The Effects of Zinc and Selenium Co-Supplementation on Resting Metabolic Rate, Thyroid Function, Physical Fitness, and Functional Capacity in Overweight and Obese People under a Hypocaloric Diet: A Randomized, Double-Blind, and Placebo-Controlled Trial
Abstract
1. Introduction
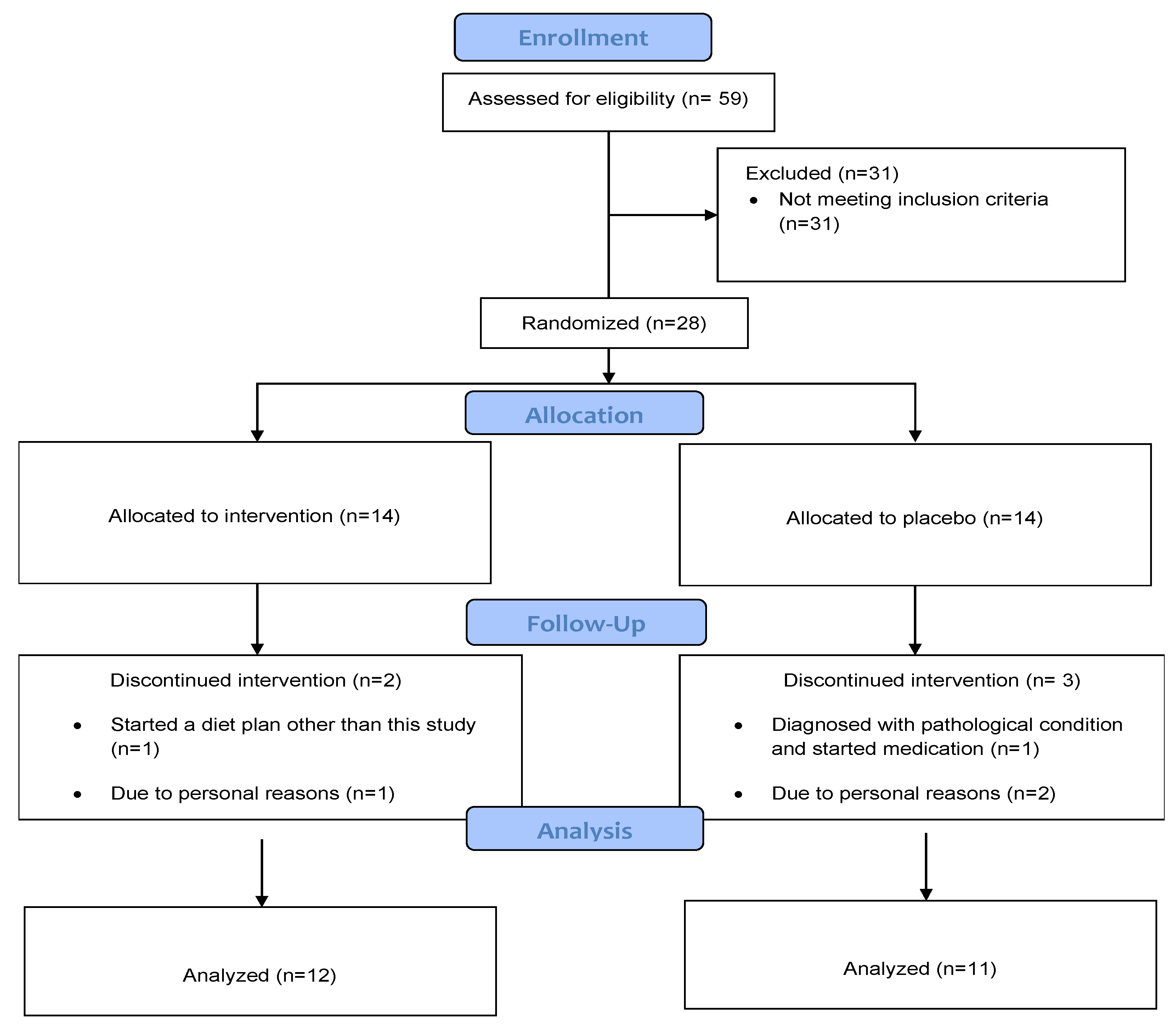
2. Materials and Methods
2.1. Study Design
2.2. Intervention
2.3. Blinding and Randomization
2.4. Anthropometry and Body Composition
2.5. Participants’ Adherence
2.6. Thyroid Function Assessment
2.7. Resting Metabolic Rate
2.8. Functional Capacity and Physical Fitness Assessment
2.9. Statistical Analysis
3. Results
3.1. Effects on Anthropometry and Body Composition
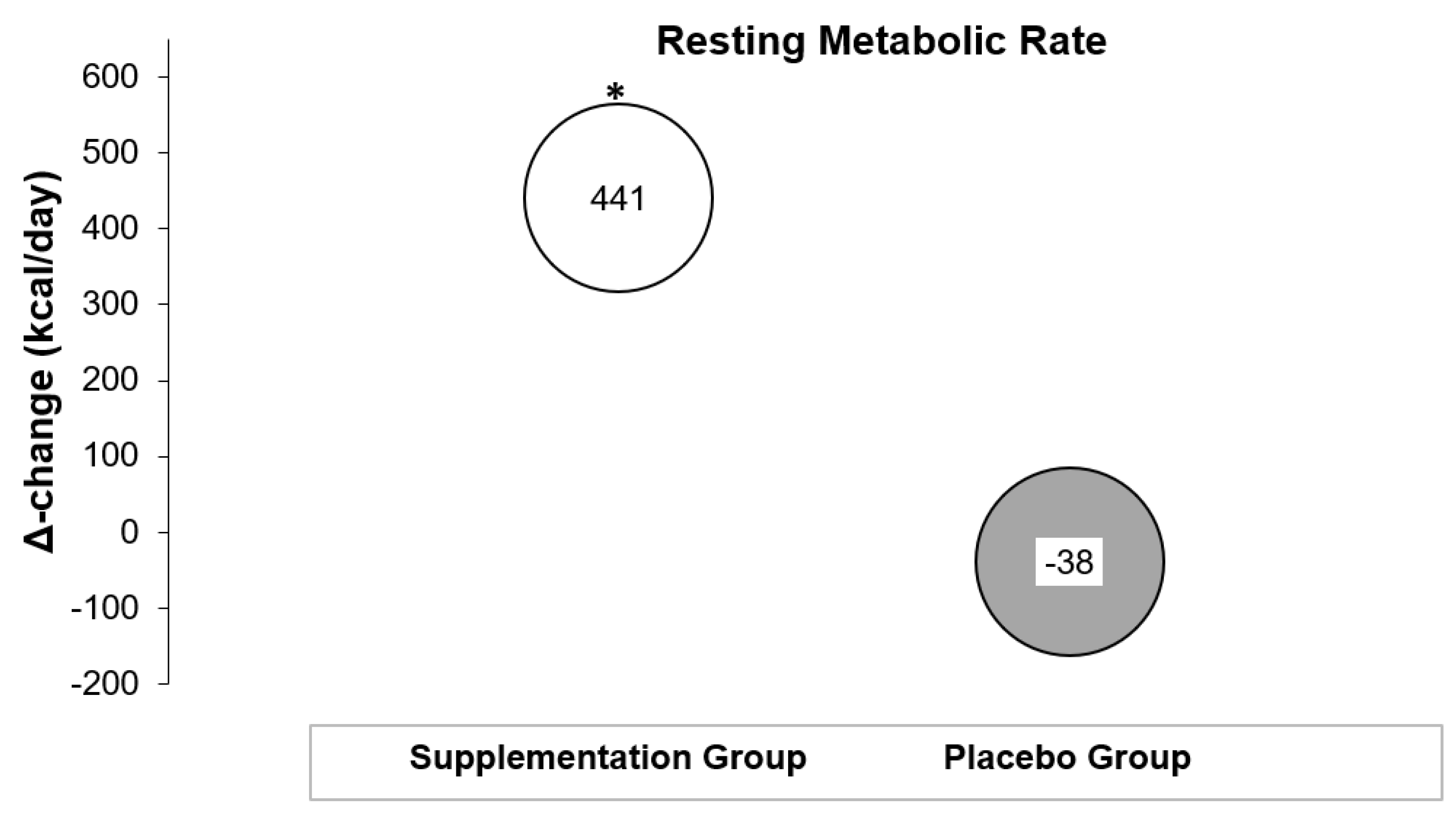
3.2. Effects on Resting Metabolic Rate
3.3. Effects on Respiratory Quotient
3.4. Effects on Thyroid Hormones
3.5. Effects on Selenium and Zinc Serum Levels
3.6. Effects on Physical Fitness and Functional Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 27 March 2021).
- Lam, B.C.C.; Lim, A.Y.L.; Chan, S.L.; Yum, M.P.S.; Koh, N.S.Y.; Finkelstein, E.A. The impact of obesity: A narrative review. Singap. Med. J. 2023, 64, 163–171. [Google Scholar] [CrossRef]
- Apovian, C.M. Obesity: Definition, comorbidities, causes, and burden. Am. J. Manag. Care 2016, 22, s176–s185. [Google Scholar] [PubMed]
- Vallgårda, S.; Nielsen, M.E.J.; Hansen, A.K.K.; Cathaoir, K.Ó.; Hartlev, M.; Holm, L.; Christensen, B.J.; Jensen, J.D.; Sørensen, T.I.A.; Sandøe, P. Should Europe follow the US and declare obesity a disease?: A discussion of the so-called utilitarian argument. Eur. J. Clin. Nutr. 2017, 71, 1263–1267. [Google Scholar] [CrossRef]
- Bays, H. Adiposopathy, “sick fat”, Ockham’s razor, and resolution of the obesity paradox. Curr. Atheroscler. Rep. 2014, 16, 409. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; González-Campoy, J.M.; Henry, R.R.; Bergman, D.A.; Kitabchi, A.E.; Schorr, A.B.; Rodbard, H.W. Adiposopathy Working Group Is adiposopathy (sick fat) an endocrine disease? Int. J. Clin. Pract. 2008, 62, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.M.; Macedo-de la Concha, L.E.; Pantoja-Meléndez, C.A. Low-grade inflammation and its relation to obesity and chronic degenerative diseases. Rev. Médica Hosp. Gen. México 2017, 80, 101–105. [Google Scholar] [CrossRef]
- Van Gaal, L.F.; Mertens, I.L.; De Block, C.E. Mechanisms linking obesity with cardiovascular disease. Nature 2006, 444, 875–880. [Google Scholar] [CrossRef]
- Astrup, A.; Bügel, S. Overfed but undernourished: Recognizing nutritional inadequacies/deficiencies in patients with overweight or obesity. Int. J. Obes. 2019, 43, 219–232. [Google Scholar] [CrossRef]
- Sajjadi, S.F.; Mirzababaei, A.; Abdollahi, A.; Shiraseb, F.; Mirzaei, K. The association between deficiency of nutrient intake and resting metabolic rate in overweight and obese women: A cross-sectional study. BMC Res. Notes 2021, 14, 179. [Google Scholar] [CrossRef]
- Fiamenghi, V.I.; Mello, E.D.d. Vitamin D deficiency in children and adolescents with obesity: A meta-analysis. J. Pediatr. 2021, 97, 273–279. [Google Scholar] [CrossRef]
- Purdy, J.C.; Shatzel, J.J. The hematologic consequences of obesity. Eur. J. Haematol. 2021, 106, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Damms-Machado, A.; Weser, G.; Bischoff, S.C. Micronutrient deficiency in obese subjects undergoing low calorie diet. Nutr. J. 2012, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- McKay, J.; Ho, S.; Jane, M.; Pal, S. Overweight & obese Australian adults and micronutrient deficiency. BMC Nutr. 2020, 6, 12. [Google Scholar]
- Via, M. The malnutrition of obesity: Micronutrient deficiencies that promote diabetes. ISRN Endocrinol. 2012, 2012, 103472. [Google Scholar] [CrossRef]
- Hosseini, B.; Saedisomeolia, A.; Allman-Farinelli, M. Association Between Antioxidant Intake/Status and Obesity: A Systematic Review of Observational Studies. Biol. Trace Elem. Res. 2017, 175, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Beserra, J.B.; Morais, J.B.S.; Severo, J.S.; Cruz, K.J.C.; de Oliveira, Ana Raquel Soares; Henriques, G.S.; do Nascimento Marreiro, D. Relation Between Zinc and Thyroid Hormones in Humans: A Systematic Review. Biol. Trace Elem. Res. 2021, 199, 4092–4100. [Google Scholar] [CrossRef] [PubMed]
- Cruz, K.J.; Morais, J.B.; de Oliveira, A.R.; Severo, J.S.; Marreiro, D.D. The Effect of Zinc Supplementation on Insulin Resistance in Obese Subjects: A Systematic Review. Biol. Trace Elem. Res. 2017, 176, 239–243. [Google Scholar] [CrossRef]
- Khorshidi, M.; Zarezadeh, M.; Sadeghi, A.; Teymouri, A.; Emami, M.R.; Kord-Varkaneh, H.; Aryaeian, N.; Rahmani, J.; Mousavi, S.M. The Effect of Zinc Supplementation on Serum Leptin Levels: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Horm. Metab. Res. 2019, 51, 503–510. [Google Scholar] [CrossRef]
- Kang, D.; Lee, J.; Wu, C.; Guo, X.; Lee, B.J.; Chun, J.; Kim, J. The role of selenium metabolism and selenoproteins in cartilage homeostasis and arthropathies. Exp. Mol. Med. 2020, 52, 1198–1208. [Google Scholar] [CrossRef]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Ajsuvakova, O.P.; Filippini, T.; Zhou, J.C.; Lei, X.G.; Gatiatulina, E.R.; Michalke, B.; Skalnaya, M.G.; Vinceti, M.; Aschner, M.; et al. Selenium and Selenoproteins in Adipose Tissue Physiology and Obesity. Biomolecules 2020, 10, 658. [Google Scholar] [CrossRef]
- Steinbrenner, H. Interference of selenium and selenoproteins with the insulin-regulated carbohydrate and lipid metabolism. Free Radic. Biol. Med. 2013, 65, 1538–1547. [Google Scholar] [CrossRef]
- Wang, N.; Tan, H.; Li, S.; Xu, Y.; Guo, W.; Feng, Y. Supplementation of Micronutrient Selenium in Metabolic Diseases: Its Role as an Antioxidant. Oxid. Med. Cell Longev. 2017, 2017, 7478523. [Google Scholar] [CrossRef]
- Li, J.; Cao, D.; Huang, Y.; Chen, B.; Chen, Z.; Wang, R.; Dong, Q.; Wei, Q.; Liu, L. Zinc Intakes and Health Outcomes: An Umbrella Review. Front. Nutr. 2022, 9, 798078. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Akbari, M.; Moosazadeh, M.; Lankarani, K.B.; Heydari, S.T.; Kolahdooz, F.; Mohammadi, A.A.; Shabani, A.; Badehnoosh, B.; Jamilian, M.; et al. The Effects of Selenium Supplementation on Glucose Metabolism and Lipid Profiles Among Patients with Metabolic Diseases: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Horm. Metab. Res. 2017, 49, 826–830. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Wathurapatha, W.S.; Ishara, M.H.; Jayawardana, R.; Galappatthy, P.; Katulanda, P.; Constantine, G.R. Effects of Zinc supplementation on serum lipids: A systematic review and meta-analysis. Nutr. Metab. 2015, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Thoen, R.U.; Barther, N.N.; Schemitt, E.; Bona, S.; Fernandes, S.; Coral, G.; Marroni, N.P.; Tovo, C.; Guedes, R.P.; Porawski, M. Zinc supplementation reduces diet-induced obesity and improves insulin sensitivity in rats. Appl. Physiol. Nutr. Metab. 2019, 44, 580–586. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, Z.; Liu, S.; Aluo, Z.; Zhang, L.; Yu, L.; Li, Y.; Song, Z.; Zhou, L. Zinc Supplementation Alleviates Lipid and Glucose Metabolic Disorders Induced by a High-Fat Diet. J. Agric. Food Chem. 2020, 68, 5189–5200. [Google Scholar] [CrossRef] [PubMed]
- Plummer, J.D.; Postnikoff, S.D.; Tyler, J.K.; Johnson, J.E. Selenium supplementation inhibits IGF-1 signaling and confers methionine restriction-like healthspan benefits to mice. eLife 2021, 10, e62483. [Google Scholar] [CrossRef]
- Hafez, L.M.; Aboudeya, H.M.; Matar, N.A.; El-Sebeay, A.S.; Nomair, A.M.; El-Hamshary, S.A.; Nomeir, H.M.; Ibrahim, F.A.R. Ameliorative effects of zinc supplementation on cognitive function and hippocampal leptin signaling pathway in obese male and female rats. Sci. Rep. 2023, 13, 5072. [Google Scholar] [CrossRef]
- Zavros, A.; Giannaki, C.D.; Aphamis, G.; Roupa, Z.; Andreou, E. The Effects of Zinc and Selenium Supplementation on Body Composition and Thyroid Function in Individuals with Overweight or Obesity: A Systematic Review. J. Diet. Suppl. 2022, 20, 643–671. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.C.; Galuska, D.A.; Kettel Khan, L.; Gillespie, C.; Serdula, M.K. Weight regain in U.S. adults who experienced substantial weight loss, 1999–2002. Am. J. Prev. Med. 2007, 33, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Sacks, G.; Chandramohan, D.; Chow, C.C.; Wang, Y.C.; Gortmaker, S.L.; Swinburn, B.A. Quantification of the effect of energy imbalance on bodyweight. Lancet 2011, 378, 826–837. [Google Scholar] [CrossRef]
- Oussaada, S.M.; van Galen, K.A.; Cooiman, M.I.; Kleinendorst, L.; Hazebroek, E.J.; van Haelst, M.M.; Ter Horst, K.W.; Serlie, M.J. The pathogenesis of obesity. Metabolism 2019, 92, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Agnihothri, R.V.; Courville, A.B.; Linderman, J.D.; Smith, S.; Brychta, R.; Remaley, A.; Chen, K.Y.; Simchowitz, L.; Celi, F.S. Moderate weight loss is sufficient to affect thyroid hormone homeostasis and inhibit its peripheral conversion. Thyroid 2014, 24, 19–26. [Google Scholar] [CrossRef]
- Marzullo, P.; Minocci, A.; Mele, C.; Fessehatsion, R.; Tagliaferri, M.; Pagano, L.; Scacchi, M.; Aimaretti, G.; Sartorio, A. The relationship between resting energy expenditure and thyroid hormones in response to short-term weight loss in severe obesity. PLoS ONE 2018, 13, e0205293. [Google Scholar] [CrossRef]
- Radetti, G.; Longhi, S.; Baiocchi, M.; Cassar, W.; Buzi, F. Changes in lifestyle improve body composition, thyroid function, and structure in obese children. J. Endocrinol. Investig. 2012, 35, 281–285. [Google Scholar]
- Saleh, V.; Afroundeh, R.; Siahkouhian, M.; Asadi, A. Relationship between Resting Metabolic rate and Body Composition Factors in Obese and Normal Weight Gymnast Children. Int. J. Pediatr. 2021, 9, 14331–14340. [Google Scholar]
- Miller, W.M.; Spring, T.J.; Zalesin, K.C.; Kaeding, K.R.; Nori Janosz, K.E.; McCullough, P.A.; Franklin, B.A. Lower than predicted resting metabolic rate is associated with severely impaired cardiorespiratory fitness in obese individuals. Obesity 2012, 20, 505–511. [Google Scholar] [CrossRef]
- Wada, L.; King, J.C. Effect of low zinc intakes on basal metabolic rate, thyroid hormones and protein utilization in adult men. J. Nutr. 1986, 116, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, C.; Volpe, S.L. Effect of zinc supplementation on thyroid hormone function. A case study of two college females. Ann. Nutr. Metab. 2007, 51, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, W.C.; Keim, N.L. Dietary selenium intake modulates thyroid hormone and energy metabolism in men. J. Nutr. 2003, 133, 3443–3448. [Google Scholar] [CrossRef]
- Hawkes, W.C.; Keim, N.L.; Diane Richter, B.; Gustafson, M.B.; Gale, B.; Mackey, B.E.; Bonnel, E.L. High-selenium yeast supplementation in free-living North American men: No effect on thyroid hormone metabolism or body composition. J. Trace Elem. Med. Biol. 2008, 22, 131–142. [Google Scholar] [CrossRef]
- Dreher, M.; Kabitz, H.J. Impact of obesity on exercise performance and pulmonary rehabilitation. Respirology 2012, 17, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Martinez, X.; Petermann, F.; Leiva, A.M.; Garrido-Mendez, A.; Salas-Bravo, C.; Martinez, M.A.; Labrana, A.M.; Duran, E.; Valdivia-Moral, P.; Zagalaz, M.L.; et al. Association of physical inactivity with obesity, diabetes, hypertension and metabolic syndrome in the chilean population. Rev. Med. Chil. 2018, 146, 585–595. [Google Scholar]
- Lankhaar, J.A.; de Vries, W.R.; Jansen, J.A.; Zelissen, P.M.; Backx, F.J. Impact of overt and subclinical hypothyroidism on exercise tolerance: A systematic review. Res. Q. Exerc. Sport. 2014, 85, 365–389. [Google Scholar] [CrossRef]
- Trouwborst, I.; Verreijen, A.; Memelink, R.; Massanet, P.; Boirie, Y.; Weijs, P.; Tieland, M. Exercise and Nutrition Strategies to Counteract Sarcopenic Obesity. Nutrients 2018, 10, 605. [Google Scholar] [CrossRef]
- Kaidar-Person, O.; Person, B.; Szomstein, S.; Rosenthal, R.J. Nutritional deficiencies in morbidly obese patients: A new form of malnutrition? Part B: Minerals. Obes. Surg. 2008, 18, 1028–1034. [Google Scholar] [CrossRef]
- McClung, J.P. Iron, Zinc, and Physical Performance. Biol. Trace Elem. Res. 2019, 188, 135–139. [Google Scholar] [CrossRef]
- Wesolowski, L.T.; Semanchik, P.L.; White-Springer, S.H. Beyond antioxidants: Selenium and skeletal muscle mitochondria. Front. Vet. Sci. 2022, 9, 1011159. [Google Scholar] [CrossRef] [PubMed]
- Baltaci, A.K.; Mogulkoc, R.; Akil, M.; Bicer, M. Review—Selenium—Its metabolism and relation to exercise. Pak. J. Pharm. Sci. 2016, 29, 1719–1725. [Google Scholar] [PubMed]
- Hashemipour, M.; Kelishadi, R.; Shapouri, J.; Sarrafzadegan, N.; Amini, M.; Tavakoli, N.; Movahedian-Attar, A.; Mirmoghtadaee, P.; Poursafa, P. Effect of zinc supplementation on insulin resistance and components of the metabolic syndrome in prepubertal obese children. Hormones 2009, 8, 279–285. [Google Scholar] [CrossRef]
- Payahoo, L.; Ostadrahimi, A.; Mobasseri, M.; Khaje, B.Y.R.; Asghari, J.M. Effects of zinc supplementation on serum leptin level and insulin sensitivity in obese people. Ann. Nutr. Metab. 2013, 63, 173. [Google Scholar] [CrossRef]
- Nazem, M.R.; Asadi, M.; Jabbari, N.; Allameh, A. Effects of zinc supplementation on superoxide dismutase activity and gene expression, and metabolic parameters in overweight type 2 diabetes patients: A randomized, double-blind, controlled trial. Clin. Biochem. 2019, 69, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Guarino, G.; Ragozzino, G.; Della Corte, T.; Fontana, S.; Strollo, F.; Cecaro, M.; Gentile, S. Selenium Supplementation in Obese Patients with Subclinical Hypothyroidism and Type 2 Diabetes. J. Nutr. Health Sci. 2018, 5, 202. [Google Scholar]
- Sheehan, M.T. Biochemical Testing of the Thyroid: TSH is the Best and, Oftentimes, Only Test Needed—A Review for Primary Care. Clin. Med. Res. 2016, 14, 83–92. [Google Scholar] [CrossRef]
- Khandelwal, D.; Tandon, N. Overt and subclinical hypothyroidism: Who to treat and how. Drugs 2012, 72, 17–33. [Google Scholar] [CrossRef]
- Diaz, A.; Lipman Diaz, E.G. Hypothyroidism. Pediatr. Rev. 2014, 35, 336–347. [Google Scholar] [CrossRef]
- Compher, C.; Frankenfield, D.; Keim, N.; Roth-Yousey, L. Evidence Analysis Working Group Best practice methods to apply to measurement of resting metabolic rate in adults: A systematic review. J. Am. Diet. Assoc. 2006, 106, 881–903. [Google Scholar] [CrossRef]
- Kear, B.M.; Guck, T.P.; McGaha, A.L. Timed Up and Go (TUG) Test: Normative Reference Values for Ages 20 to 59 Years and Relationships With Physical and Mental Health Risk Factors. J. Prim. Care Community Health 2017, 8, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bermejo, L.; Adsuar, J.C.; Mendoza-Muñoz, M.; Barrios-Fernández, S.; Garcia-Gordillo, M.A.; Pérez-Gómez, J.; Carlos-Vivas, J. Test-Retest Reliability of Five Times Sit to Stand Test (FTSST) in Adults: A Systematic Review and Meta-Analysis. Biology 2021, 10, 510. [Google Scholar] [CrossRef]
- Goele, K.; Bosy-Westphal, A.; Rumcker, B.; Lagerpusch, M.; Muller, M.J. Influence of changes in body composition and adaptive thermogenesis on the difference between measured and predicted weight loss in obese women. Obes. Facts 2009, 2, 105–109. [Google Scholar] [CrossRef]
- Schwartz, A.; Doucet, E. Relative changes in resting energy expenditure during weight loss: A systematic review. Obes. Rev. 2010, 11, 531–547. [Google Scholar] [CrossRef]
- Trexler, E.T.; Smith-Ryan, A.E.; Norton, L.E. Metabolic adaptation to weight loss: Implications for the athlete. J. Int. Soc. Sports Nutr. 2014, 11, 7. [Google Scholar] [CrossRef]
- Martins, C.; Gower, B.A.; Hunter, G.R. Metabolic adaptation delays time to reach weight loss goals. Obesity 2022, 30, 400–406. [Google Scholar] [CrossRef]
- Martins, C.; Roekenes, J.; Gower, B.A.; Hunter, G.R. Metabolic adaptation is associated with less weight and fat mass loss in response to low-energy diets. Nutr. Metab. 2021, 18, 60. [Google Scholar] [CrossRef]
- Fothergill, E.; Guo, J.; Howard, L.; Kerns, J.C.; Knuth, N.D.; Brychta, R.; Chen, K.Y.; Skarulis, M.C.; Walter, M.; Walter, P.J.; et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity 2016, 24, 1612–1619. [Google Scholar] [CrossRef]
- Federico, A.; Iodice, P.; Federico, P.; Del Rio, A.; Mellone, M.C.; Catalano, G.; Federico, P. Effects of selenium and zinc supplementation on nutritional status in patients with cancer of digestive tract. Eur. J. Clin. Nutr. 2001, 55, 293–297. [Google Scholar] [CrossRef] [PubMed]
- De Mello, A.H.; Costa, A.B.; Engel, J.D.G.; Rezin, G.T. Mitochondrial dysfunction in obesity. Life Sci. 2018, 192, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Zampino, M.; Semba, R.D.; Adelnia, F.; Spencer, R.G.; Fishbein, K.W.; Schrack, J.A.; Simonsick, E.M.; Ferrucci, L. Greater Skeletal Muscle Oxidative Capacity Is Associated With Higher Resting Metabolic Rate: Results From the Baltimore Longitudinal Study of Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 2262–2268. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Zhu, K.; Feng, R.N.; Sun, C.H. Effects of multivitamin and mineral supplementation on adiposity, energy expenditure and lipid profiles in obese Chinese women. Int. J. Obes. 2010, 34, 1070–1077. [Google Scholar] [CrossRef]
- Drabsch, T.; Holzapfel, C.; Stecher, L.; Petzold, J.; Skurk, T.; Hauner, H. Associations Between C-Reactive Protein, Insulin Sensitivity, and Resting Metabolic Rate in Adults: A Mediator Analysis. Front. Endocrinol. 2018, 9, 556. [Google Scholar] [CrossRef] [PubMed]
- Cavedon, E.; Manso, J.; Negro, I.; Censi, S.; Serra, R.; Busetto, L.; Vettor, R.; Plebani, M.; Pezzani, R.; Nacamulli, D.; et al. Selenium Supplementation, Body Mass Composition, and Leptin Levels in Patients with Obesity on a Balanced Mildly Hypocaloric Diet: A Pilot Study. Int. J. Endocrinol. 2020, 2020, 4802739. [Google Scholar] [CrossRef]
- Wang, C.; Liu, L.; Chou, S.; Lee, B. Correlation of Calcium, Copper, Iron, Magnesium and Zinc Content in Hair with Basal Metabolic Rate and Bioelectrical Impedance in Adolescent Females. Tzu Chi Med. J. 2008, 20, 58–62. [Google Scholar] [CrossRef]
- Khorsandi, H.; Nikpayam, O.; Yousefi, R.; Parandoosh, M.; Hosseinzadeh, N.; Saidpour, A.; Ghorbani, A. Zinc supplementation improves body weight management, inflammatory biomarkers and insulin resistance in individuals with obesity: A randomized, placebo-controlled, double-blind trial. Diabetol. Metab. Syndr. 2019, 11, 101. [Google Scholar] [CrossRef]
- Gómez-García, A.; Hernández-Salazar, E.; González-Ortiz, M.; Martínez-Abundis, E. Effect of oral zinc administration on insulin sensitivity, leptin and androgens in obese males. Rev. Med. Chil. 2006, 134, 279–284. [Google Scholar]
- Banaszak, M.; Górna, I.; Przysławski, J. Zinc and the Innovative Zinc-α2-Glycoprotein Adipokine Play an Important Role in Lipid Metabolism: A Critical Review. Nutrients 2021, 13, 2023. [Google Scholar] [CrossRef]
- Shinde, A.B.; Song, A.; Wang, Q.A. Brown Adipose Tissue Heterogeneity, Energy Metabolism, and Beyond. Front. Endocrinol. 2021, 12, 651763. [Google Scholar] [CrossRef] [PubMed]
- Elattar, S.; Dimri, M.; Satyanarayana, A. The tumor secretory factor ZAG promotes white adipose tissue browning and energy wasting. FASEB J. 2018, 32, 4727–4743. [Google Scholar] [CrossRef]
- Hasani, M.; Saidpour, A.; Irandoost, P.; Golab, F.; Khazdouz, M.; Qorbani, M.; Agh, F.; Mohammad Sharifi, A.; Vafa, M. Beneficial effects of Se/Zn co-supplementation on body weight and adipose tissue inflammation in high-fat diet-induced obese rats. Food Sci. Nutr. 2021, 9, 3414–3425. [Google Scholar] [CrossRef] [PubMed]
- Fatmi, W.; Kechrid, Z.; Nazıroğlu, M.; Flores-Arce, M. Selenium supplementation modulates zinc levels and antioxidant values in blood and tissues of diabetic rats fed zinc-deficient diet. Biol. Trace Elem. Res. 2013, 152, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodianfard, S.; Vafa, M.; Golgiri, F.; Khoshniat, M.; Gohari, M.; Solati, Z.; Djalali, M. Effects of Zinc and Selenium Supplementation on Thyroid Function in Overweight and Obese Hypothyroid Female Patients: A Randomized Double-Blind Controlled Trial. J. Am. Coll. Nutr. 2015, 34, 391–399. [Google Scholar] [CrossRef]
- Marreiro, D.N.; Geloneze, B.; Tambascia, M.A.; Lerario, A.C.; Halpern, A.; Cozzolino, S.M. Effect of zinc supplementation on serum leptin levels and insulin resistance of obese women. Biol. Trace Elem. Res. 2006, 112, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Karandish, M.; Mozaffari-Khosravi, H.; Mohammadi, S.M.; Cheraghian, B.; Azhdari, M. The effect of curcumin and zinc co-supplementation on glycemic parameters in overweight or obese prediabetic subjects: A phase 2 randomized, placebo-controlled trial with a multi-arm, parallel-group design. Phytother. Res. 2021, 35, 4377–4387. [Google Scholar] [CrossRef]
- Kelesidis, T.; Kelesidis, I.; Chou, S.; Mantzoros, C.S. Narrative review: The role of leptin in human physiology: Emerging clinical applications. Ann. Intern. Med. 2010, 152, 93–100. [Google Scholar] [CrossRef]
- Mahmoud, A.H.; AbdElMonem, H.A.; Abbas, M.M. The role of selenium and zinc oxide nanoparticles on mitigating side effects of obesity in rats. Braz. J. Biol. 2022, 84, e264004. [Google Scholar] [CrossRef]
- Austin, S.B.; Yu, K.; Liu, S.H.; Dong, F.; Tefft, N. Household expenditures on dietary supplements sold for weight loss, muscle building, and sexual function: Disproportionate burden by gender and income. Prev. Med. Rep. 2017, 6, 236–241. [Google Scholar] [CrossRef]
- Watanabe, M.; Risi, R.; Masi, D.; Caputi, A.; Balena, A.; Rossini, G.; Tuccinardi, D.; Mariani, S.; Basciani, S.; Manfrini, S.; et al. Current Evidence to Propose Different Food Supplements for Weight Loss: A Comprehensive Review. Nutrients 2020, 12, 2873. [Google Scholar] [CrossRef]
- Abdollahi, S.; Toupchian, O.; Jayedi, A.; Meyre, D.; Tam, V.; Soltani, S. Zinc Supplementation and Body Weight: A Systematic Review and Dose-Response Meta-analysis of Randomized Controlled Trials. Adv. Nutr. 2020, 11, 398–411. [Google Scholar] [CrossRef]
- Scott, D.; Blizzard, L.; Fell, J.; Giles, G.; Jones, G. Associations between dietary nutrient intake and muscle mass and strength in community-dwelling older adults: The Tasmanian Older Adult Cohort Study. J. Am. Geriatr. Soc. 2010, 58, 2129–2134. [Google Scholar] [CrossRef]
- Gunanti, I.R.; Al-Mamun, A.; Schubert, L.; Long, K.Z. The effect of zinc supplementation on body composition and hormone levels related to adiposity among children: A systematic review. Public. Health Nutr. 2016, 19, 2924–2939. [Google Scholar] [CrossRef]
- Hernández-Camacho, J.D.; Vicente-García, C.; Parsons, D.S.; Navas-Enamorado, I. Zinc at the crossroads of exercise and proteostasis. Redox Biol. 2020, 35, 101529. [Google Scholar] [CrossRef]
- Wang, L.; Yin, J.; Zhang, F.; Yu, H.; Chen, F.; Zhang, Z.; Zhang, X. Selenium Status Affects Hypertrophic Growth of Skeletal Muscle in Growing Zebrafish by Mediating Protein Turnover. J. Nutr. 2021, 151, 1791–1801. [Google Scholar] [CrossRef]
- Perri, G.; Mendonça, N.; Jagger, C.; Walsh, J.; Eastell, R.; Mathers, J.C.; Hill, T.R. Dietary Selenium Intakes and Musculoskeletal Function in Very Old Adults: Analysis of the Newcastle 85+ Study. Nutrients 2020, 12, 2068. [Google Scholar] [CrossRef]
- Nguyen, J.C.D.; Killcross, A.S.; Jenkins, T.A. Obesity and cognitive decline: Role of inflammation and vascular changes. Front. Neurosci. 2014, 8, 375. [Google Scholar] [CrossRef]
- Cheng, B.; Wang, J.; Meng, X.; Sun, L.; Hu, B.; Li, H.; Sheng, J.; Chen, G.; Tao, F.; Sun, Y.; et al. The association between essential trace element mixture and cognitive function in Chinese community-dwelling older adults. Ecotoxicol. Environ. Saf. 2022, 231, 113182. [Google Scholar] [CrossRef] [PubMed]
- Gau, J.; Chavan, B.; Li, Y.; Clark, B.C.; Haile, Z.T. Association between serum zinc levels and basic physical functioning: Secondary data analysis of NHANES 2011-14. BMC Nutr. 2021, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Stegemöller, E.L.; Nocera, J.; Malaty, I.; Shelley, M.; Okun, M.S.; Hass, C.J. NPF Quality Improvement Initiative Investigators Timed up and go, cognitive, and quality-of-life correlates in Parkinson’s disease. Arch. Phys. Med. Rehabil. 2014, 95, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Tah, P.C.; Lee, Z.; Poh, B.K.; Abdul Majid, H.; Hakumat-Rai, V.; Mat Nor, M.B.; Kee, C.C.; Kamarul Zaman, M.; Hasan, M.S. A Single-Center Prospective Observational Study Comparing Resting Energy Expenditure in Different Phases of Critical Illness: Indirect Calorimetry Versus Predictive Equations. Crit. Care Med. 2020, 48, e380–e390. [Google Scholar] [CrossRef] [PubMed]
| Variables | Supplementation | Placebo | Main Effects and Interactions (p-Value) | ||
|---|---|---|---|---|---|
| Weight (kg) | Time | Time × Group | Group | ||
| Baseline | 83.3 ± 20.0 | 79.3 ± 12.2 | 0.098 | 0.776 | 0.593 |
| post | 82.0 ± 18.7 | 78.4 ± 10.9 | |||
| Δ change | −1.3 ± 3.1 | −0.9 ± 2.9 | |||
| Body mass index—BMI (kg/m2) | Time | Time × Group | Group | ||
| Baseline | 29.1 ± 4.4 | 28.3 ± 4.5 | 0.111 | 0.823 | 0.663 |
| post | 28.7 ± 3.9 | 27.9 ± 3.9 | |||
| Δ Change | −0.4 ± 1.1 | −0.3 ± 1.1 | |||
| Total Body Fat (%) | Time | Time × Group | Group | ||
| Baseline | 30.4 ± 8.0 | 28.3 ± 9.1 | 0.014 | 0.144 | 0.664 |
| post | 28.9 ± 7.3 | 27.9 ± 9.0 | |||
| Δ Change | −1.5 ± 1.7 | −0.4 ± 1.7 | |||
| Fat-free mass (%) | Time | Time × Group | Group | ||
| Baseline | 57.5 ± 14.1 | 56.6 ± 9.8 | 0.493 | 0.183 | 0.812 |
| post | 58.1 ± 14.3 | 56.4 ± 9.9 | |||
| Δ Change | 0.6 ± 1.4 | −0.2 ± 1.3 | |||
| Muscle Mass (%) | Time | Time × Group | Group | ||
| Baseline | 54.6 ± 13.4 | 53.7 ± 9.4 | 0.498 | 0.186 | 0.810 |
| post | 55.2 ± 13.6 | 53.6 ± 9.5 | |||
| Δ Change | 0.5 ± 1.3 | −0.1 ± 1.2 | |||
| Variables | Supplementation | Placebo | Main Effects and Interactions (p-Value) | ||
|---|---|---|---|---|---|
| Resting Metabolic Rate (kcal/day) | Time | Time × Group | Group | ||
| Baseline | 1923 ± 440 | 2467 ± 367 | 0.086 | 0.045 | 0.078 |
| post | 2364 ± 410 | 2429 ± 484 | |||
| Δ change | 441 ± 594 | −38 ± 308 | |||
| Respiratory Quotient | Time | Time × Group | Group | ||
| Baseline | 0.88 ± 0.05 | 0.91 ± 0.07 | 0.835 | 0.436 | 0.094 |
| post | 0.86 ± 0.06 | 0.92 ± 0.11 | |||
| Δ Change | −0.02 ± 0.07 | 0.01 ± 0.12 | |||
| Free Triiodothyronine (pmol/L) | Time | Time × Group | Group | ||
| Baseline | 4.69 ± 1.02 | 4.17 ± 0.77 | 0.120 | 0.308 | 0.352 |
| post | 4.17 ± 0.65 | 4.06 ± 0.42 | |||
| Δ Change | −0.52 ± 0.84 | −0.11 ± 0.67 | |||
| Free Thyroxine (pmol/L) | Time | Time × Group | Group | ||
| Baseline | 15.22 ± 1.36 | 15.34 ± 1.82 | 0.007 | 0.810 | 0.943 |
| post | 16.04 ± 1.69 | 16.04 ± 2.51 | |||
| Δ Change | 0.82 ± 0.86 | 0.70 ± 1.16 | |||
| Thyroid-Stimulating Hormone (mIU/L) | Time | Time × Group | Group | ||
| Baseline | 1.52 ± 0.31 | 1.58 ± 0.34 | 0.083 | 0.218 | 0.650 |
| post | 1.46 ± 0.37 | 1.25 ± 0.456 | |||
| Δ Change | −0.06 ± 0.44 | −0.33 ± 0.40 | |||
| Selenium (μg/L) | Time | Time × Group | Group | ||
| Baseline | 83.04 ± 13.59 | 90.61 ± 23.23 | 0.006 | 0.004 | 0.159 |
| post | 119.40 ± 23.93 | 89.58 ± 10.61 | |||
| Δ Change | 36.36 ± 20.95 | −1.03 ± 24.47 | |||
| Zinc (umol/L) | Time | Time × Group | Group | ||
| Baseline | 11.35 ± 3.40 | 10.82 ± 3.45 | 0.087 | 0.171 | 0.525 |
| post | 11.76 ± 3.40 | 14.23 ± 4.65 | |||
| Δ change | 0.41 ± 4.75 | 3.40 ± 3.26 | |||
| Variables | Supplementation | Placebo | Main Effects and Interactions (p-Value) | ||
|---|---|---|---|---|---|
| VO2max (mL/kg/min) | Time | Time × Group | Group | ||
| Baseline | 27.2 ± 7.9 | 26.2 ± 9.1 | 0.722 | 0.695 | 0.700 |
| post | 28.0 ± 5.7 | 26.2 ± 8.6 | |||
| Δ change | 0.7 ± 5.3 | −0.1 ± 2.3 | |||
| STS-5 (s) | Time | Time × Group | Group | ||
| Baseline | 12.7 ± 2.5 | 13.6 ± 1.6 | 0.000 | 0.563 | 0.384 |
| post | 11.5 ± 2.4 | 12.2 ± 1.8 | |||
| Δ Change | −1.2 ± 0.7 | −1.4 ± 1.2 | |||
| STS-30 (rep) | Time | Time × Group | Group | ||
| Baseline | 11.7 ± 2.6 | 11.8 ± 0.7 | 0.000 | 0.376 | 0.735 |
| post | 13.9 ± 3.2 | 13.2 ± 2.2 | |||
| Δ Change | 2.1 ± 1.6 | 1.4 ± 2.3 | |||
| STS-60 (rep) | Time | Time × Group | Group | ||
| Baseline | 23.8 ± 5.1 | 23.5 ± 2.1 | 0.000 | 0.350 | 0.598 |
| post | 28.2 ± 7.2 | 26.4 ± 4.1 | |||
| Δ Change | 4.4 ± 3.7 | 2.9 ± 3.6 | |||
| Handgrip Left (kg) | Time | Time × Group | Group | ||
| Baseline | 26.4 ± 10.5 | 27.1 ± 10.9 | 0.018 | 0.616 | 0.966 |
| post | 29.5 ± 13.2 | 29.2 ± 9.3 | |||
| Δ Change | 3.1 ± 5.1 | 2.1 ± 4.1 | |||
| Handgrip Right (kg) | Time | Time × Group | Group | ||
| Baseline | 28.9 ± 11.4 | 26.1 ± 9.8 | 0.010 | 0.957 | 0.568 |
| post | 31.5 ± 14.7 | 28.7 ± 7.9 | |||
| Δ Change | 2.5 ± 4.3 | 2.6 ± 4.1 | |||
| TUG (s) | Time | Time × Group | Group | ||
| Baseline | 6.8 ± 1.1 | 6.9 ± 0.5 | 0.093 | 0.010 | 0.157 |
| post | 6.1 ± 0.9 | 7.1 ± 0.3 | |||
| Δ change | −0.6 ± 0.8 # | 0.1 ± 0.4 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavros, A.; Andreou, E.; Aphamis, G.; Bogdanis, G.C.; Sakkas, G.K.; Roupa, Z.; Giannaki, C.D. The Effects of Zinc and Selenium Co-Supplementation on Resting Metabolic Rate, Thyroid Function, Physical Fitness, and Functional Capacity in Overweight and Obese People under a Hypocaloric Diet: A Randomized, Double-Blind, and Placebo-Controlled Trial. Nutrients 2023, 15, 3133. https://doi.org/10.3390/nu15143133
Zavros A, Andreou E, Aphamis G, Bogdanis GC, Sakkas GK, Roupa Z, Giannaki CD. The Effects of Zinc and Selenium Co-Supplementation on Resting Metabolic Rate, Thyroid Function, Physical Fitness, and Functional Capacity in Overweight and Obese People under a Hypocaloric Diet: A Randomized, Double-Blind, and Placebo-Controlled Trial. Nutrients. 2023; 15(14):3133. https://doi.org/10.3390/nu15143133
Chicago/Turabian StyleZavros, Antonis, Eleni Andreou, George Aphamis, Gregory C. Bogdanis, Giorgos K. Sakkas, Zoe Roupa, and Christoforos D. Giannaki. 2023. "The Effects of Zinc and Selenium Co-Supplementation on Resting Metabolic Rate, Thyroid Function, Physical Fitness, and Functional Capacity in Overweight and Obese People under a Hypocaloric Diet: A Randomized, Double-Blind, and Placebo-Controlled Trial" Nutrients 15, no. 14: 3133. https://doi.org/10.3390/nu15143133
APA StyleZavros, A., Andreou, E., Aphamis, G., Bogdanis, G. C., Sakkas, G. K., Roupa, Z., & Giannaki, C. D. (2023). The Effects of Zinc and Selenium Co-Supplementation on Resting Metabolic Rate, Thyroid Function, Physical Fitness, and Functional Capacity in Overweight and Obese People under a Hypocaloric Diet: A Randomized, Double-Blind, and Placebo-Controlled Trial. Nutrients, 15(14), 3133. https://doi.org/10.3390/nu15143133









