Evaluation of Selected Antioxidant Parameters in Ready-to-Eat Food for Infants and Young Children
Abstract
1. Introduction
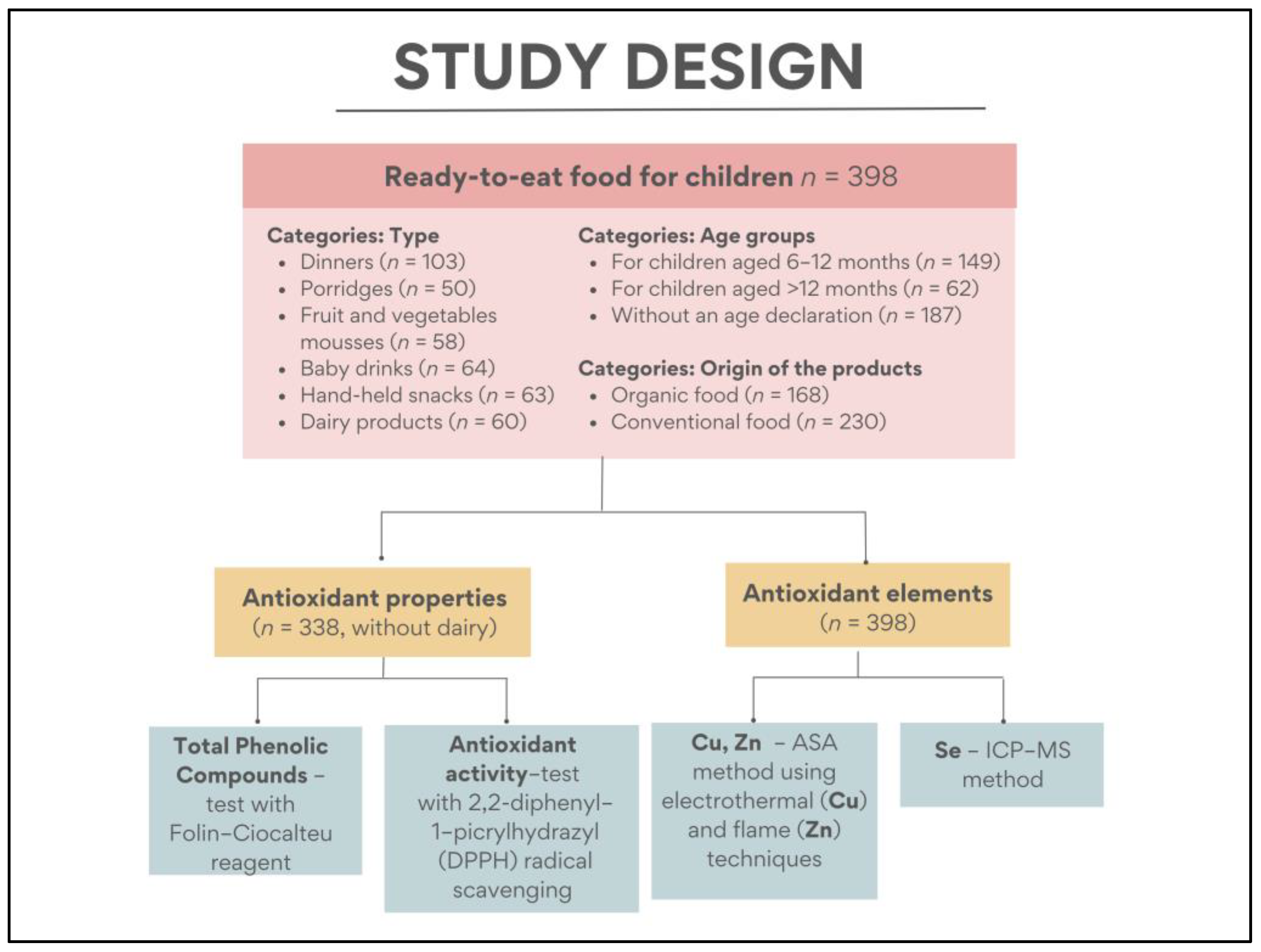
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Determination of Mineral Components
2.3. Determination of Antioxidant Properties
2.4. Statistical Analysis
3. Results
3.1. Evaluation of Antioxidant Properties: DPPH, TPC
3.2. Evaluation of Mineral Components: Cu, Se, Zn
3.3. EAR of Cu, Se, and Zn
3.4. Evaluation of the Content of Cu, Se, Zn, and DPPH, TPC in Baby Products Taking into Account the Intended Use of Products for the Age Groups
3.5. Evaluation of the Content of Cu, Se, Zn, and DPPH, TPC in Baby Products Taking into Account Food Origin
3.6. Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Strategy for Infant and Young Child Feeding. World Health Organization & United Nations Children’s Fund (UNICEF). Available online: https://www.who.int/publications/i/item/9241562218 (accessed on 6 June 2023).
- Ceballos-Rasgado, M.; Lowe, N.M.; Moran, V.H.; Clegg, A.; Mallard, S.; Harris, C.; Montez, J.; Xipsiti, M. Toward revising dietary zinc recommendations for children aged 0 to 3 years: A systematic review and meta-analysis of zinc absorption, excretion, and requirements for growth. Nutr. Rev. 2022, 81, 967–987. [Google Scholar] [CrossRef]
- Ekweagwu, E.; Agwu, A.E.; Madukwe, E. The role of micronutrients in child health: A review of the literature. Afr. J. Biotechnol. 2008, 7, 3804–3810. [Google Scholar]
- Josko Osredkar, N.S. Copper and Zinc, Biological Role and Significance of Copper/Zinc Imbalance. J. Clinic Toxicol. 2011, 3, 495. [Google Scholar] [CrossRef]
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Normy Żywienia dla Populacji Polski i ich Zastosowanie; Narodowy Instytut Zdrowia Publicznego-Państwowy Zakład Higieny Warsaw: Warsaw, Poland, 2020.
- Vetlényi, E.; Rácz, G. The physiological function of copper, the etiological role of copper excess and deficiency. Orv. Hetil. 2020, 161, 1488–1496. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Araya, M.; Koletzko, B.; Uauy, R. Copper deficiency and excess in infancy: Developing a research agenda. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Marzec, A.; Zareba, S. Copper and zinc in food products for infants and children. Rocz. Panstw. Zakl. Hig. 2005, 56, 355–359. [Google Scholar]
- Minich, W.B. Selenium Metabolism and Biosynthesis of Selenoproteins in the Human Body. Biochemistry 2022, 87, S102–S168. [Google Scholar] [CrossRef]
- Mehdi, Y.; Hornick, J.L.; Istasse, L.; Dufrasne, I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef]
- Alamed, J.; Chaiyasit, W.; McClements, D.J.; Decker, E.A. Relationships between free radical scavenging and antioxidant activity in foods. J. Agric. Food Chem. 2009, 57, 2969–2976. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Commission Directive 2006/125/EC of 5 December 2006 on processed cereal-based foods and baby foods for infants and young children. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32006L0125 (accessed on 13 July 2023).
- Foterek, K.; Hilbig, A.; Alexy, U. Breast-feeding and weaning practices in the DONALD study: Age and time trends. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 361–367. [Google Scholar] [CrossRef]
- Theurich, M.A.; Zaragoza-Jordana, M.; Luque, V.; Gruszfeld, D.; Gradowska, K.; Xhonneux, A.; Riva, E.; Verduci, E.; Poncelet, P.; Damianidi, L.; et al. Commercial complementary food use amongst European infants and children: Results from the EU Childhood Obesity Project. Eur. J. Nutr. 2020, 59, 1679–1692. [Google Scholar] [CrossRef]
- Usal, M.; Sahan, Y. In vitro evaluation of the bioaccessibility of antioxidative properties in commercially baby foods. J. Food Sci. Technol. 2020, 57, 3493–3501. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Quality parameters, bioactive compounds and their correlation with antioxidant capacity of commercial fruit-based baby foods. Food Sci. Technol. Int. 2014, 20, 479–487. [Google Scholar] [CrossRef]
- Szajdek, A.; Borowska, E.J.; Borowski, J.; Saczuk, B. Musy owocowe jako zrodlo naturalnych przeciwutleniaczy. Żywność Nauka Technol. Jakość 2007, 14, 100–108. [Google Scholar]
- Mir-Marqués, A.; González-Masó, A.; Cervera, M.L.; de la Guardia, M. Mineral profile of Spanish commercial baby food. Food Chem. 2015, 172, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Zand, N.; Chowdhry, B.Z.; Wray, D.S.; Pullen, F.S.; Snowden, M.J. Elemental content of commercial ‘ready to-feed’ poultry and fish based infant foods in the UK. Food Chem. 2012, 135, 2796–2801. [Google Scholar] [CrossRef]
- Škrbić, B.; Živančev, J.; Jovanović, G.; Farre, M. Essential and toxic elements in commercial baby food on the Spanish and Serbian market. Food Addit. Contam. Part B Surveill. 2017, 10, 27–38. [Google Scholar] [CrossRef]
- Khamoni, J.A.; Hamshaw, T.; Gardiner, P.H.E. Impact of ingredients on the elemental content of baby foods. Food Chem. 2017, 231, 309–315. [Google Scholar] [CrossRef]
- Ruiz-de-Cenzano, M.; Rochina-Marco, A.; Cervera, M.L.; de la Guardia, M. Evaluation of the Content of Antimony, Arsenic, Bismuth, Selenium, Tellurium and Their Inorganic Forms in Commercially Baby Foods. Biol. Trace Elem. Res. 2017, 180, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A.; Arabameri, M.; Moazzen, M.; Shariatifar, N.; Aeenehvand, S.; Khaniki, G.J.; Abdel-Wahhab, M.; Shahsavari, S. Probabilistic Health Risk Assessment of Trace Elements in Baby Food and Milk Powder Using ICP-OES Method. Biol. Trace Elem. Res. 2022, 200, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Vallinoto, P.; Moreira, E.G.; Maihara, V.A. Estimation of daily dietary intake of essential minerals and trace elements in commercial complementary foods marketed in Brazil. Food Chem. Adv. 2022, 1, 100039. [Google Scholar] [CrossRef]
- Weker, H.; Barańska, M.; Riahi, A.; Strucińska, M.; Więch, M.; Rowicka, G.; Dyląg, H.; Klemarczyk, W.; Bzikowska, A.; Socha, P. Nutrition of infants and young children in Poland—Pitnuts 2016. Dev. Period Med. 2017, 21, 13–28. [Google Scholar] [PubMed]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Hangun-Balkir, Y.; McKenney, M. Determination of antioxidant activities of berries and resveratrol. Green Chem. Lett. Rev. 2012, 5, 147–153. [Google Scholar] [CrossRef]
- Pérez-Lamela, C.; Franco, I.; Falqué, E. Impact of High-Pressure Processing on Antioxidant Activity during Storage of Fruits and Fruit Products: A Review. Molecules 2021, 26, 5265. [Google Scholar] [CrossRef]
- Deng, H.; Lei, J.; Yang, T.; Liu, M.; Meng, Y.; Guo, Y.; Xue, J. Effect of ultra-high pressure and high temperature short-time sterilization on the quality of NFC apple juice during storage. Sci. Agric. Sin. 2019, 52, 3903–3923. [Google Scholar]
- Szczepańska, J.; Pinto, C.A.; Skąpska, S.; Saraiva, J.A.; Marszałek, K. Effect of static and multi-pulsed high pressure processing on the rheological properties, microbial and physicochemical quality, and antioxidant potential of apple juice during refrigerated storage. LWT 2021, 150, 112038. [Google Scholar] [CrossRef]
- Zand, N.; Chowdhry, B.Z.; Zotor, F.B.; Wray, D.S.; Amuna, P.; Pullen, F.S. Essential and trace elements content of commercial infant foods in the UK. Food Chem. 2011, 128, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.F.; Fox, M.K.; Briefel, R.R.; Siega-Riz, A.M.; Dwyer, J.T.; Deming, D.M.; Reidy, K.C. Nutrient intakes of US infants, toddlers, and preschoolers meet or exceed dietary reference intakes. J. Am. Diet Assoc. 2010, 110, S27–S37. [Google Scholar] [CrossRef] [PubMed]
- Grimes, C.A.; Szymlek-Gay, E.A.; Campbell, K.J.; Nicklas, T.A. Food Sources of Total Energy and Nutrients among U.S. Infants and Toddlers: National Health and Nutrition Examination Survey 2005–2012. Nutrients 2015, 7, 6797–6836. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-Monge, M.F.; Barrado, E.; Parodi-Román, J.; Escobedo-Monge, M.A.; Torres-Hinojal, M.C.; Marugán-Miguelsanz, J.M. Copper and Copper/Zn Ratio in a Series of Children with Chronic Diseases: A Cross-Sectional Study. Nutrients 2021, 13, 3578. [Google Scholar] [CrossRef]
- Haftenberger, M.; Lehmann, F.; Lage Barbosa, C.; Brettschneider, A.K.; Mensink, G.B.M. Consumption of organic food by children in Germany—Results of EsKiMo II. J. Health Monit. 2020, 5, 19–26. [Google Scholar] [CrossRef]
- Smith-Spangler, C.; Brandeau, M.L.; Hunter, G.E.; Bavinger, J.C.; Pearson, M.; Eschbach, P.J.; Sundaram, V.; Liu, H.; Schirmer, P.; Stave, C.; et al. Are organic foods safer or healthier than conventional alternatives? A systematic review. Ann. Intern. Med. 2012, 157, 348–366. [Google Scholar] [CrossRef]
- Batra, P.; Sharma, N.; Gupta, P. Organic foods for children: Health or hype. Indian Pediatr. 2014, 51, 349–353. [Google Scholar] [CrossRef]
- Forman, J.; Silverstein, J.; Committee on Nutrition; Council on Environmental Health; Bhatia, J.J.S.; Abrams, S.A.; Corkins, M.R.; de Ferranti, S.D.; Golden, N.H.; Paulson, J.A.; et al. Organic Foods: Health and Environmental Advantages and Disadvantages. Pediatrics 2012, 130, e1406–e1415. [Google Scholar] [CrossRef]
| Type of Product (Sign) | TPC (mg GAE/100 g of Fresh Weight) | DPPH (% Free Radical Scavenging) | Cu (mg/kg of Fresh Weight) | Se (µg/kg of Fresh Weight) | Zn (mg/kg of Fresh Weight) | Cu:Zn Molar Ratio | |
|---|---|---|---|---|---|---|---|
| Dinners (D) n = 103 | M ± SD | 29.1 ± 23.3 | 59.3 ± 29.5 | 7.3 ± 2.5 | 26.7 ± 14.3 | 5.3 ± 5.1 | 2.0 ± 1.2 |
| Min-Max | (0–181.6) | (0–96.9) | (1.9–15.6) | (5.3–76.4) | (0.8–46.3) | (0.1–6.4) | |
| Me | 25.8 (14.2–37.0) | 69 (50.8–79.2) | 7 (5.7–8.5) | 24.5 (16.4–32.2) | 4.0 (2.8–6.6) | 1.7 (1.2–2.4) | |
| Q1–Q3 | *** FV, BD | *** FV, BD | *** P, FV, BD, S, DP | *** FV, BD, S, DP | *** P, FV, BD, S, D | ** S, *** P, FV | |
| Porridges (P) n = 50 | M ± SD | 44.9 ± 45.9 | 68.5 ± 27.0 | 3.8 ± 2.2 | 55.6 ± 95.5 | 30.4 ± 16.5 | 0.2 ± 0.1 |
| Min–Max | (3.6–206.0) | (0–97.0) | (0.5–9.2) | (5.2–686.6) | (3.7–93.3) | (0–0.4) | |
| Me | 33.5 (12.5–69.2) | 77.9 (59.1–86.8) | 3.4 (1.8–5.4) | 33.4 (22.3–65.1) | 27.4 (18.8–37.1) | 0.1 (0–0.2) | |
| Q1–Q3 | *** FV | *** FV, BD | *** D, FV, S, DP | *** FV, BD, S, DP | *** D, FV, BD | *** D, FV, BD, S | |
| Fruit and Vegetable–mousses (FV) n = 58 | M ± SD | 112.0 ± 65.4 | 100.0 ± 17.6 | 11.0 ± 6.7 | 80.4 ± 37.0 | 2.2 ± 1 | 6.5 ± 5.4 |
| Min–Max | (6.5–250.8) | (0–99.6) | (2.2–39.5) | (<LOQ-175.7) | (0.6–4.8) | (0.8–30.9) | |
| Me | 111.8 (55.9–162.4) | 95.3 (91.0–99.6) | 9.3 (7.3–14.2) | 79.3 (53.3–102.5) | 2.2 (1.3–2.9) | 4.8 (3.1–7.8) | |
| Q1–Q3 | *** D, P, S | *** D, P, BD, S | * D, DP, *** P, BD | *** D, P | *** D, P, S, DP | ** DP, *** D, P, BD, S | |
| Baby drinks (BD) n = 64 | M ± SD | 75.9 ± 55.8 | 22.8 ± 46.0 | 3.4 ± 2.1 | 116.7 ± 110.0 | 4.2 ± 7.8 | 3.9 ± 8.5 |
| Min–Max | (0–242.0) | (0–87.4) | (1.3–8.7) | (<LOQ-567.2) | (0.1–39.0) | (0.2–62.5) | |
| Me | 74.7 (33.0–105.4) | 34.8 (2.8–52.5) | 2.1 (1.8–4.6) | 87.7 (56.7–111) | 1.3 (0.7–2.6) | 1.9 (0.9–3.4) | |
| Q1–Q3 | *** D | *** D, P, FV, S | *** D, FV, S, DP | *** D, P | *** D, P, S | *** P, FV, S | |
| Hand-held snacks (S) n = 63 | M ± SD | 68.1 ± 103.1 | 66.6 ± 85.6 | 14.3 ± 14.7 | 76.6 ± 21.6 | 16.7 ± 10.7 | 1.1 ± 0.9 |
| Min–Max | (0–525.4) | (0–91.7) | (2.9–90.7) | (24.1–122.2) | (3.9–69.4) | (0.2–6.2) | |
| Me | 29.3 (12.4–82.0) | 77.0 (56.1–85.6) | 11.5 (8.7–13.9) | 78.9 (65.4–91.2) | 14.4 (10.4–20) | 0.8 (0.5–1.3) | |
| Q1–Q3 | *** FV | *** FV, BD | * FV *** D, P, BD | *** D, P | *** D, FV, BD | ** D *** P, FV, BD, DP | |
| Dairy products (DP) n = 60 | M ± SD | - | - | 19.4 ± 7.5 | 163.2 ± 84.8 | 16.3 ± 18.2 | 3.5 ± 2.8 |
| Min–Max | (9.1–51.7) | (47.5–454.7) | (2–63.7) | (0.3–11.2) | |||
| Me | 18.2 (14–22.9) | 134.9 (113.9–195.5) | 5.8 (4.3–38.7) | 3.4 (0.6–5.2) | |||
| Q1–Q3 | * FV *** D, P, BD | *** D, P | *** D, FV, BD | ** FV *** P, S | |||
| TOTAL n = 398 | M ± SD | 61.8 ± 67.7 | 60.5 ± 38.0 | 9.7 ± 9.1 | 81.1 ± 80.7 | 11.3 ± 15.0 | 2.8 ± 4.6 |
| Min–Max | (0–525.4) | (0–99.6) | (0.5–90.7) | (<LOQ-686.6) | (0.1–93.3) | (0–62.5) | |
| Me Q1–Q3 | 37.8 (16.6–86.7) | 71.4 (48–86.8) | 7.8 (4.4–12.1) | 65.0 (30.1–100.2) | 7.8 (4.4–12.1) | (0.6–3.3) |
| Type of Product | Elements | Dinners | Porridge | Fruit and Vegetables Mousses | Baby Drinks | Hand-Held Snacks | Dairy Products | Total |
|---|---|---|---|---|---|---|---|---|
| % EAR | Cu | 167.1 ± 89.1 | 35.1 ± 20.4 | 492.9 ± 300.0 | 58 ± 35.4 | 465.2 ± 477.3 | 196.4 ± 184.1 | 232.0 ± 291.9 |
| Zn | 12.9 ± 14.6 | 28.2 ± 15.3 | 9.7 ± 4.6 | 7.3 ± 13.4 | 54.5 ± 35 | 7.1 ± 6.3 | 19.2 ± 24.2 | |
| Se | 9.3 ± 6.6 | 7.6 ± 13 | 52.8 ± 24.4 | 29.5 ± 47.8 | 36.7 ± 10.4 | 17.6 ± 12.2 | 24.3 ± 22.9 |
| Type of Product (Sign) | Element | n | M ± SD | Min–Max | Me | Q1–Q3 |
|---|---|---|---|---|---|---|
| For infant Aged 6–12 months (A) | TPC (mg GAE/100 g of fresh weight) | 149 | 64.9 ± 59.7 | 0–234.6 | 42.6 *** B | 21.1–98.7 |
| DPPH (% Free Radical Scavenging) | 68.6 ± 31.9 | 0–99.6 | 79.9 *** C | 56.8–93.3 | ||
| Cu (mg/kg of fresh weight) | 7.5 ± 5.3 | 0.5–39.5 | 6.9 *** C | 4.1–9.2 | ||
| Se (µg/kg of fresh weight) | 54.8 ± 63.7 | 0–686.6 | 39.8 ** B, *** C | 22.5–71.6 | ||
| Zn (mg/kg of fresh weight) | 10.0 ± 14.6 | 0.6–93.3 | 3.7 | 2.2–12.0 | ||
| For children between 1 and 3 years (B) | TPC (mg GAE/100 g of fresh weight) | 62 | 33.4 ± 39.4 | 3.5–250.8 | 24.0 *** A | 12.2–38.2 |
| DPPH (% Free Radical Scavenging) | 73.9 ± 21.4 | 0–97.9 | 77.8 *** C | 67.8–88.5 | ||
| Cu (mg/kg of fresh weight) | 7.4 ± 3.2 | 2.8–20.4 | 6.9 *** C | 5.3–8.6 | ||
| Se (µg/kg of fresh weight) | 32.8 ± 23.9 | 5.2–136.9 | 26.1 ** B,*** C | 17–41.7 | ||
| Zn (mg/kg of fresh weight) | 11.1 ± 13.5 | 0.7–52.9 | 5.8 | 2.8–9.6 | ||
| Without an age declaration (C) | TPC (mg GAE/100 g of fresh weight) | 187 | 72.0 ± 82.5 | 0–525.4 | 50.8 *** B | 15.9–99.1 |
| DPPH (% Free Radical Scavenging) | 44.5 ± 44.9 | 0–91.7 | 53.4 *** A,B | 30.1–76.9 | ||
| Cu (mg/kg of fresh weight) | 12.2 ± 11.6 | 1.3–90.7 | 10.7 *** A,B | 2.2–17.6 | ||
| Se (µg/kg of fresh weight) | 118.1 ± 88.2 | 0–567.2 | 93.0 *** A,B | 68.0–127.7 | ||
| Zn (mg/kg of fresh weight) | 12.3 ± 14.1 | 0.1–69.4 | 5.5 | 2.2–17.6 |
| Type of Product (Sign) | Elements | n | M ± SD | Min–Max | Me | Q1–Q3 |
|---|---|---|---|---|---|---|
| Organic food (O) | TPC (mg GAE/100 g) | 168 | 56.5 ± 63.5 | 0–507.2 | 34.3 | 16.8–78.2 |
| DPPH (% Free Radical Scavenging) | 63.3 ± 31.7 | 0–98.3 | 72.7 | 48.6–85.5 | ||
| Cu (mg/kg) | 9.3 ± 10.5 | 1.1–90.7 | 7.2 | 5.2–10.9 | ||
| Se (µg/kg) | 84.2 ± 78.8 | 0–567.2 | 65.5 | 28.8–106 | ||
| Zn (mg/kg) | 12.2 ± 13.9 | 0.5–93.3 | 6.8 ** C | 2.8–16.3 | ||
| Conventional food (C) | TPC | 230 | 67 ± 71.3 | 0–525.4 | 44.6 | 15.8–96.8 |
| DPPH (% Free Radical Scavenging) | 57.7 ± 43.2 | 0–99.6 | 69.5 | 43.8–89.5 | ||
| Cu (mg/kg) | 10 ± 7.7 | 0.5–51.7 | 51.7 | 4.2–13.2 | ||
| Se (µg/kg) | 76.9 ± 83.3 | 2.9–686.6 | 64.4 | 30.5–91.8 | ||
| Zn (mg/kg) | 10.7 ± 14.4 | 0–75.7 | 4.3 ** C | 2.0–12.4 |
| Type of Products | Parameter 1 | Parameter 2 | r | p |
|---|---|---|---|---|
| Dinners | TPC | Cu | −0.21 | <0.05 |
| Zn | Cu | 0.35 | <0.001 | |
| Zn | DPPH | 0.23 | <0.05 | |
| DPPH | Cu | 0.27 | <0.005 | |
| Porridges | Se | Cu | 0.29 | <0.001 |
| Zn | TPC | −0.28 | <0.001 | |
| Cu | Zn | 0.21 | <0.001 | |
| Cu | DPPH | 0.38 | <0.001 | |
| DPPH | TPC | 0.17 | <0.005 | |
| Fruit and vegetable mousses | TPC | Zn | −0.32 | <0.05 |
| Baby drinks | TPC | Se | −0.28 | <0.02 |
| Se | Zn | 0.56 | <0.001 | |
| Se | Cu | 0.38 | <0.005 | |
| Zn | Cu | 0.27 | <0.05 | |
| Cu | DPPH | −0.32 | <0.05 | |
| Dairy products | Se | Zn | 0.45 | <0.001 |
| TOTAL | TPC | Se | 0.22 | <0.001 |
| TPC | Zn | −0.28 | <0.001 | |
| TPC | DPPH | 0.17 | <0.005 | |
| Se | Cu | 0.29 | <0.001 | |
| Cu | Zn | 0.21 | <0.001 | |
| Cu | DPPH | 0.38 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żmudzińska, A.; Puścion-Jakubik, A.; Soroczyńska, J.; Socha, K. Evaluation of Selected Antioxidant Parameters in Ready-to-Eat Food for Infants and Young Children. Nutrients 2023, 15, 3160. https://doi.org/10.3390/nu15143160
Żmudzińska A, Puścion-Jakubik A, Soroczyńska J, Socha K. Evaluation of Selected Antioxidant Parameters in Ready-to-Eat Food for Infants and Young Children. Nutrients. 2023; 15(14):3160. https://doi.org/10.3390/nu15143160
Chicago/Turabian StyleŻmudzińska, Anita, Anna Puścion-Jakubik, Jolanta Soroczyńska, and Katarzyna Socha. 2023. "Evaluation of Selected Antioxidant Parameters in Ready-to-Eat Food for Infants and Young Children" Nutrients 15, no. 14: 3160. https://doi.org/10.3390/nu15143160
APA StyleŻmudzińska, A., Puścion-Jakubik, A., Soroczyńska, J., & Socha, K. (2023). Evaluation of Selected Antioxidant Parameters in Ready-to-Eat Food for Infants and Young Children. Nutrients, 15(14), 3160. https://doi.org/10.3390/nu15143160








