1. Introduction
Indonesia has an estimated overall caseload of over 6 million children under 5 years of age with wasting, of whom more than 2 million are severely wasted, based on national basic health research data [
1,
2]. Severe acute malnutrition (SAM) is defined by a weight-for-height Z-score (WHZ) <−3 and/or a mid-upper arm circumference (MUAC) <115 mm, and/or nutritional oedema, regardless of anthropometric status [
3]. Children with severe wasting are up to 12 times more likely to die compared with their well-nourished peers and are susceptible to long-term negative health outcomes such as linear growth impairment, leading to stunting and sub-optimal cognitive development [
4]. Therefore, children diagnosed with SAM require immediate treatment.
The Government of Indonesia has shown great commitment to fighting wasting through a rapid expansion of integrated management of acute malnutrition (IMAM) services to prevent and treat wasting in Indonesia, including strengthening inpatient- and community-based treatments. Inpatient treatment of severe wasting has been a standard component of health services in Indonesia for many years. However, in the >85% of cases that have no complications, SAM can also be treated at home using high-energy-dense ready-to-use therapeutic foods (RUTFs). Indeed, research has shown that home treatment through IMAM is at least as effective as in-patient treatment for uncomplicated SAM [
5,
6]. While the effectiveness of RUTFs on weight gain and recovery rates has been well established, reported weight gains are often less than the 4 g/kg body weight (BW)/day required by WHO [
7]. Besides providing basic medicines such as antibiotics and deworming tablets, RUTFs are an essential part of IMAM [
4]. However, locally produced RUTFs are currently not yet available in Indonesia and the importation of RUTFs is restricted, posing major challenges to the implementation of IMAM at scale.
RUTF is defined as a high-energy, fortified, ready-to-eat, soft or crushable, non-water-based food suitable for the treatment of children with SAM from the age of six months onward [
8]. The original WHO guidelines required at least 50% of its protein content to come from dairy [
3], although this requirement has recently been adapted [
9]. The most widely used RUTF is a peanut paste with milk and oil as well as vitamins and minerals, containing approximately 540 kcal/100 g. The peanuts may be replaced with other legumes or cereals, depending on local availability, cost, and acceptability, while maintaining compliance with the recommended nutritional composition [
3]; however, the introduction of local RUTFs often requires a long development process [
10].
To support the efforts of the Government of Indonesia in scaling up IMAM to the national level, UNICEF, Savica and the French National Research Institute for Sustainable Development (IRD) conducted a combined acceptability and efficacy study on different types of RUTFs produced using local ingredients that are readily available in Indonesia. Here we report the main outcomes of the study, including acceptability and changes in weights and MUACs of children treated for SAM using standard RUTF or one of four local RUTFs.
2. Materials and Methods
2.1. Study Design
This study was designed as an individually randomized controlled trial. All children meeting the inclusion criteria were invited to participate in the intervention study. Each child was randomly allocated to receive 8 weeks of SAM treatment with one of five RUTF products. A peanut–milk paste RUTF (CON) was used as the control RUTF. Experimental RUTFs consisted of a soy–milk paste (SOY), a mungbean–milk paste (MUN1), a slightly thinner mungbean–milk paste (MUN2) or a peanut–milk paste-filled wafer roll (PEA). Details of the nutritional content of the products are provided in
Table 1. Products conforming to WHO guidelines and with certificates of analysis were submitted by all producers. According to protocol, randomization was conducted based on an automatically generated allocation list with a block size of ten; however, due to the late production of one product, the first two weeks of the trial started without this product (block randomization with eight blocks), and the product was slightly overrepresented in the later blocks to compensate.
2.2. Study Location
The study was conducted in Bogor District, West Java Province, and covered 322 villages and 81 Primary Healthcare Centres (PHC) in 31 subdistricts. This represents around 80% of this district. The remainder of the subdistricts were too remote and/or too difficult to access. The study was conducted from July to December 2021.
2.3. Subjects
Eligible for inclusion in the study were children aged 6–59 months with uncomplicated SAM (WHZ < −3 and/or MUAC < 115 mm and/or presence of bilateral pitting oedema +1 or +2 regardless of anthropometry) who had no underlying health conditions or complications of SAM, no severe anaemia (<70 g/L) or body weight less than 4 kg, who were not allergic to any of the ingredients in the RUTFs, who passed the appetite test and who had not received treatment for SAM (including not having consumed RUTF, F75, F100 or food for special medical purposes) in the last two months.
As reliable data from the PHC on the anthropometric status of children in their catchment area were not available due to the COVID-19 pandemic (no monthly growth monitoring sessions being conducted), we pre-screened children for SAM using MUAC through home visits, using a cut-off of 135 mm, to allow better detection of children with low WHZ [
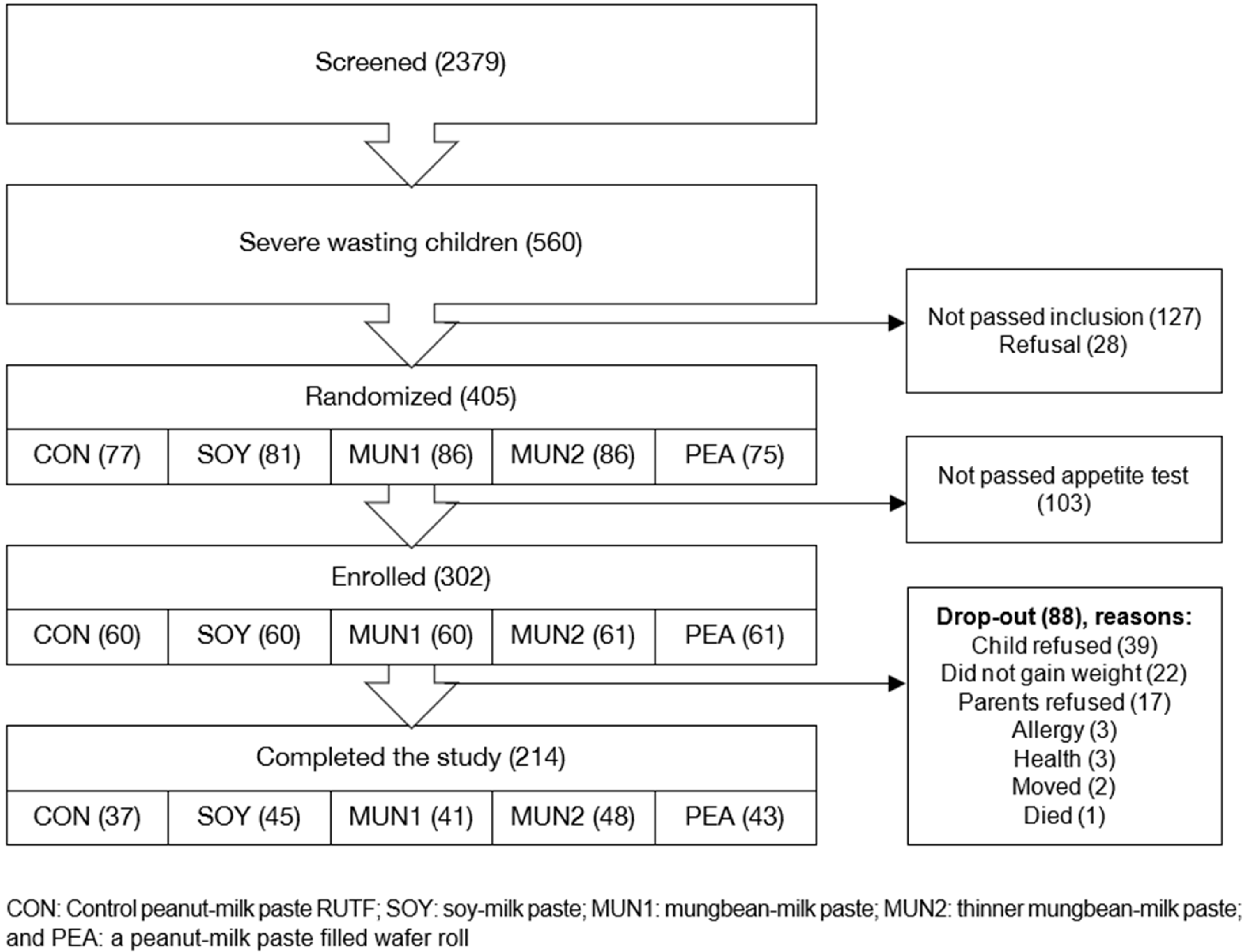
11]. This approach generated a short list of children at high risk of SAM. Children identified during the pre-screening were then re-visited at home, in close coordination with the PHC staff and with full adherence to national and local regulations and protocols related to the COVID-19 pandemic. The screening process (
Figure 1) consisted of confirming SAM based on anthropometry (WHZ and/or MUAC and/or nutritional oedema), physical examination, determination of haemoglobin concentration (HemoCue™ Angelholm, Sweden 201+) and an appetite test. Eligible children whose parents gave informed consent were enrolled in the study.
2.4. Study Procedures and Data Collection
2.4.1. Anthropometry
At recruitment, height or length was measured to the nearest 0.1 cm using a Shorrboard® (Weigh and Measures, LLC, Olney, MD, USA). Weight was measured to the nearest 0.1 kg (SECA 874 or AND UC-321 digital weighing scales). MUAC was measured to the nearest 1 mm using standardized measuring tapes (UNICEF). All measurements were performed in duplicate and repeated if they exceeded the allowable difference (>0.2 cm for height/length, >0.2 kg for weight and >2 mm for MUAC). Weight and MUAC measurements were repeated weekly, whereas heights/lengths were measured at 4 weeks (mid-term) and 8 weeks (end of study). All children were screened for oedema using standardized techniques.
2.4.2. Appetite Test
Prior to the appetite test, eligible children were randomized to one of the five intervention arms using the block randomization described above. The criteria to pass the appetite test were based on the Ministry of Health (MoH) Guidelines on SAM treatment. Each child was given one sachet of their assigned product by the mother/caregiver, and consumption was observed for up to 30 minutes, after which leftovers were weighed. Duration of consumption, the total amount of product consumed, the observed reaction of the child and information on the child’s normal appetite were recorded. If a child failed the appetite test and generally had very poor appetite, the child was excluded and referred to the PHC for follow-up and treatment. However, if the mother stated that her child usually had good appetite, they were provided with the product to consume at home for three days. If the child still did not consume the RUTF (or consumed <50% of the provided RUTF for three consecutive days), the child was dropped from the study and referred to the PHC for treatment.
2.4.3. Data Collection
At baseline, a short questionnaire on the social-economic status of the household, household food security, infant and young child feeding (IYCF) practices (including 24-h food recall), use of micronutrient supplements and supplementary feeding provided through the PHC, morbidity (history and current), gestational age, birth weight and length and immunization status was administered. All data were recorded on tablets using a digital questionnaire (Survey2 Go). At the endline, data on 24-h food recall, height, weight, MUAC, haemoglobin concentration and parents’ observation of their child’s preferences (taste, colour and smell) of the RUTF products were collected.
Mothers/caregivers were provided with a monitoring book—and an explanation of its use—to record the frequency and timing of RUTF consumption, frequency of breastfeeding and intake of any other foods on a daily basis. A field worker made weekly home visits to collect these recordings, as well as information on intra-household sharing of the RUTF and morbidity (weekly recall). The field worker counted the remaining sachets and weighed any open sachets to calculate the total amount of RUTF product consumed. During this visit, the field worker also provided counselling to the mothers/caregivers on RUTF consumption, including not sharing the RUTF with other household members and giving other foods only after the child had finished their daily dose of RUTF. In addition, field workers maintained regular communication with the mothers/caregivers through texting and phone calls. Phone credit was provided to mothers/caregivers and cadres for this purpose.
They reminded mothers/caregivers to feed their child the RUTF, fill in the monitoring book and addressed any problems that arose. On the day after the field worker’s visit, mothers/caregivers who were able to do so were asked to take a picture of the package and their entry in the monitoring book so that the field workers could provide remote feedback. Mothers/caregivers without a camera phone were visited three times in the first week to make sure they completed the monitoring book correctly.
2.4.4. Treatment
A weekly supply of RUTF was provided in a box during each of the weekly check-up visits, adjusted each week to the weight of the child according to the MoH Guidelines on SAM treatment (
Table 2).
Each sachet was kept in a zip-lock plastic bag labelled with the name of the child, the name of the mother/caregiver, week, day and date of consumption. Mothers/caregivers were given counselling on RUTF consumption. In addition, the child also received broad-spectrum antibiotics (amoxicillin) for five days in the first week of the intervention and anthelminthic therapy (pyrantel palmoate) according to the MoH Guidelines on the treatment of severe wasting, except for those who had received deworming in the past six months.
2.5. Sample Size Calculation
The main objective of the study was to compare four locally produced RUTFs with the standard peanut-based RUTF for acceptability and efficacy. For the efficacy study, we based our initial sample size estimates on an overall increase of 4 g/kg BW/day, the target set by the WHO. A sample size of 50 children per group would allow the detection of a 20% difference between control and intervention products, assuming a standard deviation (SD) of 1.6 g/kg BW, a study design effect of 1.0 (individual randomization), a significance of 0.05 and a power of 0.80.
2.6. Statistical Analyses
All data were entered into a digital questionnaire and extracted to SPSS 25 and cleaned. Data from 302 enrolled children were included in the analysis. Descriptive analyses were employed to examine the distribution of the full range of variables. All enrolled children were included in the ‘intention to treat’ analysis, while a separate analysis was conducted on the children who finished the 8-week intervention study. Repeated-measurement data were analysed using general linear models (GLM). Normality of data was checked using z-score skewness and kurtosis, and the Kolmogorov-Smirnov test. Data are presented as the mean ± standard deviation (SD) for normally distributed variables, median and interquartile range (IQR) for non-normally distributed variables and as percentages for categorical variables. Non-parametric Kruskal–Wallis test was used to test differences between non-normally distributed variables. Pearson chi-square test was used to compare categorical variables. Mann–Whitney U test was used to compare non-parametric variables between intervention groups. Data were considered significant at p < 0.05.
Minimum dietary diversity score (MDDS) and minimum acceptable diet (MAD) were calculated using WHO IYCF Practices Indicators 2010 and 2021. A dichotomous variable was created to define whether children met the MDDS (at least 4 food groups) or not (<4 food groups). Household food security was determined by applying the Household Food Insecurity Access Scale.
2.7. Quality Control
All field staff held a degree in nutrition, with experience in conducting research and taking anthropometric measurements in young children. Prior to the fieldwork, the field team was given standardized training on the study methodology, IMAM and counselling on RUTF consumption. Data were collected using a digital questionnaire, with multiple quality checks throughout the process. All equipment was cleaned and calibrated regularly using a standardized procedure. Any malfunctioning equipment was replaced with a spare. Dropouts and Adverse Events/Serious Adverse Events (AE/SAE) were recorded and reported to UNICEF and the health authorities.
3. Results
A total of 6145 children were pre-screened and 2379 were identified as being at risk of SAM. Of these, 560 children had SAM; a quarter did not meet the study criteria (
n = 127; 22%) and were referred to the PHC for treatment or their parents did not consent (
n = 28; 5%) (
Figure 1).
Among the 302 (74.6%) enrolled children, 253 (83.8%) had WHZ scores < −3, 18 (6.0%) had MUAC < 11.5 cm, and 31 (10.3%) met both inclusion criteria (
Table 3). No child had bilateral pitting oedema. There were no differences in the enrolment or the drop-out rates between the children who were enrolled based on their WHZ score, MUAC or both.
Overall drop-out rate during the intervention was high at 29.1% (
n = 88), ranging from 21.3% in the MUN2 group to 38.3% in the group receiving the control RUTF (CON). While this was not statistically significant (
p = 0.30), children in the CON group dropped out significantly earlier compared with children in the PEA group (19.0 vs. 33.6 days;
p = 0.002, GLM,
Table 4). Additionally, children in the MUN2 group stayed in the study longer compared with children in the CON group (24.3 days,
p = 0.030, Log Rank); similar trends were observed for children in the SOY group (
p = 0.078) and the PEA group (
p = 0.143).
There was no preference among the caregivers for the taste of the products (p > 0.05), although slightly more caregivers of children who received the MUN2 and PEA products indicated that their children liked the taste compared with the other products.
The amount of RUTF consumed during the appetite test (20.0 ± 14.9 g) and the velocity of RUTF consumption (0.75 g/min) were not different among the 5 intervention groups (
p > 0.05). However, intake was significantly different over the 2 months of SAM treatment, with children receiving MUN2 consuming twice as much as children receiving the CON or SOY RUTF (4.49 kg vs. 2.18 kg and 2.32 kg, respectively,
p < 0.05,
Table 5).
Mothers were encouraged to continue breastfeeding their children during the intervention. Only 7% of the children consumed other foods less than once per day during the 8-week intervention, while the rest of the children consumed other foods 1–3 times per day, despite not finishing their daily dose of RUTF.
The mean weight gain, expressed as g/kg BW/day, ranged from 1.38 ± 0.19 g in the PEA group to 1.67 ± 0.19 g in the MUN2 group over the 8-week intervention (p > 0.05). Average consumption over the course of the study was significantly different between the groups. When controlled for consumption, age and food insecurity, the estimated increase in the weight of children receiving either CON or SOY RUTFs was around 2.3 g/kg BW/day compared with around 1.6 g/kg BW/day in children receiving the other RUTF products (p > 0.05).
Weight gain (g/kg BW/day) was not statistically different (p = 0.8) between children admitted with low MUAC only (1.61 g/kg BW/day), low WHZ only (1.45 g/kg BW/day) or both low MUAC and low WHZ (1.58 g/kg BW/day). Additionally, the recovery of children included in the study based on MUAC only or WHZ only did not differ (67% and 76% recovered, respectively, p > 0.05).
At baseline, the mean haemoglobin concentration in the study population was 9.9 ± 1.3 g/dL (n = 296 children) and there were no significant differences between the groups. A slight increase in haemoglobin concentration between baseline and endline was seen in all groups (n = 210), but the increase was significantly higher in the MUN2 group compared with the PEA and MUN1 RUTF groups.
4. Discussion
To our knowledge, this study is the first large trial in Indonesia comparing the acceptability and effectiveness of RUTFs based on locally available ingredients compared with a standard RUTF. This study showed that RUTF products made from locally available ingredients were comparable to the standard peanut-based RUTF in terms of weight gain. Weight gain was between 1.3–1.6 g/kg BW/day, which is in line with findings of effectiveness trials in Cambodia and Vietnam [
12,
13,
14], but much less than the 4.0 g/kg BW/day set by the WHO. One factor affecting this lower-than-required weight gain was compliance, especially in the standard and soy-based RUTFs. The low compliance is worrisome, as caregivers and children received intensive guidance from field workers and supervision was much higher than can be expected in a standard IMAM program. We assume that weight gain would have been higher if compliance had been higher, but in real program settings, lower compliance is more likely to be encountered, yet, all products met the WHO cure rate criteria of 70%. An important finding of the present study, therefore, is that compliance was significantly better for some of the local products, clearly showing the importance of developing RUTF products adapted to local taste preferences.
More than 60% of the children in this study were less than 2 years old, indicating that severe wasting is a serious public health problem during early childhood in Indonesia. Recent evidence from Africa and South-East Asia shows that children who experience a period of wasting are at an increased risk of stunting and mortality in the period thereafter. Hence, given that the majority of severe wasting occurs within the window of opportunity to reduce stunting, early detection and treatment of severe wasting will contribute significantly to the efforts of the Government of Indonesia to reduce stunting. The importance of counselling, both on IYCF and continued severe wasting treatment, cannot be underestimated, with most dropouts in the <2 years age group and >40% of children in the control group not finishing treatment.
Most products seem to be acceptable, in particular, local products and especially the MUN2 and PEA products were well received by the younger children (6–11 months old); the PEA RUTF was crushed by the caregivers before presentation to their children when the child was below 11 months of age. The MUN2 product, which had lower viscosity than the other pastes, was less well received by older children (24–59 months). The relatively lower acceptability of CON RUTF is in line with findings in other South-East Asian countries [
10,
12,
13].
Based on the findings of this study, the successful implementation of an IMAM program in Indonesia will depend on the production and distribution of local RUTF products, because the almost 40% drop-out within the first weeks of SAM treatment observed with the current gold standard (peanut–milk paste RUTF) is untenable. Therefore, local development and production of RUTF should be strongly encouraged. Further revision of the products in this study is recommended to further increase the nutrient density and acceptability of the local products. The availability of a larger choice of products can increase the success of a larger national program as it enables catering to personal and local preferences in terms of ingredients, taste and form, and can be adapted to the age of the child.
The lack of impact for 4 of the 5 RUTFs is in line with studies from Africa and Asia, which showed little or no improvement in haemoglobin concentrations in children receiving SAM treatment with standard RUTF [
15,
16] containing 10–14 mg iron/100 g. Some researchers have argued that the amount of iron in standard RUTF should be increased to over 30 mg/100 g [
17] to see an impact on iron status in children recovering from SAM, but concerns about the negative effects of high doses of iron on e.g., human microbiome, might hamper this approach.
It is important to note that close monitoring and intensive counselling are essential for the success of SAM treatment at home. Not only should the importance of the treatment be stressed, but counselling should include infant and young child feeding, given the high proportion of children under 2 years who have SAM. Therefore, government programs should consider incorporating Social and Behavioural Change Communication (SBCC) and a ‘buddy’ system similar to that used in tuberculosis programs to motivate the parents and ensure optimal compliance.
Overall, the results of the present study confirm that the development of RUTFs adapted to the local availability of ingredients and local organoleptic preferences contribute to higher acceptance of the products and thereby can contribute to more efficient IMAM programs.
Author Contributions
Conceptualization, D.D.S., B.R.B., J.S., J.H.R. and F.T.W.; data curation, A.R. and D.D.S.; formal analysis, A.R., D.D.S. and F.T.W.; investigation, A.R., D.D.S. and F.T.W.; methodology, D.D.S., B.R.B., J.S., J.H.R. and F.T.W.; project administration, A.R.; resources, A.R. and D.D.S.; supervision, D.D.S., B.R.B., J.S., J.H.R. and F.T.W.; validation, D.D.S. and F.T.W.; visualization, A.R., D.D.S. and F.T.W.; writing—original draft, A.R., D.D.S. and F.T.W.; writing—review and editing, B.R.B., J.S., R.N. and J.H.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by UNICEF Indonesia and IRD.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and the protocol was approved by the ethical committee of Gajah Mada University (KE/FK/137/EC/2020). Research permits were obtained from the appropriate local government institutions.
Informed Consent Statement
Parents were informed of the purpose, risks and benefits of the study and asked to sign the informed consent forms prior to inclusion of their children in the study.
Data Availability Statement
The data that support the findings of this study are available from UNICEF Indonesia, but restrictions apply to the availability of the data, which were used under license for the current study, and therefore are not publicly available. Data are available from the authors upon reasonable request and with permission from UNICEF Indonesia.
Acknowledgments
The authors acknowledge the field team led by Ilyatun Niswah, Fadhillah Dzaky, Fajar Abdillah and Asri Ismiyani, and the Savica team for their support to this study. This study would not have been possible without the participating mothers and children. We also thank the staff at the Bogor District Health Office, the local health centres, village midwives and cadres for their kind collaboration. We would like to thank the Nutrition Research and Development Agency of the Government of Indonesia, SEAFAST IPB, PT Indofood and Nutriset for contributing their products for this study. Support from the following high-level government officials in the study and advancement of wasting management in Indonesia is of utmost importance: From the Ministry of National Development Planning (Bappenas), Pungkas Bahjuri Ali(Director of Community Health and Nutrition), Sidayu Ariteja (Junior Planner), Nurul Azma Ahmad Tarmizi (Scaling Up Nutrition Program Assistant). From the Ministry of Health: Erna Mulati (Director of Nutrition and Maternal Child Health), Ni Made Diah (officer in charge for the Director of Nutrition and Maternal Child Health), and Nida Rohmawati (Coordinator of the Taskforce on children under five and pre-school children, Directorate of Nutrition and Maternal Child Health).
Conflicts of Interest
All authors declare no conflicts of interest. The producers of the products were not involved in study design, data collection or analysis.
References
- Laporan Nasional Riskesdas 2018. Jakarta: Badan Penelitian dan Pengembangan Kesehatan. 2019. Available online: http://repository.bkpk.kemkes.go.id/3514/1/Laporan%20Riskesdas%202018%20Nasional.pdf (accessed on 5 September 2022).
- UNICEF. Levels and Trends in Child Malnutrition: Key Findings of the 2021 Edition of the Joint Child Malnutrition Estimates; United Nations Children’s Fund (UNICEF): New York, NY, USA; World Health Organization: Geneva, Switzerland; International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2021. [Google Scholar]
- World Health Organization. Guideline: Updates on the Management of Severe Acute Malnutrition in Infants and Children; World Health Organization: Geneva, Switzerland, 2013; Available online: https://apps.who.int/iris/handle/10665/95584 (accessed on 5 September 2022).
- UNICEF. Community-Based Management of Severe Acute Malnutrition: A Joint Statement by the World Health Organization, the World Food Programme, the United Nations System Standing Committee on Nutrition and the United Nations Children’s Fund; UNICEF: Geneva, Switzerland, 2007. [Google Scholar]
- Maleta, K.; Amadi, B. Community-Based Management of Acute Malnutrition (CMAM) in Sub-Saharan Africa: Case Studies from Ghana, Malawi, and Zambia. Food Nutr. Bull. 2014, 35, S34–S38. [Google Scholar] [CrossRef] [PubMed]
- Ciliberto, M.A.; Sandige, H.; Ndekha, M.J.; Ashorn, P.; Briend, A.; Ciliberto, H.M.; Manary, M.J. Comparison of home-based therapy with ready-to-use therapeutic food with standard therapy in the treatment of malnourished Malawian children: A controlled, clinical effectiveness trial. Am. J. Clin. Nutr. 2005, 81, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Schoonees, A.; Lombard, M.J.; Musekiwa, A.; Nel, E.; Volmink, J. Ready-to-use therapeutic food (RUTF) for home-based nutritional rehabilitation of severe acute malnutrition in children from six months to five years of age. Cochrane Database Syst. Rev. 2019, 5, CD009000. [Google Scholar] [PubMed]
- Manary, M.J. Local Production and Provision of Ready-To-Use Therapeutic Food (Rutf) Spread for the Treatment of Severe Childhood Malnutrition. Food Nutr. Bull. 2006, 27, S83–S89. [Google Scholar] [CrossRef] [PubMed]
- FAO. A Categorical Breakthrough New Codex Alimentarius Guideline Means Ready-to-Use Therapeutic Foods Can be Manufactured and Incorporated into More Countries’ Health Plans. 2022. Available online: https://www.fao.org/fao-stories/article/en/c/1619824/ (accessed on 8 February 2023).
- Borg, B.; Mihrshahi, S.; Laillou, A.; Sigh, S.; Sok, D.; Peters, R.; Chamnan, C.; Berger, J.; Prak, S.; Roos, N.; et al. Development and testing of locally-produced ready-to-use therapeutic and supplementary foods (RUTFs and RUSFs) in Cambodia: Lessons learned. BMC Public Health 2019, 19, 1200. [Google Scholar] [CrossRef] [PubMed]
- Laillou, A.; Prak, S.; de Groot, R.; Whitney, S.; Conkle, J.; Horton, L.; Un, S.O.; Dijkhuizen, M.A.; Wieringa, F.T. Optimal screening of children with acute malnutrition requires a change in current WHO guidelines as MUAC and WHZ identify different patient groups. PLoS ONE 2014, 9, e101159. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Nga, T.T.; Hoang, M.-A.; Maalouf-Manasseh, Z.; Hammond, W.; Thuc, T.M.L.; Minh, T.H.N.; Hop, T.L.; Berger, J.; Wieringa, F.T. Acceptability of Two Ready-to-Use Therapeutic Foods by HIV-Positive Patients in Vietnam. Food Nutr. Bull. USA 2015, 36, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Nga, T.T.; Nguyen, M.; Mathisen, R.; Hoa, D.T.B.; Minh, N.H.; Berger, J.; Wieringa, F.T. Acceptability and impact on anthropometry of a locally developed ready-to-use therapeutic food in pre-school children in Vietnam. Nutr. J. 2013, 12, 120. [Google Scholar] [CrossRef] [PubMed]
- Sigh, S.; Roos, N.; Chamnan, C.; Laillou, A.; Prak, S.; Wieringa, F.T. Effectiveness of a Locally Produced, Fish-Based Food Product on Weight Gain among Cambodian Children in the Treatment of Acute Malnutrition: A Randomized Controlled Trial. Nutrients 2018, 10, 909. [Google Scholar] [CrossRef] [PubMed]
- Kangas, S.T.; Salpéteur, C.; Nikièma, V.; Talley, L.; Briend, A.; Ritz, C.; Friis, H.; Kaestel, P. Vitamin A and iron status of children before and after treatment of uncomplicated severe acute malnutrition. Clin. Nutr. 2020, 39, 3512–3519. [Google Scholar] [CrossRef] [PubMed]
- Sigh, S.; Roos, N.; Chamnan, C.; Laillou, A.; Wieringa, F.T. Ready-to-use-therapeutic foods fail to improve vitamin A and iron status meaningfully during treatment for severe acute malnutrition in 6-59-months-old Cambodian children. Nutrients 2023, 15, 905. [Google Scholar] [CrossRef] [PubMed]
- Akomo, P.; Bahwere, P.; Murakami, H.; Banda, C.; Maganga, E.; Kathumba, S.; Sadler, K.; Collins, S. Soya, maize and sorghum ready-to-use therapeutic foods are more effective in correcting anaemia and iron deficiency than the standard ready-to-use therapeutic food: Randomized controlled trial. BMC Public Health 2019, 19, 806. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).