Association between the Consumption of Ultra-Processed Foods and Asthma in Adults from Ribeirão Preto, São Paulo, Brazil
Abstract
1. Introduction
2. Methods
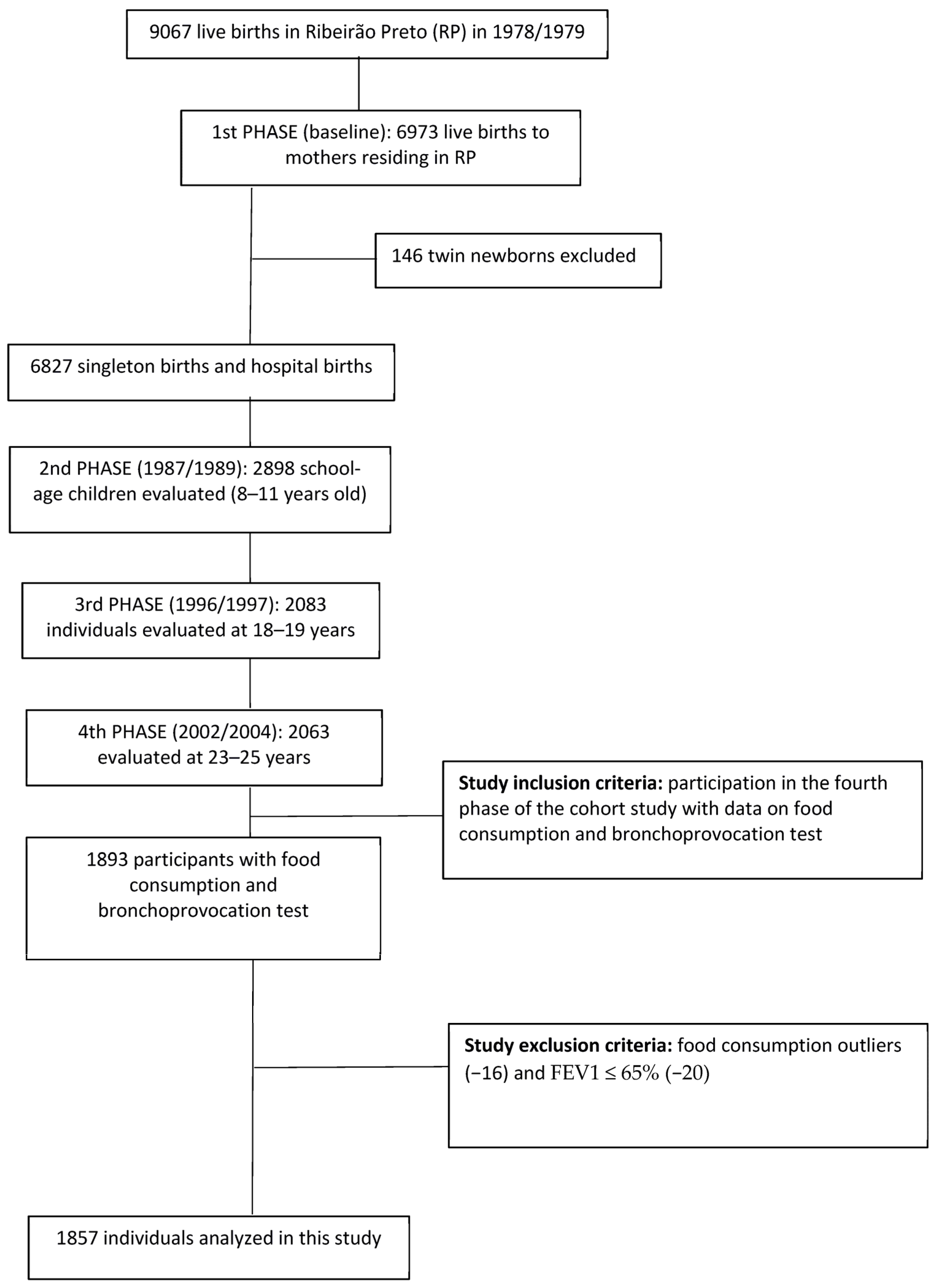
2.1. Study Design and Sample
2.2. Food Intake and Consumption of Ultraprocessed Foods
2.3. Asthma
2.4. Confounding Variables
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vos, T.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abate, K.H.; Abd-Allah, F.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; Aboyans, V.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 328 Diseases and Injuries for 195 Countries, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; Gupta, V.; Abrams, E.M.; Adedoyin, R.A.; Adhikari, T.B.; Advani, S.M.; Agrawal, A.; Ahmadian, E.; et al. Prevalence and Attributable Health Burden of Chronic Respiratory Diseases, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef] [PubMed]
- IBGE Instituto Brasileiro de Geografia e Estatística. Pesquisa Nacional de Saúde: 2019: Percepção do Estado de Saúde, Estilos de Vida, Doenças Crônicas e Saúde Bucal: Brasil e Grandes Regiões. Available online: https://biblioteca.ibge.gov.br/visualizacao/livros/liv101764.pdf (accessed on 7 January 2021).
- GINA. Global Initiative for Asthma Management and Prevention: Pocket Guide For Asthma Management and Prevention (Updated 2020); 2020. Available online: https://ginasthma.org/wp-content/uploads/2020/04/Main-pocket-guide_2020_04_03-final-wms.pdf (accessed on 8 January 2021).
- Sociedade Brasileira de Pneumologia e Tisiologia Diretrizes Da Sociedade Brasileira de Pneumologia e Tisiologia Para o Manejo Da Asma -2012. J. Bras. Pneumol. v. J. Bras. Pneumol. Abril 2012, 38, S155–S165.
- World Health Organization WHO Announces COVID-19 Outbreak a Pandemic. Available online: http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic (accessed on 21 May 2020).
- Kim, J.H.; Ellwood, P.E.; Asher, M.I. Diet and Asthma: Looking Back, Moving Forward. Respir. Res. 2009, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Guilleminault, L.; Williams, E.J.; Scott, H.A.; Berthon, B.S.; Jensen, M.; Wood, L.G. Diet and Asthma: Is It Time to Adapt Our Message? Nutrients 2017, 9, 1227. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Levy, R.B.; Claro, R.M.; de Castro, I.R.R.; Cannon, G. Uma Nova Classifi Cação de Alimentos Baseada Na Extensão e Propósito Do Seu Processamento. Cad. Saude Publica 2010, 26, 2039–2049. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Levy, R.B.; Claro, R.M.; De Castro, I.R.R.; Cannon, G. Increasing Consumption of Ultra-Processed Foods and Likely Impact on Human Health: Evidence from Brazil. Public Health Nutr. 2011, 14, 5–13. [Google Scholar] [CrossRef]
- Martins, A.P.B.; Levy, R.B.; Claro, R.M.; Moubarac, J.C.; Monteiro, C.A. Increased Contribution of Ultra-Processed Food Products in the Brazilian Diet (1987–2009). Rev. Saude Publica 2013, 47, 656–665. [Google Scholar] [CrossRef]
- Hancu, A.; Mihaltan, F.; Radulian, G. Asthma and Ultra-Processed Food. Maedica 2019, 14, 402–407. [Google Scholar] [CrossRef]
- Li, Z.; Rava, M.; Bédard, A.; Dumas, O.; Garcia-Aymerich, J.; Leynaert, B.; Pison, C.; Le Moual, N.; Romieu, I.; Siroux, V.; et al. Cured Meat Intake Is Associated with Worsening Asthma Symptoms. Thorax 2017, 72, 206–212. [Google Scholar] [CrossRef]
- Garcia-Larsen, V.; Del Giacco, S.R.; Moreira, A.; Bonini, M.; Charles, D.; Reeves, T.; Carlsen, K.H.; Haahtela, T.; Bonini, S.; Fonseca, J.; et al. Asthma and Dietary Intake: An Overview of Systematic Reviews. Allergy Eur. J. Allergy Clin. Immunol. 2016, 71, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.; Moreira, A.; Padrão, P.; Teixeira, V.H.; Carvalho, P.; Delgado, L.; Lopes, C.; Severo, M.; Moreira, P. Dietary Patterns and Asthma Prevalence, Incidence and Control. Clin. Exp. Allergy 2015, 45, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Bielemann, R.M.; Santos Motta, J.V.; Minten, G.C.; Horta, B.L.; Gigante, D.P. Consumption of Ultra-Processed Foods and Their Impact on the Diet of Young Adults. Rev. Saude Publica 2015, 49, 28. [Google Scholar] [CrossRef] [PubMed]
- Juul, F.; Parekh, N.; Martinez-Steele, E.; Monteiro, C.A.; Chang, V.W. Ultra-Processed Food Consumption among US Adults from 2001 to 2018. Am. J. Clin. Nutr. 2022, 115, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, C.M.; Cortese, M.; Costa, C.D.S.; Bjornevik, K.; Barros, A.J.D.; Barros, F.C.; Santos, I.S.; Matijasevich, A. Ultra-Processed Food Consumption during Childhood and Asthma in Adolescence: Data from the 2004 Pelotas Birth Cohort Study. Pediatr. Allergy Immunol. 2020, 31, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Melo, B.; Rezende, L.; Machado, P.; Gouveia, N.; Levy, R. Associations of Ultra-Processed Food and Drink Products with Asthma and Wheezing among Brazilian Adolescents. Pediatr. Allergy Immunol. 2018, 29, 504–511. [Google Scholar] [CrossRef]
- Moreno-Galarraga, L.; Martín-Álvarez, I.; Fernández-Montero, A.; Santos Rocha, B.; Ciriza Barea, E.; Martín-Calvo, N. Consumption of Ultra-Processed Products and Wheezing Respiratory Diseases in Children: The SENDO Project. An. Pediatría English Ed. 2021, 95, 18–25. [Google Scholar] [CrossRef]
- Li, H.; Li, S.; Yang, H.; Zhang, Y.; Ma, Y.; Hou, Y.; Zhang, X.; Sun, L.; Borné, Y.; Wang, Y. Association of Ultra-Processed Food Intake with Cardiovascular Disease and Respiratory Disease: A Prospective Cohort Study. SSRN Electron. J. 2022, 67, 2200628. [Google Scholar] [CrossRef]
- Kong, W.; Xie, Y.; Zhong, J.; Cao, C. Ultra-Processed Foods and Allergic Symptoms among Children and Adults in the United States: A Population-Based Analysis of NHANES 2005–2006. Front. Public Health 2022, 10, 1038141. [Google Scholar] [CrossRef]
- Barbieri, M.A.; Ferraro, A.A.; Simões, V.M.F.; Goldani, M.Z.; Cardoso, V.C.; Moura Da Silva, A.A.; Bettiol, H. Cohort Profile: The 1978-79 Ribeirao Preto (Brazil) Birth Cohort Study. Int. J. Epidemiol. 2022, 51, 27G–28G. [Google Scholar] [CrossRef]
- Arruda, S.P.M.; Da Silva, A.A.M.; Kac, G.; Goldani, M.Z.; Bettiol, H.; Barbieri, M.A. Socioeconomic and Demographic Factors Are Associated with Dietary Patterns in a Cohort of Young Brazilian Adults. BMC Public Health 2014, 14, 654. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.A.; Stocco, P.R. Development of a Quantitative Questionnaire of Food Intake in Japanese Immigrants and Their Descendants Residents in Sao Paulo, Brazil. Cad. Saúde Pública 2000, 16, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.B.; Cardoso, M.A. Development of a Food Frequency Questionnaire as a Tool for Programs of Chronic Diseases Prevention. Rev. Nutr. 2002, 15, 239–245. [Google Scholar] [CrossRef]
- Molina, M.C.; Bettiol, H.; Barbieri, M.A.; da Silva, A.A.M.; Conceição, S.I.O.; Dos-Santos, J.E. Food Consumption by Young Adults Living in Ribeirão Preto, SP, 2002/2004. Braz. J. Med. Biol. Res. 2007, 40, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Zabotto, C.B.; de Toledo, V.R.P.; de Fatima, G.M. Registro Fotográfico Para Inqueritos Dietéticos: Utensilios e Porçöes; Universidade Federal de Goiás: Goiânia, Brazil, 1996; 74p. [Google Scholar]
- Machado Arruda, S.P.; da Silva, A.A.M.; Kac, G.; Vilela, A.A.F.; Goldani, M.; Bettiol, H.; Barbieri, M.A. Dietary Patterns Are Associated with Excess Weight and Abdominal Obesity in a Cohort of Young Brazilian Adults. Eur. J. Nutr. 2016, 55, 2081–2091. [Google Scholar] [CrossRef]
- United States Department of Agriculture-USDA Nutrient Database for Standard Reference-SR14. Available online: http://ndb.nal.usda.gov/ndb/foods (accessed on 21 May 2020).
- IBGE Instituto Brasileiro de Geografia e Estatística. Pesquisa de Orçamentos Familiares 2008/2009 Tabelas de Composição Nutricional Dos Alimentos Consumidos No Brasil. Available online: https://biblioteca.ibge.gov.br/visualizacao/livros/liv50002.pdf (accessed on 5 November 2022).
- Núcleo de estudos e pesquisas em alimentação da Universidade Estadual de Campinas, Tabela Brasileira de Composicao de Alimentos—TACO 4 Edicao Ampliada e Revisada. 2011. Available online: https://www.nepa.unicamp.br/taco/contar/taco_4_edicao_ampliada_e_revisada.pdf?arquivo=1 (accessed on 10 March 2021).
- da Costa Louzada, M.L.; Canella, D.S.; Jaime, P.C.; Monteiro, C.A. Alimentação e Saúde: A Fundamentação Científica Do Guia Alimentar Para a População Brasileira; Universidade de São Paulo: São Paulo, Brazil, 2019. [Google Scholar] [CrossRef]
- IBGE Instituto Brasileiro de Geografia e Estatística. Pesquisa de Orçamentos Familiares 2002/2003: Análise Da Disponibilidade Domiciliar de Alimentos e Do Estado Nutricional No Brasil. Available online: https://biblioteca.ibge.gov.br/visualizacao/periodicos/94/cd_2010_religiao_deficiencia.pdf (accessed on 25 October 2022).
- Crapo, R.O.; Casaburi, R.; Coates, A.L.; Enright, P.L.; Hankinson, J.L.; Irvin, C.G.; MacIntyre, N.R.; McKay, R.T.; Wanger, J.S.; Anderson, S.D.; et al. Guidelines for Methacholine and Exercise Challenge Testing—1999. Am. J. Respir. Crit. Care Med. 2000, 161, 309–329. [Google Scholar]
- Pereira, L.P. Valor da Espirometria Para Detecção de Asma em Estudos Epidemiológicos; Biblioteca Digital de Teses e Dissertações da Universidade de São Paulo: Ribeirão Preto, Brazil, 2013. [Google Scholar]
- Cortes, T.R.; Faerstein, E.; Struchiner, C.J. Utilização de Diagramas Causais Em Epidemiologia: Um Exemplo de Aplicação Em Situação de Confusão. Cad. Saude Publica 2016, 32, e00103115. [Google Scholar] [CrossRef]
- Robins, J.M.; Hernán, M.Á.; Brumback, B. Marginal Structural Models and Causal Inference in Epidemiology. Epidemiology 2000, 11, 550–560. [Google Scholar] [CrossRef]
- Matsudo, S.; Araújo, T.; Matsudo, V.; Andrade, D.; Andrade, E.; Oliveira, L.C.; Braggion, G. Questionário Internacional De Atividade Física (Ipaq): Estupo De Validade E Reprodutibilidade No Brasil. Rev. Bras. Atividade Física Saúde 2012, 6, 5–18. [Google Scholar] [CrossRef]
- Coutinho, L.M.S.; Scazufca, M.; Menezes, P.R. Methods for Estimating Prevalence Ratios in Cross-Sectional Studies. Rev. Saude Publica 2008, 42, 992–998. [Google Scholar] [CrossRef]
- Dos Santos Costa, C.; Assunção, M.C.F.; Dos Santos Vaz, J.; Rauber, F.; Oliveira Bierhals, I.; Matijasevich, A.; Horta, B.L.; Gonçalves, H.; Wehrmeister, F.C.; Santos, I.S. Consumption of Ultra-Processed Foods at 11, 22 and 30 Years at the 2004, 1993 and 1982 Pelotas Birth Cohorts. Public Health Nutr. 2020, 24, 299–308. [Google Scholar] [CrossRef]
- da Silva, C.L.; Sousa, A.G.; Borges, L.P.S.L.; da Costa, T.H.M. Usual Consumption of Ultra-Processed Foods and Its Association with Sex, Age, Physical Activity, and Body Mass Index in Adults Living in Brasília City, Brazil. Rev. Bras. Epidemiol. 2021, 24, e210033. [Google Scholar] [CrossRef]
- Menezes, A.M.B.; Schneider, B.C.; Oliveira, V.P.; Prieto, F.B.; Silva, D.L.R.; Lerm, B.R.; da Costa, T.B.; Bouilly, R.; Wehrmeister, F.C.; Gonçalves, H.; et al. Longitudinal Association between Diet Quality and Asthma Symptoms in Early Adult Life in a Brazilian Birth Cohort. J. Asthma Allergy 2020, 13, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Maranhão, A.L.A.; Da Silva Carvalho, S.R. Aplicação Dos Testes de Função Pulmonar No Diagnóstico e Manejo Da Asma. Pulmão 2018, 27, 89–92. [Google Scholar]
- Zein, J.G.; Erzurum, S.C. Asthma Is Different in Women. Curr. Allergy Asthma Rep. 2015, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- da Costa Louzada, M.L.; Martins, A.P.B.; Canella, D.S.; Baraldi, L.G.; Levy, R.B.; Claro, R.M.; Moubarac, J.C.; Cannon, G.; Monteiro, C.A. Ultra-Processed Foods and the Nutritional Dietary Profile in Brazil. Rev. Saude Publica 2015, 49, 38. [Google Scholar] [CrossRef]
- Julia, V.; Macia, L.; Dombrowicz, D. The Impact of Diet on Asthma and Allergic Diseases. Nat. Rev. Immunol. 2015, 15, 308–322. [Google Scholar] [CrossRef]
- Freedman, B.J. Sulphur Dioxide in Foods and Beverages: Its Use as a Preservative and Its Effect on Asthma. Br. J. Dis. Chest 1980, 74, 128–134. [Google Scholar] [CrossRef]
- Steinman, H.A.; Weinberg, E.G. The Effects of Soft-Drink Preservatives on Asthmatic Children. S. Afr. Med. J. 1986, 70, 404–406. [Google Scholar]
- Sugiura, H.; Ichinose, M. Nitrative Stress in Inflammatory Lung Diseases. Nitric Oxide—Biol. Chem. 2011, 25, 138–144. [Google Scholar] [CrossRef]
- Cade, J.; Thompson, R.; Burley, V.; Warm, D. Development, Validation and Utilisation of Food-Frequency Questionnaires—A Review. Public Health Nutr. 2002, 5, 567–587. [Google Scholar] [CrossRef] [PubMed]

| Variables | Total | Asthma * | ||
|---|---|---|---|---|
| n (%) | No (n, %) | Yes (n, %) | p-Value # | |
| Sex | <0.001 | |||
| Male | 895 (48.2) | 814 (90.9) | 81 (9.1) | |
| Female | 962 (51.8) | 798 (82.9) | 164 (17.1) | |
| Age | 0.008 | |||
| 23 years | 521 (28.0) | 432 (82.9) | 89 (17.1) | |
| 24 years | 930 (50.1) | 821 (88.3) | 109 (11.7) | |
| 25 years | 406 (21.9) | 359 (88.4) | 47 (11.6) | |
| Household income ** | 0.002 | |||
| <5 MW | 605 (32.6) | 504(83.3) | 101 (16.7) | |
| 5–9.9 MW | 568 (30.6) | 499 (87.8) | 69 (12.2) | |
| >9.9 MW | 553 (29.8) | 500 (90.4) | 53 (9.6) | |
| No information | 131 (7.0) | 109 (83.2) | 22 (16.8) | |
| Physical activity level | 0.094 | |||
| High | 873 (47.0) | 767 (87.9) | 106 (12.1) | |
| Moderate | 590 (31.7) | 518 (87.8) | 72 (12.2) | |
| Low | 389 (21.0) | 323 (83.0) | 66 (17.0) | |
| No information | 5 (0.3) | 4 (80.0) | 1 (20.0) | |
| Smoking | 0.001 | |||
| No | 1548 (83.4) | 1360 (87.9) | 188 (12.1) | |
| Yes | 309 (16.6) | 252 (81.6) | 57 (18.4) | |
| Total | 1857 (100.0) | 1612 (86.8) | 245 (13.2) | |
| Food Groups | Total (n = 1857) | Asthma | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| No (n = 1612) | Yes (n = 245) | |||||||
| Mean | SD | Mean | SD | Mean | SD | |||
| Total energy kcal/day | 2237.3 | 705.8 | 2232.3 | 701.8 | 2270.1 | 732.0 | 0.435 | |
| Total intake | 2135.4 | 751.8 | 2132.9 | 761.0 | 2152.0 | 695.3 | 0.712 | |
| Unprocessed/minimally processed | kcal of diet | 1120.0 | 380.2 | 1120.0 | 379.0 | 1118.5 | 388.2 | 0.961 |
| %TCV | 50.9 | 11.2 | 51.1 | 11.3 | 50.1 | 11.0 | 0.196 | |
| g/day | 1191.5 | 444.5 | 1190.5 | 446.1 | 1198.2 | 435.0 | 0.802 | |
| %g/day | 57.6 | 15.2 | 57.6 | 15.3 | 56.9 | 14.4 | 0.502 | |
| Processed | kcal of diet | 246.0 | 142.4 | 244.0 | 139.0 | 257.3 | 163.0 | 0.167 |
| %TCV | 11.0 | 5.4 | 11.0 | 5.2 | 11.2 | 6.1 | 0.455 | |
| g/day | 155.6 | 136.3 | 153.9 | 133.9 | 166.6 | 151.0 | 0.177 | |
| %g/day | 7.3 | 5.6 | 7.3 | 5.5 | 7.6 | 6.3 | 0.372 | |
| Ultraprocessed | kcal of diet | 869.3 | 434.1 | 866.0 | 433.3 | 892.0 | 439.3 | 0.386 |
| %TCV | 37.9 | 11.2 | 37.8 | 11.3 | 38.6 | 10.8 | 0.333 | |
| g/day | 788.3 | 580.0 | 788.5 | 593.0 | 787.2 | 487.2 | 0.975 | |
| %g/day | 35.1 | 15.1 | 35.0 | 15.2 | 35.4 | 15.2 | 0.729 | |
| Macronutrients and alcohol | ||||||||
| Carbohydrates | %TCV | 55.7 | 6.7 | 55.8 | 6.6 | 55.5 | 6.8 | 0.544 |
| g/1000 kcal | 139.4 | 16.6 | 139.5 | 16.6 | 138.8 | 17.0 | ||
| Proteins | %TCV | 16.8 | 3.2 | 16.9 | 3.2 | 16.3 | 3.2 | 0.008 |
| g/1000 kcal | 42.1 | 7.9 | 42.3 | 7.9 | 40.8 | 7.9 | ||
| Lipids | %TCV | 26.1 | 4.9 | 26.0 | 4.8 | 26.7 | 5.1 | 0.037 |
| g/1000 kcal | 29.0 | 5.4 | 28.9 | 5.4 | 29.7 | 5.7 | ||
| Fibers | g/1000 kcal | 11.1 | 3.0 | 11.1 | 3.0 | 11.0 | 2.8 | 0.704 |
| Alcohol | %TCV | 1.3 | 1.8 | 1.3 | 1.7 | 1.4 | 2.3 | 0.215 |
| g/1000 kcal | 1.8 | 2.6 | 1.8 | 2.5 | 2.0 | 3.2 | ||
| Ultraprocessed Foods | Total | Asthma | p-Value * | ||||
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| Mean | SD | Mean | SD | Mean | SD | ||
| %TCV | |||||||
| Sugar-sweetened beverages | 9.9 | 7.8 | 9.9 | 8.0 | 9.8 | 7.0 | 0.900 |
| Savory snacks | 8.2 | 7.0 | 8.2 | 7.0 | 7.5 | 6.3 | 0.124 |
| Sweets/desserts | 4.4 | 4.0 | 4.4 | 4.0 | 4.4 | 4.0 | 0.803 |
| Cookies | 3.0 | 3.2 | 2.9 | 3.1 | 3.4 | 3.9 | 0.012 |
| Breads | 2.1 | 2.0 | 2.1 | 2.0 | 2.0 | 2.0 | 0.380 |
| Sausages | 1.9 | 1.8 | 1.9 | 1.7 | 2.0 | 1.9 | 0.213 |
| Cakes | 1.9 | 2.6 | 1.9 | 2.4 | 2.3 | 3.6 | 0.013 |
| Chips | 1.7 | 2.6 | 1.7 | 2.6 | 2.2 | 2.8 | 0.005 |
| Dairy products | 1.6 | 2.1 | 1.6 | 2.1 | 1.8 | 2.2 | 0.223 |
| Ramen noodles | 1.0 | 0.8 | 1.0 | 0.8 | 1.0 | 0.7 | 0.679 |
| Margarine | 0.8 | 1.1 | 0.8 | 1.1 | 0.9 | 1.0 | 0.279 |
| Granola | 0.6 | 1.6 | 0.6 | 1.6 | 0.3 | 1.0 | 0.008 |
| Mayonnaise | 0.5 | 0.8 | 0.5 | 0.8 | 0.6 | 1.1 | 0.597 |
| Hard liquors | 0.1 | 0.6 | 0.2 | 0.6 | 0.1 | 0.5 | 0.442 |
| %grams | |||||||
| Sugar-sweetened beverages | 23.8 | 15.0 | 23.7 | 15.1 | 24.0 | 14.1 | 0.918 |
| Savory snacks | 3.5 | 3.4 | 3.6 | 3.4 | 3.3 | 3.0 | 0.164 |
| Sweets/desserts | 1.4 | 1.3 | 1.4 | 1.3 | 1.4 | 1.3 | 0.621 |
| Dairy products | 1.4 | 1.9 | 1.4 | 1.9 | 1.5 | 1.9 | 0.494 |
| Sausages | 1.1 | 1.0 | 1.1 | 0.9 | 1.2 | 1.1 | 0.199 |
| Cakes | 0.9 | 1.2 | 0.8 | 1.1 | 1.0 | 1.7 | 0.013 |
| Breads | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.591 |
| Cookies | 0.7 | 0.8 | 0.7 | 0.8 | 0.8 | 1.0 | 0.026 |
| Chips | 0.4 | 0.7 | 0.4 | 0.7 | 0.5 | 0.7 | 0.030 |
| Ramen noodles | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.3 | 0.705 |
| Granola | 0.2 | 0.4 | 0.2 | 0.5 | 0.1 | 0.3 | 0.015 |
| Mayonnaise | 0.2 | 0.3 | 0.2 | 0.3 | 0.2 | 0.5 | 0.296 |
| Margarine | 0.1 | 0.2 | 0.1 | 0.2 | 0.2 | 0.2 | 0.310 |
| Hard liquors | 0.1 | 0.2 | 0.1 | 0.2 | 0.1 | 0.2 | 0.496 |
| Unadjusted Analysis | Adjusted Analysis a,b | ||||
|---|---|---|---|---|---|
| Ultraprocessed Food Consumption | n | PR (95% CI) | p-Value | PR (95% CI) | p-Value |
| Tertile (% kcal) | 0.932 c | 0.928 c | |||
| 1st tertile | 619 | Reference | - | Reference | - |
| 2nd tertile | 619 | 1.17 (0.88–1.55) | 0.280 | 1.19 (0.90–1.58) | 0.212 |
| 3rd tertile | 619 | 1.01 (0.75–1.36) | 0.932 | 0.99 (0.74–1.32) | 0.935 |
| % kcal | 1857 | 1.00 (0.99–1.01) | 0.315 | 1.00 (0.99–1.01) | 0.466 |
| Tertile (% g) | 0.934 c | 0.942 c | |||
| 1st tertile | 619 | Reference | - | Reference | - |
| 2nd tertile | 619 | 0.94 (0.70–1.25) | 0.673 | 0.98 (0.74–1.32) | 0.925 |
| 3rd tertile | 619 | 1.01 (0.76–1.34) | 0.934 | 0.99 (0.73–1.32) | 0.942 |
| % g | 1857 | 1.00 (0.99–1.00) | 0.714 | 1.00 (0.99–1.00) | 0.937 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, H.C.O.A.; Rudakoff, L.C.S.; Muniz, A.K.O.A.; Magalhães, E.I.d.S.; Bragança, M.L.B.M.; Silva, A.A.M.d.; Vianna, E.d.S.O.; Bettiol, H.; Barbieri, M.A. Association between the Consumption of Ultra-Processed Foods and Asthma in Adults from Ribeirão Preto, São Paulo, Brazil. Nutrients 2023, 15, 3165. https://doi.org/10.3390/nu15143165
Serra HCOA, Rudakoff LCS, Muniz AKOA, Magalhães EIdS, Bragança MLBM, Silva AAMd, Vianna EdSO, Bettiol H, Barbieri MA. Association between the Consumption of Ultra-Processed Foods and Asthma in Adults from Ribeirão Preto, São Paulo, Brazil. Nutrients. 2023; 15(14):3165. https://doi.org/10.3390/nu15143165
Chicago/Turabian StyleSerra, Hellen Cristina Oliveira Amorim, Lívia Carolina Sobrinho Rudakoff, Alessandra Karla Oliveira Amorim Muniz, Elma Izze da Silva Magalhães, Maylla Luanna Barbosa Martins Bragança, Antônio Augusto Moura da Silva, Elcio dos Santos Oliveira Vianna, Heloisa Bettiol, and Marco Antonio Barbieri. 2023. "Association between the Consumption of Ultra-Processed Foods and Asthma in Adults from Ribeirão Preto, São Paulo, Brazil" Nutrients 15, no. 14: 3165. https://doi.org/10.3390/nu15143165
APA StyleSerra, H. C. O. A., Rudakoff, L. C. S., Muniz, A. K. O. A., Magalhães, E. I. d. S., Bragança, M. L. B. M., Silva, A. A. M. d., Vianna, E. d. S. O., Bettiol, H., & Barbieri, M. A. (2023). Association between the Consumption of Ultra-Processed Foods and Asthma in Adults from Ribeirão Preto, São Paulo, Brazil. Nutrients, 15(14), 3165. https://doi.org/10.3390/nu15143165






