A Mango Leaf Extract (Zynamite®) Combined with Quercetin Has Exercise-Mimetic Properties in Human Skeletal Muscle
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. General Overview
2.3. Pre-Tests and Familiarization
2.4. Main Experiments and Supplement Administration
2.5. Muscle Protein Extraction and Western Blotting
2.6. Statistical Analysis
3. Results
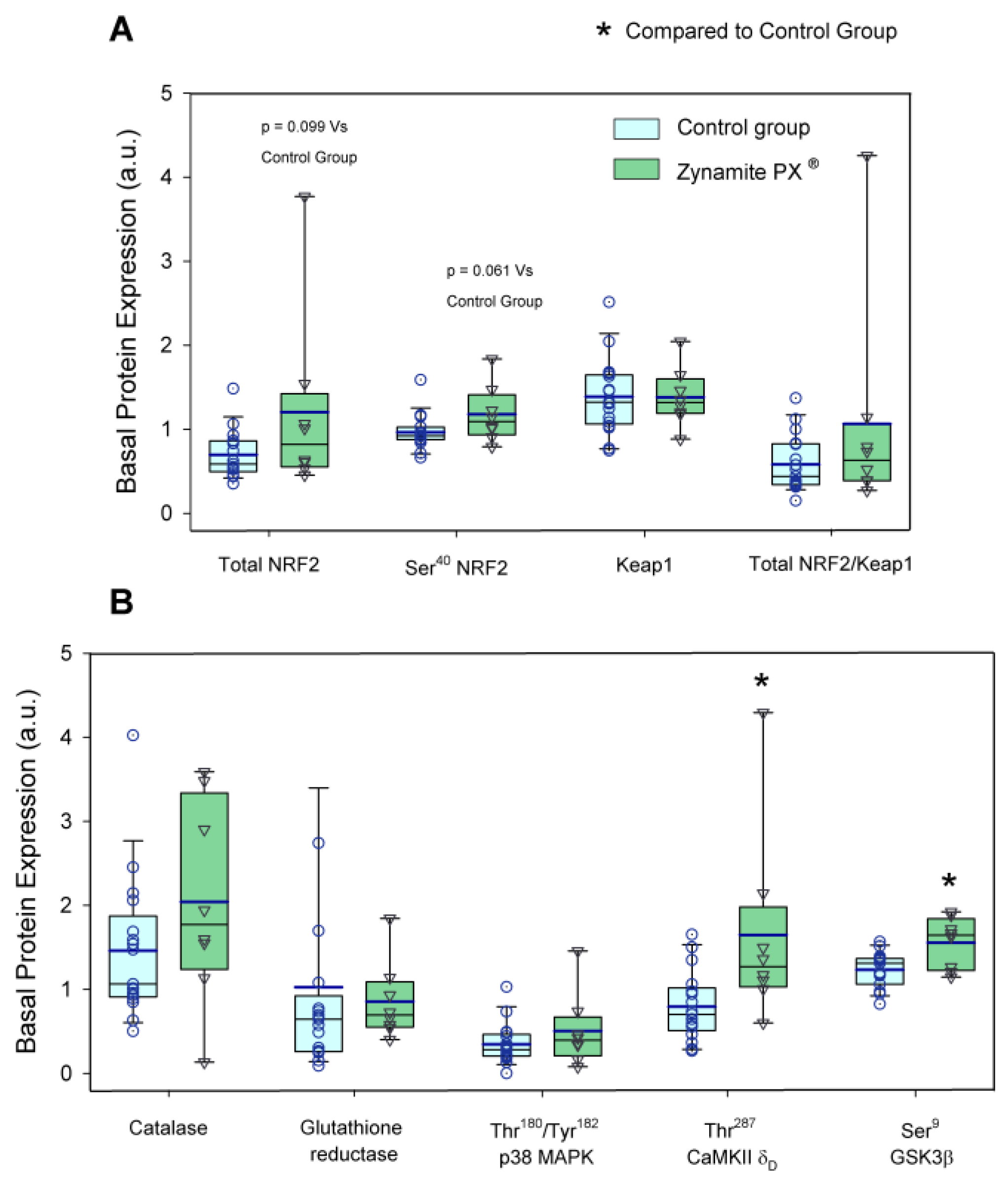
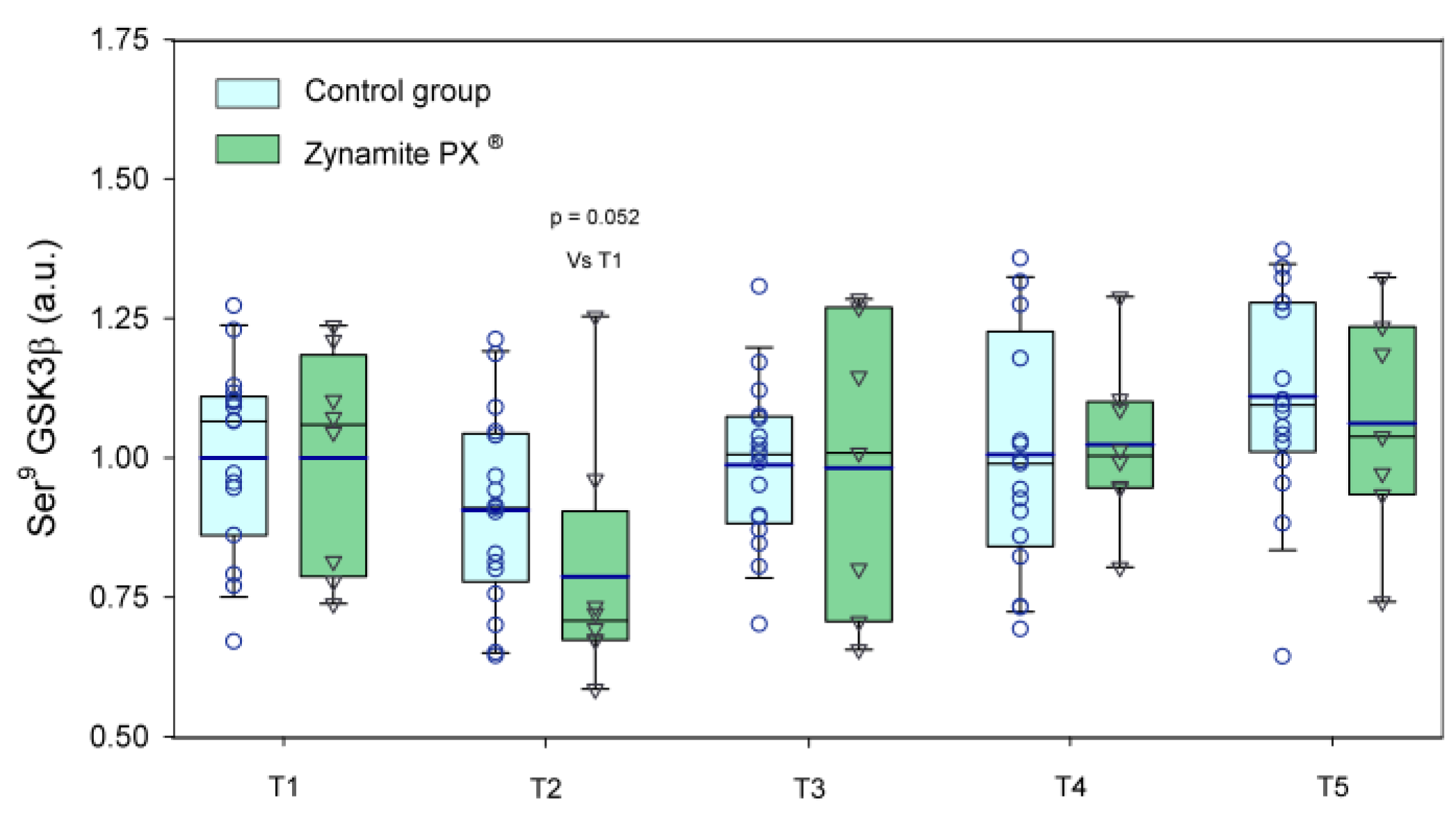
3.1. Effects of Zynamite PX® Supplementation on Basal Signalling
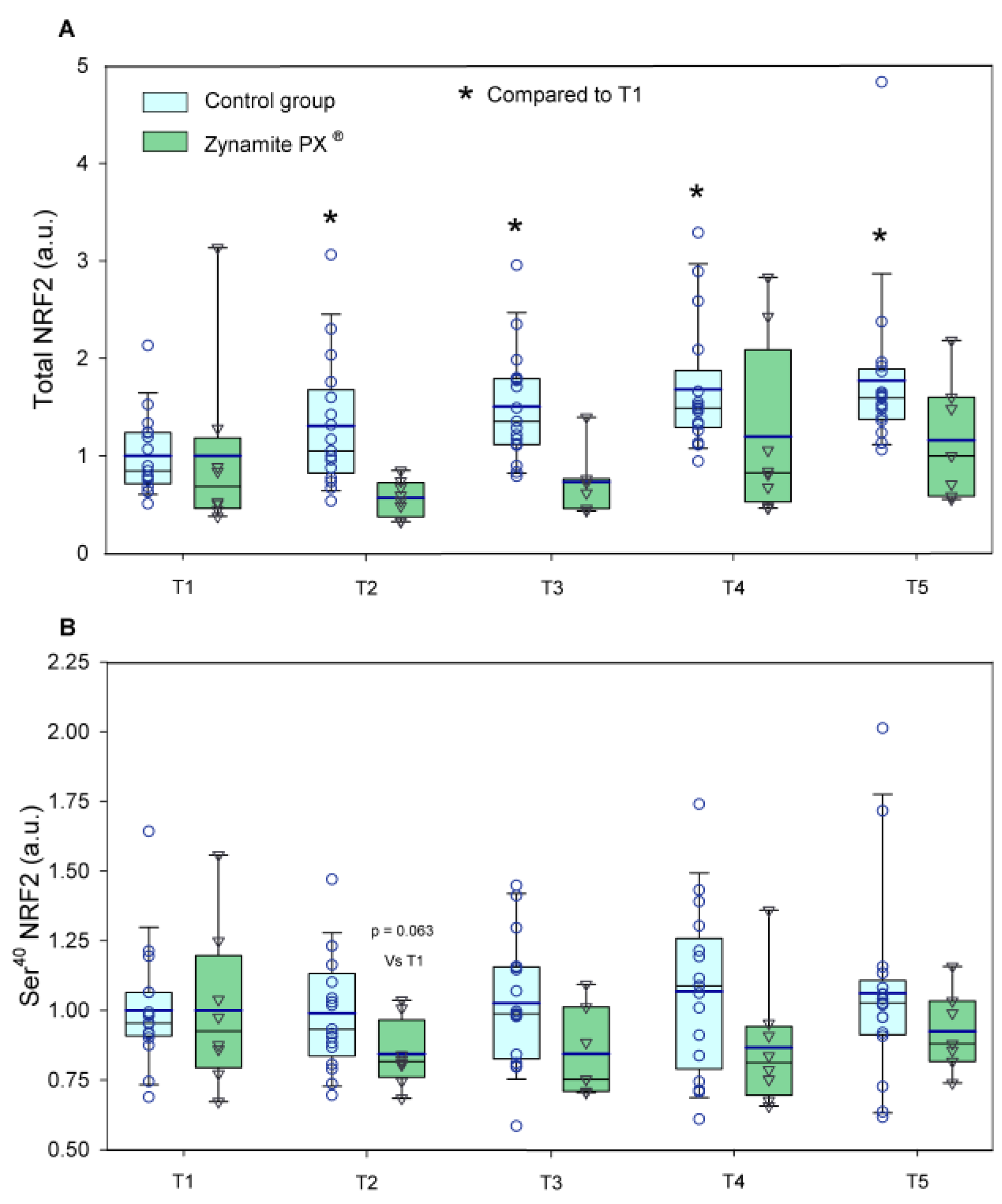
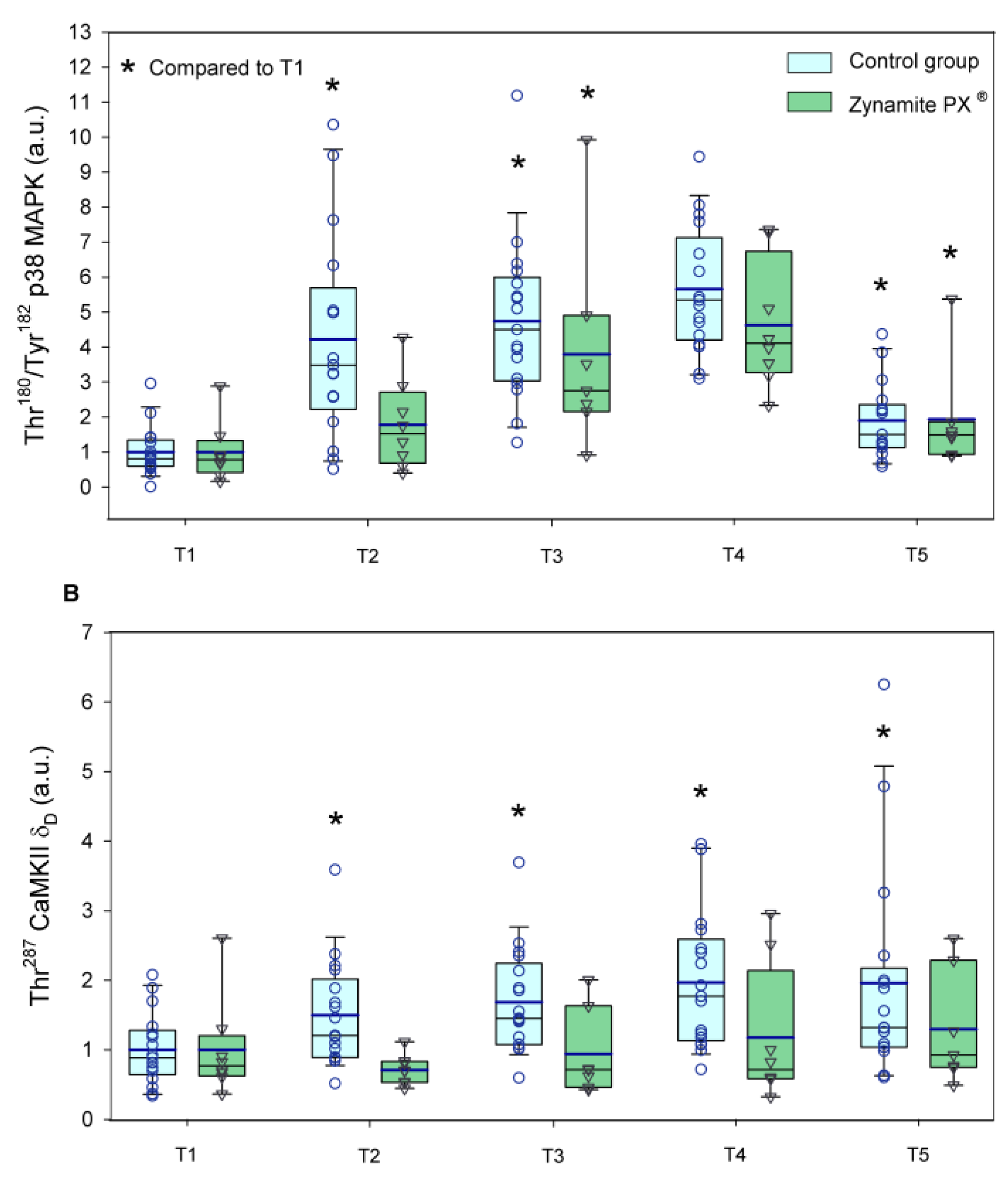
3.2. Effects of Zynamite PX® Supplementation Signalling Response to Exercise
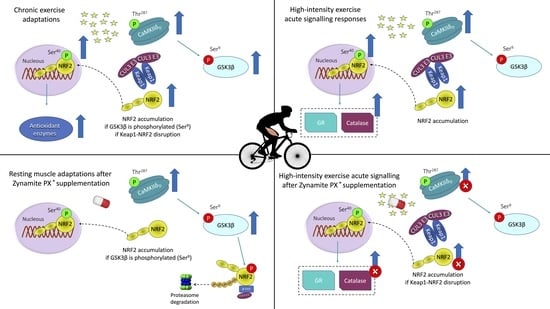
4. Discussion
4.1. Potential Benefits Associated with Increased Inhibition of GSK3β
4.2. Zynamite PX® Increases Basal Levels of CaMKII Phosphorylation (Thr287-CaMKIIδD)
4.3. Zynamite PX® Supplementation Attenuates the Activation of Stress Kinases and Redox Signalling in Response to High-Intensity Exercise to Exhaustion
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bowtell, J.; Kelly, V. Fruit-Derived Polyphenol Supplementation for Athlete Recovery and Performance. Sports Med. 2019, 49, 3–23. [Google Scholar] [CrossRef]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015, 88, 108–146. [Google Scholar] [CrossRef]
- Martin-Rincon, M.; Gelabert-Rebato, M.; Galvan-Alvarez, V.; Gallego-Selles, A.; Martinez-Canton, M.; Lopez-Rios, L.; Wiebe, J.C.; Martin-Rodriguez, S.; Arteaga-Ortiz, R.; Dorado, C.; et al. Supplementation with a Mango Leaf Extract (Zynamite(R)) in Combination with Quercetin Attenuates Muscle Damage and Pain and Accelerates Recovery after Strenuous Damaging Exercise. Nutrients 2020, 12, 614. [Google Scholar] [CrossRef] [PubMed]
- Gelabert-Rebato, M.; Wiebe, J.C.; Martin-Rincon, M.; Gericke, N.; Perez-Valera, M.; Curtelin, D.; Galvan-Alvarez, V.; Lopez-Rios, L.; Morales-Alamo, D.; Calbet, J.A.L. Mangifera indica L. leaf extract in combination with luteolin or quercetin enhances VO2peak and peak power output, and preserves skeletal muscle function during ischemia-reperfusion in humans. Front. Physiol. 2018, 9, 740. [Google Scholar] [CrossRef] [PubMed]
- Gelabert-Rebato, M.; Wiebe, J.C.; Martin-Rincon, M.; Galvan-Alvarez, V.; Curtelin, D.; Perez-Valera, M.; Habib, J.J.; Pérez-López, A.; Vega, T.; Morales-Alamo, D.; et al. Enhancement of exercise performance by 48 hours, and 15-day supplementation with mangiferin and luteolin in men. Nutrients 2019, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- Gelabert-Rebato, M.; Martin-Rincon, M.; Galvan-Alvarez, V.; Gallego-Selles, A.; Martinez-Canton, M.; Vega-Morales, T.; Wiebe, J.C.; Fernandez-Del Castillo, C.; Castilla-Hernandez, E.; Diaz-Tiberio, O.; et al. A single dose of the mango leaf extract Zynamite((R)) in combination with quercetin enhances peak power output during repeated sprint exercise in men and women. Nutrients 2019, 11, 2592. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rios, L.; Wiebe, J.C.; Vega-Morales, T.; Gericke, N. Central nervous system activities of extract Mangifera indica L. J. Ethnopharmacol. 2020, 260, 112996. [Google Scholar] [CrossRef]
- Morales-Alamo, D.; Calbet, J.A. Free radicals and sprint exercise in humans. Free Radic. Res. 2014, 48, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Ghosh, J.; Roy, A.; Sil, P.C. Mangiferin exerts hepatoprotective activity against D-galactosamine induced acute toxicity and oxidative/nitrosative stress via Nrf2-NFkappaB pathways. Toxicol. Appl. Pharmacol. 2012, 260, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.Y.; Zhang, F.; Zhang, L.; Liu, H.Z.; Zhao, Z.M.; Wen, X.R.; Wu, J.; Qi, D.S.; Sun, Y.; Du, Y.; et al. Mangiferin regulates interleukin-6 and cystathionine-b-synthase in lipopolysaccharide-induced brain injury. Cell. Mol. Neurobiol. 2014, 34, 651–657. [Google Scholar] [CrossRef]
- Luczkiewicz, P.; Kokotkiewicz, A.; Dampc, A.; Luczkiewicz, M. Mangiferin: A promising therapeutic agent for rheumatoid arthritis treatment. Med. Hypotheses 2014, 83, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Chaudhary, M.J.; Tiwari, P.C.; Nath, R.; Pant, K.K. Pharmacological and biochemical studies on protective effects of mangiferin and its interaction with nitric oxide (NO) modulators in adjuvant-induced changes in arthritic parameters, inflammatory, and oxidative biomarkers in rats. Inflammopharmacology 2019, 27, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Suchal, K.; Malik, S.; Khan, S.I.; Malhotra, R.K.; Goyal, S.N.; Bhatia, J.; Kumari, S.; Ojha, S.; Arya, D.S. Protective effect of mangiferin on myocardial ischemia-reperfusion injury in streptozotocin-induced diabetic rats: Role of AGE-RAGE/MAPK pathways. Sci. Rep. 2017, 7, 42027. [Google Scholar] [CrossRef]
- Niu, Y.; Liu, J.; Liu, H.Y.; Gao, L.H.; Feng, G.H.; Liu, X.; Li, L. Hypouricaemic action of mangiferin results from metabolite norathyriol via inhibiting xanthine oxidase activity. Pharm. Biol. 2016, 54, 1680–1686. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Beara, I.; Simin, N.; Pintac, D.; Majkic, T.; Bekvalac, K.; Orcic, D.; Mimica-Dukic, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Braakhuis, A.J.; Hopkins, W.G. Impact of Dietary Antioxidants on Sport Performance: A Review. Sports Med. 2015, 45, 939–955. [Google Scholar] [CrossRef]
- Gendy, A.M.; El-Gazar, A.A.; Ragab, G.M.; Al-Mokaddem, A.K.; El-Haddad, A.E.; Selim, H.; Yousef, E.M.; Hamed, N.O.; Ibrahim, S.S.A. Possible Implication of Nrf2, PPAR-gamma and MAPKs Signaling in the Protective Role of Mangiferin against Renal Ischemia/Reperfusion in Rats. Pharmaceuticals 2022, 16, 6. [Google Scholar] [CrossRef]
- Cheng, J.; Ren, C.; Cheng, R.; Li, Y.; Liu, P.; Wang, W.; Liu, L. Mangiferin ameliorates cardiac fibrosis in D-galactose-induced aging rats by inhibiting TGF-beta/p38/MK2 signaling pathway. Korean J. Physiol. Pharmacol. 2021, 25, 131–137. [Google Scholar] [CrossRef] [PubMed]
- El-Sayyad, S.M.; Soubh, A.A.; Awad, A.S.; El-Abhar, H.S. Mangiferin protects against intestinal ischemia/reperfusion-induced liver injury: Involvement of PPAR-gamma, GSK-3beta and Wnt/beta-catenin pathway. Eur. J. Pharmacol. 2017, 809, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Kampa, R.P.; Sek, A.; Bednarczyk, P.; Szewczyk, A.; Calderone, V.; Testai, L. Flavonoids as new regulators of mitochondrial potassium channels: Contribution to cardioprotection. J. Pharm. Pharmacol. 2023, 75, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.; Radman, G.; Aria, N.; Rezaei, F.; Khajenouri, M.; Ghiabi, S.; Bagheri, Y. The Effects of Quercetin on Apoptosis and Antioxidant Activity in a Renal Ischemia/Reperfusion Injury Animal Model. Drug. Res. 2023, 73, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Shen, Y.J.; Chen, M.; Zhao, J.Y.; Chen, S.H.; Zhang, W.; Song, J.K.; Li, L.; Du, G.H. Quercetin attenuates ischemia reperfusion injury by protecting the blood-brain barrier through Sirt1 in MCAO rats. J. Asian Nat. Prod. Res. 2022, 24, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Ekinci Akdemir, F.N.; Gulcin, I.; Karagoz, B.; Soslu, R. Quercetin protects rat skeletal muscle from ischemia reperfusion injury. J. Enzyme Inhib. Med. Chem. 2016, 31, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Ozyurek, M.; Bektasoglu, B.; Guclu, K.; Apak, R. Measurement of xanthine oxidase inhibition activity of phenolics and flavonoids with a modified cupric reducing antioxidant capacity (CUPRAC) method. Anal. Chim. Acta 2009, 636, 42–50. [Google Scholar] [CrossRef]
- Holland, J.A.; O’Donnell, R.W.; Chang, M.M.; Johnson, D.K.; Ziegler, L.M. Endothelial cell oxidant production: Effect of NADPH oxidase inhibitors. Endothelium 2000, 7, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Ji, L.L.; Kavazis, A.N.; Jackson, M.J. Reactive oxygen species: Impact on skeletal muscle. Compr. Physiol. 2011, 1, 941–969. [Google Scholar] [CrossRef] [PubMed]
- Calbet, J.A.L.; Martin-Rodriguez, S.; Martin-Rincon, M.; Morales-Alamo, D. An integrative approach to the regulation of mitochondrial respiration during exercise: Focus on high-intensity exercise. Redox Biol. 2020, 35, 101478. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.V.; Vasilaki, A.; Larkin, L.M.; McArdle, A.; Jackson, M.J. Repeated bouts of aerobic exercise lead to reductions in skeletal muscle free radical generation and nuclear factor kappaB activation. J. Physiol. 2008, 586, 3979–3990. [Google Scholar] [CrossRef]
- Morales-Alamo, D.; Ponce-Gonzalez, J.G.; Guadalupe-Grau, A.; Rodriguez-Garcia, L.; Santana, A.; Cusso, M.R.; Guerrero, M.; Guerra, B.; Dorado, C.; Calbet, J.A. Increased oxidative stress and anaerobic energy release, but blunted Thr172-AMPKalpha phosphorylation, in response to sprint exercise in severe acute hypoxia in humans. J. Appl. Physiol. 2012, 113, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.J.; Yamada, T.; Rassier, D.E.; Andersson, D.C.; Westerblad, H.; Lanner, J.T. Reactive oxygen/nitrogen species and contractile function in skeletal muscle during fatigue and recovery. J. Physiol. 2016, 594, 5149–5160. [Google Scholar] [CrossRef]
- Islam, H.; Bonafiglia, J.T.; Turnbull, P.C.; Simpson, C.A.; Perry, C.G.R.; Gurd, B.J. The impact of acute and chronic exercise on Nrf2 expression in relation to markers of mitochondrial biogenesis in human skeletal muscle. Eur. J. Appl. Physiol. 2020, 120, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, M.; Gunnarsson, T.P.; Hostrup, M.; Iaia, F.M.; Schena, F.; Pilegaard, H.; Bangsbo, J. Metabolic stress-dependent regulation of the mitochondrial biogenic molecular response to high-intensity exercise in human skeletal muscle. J. Physiol. 2018, 596, 2823–2840. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Radak, Z.; Ji, L.L. Exercise-induced oxidative stress: Past, present and future. J. Physiol. 2016, 594, 5081–5092. [Google Scholar] [CrossRef] [PubMed]
- Schlittler, M.; Neyroud, D.; Tanga, C.; Zanou, N.; Kamandulis, S.; Skurvydas, A.; Kayser, B.; Westerblad, H.; Place, N.; Andersson, D.C. Three weeks of sprint interval training improved high-intensity cycling performance and limited ryanodine receptor modifications in recreationally active human subjects. Eur. J. Appl. Physiol. 2019, 119, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Morales-Alamo, D.; Ponce-Gonzalez, J.G.; Guadalupe-Grau, A.; Rodriguez-Garcia, L.; Santana, A.; Cusso, R.; Guerrero, M.; Dorado, C.; Guerra, B.; Calbet, J.A. Critical role for free radicals on sprint exercise-induced CaMKII and AMPKalpha phosphorylation in human skeletal muscle. J. Appl. Physiol. 2013, 114, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Selles, A.; Martin-Rincon, M.; Martinez-Canton, M.; Perez-Valera, M.; Martin-Rodriguez, S.; Gelabert-Rebato, M.; Santana, A.; Morales-Alamo, D.; Dorado, C.; Calbet, J.A.L. Regulation of Nrf2/Keap1 signalling in human skeletal muscle during exercise to exhaustion in normoxia, severe acute hypoxia and post-exercise ischaemia: Influence of metabolite accumulation and oxygenation. Redox Biol. 2020, 36, 101627. [Google Scholar] [CrossRef]
- Merry, T.L.; Ristow, M. Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and the anti-oxidant response in mice. J. Physiol. 2016, 594, 5195–5207. [Google Scholar] [CrossRef] [PubMed]
- Merry, T.L.; MacRae, C.; Pham, T.; Hedges, C.P.; Ristow, M. Deficiency in ROS-sensing nuclear factor erythroid 2-like 2 causes altered glucose and lipid homeostasis following exercise training. Am. J. Physiol. Cell Physiol. 2020, 318, C337–C345. [Google Scholar] [CrossRef]
- Done, A.J.; Traustadottir, T. Nrf2 mediates redox adaptations to exercise. Redox Biol. 2016, 10, 191–199. [Google Scholar] [CrossRef]
- Williamson, D.; Gallagher, P.; Harber, M.; Hollon, C.; Trappe, S. Mitogen-activated protein kinase (MAPK) pathway activation: Effects of age and acute exercise on human skeletal muscle. J. Physiol. 2003, 547, 977–987. [Google Scholar] [CrossRef]
- Guerra, B.; Olmedillas, H.; Guadalupe-Grau, A.; Ponce-Gonzalez, J.G.; Morales-Alamo, D.; Fuentes, T.; Chapinal, E.; Fernandez-Perez, L.; De Pablos-Velasco, P.; Santana, A.; et al. Is sprint exercise a leptin signaling mimetic in human skeletal muscle? J. Appl. Physiol. 2011, 111, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Crilly, M.J.; Tryon, L.D.; Erlich, A.T.; Hood, D.A. The role of Nrf2 in skeletal muscle contractile and mitochondrial function. J. Appl. Physiol. 2016, 121, 730–740. [Google Scholar] [CrossRef]
- Bhat, A.; Abu, R.; Jagadesan, S.; Vellichirammal, N.N.; Pendyala, V.V.; Yu, L.; Rudebush, T.L.; Guda, C.; Zucker, I.H.; Kumar, V.; et al. Quantitative Proteomics Identifies Novel Nrf2-Mediated Adaptative Signaling Pathways in Skeletal Muscle following Exercise Training. Antioxidants 2023, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Rada, P.; Rojo, A.I.; Chowdhry, S.; McMahon, M.; Hayes, J.D.; Cuadrado, A. SCF/beta-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol. Cell. Biol. 2011, 31, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Rada, P.; Rojo, A.I.; Evrard-Todeschi, N.; Innamorato, N.G.; Cotte, A.; Jaworski, T.; Tobon-Velasco, J.C.; Devijver, H.; Garcia-Mayoral, M.F.; Van Leuven, F.; et al. Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/beta-TrCP axis. Mol. Cell. Biol. 2012, 32, 3486–3499. [Google Scholar] [CrossRef] [PubMed]
- Jayasuriya, R.; Ramkumar, K.M. Mangiferin alleviates hyperglycemia-induced endothelial impairment via Nrf2 signaling pathway. Eur. J. Pharmacol. 2022, 936, 175359. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Sheng, Y.C.; Zheng, Z.Y.; Shi, L.; Wang, Z.T. The involvement of p62-Keap1-Nrf2 antioxidative signaling pathway and JNK in the protection of natural flavonoid quercetin against hepatotoxicity. Free Radic. Biol. Med. 2015, 85, 12–23. [Google Scholar] [CrossRef]
- Powers, S.K.; Schrager, M. Redox signaling regulates skeletal muscle remodeling in response to exercise and prolonged inactivity. Redox Biol. 2022, 54, 102374. [Google Scholar] [CrossRef]
- Bojarczuk, A.; Dzitkowska-Zabielska, M. Polyphenol Supplementation and Antioxidant Status in Athletes: A Narrative Review. Nutrients 2022, 15, 158. [Google Scholar] [CrossRef]
- Gao, L.; Kumar, V.; Vellichirammal, N.N.; Park, S.Y.; Rudebush, T.L.; Yu, L.; Son, W.M.; Pekas, E.J.; Wafi, A.M.; Hong, J.; et al. Functional, proteomic and bioinformatic analyses of Nrf2- and Keap1- null skeletal muscle. J. Physiol. 2020, 598, 5427–5451. [Google Scholar] [CrossRef]
- Song, B.; Lai, B.; Zheng, Z.; Zhang, Y.; Luo, J.; Wang, C.; Chen, Y.; Woodgett, J.R.; Li, M. Inhibitory phosphorylation of GSK-3 by CaMKII couples depolarization to neuronal survival. J. Biol. Chem. 2010, 285, 41122–41134. [Google Scholar] [CrossRef] [PubMed]
- Martin-Rincon, M.; Gelabert-Rebato, M.; Perez-Valera, M.; Galvan-Alvarez, V.; Morales-Alamo, D.; Dorado, C.; Boushel, R.; Hallen, J.; Calbet, J.A.L. Functional reserve and sex differences during exercise to exhaustion revealed by post-exercise ischaemia and repeated supramaximal exercise. J. Physiol. 2021, 599, 3853–3878. [Google Scholar] [CrossRef] [PubMed]
- Morales-Alamo, D.; Losa-Reyna, J.; Torres-Peralta, R.; Martin-Rincon, M.; Perez-Valera, M.; Curtelin, D.; Ponce-Gonzalez, J.G.; Santana, A.; Calbet, J.A. What limits performance during whole-body incremental exercise to exhaustion in humans? J. Physiol. 2015, 593, 4631–4648. [Google Scholar] [CrossRef] [PubMed]
- Martin-Rincon, M.; Gonzalez-Henriquez, J.J.; Losa-Reyna, J.; Perez-Suarez, I.; Ponce-Gonzalez, J.G.; de La Calle-Herrero, J.; Perez-Valera, M.; Perez-Lopez, A.; Curtelin, D.; Cherouveim, E.D.; et al. Impact of data averaging strategies on VO2max assessment: Mathematical modeling and reliability. Scand. J. Med. Sci. Sports 2019, 29, 1473–1488. [Google Scholar] [CrossRef]
- Perez-Suarez, I.; Martin-Rincon, M.; Gonzalez-Henriquez, J.J.; Fezzardi, C.; Perez-Regalado, S.; Galvan-Alvarez, V.; Juan-Habib, J.W.; Morales-Alamo, D.; Calbet, J.A.L. Accuracy and Precision of the COSMED K5 Portable Analyser. Front. Physiol. 2018, 9, 1764. [Google Scholar] [CrossRef] [PubMed]
- Guerra, B.; Gomez-Cabrera, M.C.; Ponce-Gonzalez, J.G.; Martinez-Bello, V.E.; Guadalupe-Grau, A.; Santana, A.; Sebastia, V.; Vina, J.; Calbet, J.A. Repeated muscle biopsies through a single skin incision do not elicit muscle signaling, but IL-6 mRNA and STAT3 phosphorylation increase in injured muscle. J. Appl. Physiol. 2011, 110, 1708–1715. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Gallego-Selles, A.; Galvan-Alvarez, V.; Martinez-Canton, M.; Garcia-Gonzalez, E.; Morales-Alamo, D.; Santana, A.; Gonzalez-Henriquez, J.J.; Dorado, C.; Calbet, J.A.L.; Martin-Rincon, M. Fast regulation of the NF-kappaB signalling pathway in human skeletal muscle revealed by high-intensity exercise and ischaemia at exhaustion: Role of oxygenation and metabolite accumulation. Redox Biol. 2022, 55, 102398. [Google Scholar] [CrossRef]
- Martinez-Canton, M.; Gallego-Selles, A.; Gelabert-Rebato, M.; Martin-Rincon, M.; Pareja-Blanco, F.; Rodriguez-Rosell, D.; Morales-Alamo, D.; Sanchis-Moysi, J.; Dorado, C.; Jose Gonzalez-Badillo, J.; et al. Role of CaMKII and sarcolipin in muscle adaptations to strength training with different levels of fatigue in the set. Scand. J. Med. Sci. Sports 2021, 31, 91–103. [Google Scholar] [CrossRef]
- Chowdhry, S.; Zhang, Y.; McMahon, M.; Sutherland, C.; Cuadrado, A.; Hayes, J.D. Nrf2 is controlled by two distinct beta-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene 2013, 32, 3765–3781. [Google Scholar] [CrossRef] [PubMed]
- Cross, D.A.; Alessi, D.R.; Cohen, P.; Andjelkovich, M.; Hemmings, B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995, 378, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Doble, B.W.; Woodgett, J.R. GSK-3: Tricks of the trade for a multi-tasking kinase. J. Cell Sci. 2003, 116, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Mascher, H.; Andersson, H.; Nilsson, P.A.; Ekblom, B.; Blomstrand, E. Changes in signalling pathways regulating protein synthesis in human muscle in the recovery period after endurance exercise. Acta Physiol. 2007, 191, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Hanakita, H.; Uemichi, K.; Takemasa, T. Effect of the order of concurrent training combined with resistance and high-intensity interval exercise on mTOR signaling and glycolytic metabolism in mouse skeletal muscle. Physiol. Rep. 2021, 9, e14770. [Google Scholar] [CrossRef] [PubMed]
- Drescher, C.; Konishi, M.; Ebner, N.; Springer, J. Loss of muscle mass: Current developments in cachexia and sarcopenia focused on biomarkers and treatment. J. Cachexia Sarcopenia Muscle 2015, 6, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Drescher, C.; Konishi, M.; Ebner, N.; Springer, J. Loss of muscle mass: Current developments in cachexia and sarcopenia focused on biomarkers and treatment. Int. J. Cardiol. 2016, 202, 766–772. [Google Scholar] [CrossRef]
- Martin-Rincon, M.; Perez-Suarez, I.; Perez-Lopez, A.; Ponce-Gonzalez, J.G.; Morales-Alamo, D.; de Pablos-Velasco, P.; Holmberg, H.C.; Calbet, J.A.L. Protein synthesis signaling in skeletal muscle is refractory to whey protein ingestion during a severe energy deficit evoked by prolonged exercise and caloric restriction. Int. J. Obes. 2019, 43, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Schakman, O.; Kalista, S.; Bertrand, L.; Lause, P.; Verniers, J.; Ketelslegers, J.M.; Thissen, J.P. Role of Akt/GSK-3beta/beta-catenin transduction pathway in the muscle anti-atrophy action of insulin-like growth factor-I in glucocorticoid-treated rats. Endocrinology 2008, 149, 3900–3908. [Google Scholar] [CrossRef]
- Kurgan, N.; Whitley, K.C.; Maddalena, L.A.; Moradi, F.; Stoikos, J.; Hamstra, S.I.; Rubie, E.A.; Kumar, M.; Roy, B.D.; Woodgett, J.R.; et al. A Low-Therapeutic Dose of Lithium Inhibits GSK3 and Enhances Myoblast Fusion in C2C12 Cells. Cells 2019, 8, 1340. [Google Scholar] [CrossRef]
- Shin, S.; Wolgamott, L.; Yu, Y.; Blenis, J.; Yoon, S.O. Glycogen synthase kinase (GSK)-3 promotes p70 ribosomal protein S6 kinase (p70S6K) activity and cell proliferation. Proc. Natl. Acad. Sci. USA 2011, 108, E1204-1213. [Google Scholar] [CrossRef]
- Ciaraldi, T.P.; Carter, L.; Mudaliar, S.; Henry, R.R. GSK-3beta and control of glucose metabolism and insulin action in human skeletal muscle. Mol. Cell. Endocrinol. 2010, 315, 153–158. [Google Scholar] [CrossRef]
- McManus, E.J.; Sakamoto, K.; Armit, L.J.; Ronaldson, L.; Shpiro, N.; Marquez, R.; Alessi, D.R. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005, 24, 1571–1583. [Google Scholar] [CrossRef]
- Ivy, J.L.; Ding, Z.; Hwang, H.; Cialdella-Kam, L.C.; Morrison, P.J. Post exercise carbohydrate-protein supplementation: Phosphorylation of muscle proteins involved in glycogen synthesis and protein translation. Amino Acids 2008, 35, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Salih, M.; Leddy, J.J.; Tuana, B.S. The muscle-specific calmodulin-dependent protein kinase assembles with the glycolytic enzyme complex at the sarcoplasmic reticulum and modulates the activity of glyceraldehyde-3-phosphate dehydrogenase in a Ca2+/calmodulin-dependent manner. J. Biol. Chem. 2004, 279, 35176–35182. [Google Scholar] [CrossRef] [PubMed]
- Sacchetto, R.; Bovo, E.; Salviati, L.; Damiani, E.; Margreth, A. Glycogen synthase binds to sarcoplasmic reticulum and is phosphorylated by CaMKII in fast-twitch skeletal muscle. Arch. Biochem. Biophys. 2007, 459, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.S.; Paglialunga, S.; Vigna, C.; Ludzki, A.; Herbst, E.A.; Lally, J.S.; Schrauwen, P.; Hoeks, J.; Tupling, A.R.; Bonen, A.; et al. High-fat diet-induced mitochondrial biogenesis is regulated by mitochondrial-derived reactive oxygen species activation of CaMKII. Diabetes 2014, 63, 1907–1913. [Google Scholar] [CrossRef]
- Vila-Petroff, M.; Mundina-Weilenmann, C.; Lezcano, N.; Snabaitis, A.K.; Huergo, M.A.; Valverde, C.A.; Avkiran, M.; Mattiazzi, A. Ca(2+)/calmodulin-dependent protein kinase II contributes to intracellular pH recovery from acidosis via Na(+)/H(+) exchanger activation. J. Mol. Cell. Cardiol. 2010, 49, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Danila, C.I.; Hamilton, S.L. Phosphorylation of ryanodine receptors. Biol. Res. 2004, 37, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Joiner, M.L.; Koval, O.M.; Li, J.; He, B.J.; Allamargot, C.; Gao, Z.; Luczak, E.D.; Hall, D.D.; Fink, B.D.; Chen, B.; et al. CaMKII determines mitochondrial stress responses in heart. Nature 2012, 491, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Chin, E.R. The role of calcium and calcium/calmodulin-dependent kinases in skeletal muscle plasticity and mitochondrial biogenesis. Proc. Nutr. Soc. 2004, 63, 279–286. [Google Scholar] [CrossRef]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999, 98, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.R.; Joiner, M.L.; Guan, X.; Kutschke, W.; Yang, J.; Oddis, C.V.; Bartlett, R.K.; Lowe, J.S.; O’Donnell, S.E.; Aykin-Burns, N.; et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 2008, 133, 462–474. [Google Scholar] [CrossRef]
- Illario, M.; Monaco, S.; Cavallo, A.L.; Esposito, I.; Formisano, P.; D’Andrea, L.; Cipolletta, E.; Trimarco, B.; Fenzi, G.; Rossi, G.; et al. Calcium-calmodulin-dependent kinase II (CaMKII) mediates insulin-stimulated proliferation and glucose uptake. Cell. Signal. 2009, 21, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Ojuka, E.O.; Goyaram, V.; Smith, J.A. The role of CaMKII in regulating GLUT4 expression in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E322-331. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.T.; Shaw, C. Increased expression of deltaCaMKII isoforms in skeletal muscle regeneration: Implications in dystrophic muscle disease. J. Cell. Biochem. 2006, 97, 621–632. [Google Scholar] [CrossRef]
- Rose, A.J.; Kiens, B.; Richter, E.A. Ca2+-calmodulin-dependent protein kinase expression and signalling in skeletal muscle during exercise. J. Physiol. 2006, 574, 889–903. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, M.; Gunnarsson, T.P.; Christensen, P.M.; Pavlovic, D.; Shattock, M.J.; Bangsbo, J. Intensive training and reduced volume increases muscle FXYD1 expression and phosphorylation at rest and during exercise in athletes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R659–R669. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Blomstrand, E.; Chibalin, A.V.; Krook, A.; Zierath, J.R. Marathon running increases ERK1/2 and p38 MAP kinase signalling to downstream targets in human skeletal muscle. J. Physiol. 2001, 536, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.; Carson, B.P.; Garcia-Roves, P.M.; Chibalin, A.V.; Sarsfield, F.M.; Barron, N.; McCaffrey, N.; Moyna, N.M.; Zierath, J.R.; O’Gorman, D.J. Exercise intensity-dependent regulation of peroxisome proliferator-activated receptor coactivator-1 mRNA abundance is associated with differential activation of upstream signalling kinases in human skeletal muscle. J. Physiol. 2010, 588, 1779–1790. [Google Scholar] [CrossRef] [PubMed]
- Coffey, V.G.; Zhong, Z.; Shield, A.; Canny, B.J.; Chibalin, A.V.; Zierath, J.R.; Hawley, J.A. Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB J. 2006, 20, 190–192. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Borras, C.; Pallardo, F.V.; Sastre, J.; Ji, L.L.; Vina, J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J. Physiol. 2005, 567, 113–120. [Google Scholar] [CrossRef]
- Yfanti, C.; Akerstrom, T.; Nielsen, S.; Nielsen, A.R.; Mounier, R.; Mortensen, O.H.; Lykkesfeldt, J.; Rose, A.J.; Fischer, C.P.; Pedersen, B.K. Antioxidant supplementation does not alter endurance training adaptation. Med. Sci. Sports Exerc. 2010, 42, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M.; Zarse, K.; Oberbach, A.; Kloting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Bluher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef]
- Paulsen, G.; Cumming, K.T.; Holden, G.; Hallen, J.; Ronnestad, B.R.; Sveen, O.; Skaug, A.; Paur, I.; Bastani, N.E.; Ostgaard, H.N.; et al. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: A double-blind, randomised, controlled trial. J. Physiol. 2014, 592, 1887–1901. [Google Scholar] [CrossRef] [PubMed]
- Martin-Rincon, M.; Perez-Lopez, A.; Morales-Alamo, D.; Perez-Suarez, I.; de Pablos-Velasco, P.; Perez-Valera, M.; Perez-Regalado, S.; Martinez-Canton, M.; Gelabert-Rebato, M.; Juan-Habib, J.W.; et al. Exercise Mitigates the Loss of Muscle Mass by Attenuating the Activation of Autophagy during Severe Energy Deficit. Nutrients 2019, 11, 2824. [Google Scholar] [CrossRef] [PubMed]







| Control Group (n = 17) | Zynamite PX® Group (n = 8) | p | |
|---|---|---|---|
| Age (years) | 22.5 ± 2.4 | 21.6 ± 1.2 | 0.350 |
| Height (cm) | 178 ± 8 | 177 ± 9 | 0.786 |
| Weight (kg) | 72.7 ± 7.6 | 71.6 ± 7.4 | 0.730 |
| Body fat (%) | 18.6 ± 5.8 | 18.8 ± 3.6 | 0.930 |
| Fat body mass (kg) | 13.7 ± 5.3 | 13.6 ± 3.7 | 0.930 |
| Lean body mass (kg) | 55.8 ± 5.1 | 54.9 ± 5.1 | 0.698 |
| VO2max (mL min−1) | 3432 ± 489 | 3281 ± 367 | 0.448 |
| VO2max (mL kg−1 min−1) | 47.5 ± 7.1 | 46.0 ± 4.7 | 0.594 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Canton, M.; Galvan-Alvarez, V.; Garcia-Gonzalez, E.; Gallego-Selles, A.; Gelabert-Rebato, M.; Garcia-Perez, G.; Santana, A.; Lopez-Rios, L.; Vega-Morales, T.; Martin-Rincon, M.; et al. A Mango Leaf Extract (Zynamite®) Combined with Quercetin Has Exercise-Mimetic Properties in Human Skeletal Muscle. Nutrients 2023, 15, 2848. https://doi.org/10.3390/nu15132848
Martinez-Canton M, Galvan-Alvarez V, Garcia-Gonzalez E, Gallego-Selles A, Gelabert-Rebato M, Garcia-Perez G, Santana A, Lopez-Rios L, Vega-Morales T, Martin-Rincon M, et al. A Mango Leaf Extract (Zynamite®) Combined with Quercetin Has Exercise-Mimetic Properties in Human Skeletal Muscle. Nutrients. 2023; 15(13):2848. https://doi.org/10.3390/nu15132848
Chicago/Turabian StyleMartinez-Canton, Miriam, Victor Galvan-Alvarez, Eduardo Garcia-Gonzalez, Angel Gallego-Selles, Miriam Gelabert-Rebato, Giovanni Garcia-Perez, Alfredo Santana, Laura Lopez-Rios, Tanausu Vega-Morales, Marcos Martin-Rincon, and et al. 2023. "A Mango Leaf Extract (Zynamite®) Combined with Quercetin Has Exercise-Mimetic Properties in Human Skeletal Muscle" Nutrients 15, no. 13: 2848. https://doi.org/10.3390/nu15132848
APA StyleMartinez-Canton, M., Galvan-Alvarez, V., Garcia-Gonzalez, E., Gallego-Selles, A., Gelabert-Rebato, M., Garcia-Perez, G., Santana, A., Lopez-Rios, L., Vega-Morales, T., Martin-Rincon, M., & Calbet, J. A. L. (2023). A Mango Leaf Extract (Zynamite®) Combined with Quercetin Has Exercise-Mimetic Properties in Human Skeletal Muscle. Nutrients, 15(13), 2848. https://doi.org/10.3390/nu15132848