-lagged correlations, beta coefficients, means and standard deviations) to address how sleep variables, anthropometric indices, eating behaviors, and nutritional aspects were longitudinally related (see Section 2.6). If only standardized beta regression coefficients were reported, the correlation coefficients were estimated based on Peterson and Brown’s formula []. If it was not possible to obtain data for effect size computation from the primary studies, authors were contacted by e-mail to request them. Specifically, a total of 26 authors were contacted; if they did not answer, one reminder (after two weeks) was scheduled. Seven authors replied by providing the requested data; seven replied that they could not provide data (e.g., they did not have the access to the data); and 12 did not respond. The total number of studies included in the review accounts for 15 that were excluded because of insufficient data, as indicated in the PRISMA diagram (Figure 2).
2.5. Methodological Quality Assessment and Risk of Bias
In line with the PRISMA guidelines [], each study’s methodology quality and risk of bias were evaluated. The quality assessment of studies was performed by the first two authors (the percentage of agreement based on the 20% of the included studies was 83%) by using an adapted version of the Newcastle–Ottawa Scale (NOS) for cohort studies following previous procedures (e.g., []). Specifically, the adapted version of the NOS included six items categorized into three dimensions: Selection, Comparability, and Outcome. A series of response options are provided for each item, and a star system is used, assigning a maximum of one star for each Selection and Outcome item and a maximum of two stars for the Comparability item to high-quality studies. Document S5 of the Supplemental Materials shows the full list of items.
2.6. Strategy of Analysis
Data related to sleep variables measured at one time point (e.g., sleep duration at T1) and anthropometric indices, eating behaviors, and nutritional aspect variables at the last time-point considered in the study (T2), or anthropometric indices, eating behaviors, and nutritional aspects variables at one time point (e.g., BMI at T1) and sleep at the last time-point considered in the study (T2) were coded. Concerning the bidirectional relation between sleep, anthropometric indices (i.e., BMI and fat%), eating behavior, and nutritional aspect variables, the effect sizes were converted into Pearson’s correlations to compare the effects across studies and compute overall summary statistics through meta-analytic techniques. Pearson’s correlations were converted into Fisher’s Z-scores for computational purposes and converted back into correlations for presentation []. Correlations of |.10| were considered small, |.30| moderate, and |.50| large effect sizes [,]. Moreover, to estimate the quantitative relation between sleep and risk for obesity, the estimated odds ratio from each study comparing the shortest sleep duration and lowest sleep quality to the normative sleep duration and the highest level of sleep quality on the risk of obesity were used.
The computation of variance, standard error, 95% confidence interval, and statistical significance were applied for each effect size. When at least three studies [,] were available on the same association, a meta-analysis was conducted using the software ProMeta3 to obtain an overall estimate. The random-effect model was used as a conservative approach to account for different sources of variation among studies (i.e., within-study and between-studies variance; []).
Furthermore, in order to assess heterogeneity through included studies the Q statistic (to test the significance) and the I2 (for the estimation, considering 25% as low, 50% as moderate, and 75% as high proportion of dispersion) were used []. Moderator analyses were performed to examine which factors can account for the heterogeneity [] when at least three studies for each moderator level were available []. Specifically, subgroup analysis (for categorical moderators, i.e., method used to assess sleep) and meta-regression (for numerical moderators, i.e., age of participants and time-lag between waves). Finally, the evaluation of the publication bias was performed through the visualization of the funnel plot (i.e., a scatter plot of the effect sizes estimated from individual studies against a measure of their precision, such as their standard errors). When the plot appears as a symmetrical inverted funnel, it indicates no presence of bias. Nevertheless, since smaller or non-significant studies are less likely to be published, studies in the bottom left-hand corner of the plot are frequently absent. Together with the visualization of the funnel plot, in order to test the statistical asymmetry of the funnel plot, Egger’s regression method [] was used (with non-significant results indicating the absence of publication bias).
3. Results
3.1. Study Characteristics and Quality Assessment
Twenty-eight studies were included in the systematic review. A summary of the characteristics of the included studies is reported in Table 1. For what concerns the publications’ characteristics, most of the studies were articles published in peer-reviewed journals (n = 27, 96.4%), and only one [] was a dissertation. All studies were published in English, and approximately half of them (n = 15, 53.5%) were published recently between 2019 and 2023, with the remaining published before 2019. Concerning the study design, one study [] was a daily actigraphic study with 7 days of assessment. The remaining studies mostly used two time points (n = 21, 75%), with only a small percentage that used three (n = 2, 7.1%), or more than three (n = 4, 14.3%), time points. The average time lag between adjacent waves was about two years (M = 28.0 months, SD = 23.1 months), ranging from 6 months to 7 years. Most of the studies (n = 20, 71.4%) evaluated sleep variables through subjective measures (i.e., ad hoc questions or questionnaires), the remaining (n = 7, 25%) assessed sleep using objective evaluation (i.e., actigraphy), and one used both methods of assessment (n = 1, 3.6%). Moreover, regarding the considered sleep variables, most of the studies (n= 17, 61%) focused only on the sleep duration dimension, seven studies evaluated sleep timing, seven evaluated sleep quality variables, and only one evaluated insomnia symptoms. For what concerns the anthropometric indices, eating behaviors, and nutritional aspect variables, most of the studies focused only on the BMI assessment (n = 17), three focused also on food intake, six on the type of diet, and one on the energy expenditure. Most studies (n = 25, 89.3%) reported one or multiple funding sources. The total number of participants was 118,291 (M = 3960, SD = 5823, range 59–20,745). Most samples were gender balanced (the average percentage of females across samples was 51.6%; range 42.6–65.3%), and the average age of sample participants at baseline was 13.4 years (SD = 2.5, range: 8.1–18 years). With regards to the geographic context of the studies, most of them were conducted in the USA (n = 11, 39.3%). The remaining studies were conducted in Europe (n = 7, 25%), Australia (n = 3, 10.7%), China (n = 3, 10.7%), Canada (n = 2, 7.1%), Brazil (n = 1, 3.6%), and Mexico (n = 1, 3.6%). Results of the methodological quality and risk of bias assessment are reported in Document S6 of the Supplemental Materials. The overall quality of the studies was high, with a consequent low risk of bias.

Table 1.
Characteristics of studies included in the systematic review.
3.2. Longitudinal Interplay between Adolescents’ Sleep, Anthropometric Indices, Eating Behaviors, and Nutritional Aspects
In Table 2, every effect size of the included studies is reported. Twenty studies reported the needed effect sizes for the meta-analytic calculations. Results of the meta-analytic calculations for the longitudinal interplay between sleep variables, anthropometric indices, obesity risk, eating behaviors, and nutritional aspect variables are reported below.

Table 2.
Effect sizes of the included studies.
3.2.1. The Interplay between Sleep, Anthropometric Indices, and Risk of Obesity
Regarding the interplay between sleep and anthropometric indices (i.e., BMI, fat%), 18 studies examined this link either unidirectionally or bidirectionally. Regarding the longitudinal association between sleep variables at one time point (T1) and anthropometric indices at a later time (T2), 15 studies [,,,,,,,,,,,,,,] were included in the meta-analytic results, while three studies [,,] reported only qualitative information. Results of the meta-analysis, summarized in Table 3 (See Document S7 for the forest plot), showed that the overall effect size was significant, albeit small (r = −0.06, p < 0.001). Specifically, longer sleep duration, higher sleep quality, and lower presence of sleep disturbances (i.e., insomnia symptoms) were related to lower BMI and fat percentage in adolescents over time. Heterogeneity statistics were high and significant. Nevertheless, the results were not moderated by the characteristics of the participants (i.e., mean age at T1, B = 0.00, p = 0.76), by characteristics of the studies (i.e., time-lag between waves, B = 0.00, p = 0.98), or by sleep assessment method (Q = 0.29, p = 0.59). The visual investigation of the funnel plot suggested a risk for publication bias, supported by the results of the Egger’s test. Results of the studies not included in the meta-analysis confirmed this association [] when considering sleep duration but not when considering the sleep trajectories over time as predictors [,].

Table 3.
Overall meta-analytic calculations of the bidirectional relations between sleep and physical health status indicators.
Examining the opposite direction, seven studies [,,,,,,] focused on the association between anthropometric indices at one point (T1) and sleep variables at a later time (T2), which allowed for a meta-analytical calculation. As shown in Table 3 (see Document S8 for the forest plot), anthropometric indices were not significantly related to sleep quality and duration over time. This result was not moderated by participants’ age at T1 (B = −0.00, p = 0.89) or by the time-lag between waves (B = −0.00, p = 0.38), and was not affected by publication bias, as evident from the non-significant Egger’s test (p = 0.18).
Regarding the interplay between sleep and the risk of obesity over time, seven studies [,,,,,,] examined this link either unidirectionally or bidirectionally. Regarding the longitudinal association between sleep variables at one time point (T1) and obesity risk at a later time (T2), six studies [,,,,,] were included in the meta-analytic results, while one study [] reported only qualitative information. As shown in Table 3 (see Document S9 for the forest plot), the meta-analytic result was significant but small (OR: 1.30 [1.08,1.56], p < 0.01). Specifically, shorter sleep duration (<7 h) and lower sleep quality were associated with higher risk of obesity in adolescents over time. This result was not moderated by participants’ age at T1 (B = 0.02, p = 0.75) or by the time-lag between waves (B = −0.01, p = 0.06), and was not affected by publication bias, as evident from the non-significant Egger’s test (p = 0.11). Results of the study not included in the meta-analysis confirmed these findings []. Finally, only one study [] evaluated this connection bidirectionally, showing no significant association between obesity at one time point and short sleep duration at a later time point.
3.2.2. The Interplay between Sleep, Eating Behaviors, and Nutritional Aspects
Four studies [,,,] evaluated the link between sleep, different aspects of eating behaviors, and nutritional aspects (i.e., type of diet related to the number of specific foods and nutrients, amount and timing of food consumption, and energy expenditure). Given their heterogeneity in considering different eating behavior and nutritional aspect indicators, only a qualitative review of the findings was conducted. The main findings are summarized in Table 2. Regarding the association between sleep variables at one time point and eating behaviors and nutritional aspects at a later time point, one study [] showed that longer sleep duration was associated with junk food consumption over time. This result was not confirmed by the other study that evaluated this link [], which highlighted no association between sleep quality and healthy dietary quality as assessed by the Healthy Eating Index 2015 [] over time. Moreover, one study evaluated the association between sleep duration and caloric intake over time, showing no significant association []. Finally, regarding the relation between sleep and energy expenditure over time, only one study [] found a negative association between sleep duration with energy expenditure over time. Examining the opposite direction, only one study [] evaluated the association between junk food consumption at one time point and sleep at a later time point. Results showed a significant association between unhealthier eating behaviors and longer sleep duration over time.
4. Discussion
Nowadays, an alarming decline in adolescents’ sleep duration and disruption of their sleep quality and healthy habits was highlighted [,], and at the same time, adolescents’ obesity has become a global public health issue []. However, there is a lack of awareness of the possible bidirectional link between these variables over time. For these reasons, the present systematic review with meta-analysis aimed to improve the knowledge of the interplay between youth’ sleep on the one hand and physical health status indicators, eating behaviors, and nutritional aspects on the other by systematically evaluating longitudinal studies specifically focused on adolescents’ samples. Overall, the findings of this study highlighted composite association patterns between sleep and physical health variables in adolescents. Such knowledge sheds new light on the role of sleep as an essential component of health, especially during adolescence, and shows up new directions for future research examining how healthy eating behaviors and good sleep quality could bidirectionally influence each other.
4.1. Is Sleeping Well Longitudinally Related to Anthropometric Indices?
Meta-analytic results showed a small and negative association between different aspects of sleep (i.e., sleep duration, timing, quality, and insomnia symptoms) and anthropometric indices over time. Specifically, longer sleep duration, higher sleep quality, and lower presence of sleep disturbances (i.e., insomnia symptoms) were related to lower BMI and fat percentage in adolescents over time, assessed with subjective and objective measures. Despite being small, this effect aligns with prior cross-sectional research (for reviews, see [,]). Moreover, this result is partially in line with previous cross-sectional studies among the adult population in which longer sleep duration was also associated with higher BMI [,,].
On the contrary, only a few studies investigated the other direction of this link, mainly considering BMI levels and subsequent sleep duration. The reviewed findings highlighted the absence of a significant association between these factors, as higher BMI was not associated with lower sleep duration and quality. Nevertheless, it is important to underline the potentially crucial role that BMI might play in the link between poor sleep and the risk of obesity. Specifically, on the one hand, poor sleep quality can be associated with a higher level of BMI, and on the other hand, higher levels of BMI and fat percentage in adolescents can be associated with lower levels of physical activity, leading to an impairment in physical health that can, in turn, be related to youth’ sleep, suggesting the establishment of a possible vicious circle. For this reason, more research is needed considering several aspects of adolescents’ physical health to understand the longitudinal interplay between sleep and anthropometric indices.
Additionally, considering the association between adolescents’ sleep and the risk of obesity, meta-analytic results showed that short sleep duration and poor sleep quality had a small but significant effect on the future risk of obesity over time. This result reflects previous cross-sectional evidence suggesting a possible link between poor sleep quality and overweight/obesity in young subjects (for a review, see []). Moreover, this aligns with previous studies among the adult population (for reviews, see [,]. This result can be explained through several mechanisms. First, short-term sleep duration has been shown to increase daytime sleepiness and related sedentary behaviors, which can, in turn, reduce daytime physical activity []. Second, adolescents’ biological phase delay is misaligned with their early school start time, leading to the implementation of compensative behavior for their sleep debt during the weekends. One of these typical behaviors is the delay of bedtime and wake-up time during weekends compared to weekdays, known as social jet lag []. This phenomenon was found to be strongly related to an increased risk of developing obesity and metabolic syndrome (e.g., []). Third, sleep deprivation can be related to increased energy expenditure that, in turn, results in compensatory behaviors such as increased food intake []. For these reasons, it is of utmost importance to consider all these mechanisms when evaluating the relation between sleep and obesity risk over time to provide a comprehensive understanding of sleep’s potential role during development.
The small effect found should be read in light of the fact that most of the included studies considered sleep duration as a predictor of a high risk of obesity (considering “short sleep” category as a duration of around 6–7 h, compared to the “normal” sleep duration category for adolescents of 8–10 h, as suggested by the National Sleep Foundation guidelines). Since, as stated above, adolescents are normally sleep deprived, this comparison could not be so effective in predicting the risk of obesity over time for this specific age group. Conversely, other sleep dimensions can be crucial in detecting this risk. Moreover, the average time-lag between the assessments was approximately two years so several factors might have interceded. Future studies could benefit from including multiple evaluations of sleep, considering additional more qualitative aspects of sleep when evaluating this relation (i.e., sleep latency and maintenance), and using multiple time points of assessment in order to clarify the role of time in this association.
4.2. Is Sleeping Well Longitudinally Related to Eating Behaviors and Nutritional Aspects?
The results of this systematic review suggest that there is not a clear picture of the bidirectional association between adolescents’ sleep, their eating behavior, and nutritional aspects. This is in line with previous findings [], highlighting a mixed picture of the relation between sleep quality, eating behaviors, and nutritional aspects over time. Of the included studies, only four examined this link, but considering different indicators; therefore, only a qualitative review of the findings could be conducted. Most of the studies evaluated the association between sleep at one time point and eating behaviors and nutritional aspects at a later time, and only one explored this association bidirectionally, highlighting a significant positive association between longer sleep duration and junk food consumption []. One study [] evaluated how sleep duration and caloric intake were related over time, showing no significant associations. Moreover, one study [] assessed the association between sleep quality and energy expenditure, finding a negative association over time.
Considering the limited literature on the longitudinal association between different aspects of sleep, eating behaviors, and nutritional aspects in adolescents, there is a need to deepen the knowledge of this interplay. On the one hand, previous cross-sectional studies highlighted the importance of considering a wider framework of sleep factors (e.g., sleep regularity) associated with eating behaviors (e.g., []) and not only sleep duration. On the other hand, previous evidence mainly focused on the pathological expression of eating behaviors (e.g., []) on youths’ general adjustment, but less is known about the role of a non-disordered eating on sleep.
4.3. Limitations and Future Research Directions
The results of this systematic review with meta-analysis are not free from limitations. The first one concerns the factthat despite sleep being a multidimensional factor, most of the included studies measured it as a unidimensional variable, mostly considering sleep duration as an indicator of good sleep quality. Future studies need to uncover the relative impact of each aspect considering sleep’s multidimensionality (considering both healthy and pathological aspects and typical behaviors that adolescents implement to compensate for their sleep debt). Moreover, most of the examined studies evaluated the link between sleep and physical health in a unidirectional way. Only eight studies (e.g., []) considered the bidirectionality of the relation. Therefore, future prospective studies are needed to elucidate how the different facets of sleep are related to different physical health variables over time and vice versa.
Second, most studies included employed self-report measures (e.g., [,], specifically, only seven used actigraphy to assess sleep objectively (e.g., [,]), and only nine used objective methods to assess anthropometric indices (e.g., [,]. Thus, self-report data should be considered cautiously because they may be affected by social desirability, memory biases, and shared variance issues []. Future studies should integrate objective and subjective measures to assess physical health variables: along with objective information about anthropometric indices obtained from digital scaling and a stadiometer [], day-to-day variability intake or patterns of intake frequency and timing could allow for more valid data []. Similarly, it is of utmost importance to evaluate sleep parameters through actigraphy in future studies [] in order to provide a reliable and more nuanced picture of healthy adolescents’ sleep compared to self-report, which might overestimate sleep duration []. Moreover, a greater consistency between studies regarding methodology and assessment methods is required in order to better compare them in terms of sample considered, assessment method, statistical analyses, and study design.
Third, most included studies used two time points in their study designs. Future studies should evaluate the longitudinal link between sleep and physical health in adolescents with at least three assessments to better understand how (i.e., underlying mechanisms) they are related over time and for whom (i.e., moderations) this association is stronger. In this way, it would be possible to identify relevant mediators (e.g., physical activity) playing a role in the interplay between sleep quality and physical health variables in adolescents (e.g., higher BMI → higher sedentariness → shorter sleep duration, []). Furthermore, it is crucial to clarify the short-, medium-, and long-term nature of these effects. This understanding is of great importance to developing evidence-based interventions.
Finally, the included studies mainly focused on the interplay between adolescents’ sleep and physical health status indices. This highlights a gap in the literature on the specific role of eating behaviors and nutritional aspects in relation to adolescents’ sleep. Future studies should focus more on the multidimensionality of human eating behaviors and nutrition variables, considering the combined role of anthropometric indices and other aspects of eating behaviors and nutrition, such as the intake of specific foods and time of food consumption.
5. Conclusions
This is the first systematic review with a meta-analysis that provided a comprehensive synthesis of the literature regarding the longitudinal research on the association between sleep, physical health status indicators, eating behaviors, and nutritional aspects in adolescents. First, meta-analytic results revealed a weak but significant association between sleep at one time point and anthropometric indices and obesity risk at a later time. Moreover, meta-analytic results in the opposite direction showed a non-significant effect. These results suggest that future studies should clarify the existence of a vicious circle between sleep and physical health variables, also exploring their effect on sleep, considering all the dimensions.
From a theoretical perspective, this review calls attention to some gaps in the present literature. To address them, future studies should highlight the importance of considering the bidirectionality of the relation between adolescents’ sleep quality and physical health, conducting longitudinal studies that examine both qualitative and quantitative aspects of all the considered variables. Likewise, this study has important practical implications. Considering the bidirectionality of this relation, it will be possible to implement evidence-based interventions aimed at promoting well-being in adolescence by educating about sleep hygiene rules and practices to improve one’s sleep health and encouraging healthier eating patterns.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nu15143179/s1. Document S1: PRISMA checklist. Document S2: Full search strategy for each database. Document S3: Full list of screened journals. Document S4: Full list of most relevant published systematic reviews and meta-analyses. Document S5: Quality and Risk of Bias Assessment Method (adapted from the Newcastle-Ottawa Scale for Cohort Studies). Document S6: Risk of Bias Assessment Results. Document S7: Forest plot for the association between sleep variables at one time point (T1) and anthropometric indices at a later time (T2). Document S8: Forest plot for the association between anthropometric indices at one point (T1) and sleep variables at a later time (T2). Document S9: Forest plot for the association between sleep variables at one time point (T1) and obesity risk at a later time (T2). References [,,,,,,,,,,,,,] are cited in supplementary file.
Author Contributions
M.G. conceived the study, coded the papers included in the review, wrote the manuscript, and participated in the interpretation of the results; V.B. conceived the study, coded the papers included in the review, performed the statistical analyses, wrote the manuscript, and participated in the interpretation of the results; V.N., L.T. and E.C. conceived the study, wrote the manuscript, and participated in the interpretation of the results. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (ERC-CoG IDENTITIES grant agreement No. [101002163]; principal investigator: Elisabetta Crocetti).
Institutional Review Board Statement
No ethical approval was needed because data from previously published studies in which informed consent was obtained by primary investigators were retrieved and analyzed.
Informed Consent Statement
No applicable.
Data Availability Statement
Data from previously published studies were retrieved and analyzed. Data sharing is not applicable to this article.
Acknowledgments
We are grateful to Beatrice Bobba, Liesbeth Carpentier, Francesca De Lise, Francesca Golfieri, Savaş Karataş, Fabio Maratia, and Maria Pagano who helped in the selection of the studies for this systematic review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McGlinchey, E.L. Sleep and Adolescents. In Sleep and Affect; Academic Press: Cambridge, MA, USA, 2015; pp. 421–439. [Google Scholar] [CrossRef]
- Buysse, D.J. Sleep Health: Can We Define It? Does It Matter? Sleep 2014, 37, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Katz, E.S.; Kheirandish-Gozal, L.; et al. National sleep foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health 2015, 1, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Gruber, R.; Carrey, N.; Weiss, S.K.; Frappier, J.Y.; Rourke, L.; Brouillette, R.T.; Wise, M.S. Position statement on pediatric sleep for psychiatrists. J. Can. Acad. Child Adolesc. Psychiatry 2014, 23, 174–195. [Google Scholar] [PubMed]
- Fredriksen, K.; Rhodes, J.; Reddy, R.; Way, N. Sleepless in Chicago: Tracking the Effects of Adolescent Sleep Loss During the Middle School Years. Child Dev. 2004, 75, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.-P.; Janssen, I. Sleep duration estimates of Canadian children and adolescents. J. Sleep Res. 2016, 25, 541–548. [Google Scholar] [CrossRef]
- Matricciani, L.; Olds, T.; Petkov, J. In search of lost sleep: Secular trends in the sleep time of school-aged children and adolescents. Sleep Med. Rev. 2012, 16, 203–211. [Google Scholar] [CrossRef]
- Carskadon, M.A. Sleep in Adolescents: The Perfect Storm. Pediatr. Clin. N. Am. 2011, 58, 637–647. [Google Scholar] [CrossRef]
- Cain, N.; Gradisar, M. Electronic media use and sleep in school-aged children and adolescents: A review. Sleep Med. 2010, 11, 735–742. [Google Scholar] [CrossRef]
- Pagano, M.; Bacaro, V.; Crocetti, E. “Using digital media or sleeping … that is the question”. A meta-analysis on digital media use and unhealthy sleep in adolescence. Comput. Hum. Behav. 2023, 146, 107813. [Google Scholar] [CrossRef]
- Doan, N.; Parker, A.; Rosati, K.; van Beers, E.; Ferro, M.A. Sleep duration and eating behaviours among adolescents: A scoping review. Health Promot. Chronic Dis. Prev. Can. 2022, 42, 384–397. [Google Scholar] [CrossRef]
- Fatima, Y.; Doi, S.A.R.; Mamun, A.A. Sleep quality and obesity in young subjects: A meta-analysis. Obes. Rev. 2016, 17, 1154–1166. [Google Scholar] [CrossRef]
- Godos, J.; Grosso, G.; Castellano, S.; Galvano, F.; Caraci, F.; Ferri, R. Association between diet and sleep quality: A systematic review. Sleep Med. Rev. 2021, 57, 101430. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Report of the First Meeting of the ad hoc Working Group on Science and Evidence for Ending Childhood Obesity; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Ruiz, L.D.; Zuelch, M.L.; Dimitratos, S.M.; Scherr, R.E. Adolescent Obesity: Diet Quality, Psychosocial Health, and Cardiometabolic Risk Factors. Nutrients 2019, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.E.; Cohen, P.; Naumova, E.N.; Jacques, P.F.; Must, A. Adolescent Obesity and Risk for Subsequent Major Depressive Disorder and Anxiety Disorder: Prospective Evidence. Psychosom. Med. 2007, 69, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Rupp, K.; McCoy, S.M. Bullying Perpetration and Victimization among Adolescents with Overweight and Obesity in a Nationally Representative Sample. Child. Obes. 2019, 15, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Doggui, R.; Ward, S.; Johnson, C.; Bélanger, M. Trajectories of Eating Behaviour Changes during Adolescence. Nutrients 2021, 13, 1313. [Google Scholar] [CrossRef]
- Moreno, L.A.; Rodríguez, G.; Fleta, J.; Bueno-Lozano, M.; Lázaro, A.; Bueno, G. Trends of Dietary Habits in Adolescents. Crit. Rev. Food Sci. Nutr. 2010, 50, 106–112. [Google Scholar] [CrossRef]
- Samuelson, G. Dietary habits and nutritional status in adolescents over Europe. An overview of current studies in the Nordic countries. Eur. J. Clin. Nutr. 2000, 54, S21–S28. [Google Scholar] [CrossRef]
- Simmonds, M.; Llewellyn, A.; Owen, C.G.; Woolacott, N. Simple tests for the diagnosis of childhood obesity: A systematic review and meta-analysis. Obes. Rev. 2016, 17, 1301–1315. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Horesh, A.; Tsur, A.M.; Bardugo, A.; Twig, G. Adolescent and Childhood Obesity and Excess Morbidity and Mortality in Young Adulthood—A Systematic Review. Curr. Obes. Rep. 2021, 10, 301–310. [Google Scholar] [CrossRef]
- Jebeile, H.; Cardel, M.I.; Kyle, T.K.; Jastreboff, A.M. Addressing psychosocial health in the treatment and care of adolescents with obesity. Obesity 2021, 29, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P.; Taggart, F.M.; Kandala, N.-B.; Currie, A.; Peile, E.; Stranges, S.; Miller, M.A. Meta-Analysis of Short Sleep Duration and Obesity in Children and Adults. Sleep 2008, 31, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Beydoun, M.A.; Wang, Y. Is Sleep Duration Associated with Childhood Obesity? A Systematic Review and Meta-analysis. Obesity 2008, 16, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, S.; Huang, Y.; Chen, K. Sleep duration and obesity in children: A systematic review and meta-analysis of prospective cohort studies. J. Paediatr. Child Health 2017, 53, 378–385. [Google Scholar] [CrossRef]
- Wu, Y.; Gong, Q.; Zou, Z.; Li, H.; Zhang, X. Short sleep duration and obesity among children: A systematic review and meta-analysis of prospective studies. Obes. Res. Clin. Pract. 2017, 11, 140–150. [Google Scholar] [CrossRef]
- Al-Disi, D.; Al-Daghri, N.; Khanam, L.; Al-Othman, A.; Al-Saif, M.; Sabico, S.; Chrousos, G. Subjective sleep duration and quality influence diet composition and circulating adipocytokines and ghrelin levels in teen-age girls. Endocr. J. 2010, 57, 915–923. [Google Scholar] [CrossRef]
- Boeke, C.E.; Storfer-Isser, A.; Redline, S.; Taveras, E.M. Childhood Sleep Duration and Quality in Relation to Leptin Concentration in Two Cohort Studies. Sleep 2014, 37, 613–620. [Google Scholar] [CrossRef]
- Hagen, E.W.; Starke, S.J.; Peppard, P.E. The Association Between Sleep Duration and Leptin, Ghrelin, and Adiponectin Among Children and Adolescents. Curr. Sleep Med. Rep. 2015, 1, 185–194. [Google Scholar] [CrossRef]
- Hitze, B.; Bosy-Westphal, A.; Bielfeldt, F.; Settler, U.; Plachta-Danielzik, S.; Pfeuffer, M.; Schrezenmeir, J.; Mönig, H.; Müller, M.J. Determinants and impact of sleep duration in children and adolescents: Data of the Kiel Obesity Prevention Study. Eur. J. Clin. Nutr. 2009, 63, 739–746. [Google Scholar] [CrossRef]
- Kjeldsen, J.S.; Hjorth, M.F.; Andersen, R.; Michaelsen, K.F.; Tetens, I.; Astrup, A.; Chaput, J.-P.; Sjödin, A. Short sleep duration and large variability in sleep duration are independently associated with dietary risk factors for obesity in Danish school children. Int. J. Obes. 2014, 38, 32–39. [Google Scholar] [CrossRef]
- Carskadon, M.A.; Vieira, C.; Acebo, C. Association between Puberty and Delayed Phase Preference. Sleep 1993, 16, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Carskadon, M.A.; Acebo, C.; Richardson, G.S.; Tate, B.A.; Seifer, R. An Approach to Studying Circadian Rhythms of Adolescent Humans. J. Biol. Rhythm. 1997, 12, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Olds, T.S.; Maher, C.A.; Matricciani, L. Sleep Duration or Bedtime? Exploring the Relationship between Sleep Habits and Weight Status and Activity Patterns. Sleep 2011, 34, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Kuehnle, T.; Pramstaller, P.P.; Ricken, J.; Havel, M.; Guth, A.; Merrow, M. A marker for the end of adolescence. Curr. Biol. 2004, 14, R1038–R1039. [Google Scholar] [CrossRef]
- Tonetti, L.; Fabbri, M.; Natale, V. Sex Difference in Sleep-Time Preference and Sleep Need: A Cross-Sectional Survey among Italian Pre-Adolescents, Adolescents, and Adults. Chrono-Int. 2008, 25, 745–759. [Google Scholar] [CrossRef]
- Bacaro, V.; Carpentier, L.; Crocetti, E. Sleep Well, Study Well: A Systematic Review of Longitudinal Studies on the Interplay between Sleep and School Experience in Adolescence. Int. J. Environ. Res. Public Health 2023, 20, 4829. [Google Scholar] [CrossRef]
- Tarokh, L.; Saletin, J.M.; Carskadon, M.A. Sleep in adolescence: Physiology, cognition and mental health. Neurosci. Biobehav. Rev. 2016, 70, 182–188. [Google Scholar] [CrossRef]
- Wittmann, M.; Dinich, J.; Merrow, M.; Roenneberg, T. Social Jetlag: Misalignment of Biological and Social Time. Chronobiol. Int. 2006, 23, 497–509. [Google Scholar] [CrossRef]
- Taheri, S. The link between short sleep duration and obesity: We should recommend more sleep to prevent obesity. Arch. Dis. Child. 2006, 91, 881–884. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Annunziata, G.; Di Somma, C.; Laudisio, D.; Colao, A.; Savastano, S. Obesity and sleep disturbance: The chicken or the egg? Crit. Rev. Food Sci. Nutr. 2019, 59, 2158–2165. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Sinclair, K.B.; Pereira, M.A.; Garcia-Lago, E.; Feldman, H.A.; Ludwig, D.S. Compensation for Energy Intake from Fast Food Among Overweight and Lean Adolescents. JAMA 2004, 291, 2828–2833. [Google Scholar] [CrossRef] [PubMed]
- French, S.A.; Story, M.; Neumark-Sztainer, D.; Fulkerson, J.A.; Hannan, P. Fast food restaurant use among adolescents: Associations with nutrient intake, food choices and behavioral and psychosocial variables. Int. J. Obes. 2001, 25, 1823–1833. [Google Scholar] [CrossRef]
- Guthrie, J.F.; Lin, B.-H.; Frazao, E. Role of Food Prepared Away from Home in the American Diet, 1977–1978 versus 1994-96: Changes and Consequences. J. Nutr. Educ. Behav. 2002, 34, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Arcan, C.; Neumark-Sztainer, D.; Hannan, P.; van Den Berg, P.; Story, M.; Larson, N. Parental eating behaviours, home food environment and adolescent intakes of fruits, vegetables and dairy foods: Longitudinal findings from Project EAT. Public Health Nutr. 2007, 10, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Larson, N.I.; Neumark-Sztainer, D.; Story, M. Weight Control Behaviors and Dietary Intake among Adolescents and Young Adults: Longitudinal Findings from Project EAT. J. Am. Diet. Assoc. 2009, 109, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.C.; Dumith, S.D.C.; Lopes, C.; Severo, M.; Assunção, M.C.F. How Do Tracking and Changes in Dietary Pattern during Adolescence Relate to the Amount of Body Fat in Early Adulthood? PLoS ONE 2016, 11, e0149299. [Google Scholar] [CrossRef]
- Duraccio, K.M.; Krietsch, K.N.; Chardon, M.L.; Van Dyk, T.R.; Beebe, D.W. Poor sleep and adolescent obesity risk: A narrative review of potential mechanisms. Adolesc. Health Med. Ther. 2019, 10, 117–130. [Google Scholar] [CrossRef]
- Dweck, J.S.; Jenkins, S.M.; Nolan, L.J. The role of emotional eating and stress in the influence of short sleep on food consumption. Appetite 2014, 72, 106–113. [Google Scholar] [CrossRef]
- Binks, H.; Vincent, G.E.; Gupta, C.; Irwin, C.; Khalesi, S. Effects of Diet on Sleep: A Narrative Review. Nutrients 2020, 12, 936. [Google Scholar] [CrossRef]
- Zuraikat, F.M.; Wood, R.A.; Barragán, R.; St-Onge, M.-P. Sleep and Diet: Mounting Evidence of a Cyclical Relationship. Annu. Rev. Nutr. 2021, 41, 309–332. [Google Scholar] [CrossRef]
- Beebe, D.W.; Simon, S.; Summer, S.; Hemmer, S.; Strotman, D.; Dolan, L.M. Dietary Intake Following Experimentally Restricted Sleep in Adolescents. Sleep 2013, 36, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.L.; Field, J.; Miller, L.E.; DiFrancesco, M.; Beebe, D.W. Sweet/Dessert Foods Are More Appealing to Adolescents after Sleep Restriction. PLoS ONE 2015, 10, e0115434. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Xu, F.; Storfer-Isser, A.; Thomas, A.; Ievers-Landis, C.E.; Redline, S. The Association of Sleep Duration with Adolescents’ Fat and Carbohydrate Consumption. Sleep 2010, 33, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Fleig, D.; Randler, C. Association between chronotype and diet in adolescents based on food logs. Eat. Behav. 2009, 10, 115–118. [Google Scholar] [CrossRef]
- Golley, R.K.; A Maher, C.; Matricciani, L.; Olds, T.S. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int. J. Obes. 2013, 37, 546–551. [Google Scholar] [CrossRef]
- Garaulet, M.; Gómez-Abellán, P. Timing of food intake and obesity: A novel association. Physiol. Behav. 2014, 134, 44–50. [Google Scholar] [CrossRef]
- Garaulet, M.; Ortega, F.B.; Ruiz, J.R.; Rey-López, J.P.; Béghin, L.; Manios, Y.; Cuenca-García, M.; Plada, M.; Diethelm, K.; Kafatos, A.; et al. Short sleep duration is associated with increased obesity markers in European adolescents: Effect of physical activity and dietary habits. The HELENA study. Int. J. Obes. 2011, 35, 1308–1317. [Google Scholar] [CrossRef]
- Delpino, F.M.; Figueiredo, L.M.; Flores, T.R.; Silveira, E.A.; dos Santos, F.S.; Werneck, A.O.; da Costa Louzada, M.L.; Arcêncio, R.A.; Nunes, B.P. Intake of ultra-processed foods and sleep-related outcomes: A systematic review and meta-analysis. Nutrition 2023, 106, 111908. [Google Scholar] [CrossRef]
- Lane, K.E.; Davies, I.G.; Darabi, Z.; Ghayour-Mobarhan, M.; Khayyatzadeh, S.S.; Mazidi, M. The Association between Ultra-Processed Foods, Quality of Life and Insomnia among Adolescent Girls in Northeastern Iran. Int. J. Environ. Res. Public Health 2022, 19, 6338. [Google Scholar] [CrossRef]
- de Sousa, R.S.; Bragança, M.L.B.M.; de Oliveira, B.R.; Coelho, C.C.N.d.S.; da Silva, A.A.M. Association between the Degree of Processing of Consumed Foods and Sleep Quality in Adolescents. Nutrients 2020, 12, 462. [Google Scholar] [CrossRef]
- St-Onge, M.-P.; Mikic, A.; E Pietrolungo, C. Effects of Diet on Sleep Quality. Adv. Nutr. Int. Rev. J. 2016, 7, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Tambalis, K.D.; Panagiotakos, D.B.; Psarra, G.; Sidossis, L.S. Breakfast skipping in Greek schoolchildren connected to an unhealthy lifestyle profile. Results from the National Action for Children’s Health program. Nutr. Diet. 2019, 76, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef] [PubMed]
- López-Gil, J.F.; Smith, L.; Victoria-Montesinos, D.; Gutiérrez-Espinoza, H.; Tárraga-López, P.J.; Mesas, A.E. Mediterranean Dietary Patterns Related to Sleep Duration and Sleep-Related Problems among Adolescents: The EHDLA Study. Nutrients 2023, 15, 665. [Google Scholar] [CrossRef]
- Rosi, A.; Giopp, F.; Milioli, G.; Melegari, G.; Goldoni, M.; Parrino, L.; Scazzina, F. Weight Status, Adherence to the Mediterranean Diet, Physical Activity Level, and Sleep Behavior of Italian Junior High School Adolescents. Nutrients 2020, 12, 478. [Google Scholar] [CrossRef]
- Sunwoo, J.-S.; Yang, K.I.; Kim, J.H.; Koo, D.L.; Kim, D.; Hong, S.B. Sleep duration rather than sleep timing is associated with obesity in adolescents. Sleep Med. 2020, 68, 184–189. [Google Scholar] [CrossRef]
- Jarrin, D.C.; McGrath, J.J.; Drake, C.L. Beyond sleep duration: Distinct sleep dimensions are associated with obesity in children and adolescents. Int. J. Obes. 2013, 37, 552–558. [Google Scholar] [CrossRef]
- Sluggett, L.; Wagner, S.L.; Harris, R.L. Sleep Duration and Obesity in Children and Adolescents. Can. J. Diabetes 2019, 43, 146–152. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Bobba, B.; Bacaro, V.; Crocetti, E. Embedded in Contexts: A Systematic Review of the Longitudinal Associations Between Contextual Factors and Sleep. Adolesc. Res. Rev. 2023. [Google Scholar] [CrossRef]
- De Lise, F.; Bacaro, V.; Crocetti, E. The Social Side of Sleep: A Systematic Review of the Longitudinal Associations between Peer Relationships and Sleep Quality. Int. J. Environ. Res. Public Health 2023, 20, 2017. [Google Scholar] [CrossRef] [PubMed]
- Maratia, F.; Bacaro, V.; Crocetti, E. Sleep Is a Family Affair: A Systematic Review and Meta-Analysis of Longitudinal Studies on the Interplay between Adolescents’ Sleep and Family Factors. Int. J. Environ. Res. Public Health 2023, 20, 4572. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C.J.; Brannick, M.T. Publication bias in psychological science: Prevalence, methods for identifying and controlling, and implications for the use of meta-analyses. Psychol. Methods 2012, 17, 120–128. [Google Scholar] [CrossRef]
- Scherrer, V.; Preckel, F. Circadian preference and academic achievement in school-aged students: A systematic review and a longitudinal investigation of reciprocal relations. Chronobiol. Int. 2021, 38, 1195–1214. [Google Scholar] [CrossRef]
- Peterson, R.A.; Brown, S.P. On the Use of Beta Coefficients in Meta-Analysis. J. Appl. Psychol. 2005, 90, 175–181. [Google Scholar] [CrossRef]
- Lipsey, M.W.; Wilson, D.B. Practical Meta-Analysis; Sage Publications, Inc.: Thousand Oaks, CA, USA, 2000; Volume 49. [Google Scholar]
- Cohen, J. The analysis of variance. In Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1988; pp. 273–406. [Google Scholar]
- Ellis, P.D. The Essential Guide to Effect Sizes: Statistical Power, Meta-Analysis, and the Interpretation of Research Results; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Cheung, M.W.-L.; Vijayakumar, R. A Guide to Conducting a Meta-Analysis. Neuropsychol. Rev. 2016, 26, 121–128. [Google Scholar] [CrossRef]
- Crocetti, E. Systematic Reviews with Meta-Analysis: Why, when, and how? Emerg. Adulthood 2016, 4, 3–18. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Viechtbauer, W. Accounting for Heterogeneity via Random-Effects Models and Moderator Analyses in Meta-Analysis. J. Psychol. 2007, 215, 104–121. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Fairborn, S.K. The Effects of Community Violence Exposure on Adolescents’ Health; University of California: Riverside, CA, USA, 2010. [Google Scholar]
- Jindal, I.; Puyau, M.; Adolph, A.; Butte, N.; Musaad, S.; Bacha, F. The relationship of sleep duration and quality to energy expenditure and physical activity in children. Pediatr. Obes. 2021, 16, e12751. [Google Scholar] [CrossRef] [PubMed]
- Ames, M.E.; Holfeld, B.; Leadbeater, B.J. Sex and age group differences in the associations between sleep duration and BMI from adolescence to young adulthood. Psychol. Health 2016, 31, 976–992. [Google Scholar] [CrossRef]
- Araújo, J.; Severo, M.; Ramos, E. Sleep Duration and Adiposity During Adolescence. Pediatrics 2012, 130, e1146–e1154. [Google Scholar] [CrossRef]
- Lopes, C. Reproducibility and Validation of a Food Frequency Questionnaire; University of Porto Medical School: Porto, Portugal, 2000; pp. 79–115. [Google Scholar]
- Bagley, E.J.; Kelly, R.J.; El-Sheikh, M. Longitudinal relations between children’s sleep and body mass index: The moderating role of socioeconomic risk. Sleep Health 2015, 1, 44–49. [Google Scholar] [CrossRef]
- Calamaro, C.J.; Park, S.; Mason, T.B.A.; Marcus, C.L.; Weaver, T.E.; Pack, A.; Ratcliffe, S.J. Shortened sleep duration does not predict obesity in adolescents. J. Sleep Res. 2010, 19, 559–566. [Google Scholar] [CrossRef]
- Cao, M.; Zhu, Y.; Li, X.; Chen, Y.; Ma, J.; Jing, J. Gender-dependent association between sleep duration and overweight incidence in CHINESE school children: A national follow-up study. BMC Public Health 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Chong, K.H.; Parrish, A.-M.; Cliff, D.P.; Dumuid, D.; Okely, A.D. Cross-Sectional and Longitudinal Associations between 24-Hour Movement Behaviours, Recreational Screen Use and Psychosocial Health Outcomes in Children: A Compositional Data Analysis Approach. Int. J. Environ. Res. Public Health 2021, 18, 5995. [Google Scholar] [CrossRef]
- Collings, P.J.; Wijndaele, K.; Corder, K.; Westgate, K.; Ridgway, C.L.; Sharp, S.J.; Atkin, A.J.; Bamber, D.; Goodyer, I.; Brage, S.; et al. Prospective associations between sedentary time, sleep duration and adiposity in adolescents. Sleep Med. 2015, 16, 717–722. [Google Scholar] [CrossRef]
- Wolfson, A.R.; Carskadon, M.A.; Acebo, C.; Seifer, R.; Fallone, G.; Labyak, S.E.; Martin, J.L. Evidence for the Validity of a Sleep Habits Survey for Adolescents. Sleep 2003, 26, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, Y.S.; Pallesen, S.; Sivertsen, B.; Stormark, K.M.; Hysing, M. Weekday time in bed and obesity risk in adolescence. Obes. Sci. Pract. 2020, 7, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Full, K.M.; Berger, A.T.; Erickson, D.; Berry, K.M.; Laska, M.N.; Lenk, K.M.; Iber, C.; Redline, S.; Widome, R. Assessing Changes in Adolescents’ Sleep Characteristics and Dietary Quality in the START Study, a Natural Experiment on Delayed School Start Time Policies. J. Nutr. 2021, 151, 2808–2815. [Google Scholar] [CrossRef] [PubMed]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef]
- Fung, H.; Yeo, B.T.; Chen, C.; Lo, J.C.; Chee, M.W.; Ong, J.L. Adherence to 24-Hour Movement Recommendations and Health Indicators in Early Adolescence: Cross-Sectional and Longitudinal Associations in the Adolescent Brain Cognitive Development Study. J. Adolesc. Health 2022, 72, 460–470. [Google Scholar] [CrossRef]
- Gardner, L.A.; Debenham, J.; Newton, N.C.; Chapman, C.; Wylie, F.E.; Osman, B.; Teesson, M.; Champion, K.E. Lifestyle risk behaviours among adolescents: A two-year longitudinal study of the impact of the COVID-19 pandemic. BMJ Open 2022, 12, e060309. [Google Scholar] [CrossRef]
- Short, M.A.; Gradisar, M.; Lack, L.C.; Wright, H.R.; Chatburn, A. Estimating adolescent sleep patterns: Parent reports versus adolescent self-report surveys, sleep diaries, and actigraphy. Nat. Sci. Sleep 2013, 5, 23–26. [Google Scholar] [CrossRef]
- Hardy, L.L.; Mihrshahi, S.; Drayton, B.A.; Bauman, A.E. NSW Schools Physical Activity and Nutrition Survey (SPANS); NSW Department of Health: St. Leonards, NSW, Australia, 2017. [Google Scholar]
- Gong, Q.-H.; Li, S.-X.; Wang, S.-J.; Wu, Y.-H.; Han, L.-Y.; Li, H. Sleep duration and overweight in Chinese adolescents: A prospective longitudinal study with 2-year follow-up. Sleep Breath. 2020, 24, 321–328. [Google Scholar] [CrossRef]
- Jansen, E.C.; Baylin, A.; Cantoral, A.; Rojo, M.M.T.; Burgess, H.J.; O’Brien, L.M.; Olascoaga, L.T.; Peterson, K.E. Dietary Patterns in Relation to Prospective Sleep Duration and Timing among Mexico City Adolescents. Nutrients 2020, 12, 2305. [Google Scholar] [CrossRef]
- Denova-Gutiérrez, E.; Ramírez-Silva, I.; Rodríguez-Ramírez, S.; Jiménez-Aguilar, A.; Shamah-Levy, T.; A Rivera-Dommarco, J. Validity of a food frequency questionnaire to assess food intake in Mexican adolescent and adult population. Salud Pública Mex. 2016, 58, 617–628. [Google Scholar] [CrossRef]
- Kracht, C.L.; Katzmarzyk, P.T.; Champagne, C.M.; Broyles, S.T.; Hsia, D.S.; Newton, R.L.; Staiano, A.E. Association between Sleep, Sedentary Time, Physical Activity, and Adiposity in Adolescents: A Prospective Observational Study. Med. Sci. Sports Exerc. 2022, 55, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Subar, A.F.; Kirkpatrick, S.I.; Mittl, B.; Zimmerman, T.P.; Thompson, F.E.; Bingley, C.; Willis, G.; Islam, N.G.; Baranowski, T.; McNutt, S.; et al. The automated self-administered 24-h dietary recall (ASA24): A resource for researchers, clinicians, and educators from the national cancer institute. J. Acad. Nutr. Diet. 2012, 112, 1134–1137. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.; Brand, S.; Colledge, F.; Ludyga, S.; Pühse, U.; Gerber, M. Adolescents’ personal beliefs about sufficient physical activity are more closely related to sleep and psychological functioning than self-reported physical activity: A prospective study. J. Sport Health Sci. 2019, 8, 280–288. [Google Scholar] [CrossRef]
- Gerber, M.; Lang, C.; Lemola, S.; Colledge, F.; Kalak, N.; Holsboer-Trachsler, E.; Pühse, U.; Brand, S. Validation of the German version of the insomnia severity index in adolescents, young adults and adult workers: Results from three cross-sectional studies. BMC Psychiatry 2016, 16, 1–14. [Google Scholar] [CrossRef]
- Lim, L.-L.; Tse, G.; Choi, K.C.; Zhang, J.; Luk, A.O.Y.; Chow, E.; Ma, R.C.W.; Chan, M.H.M.; Wing, Y.K.; Kong, A.P.S.; et al. Temporal changes in obesity and sleep habits in Hong Kong Chinese school children: A prospective study. Sci. Rep. 2019, 9, 5881. [Google Scholar] [CrossRef]
- Maume, D.J. Social relationships and the sleep-health nexus in adolescence: Evidence from a comprehensive model with bi-directional effects. Sleep Health 2017, 3, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Merikanto, I.; Kuula, L.; Lahti, J.; Räikkönen, K.; Pesonen, A.-K. Eveningness associates with lower physical activity from pre- to late adolescence. Sleep Med. 2020, 74, 189–198. [Google Scholar] [CrossRef]
- Mitchell, J.A.; Rodriguez, D.; Schmitz, K.H.; Audrain-McGovern, J. Sleep Duration and Adolescent Obesity. Pediatrics 2013, 131, e1428–e1434. [Google Scholar] [CrossRef]
- Roberts, R.E.; Duong, H.T. Is there an association between adolescent sleep restriction and obesity. J. Psychosom. Res. 2015, 79, 651–656. [Google Scholar] [CrossRef]
- Saelee, R.; Gazmararian, J.A.; Haardörfer, R.; Suglia, S.F. Associations between the neighborhood social environment and obesity among adolescents: Do physical activity, screen time, and sleep play a role? Health Place 2020, 64, 102380. [Google Scholar] [CrossRef]
- Schäfer, A.A.; Domingues, M.; Dahly, D.L.; Meller, F.O.; Gonçalves, H.; Wehrmeister, F.C.; Assunção, M.C.F. Sleep Duration Trajectories and Body Composition in Adolescents: Prospective Birth Cohort Study. PLoS ONE 2016, 11, e0152348. [Google Scholar] [CrossRef] [PubMed]
- Seegers, V.; Petit, D.; Falissard, B.; Vitaro, F.; Tremblay, R.E.; Montplaisir, J.; Touchette, E. Short Sleep Duration and Body Mass Index: A Prospective Longitudinal Study in Preadolescence. Am. J. Epidemiology 2011, 173, 621–629. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.C.; Eisenmann, J.C.; Santos, D.; De Chaves, R.N.; de Moraes Forjaz, C.; Maia, J.A.R. Modeling the dynamics of BMI changes during adolescence. The Oporto Growth, Health and Performance Study. Int. J. Obes. 2015, 39, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Stefansdottir, R.; Rognvaldsdottir, V.; Gestsdottir, S.; Gudmundsdottir, S.L.; Chen, K.Y.; Brychta, R.J.; Johannsson, E. Changes in sleep and activity from age 15 to 17 in students with traditional and college-style school schedules. Sleep Health 2020, 6, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Wake, M.; Canterford, L.; Patton, G.C.; Hesketh, K.; Hardy, P.; Williams, J.; Waters, E.; Carlin, J.B. Comorbidities of overweight/obesity experienced in adolescence: Longitudinal study. Arch. Dis. Child. 2009, 95, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Jakobsson, M.; Sundin, K.; Högberg, K.; Josefsson, K. “I Want to Sleep, but I Can’t”: Adolescents’ Lived Experience of Sleeping Difficulties. J. Sch. Nurs. 2022, 38, 449–458. [Google Scholar] [CrossRef]
- Krističević, T.; Štefan, L.; Sporiš, G. The Associations between Sleep Duration and Sleep Quality with Body-Mass Index in a Large Sample of Young Adults. Int. J. Environ. Res. Public Health 2018, 15, 758. [Google Scholar] [CrossRef]
- Lin, C.-L.; Lin, C.-P.; Chen, S.-W.; Wu, H.-C.; Tsai, Y.-H. The association between sleep duration and overweight or obesity in Taiwanese adults: A cross-sectional study. Obes. Res. Clin. Pract. 2018, 12, 384–388. [Google Scholar] [CrossRef]
- Meyer, K.A.; Wall, M.M.; Larson, N.I.; Laska, M.N.; Neumark-Sztainer, D. Sleep Duration and BMI in a Sample of Young Adults. Obesity 2012, 20, 1279–1287. [Google Scholar] [CrossRef]
- Bacaro, V.; Ballesio, A.; Cerolini, S.; Vacca, M.; Poggiogalle, E.; Donini, L.M.; Lucidi, F.; Lombardo, C. Sleep duration and obesity in adulthood: An updated systematic review and meta-analysis. Obes. Res. Clin. Pract. 2020, 14, 301–309. [Google Scholar] [CrossRef]
- Wu, Y.; Zhai, L.; Zhang, D. Sleep duration and obesity among adults: A meta-analysis of prospective studies. Sleep Med. 2014, 15, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.M.; Hallschmid, M.; Jauch-Chara, K.; Wilms, B.; Benedict, C.; Lehnert, H.; Born, J.; Schultes, B. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am. J. Clin. Nutr. 2009, 90, 1476–1482. [Google Scholar] [CrossRef] [PubMed]
- Burman, D. Sleep Disorders: Circadian Rhythm Sleep-Wake Disorders. FP Essent. 2017, 460, 33–36. [Google Scholar] [CrossRef]
- Broussard, J.L.; Van Cauter, E. Disturbances of sleep and circadian rhythms: Novel risk factors for obesity. Curr. Opin. Endocrinol. Diabetes 2016, 23, 353–359. [Google Scholar] [CrossRef]
- He, F.; Bixler, E.O.; Liao, J.; Berg, A.; Kawasawa, Y.I.; Fernandez-Mendoza, J.; Vgontzas, A.N.; Liao, D. Habitual sleep variability, mediated by nutrition intake, is associated with abdominal obesity in adolescents. Sleep Med. 2015, 16, 1489–1494. [Google Scholar] [CrossRef]
- Swanson, S.A.; Crow, S.J.; Le Grange, D.; Swendsen, J.; Merikangas, K.R. Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement. Arch. Gen. Psychiatry 2011, 68, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Isomaa, R.; Isomaa, A.-L.; Marttunen, M.; Kaltiala-Heino, R.; Björkqvist, K. Longitudinal concomitants of incorrect weight perception in female and male adolescents. Body Image 2011, 8, 58–63. [Google Scholar] [CrossRef]
- Casadei, K.; Kiel, J. Anthropometric Measurement. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Tucker, K.L. Assessment of usual dietary intake in population studies of gene–diet interaction. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 74–81. [Google Scholar] [CrossRef]
- Sadeh, A. The role and validity of actigraphy in sleep medicine: An update. Sleep Med. Rev. 2011, 15, 259–267. [Google Scholar] [CrossRef]
- Arora, T.; Broglia, E.; Pushpakumar, D.; Lodhi, T.; Taheri, S. An Investigation into the Strength of the Association and Agreement Levels between Subjective and Objective Sleep Duration in Adolescents. PLoS ONE 2013, 8, e72406. [Google Scholar] [CrossRef] [PubMed]
- Carneiro-Barrera, A.; Amaro-Gahete, F.J.; Acosta, F.M.; Ruiz, J.R. Body Composition Impact on Sleep in Young Adults: The Mediating Role of Sedentariness, Physical Activity, and Diet. J. Clin. Med. 2020, 9, 1560. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Beisbier, S.; Laverdure, P. Occupation-and activity-based interventions to improve performance of instrumental activities of daily living and rest and sleep for children and youth ages 5–21: A systematic review. Am. J. Occup. Ther. 2020, 74, 7402180040p1–7402180040p32. [Google Scholar] [CrossRef]
- Belmon, L.S.; van Stralen, M.M.; Busch, V.; Harmsen, I.A.; Chinapaw, M.J. What are the determinants of children’s sleep behavior? A systematic review of longitudinal studies. Sleep Med. Rev. 2019, 43, 60–70. [Google Scholar] [CrossRef]
- Costa, S.; Benjamin-Neelon, S.E.; Winpenny, E.; Phillips, V.; Adams, J. Relationship between early childhood non-parental childcare and diet, physical activity, sedentary behaviour, and sleep: A systematic review of longitudinal studies. Int. J. Environ. Res. Public Health 2019, 16, 4652. [Google Scholar] [CrossRef]
- Ehsan, Z.; Ishman, S.L.; Kimball, T.R.; Zhang, N.; Zou, Y.; Amin, R.S. Longitudinal cardiovascular outcomes of sleep disordered breathing in children: A meta-analysis and systematic review. Sleep 2017, 40, zsx015. [Google Scholar] [CrossRef]
- Fatima, Y.; Doi, S.A.R.; Mamun, A.A. Longitudinal impact of sleep on overweight and obesity in children and adolescents: A systematic review and bias-adjusted meta-analysis. Obes. Rev. 2015, 16, 137–149. [Google Scholar] [CrossRef]
- Gronski, M.; Doherty, M. Interventions within the scope of occupational therapy practice to improve activities of daily living, rest, and sleep for children ages 0–5 years and their families: A systematic review. Am. J. Occup. Ther. 2020, 74, 7402180010p1–7402180010p33. [Google Scholar] [CrossRef]
- Guo, Y.; Miller, M.A.; Cappuccio, F.P. Short duration of sleep and incidence of overweight or obesity in Chinese children and adolescents: A systematic review and meta-analysis of prospective studies. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 363–371. [Google Scholar] [CrossRef]
- Miller, M.A.; Kruisbrink, M.; Wallace, J.; Ji, C.; Cappuccio, F.P. Sleep duration and incidence of obesity in infants, children, and adolescents: A systematic review and meta-analysis of prospective studies. Sleep 2018, 41, zsy018. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Kruisbrink, M.; Wallace, J.; O’Keeffe, A.; Valint, S.; Ji, C.; Cappuccio, F.P. Abstract MP090: Sleep duration predict incident obesity in childhood and adolescence: Meta-analysis of prospective studies. Circulation 2017, 135 (Suppl. S1). [Google Scholar] [CrossRef]
- Ruan, H.; Xun, P.; Cai, W.; He, K.; Tang, Q. Habitual sleep duration and risk of childhood obesity: Systematic review and dose-response meta-analysis of prospective cohort studies. Sci. Rep. 2015, 5, 16160. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).