LRG1 Associates with Iron Deficiency Anemia Markers in Adolescents
Abstract
1. Introduction
2. Material and Methods
2.1. Study Participants, Ethics, Consent, and Permissions
2.2. Blood Collection and Biochemical Analyses
2.3. ELISA Assays for LRG1
2.4. Statistical Analysis
3. Results
3.1. Description of the Study Group in School Adolescents
3.2. Elevation of Circulatory LRG1 Level in Anemia
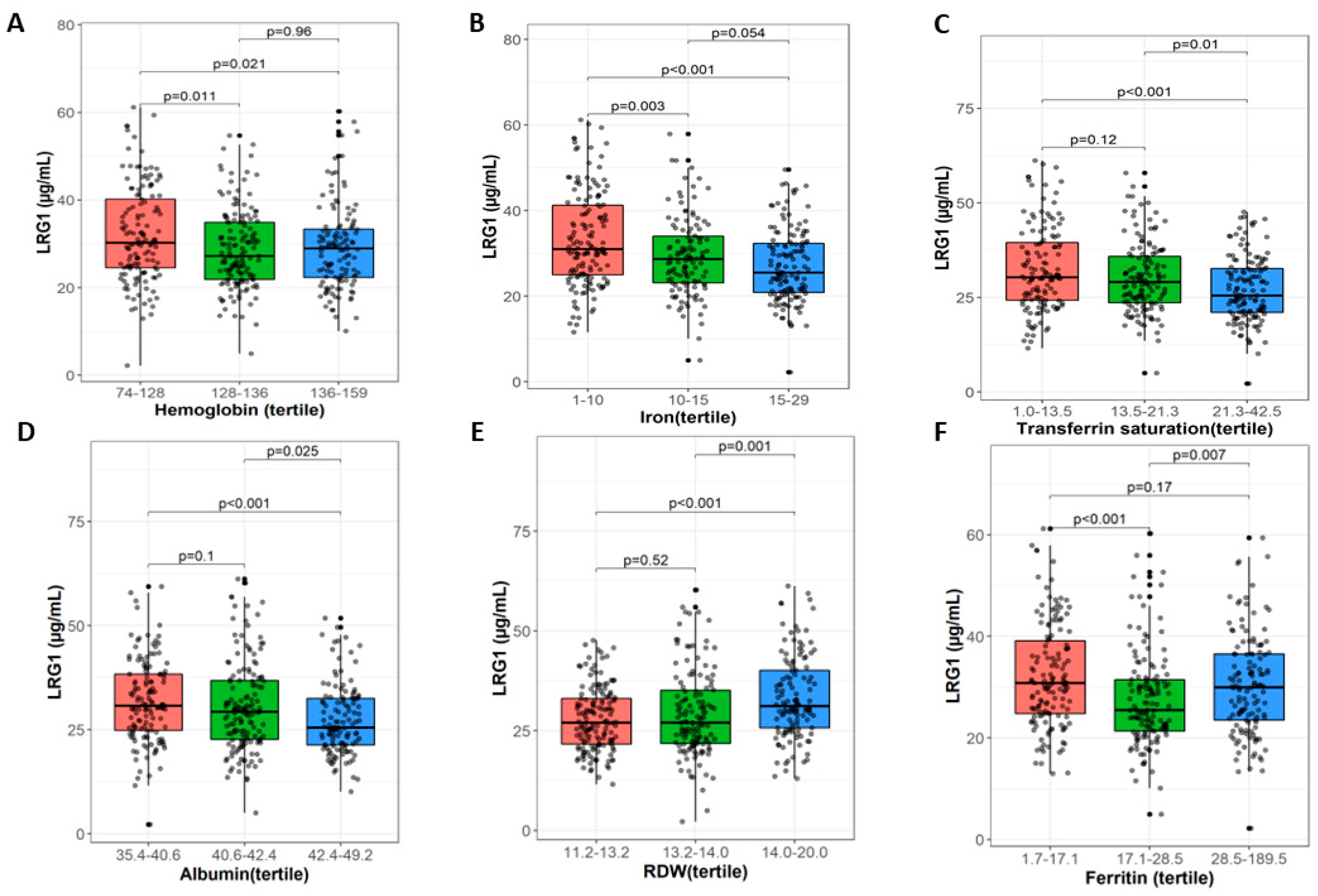
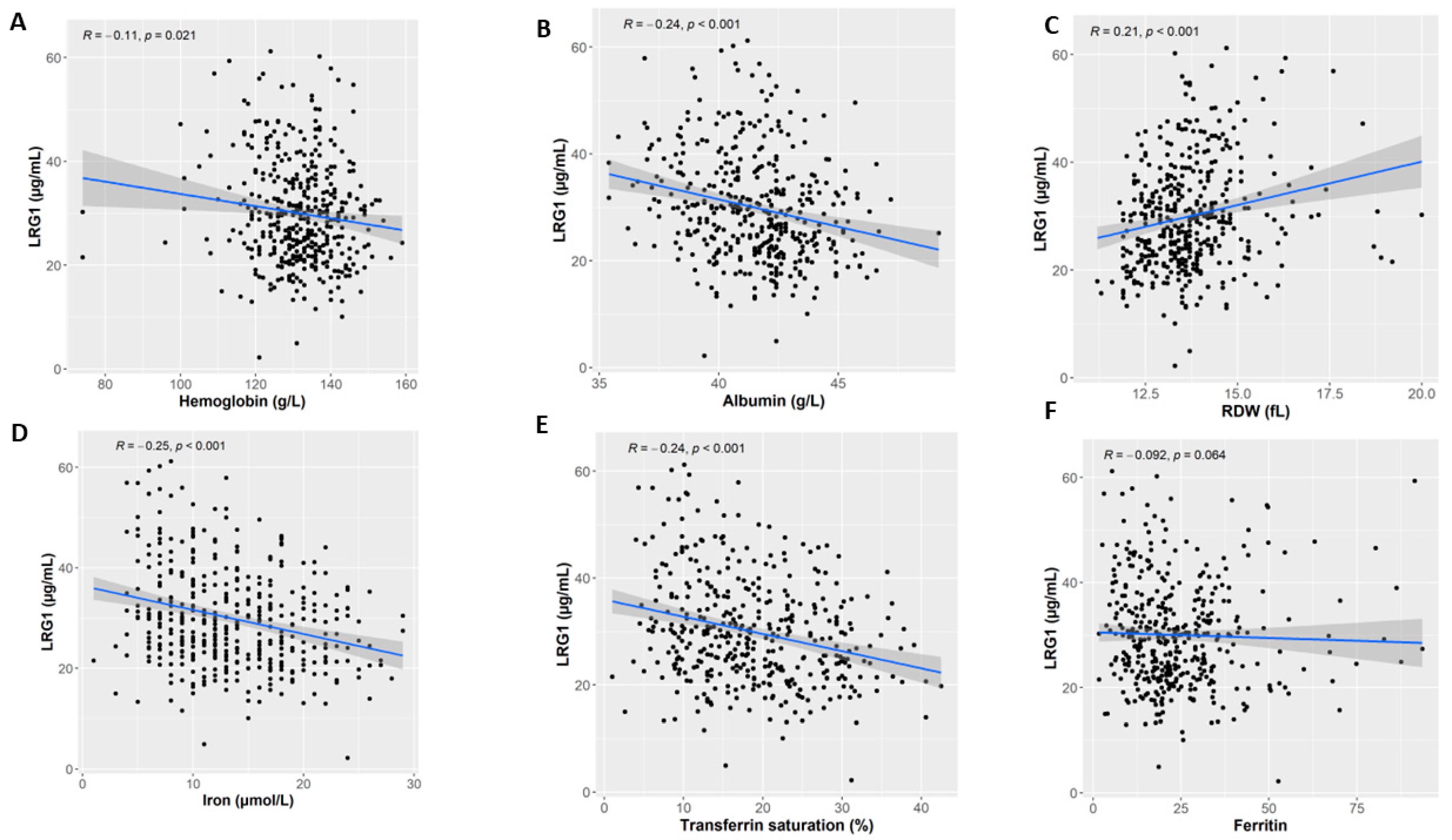
3.3. LRG1 Associates with Iron Deficiency Anemia Markers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Safiri, S.; Kolahi, A.-A.; Noori, M.; Nejadghaderi, S.A.; Karamzad, N.; Bragazzi, N.L.; Sullman, M.J.M.; Abdollahi, M.; Collins, G.S.; Kaufman, J.S.; et al. Burden of anemia and its underlying causes in 204 countries and territories, 1990–2019: Results from the Global Burden of Disease Study 2019. J. Hematol. Oncol. 2021, 14, 185. [Google Scholar] [CrossRef]
- Haas, J.D.; Brownlie, T., IV. Iron deficiency and reduced work capacity: A critical review of the research to determine a causal relationship. J. Nutr. 2001, 131, 676S–688S, discussion 688S–690S. [Google Scholar] [CrossRef]
- Jáuregui-Lobera, I. Iron deficiency and cognitive functions. Neuropsychiatr. Dis. Treat. 2014, 10, 2087–2095. [Google Scholar] [CrossRef]
- Chaparro, C.M.; Suchdev, P.S. Anemia epidemiology, pathophysiology, and etiology in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2019, 1450, 15–31. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Waldvogel-Abramowski, S.; Waeber, G.; Gassner, C.; Buser, A.; Frey, B.M.; Favrat, B.; Tissot, J.-D. Physiology of Iron Metabolism. Transfus. Med. Hemotherapy 2014, 41, 213–221. [Google Scholar] [CrossRef]
- Mackenzie, E.L.; Iwasaki, K.; Tsuji, Y.; Kalanaky, S.; Hafizi, M.; Safari, S.; Mousavizadeh, K.; Kabiri, M.; Farsinejad, A.; Fakharzadeh, S.; et al. Intracellular Iron Transport and Storage: From Molecular Mechanisms to Health Implications. Antioxid. Redox Signal. 2008, 10, 997–1030. [Google Scholar] [CrossRef]
- Ewilkinson, N.; Epantopoulos, K. The IRP/IRE system in vivo: Insights from mouse models. Front. Pharmacol. 2014, 5, 176. [Google Scholar] [CrossRef]
- Camilli, C.; Hoeh, A.E.; De Rossi, G.; Moss, S.E.; Greenwood, J. LRG1: An emerging player in disease pathogenesis. J. Biomed. Sci. 2022, 29, 6. [Google Scholar] [CrossRef]
- Alhammad, R.; Abu-Farha, M.; Hammad, M.M.; Thanaraj, T.A.; Channanath, A.; Alam-Eldin, N.; Al-Sabah, R.; Shaban, L.; Alduraywish, A.; Al-Mulla, F.; et al. Increased LRG1 Levels in Overweight and Obese Adolescents and Its Association with Obesity Markers, Including Leptin, Chemerin, and High Sensitivity C-Reactive Protein. Int. J. Mol. Sci. 2022, 23, 8564. [Google Scholar] [CrossRef]
- Zhang, A.; Fang, H.; Chen, J.; He, L.; Chen, Y. Role of VEGF-A and LRG1 in Abnormal Angiogenesis Associated with Diabetic Nephropathy. Front. Physiol. 2020, 11, 1064. [Google Scholar] [CrossRef]
- Mundo, L.; Tosi, G.M.; Lazzi, S.; Pertile, G.; Parolini, B.; Neri, G.; Posarelli, M.; De Benedetto, E.; Bacci, T.; Silvestri, E.; et al. LRG1 Expression Is Elevated in the Eyes of Patients with Neovascular Age-Related Macular Degeneration. Int. J. Mol. Sci. 2021, 22, 8879. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, L.; Fang, J.; Ge, Z.; Li, X. LRG1 modulates epithelial-mesenchymal transition and angiogenesis in colorectal cancer via HIF-1α activation. J. Exp. Clin. Cancer Res. 2016, 35, 29. [Google Scholar] [CrossRef]
- Feng, J.; Zhan, J.; Ma, S. LRG1 promotes hypoxia-induced cardiomyocyte apoptosis and autophagy by regulating hypoxia-inducible factor-1α. Bioengineered 2021, 12, 8897–8907. [Google Scholar] [CrossRef]
- Eckard, J.; Dai, J.; Wu, J.; Jian, J.; Yang, Q.; Chen, H.; Costa, M.; Frenkel, K.; Huang, X. Effects of cellular iron deficiency on the formation of vascular endothelial growth factor and angiogenesis. Cancer Cell Int. 2010, 10, 28. [Google Scholar] [CrossRef]
- Rahman, A.; Al-Taiar, A.; Shaban, L.; Al-Sabah, R.; Al-Harbi, A.; Mojiminiyi, O. Plasma 25-Hydroxy Vitamin D Is Not Associated with Either Cognitive Function or Academic Performance in Adolescents. Nutrients 2018, 10, 1197. [Google Scholar] [CrossRef]
- Al-Taiar, A.; Rahman, A.; Al-Sabah, R.; Shaban, L.; Al-Harbi, A. Vitamin D status among adolescents in Kuwait: A cross-sectional study. BMJ Open 2018, 8, e021401. [Google Scholar] [CrossRef]
- Mantadakis, E. Iron Deficiency Anemia in Children Residing in High and Low-Income Countries: Risk Factors, Prevention, Diagnosis and Therapy. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020041. [Google Scholar] [CrossRef]
- Choi, H.-J.; Lee, H.-J.; Jang, H.B.; Park, J.Y.; Kang, J.-H.; Park, K.-H.; Song, J. Effects of maternal education on diet, anemia, and iron deficiency in Korean school-aged children. BMC Public Health 2011, 11, 870. [Google Scholar] [CrossRef]
- Lutter, C.K. Iron Deficiency in Young Children in Low-Income Countries and New Approaches for Its Prevention. J. Nutr. 2008, 138, 2523–2528. [Google Scholar] [CrossRef]
- Wish, J.B. Assessing Iron Status: Beyond Serum Ferritin and Transferrin Saturation. Clin. J. Am. Soc. Nephrol. 2006, 1, S4–S8. [Google Scholar] [CrossRef]
- Bermejo, F.; Garcia-López, S. A guide to diagnosis of iron deficiency and iron deficiency anemia in digestive diseases. World J. Gastroenterol. 2009, 15, 4638–4643. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, E.; Marley, A.; A Samaan, M.; Brookes, M.J. Iron deficiency anaemia: Pathophysiology, assessment, practical management. BMJ Open Gastroenterol. 2022, 9, e000759. [Google Scholar] [CrossRef]
- Agarwal, R.; Davis, J.L.; Smith, L. Serum Albumin Is Strongly Associated with Erythropoietin Sensitivity in Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2007, 3, 98–104. [Google Scholar] [CrossRef]
- Heidari, B.; Taheri, H.; Hajian-Tilaki, K.; Yolmeh, M.; Akbari, R. Low baseline serum albumin as a predictor of anemia in chronic hemodialysis patients. Casp. J. Intern. Med. 2014, 6, 161–164. [Google Scholar]
- Pieroni, L.; Jardel, C.; Merle-Beral, M.H.; Azgui, Z.; Baudet, S.; Tostivint, I.; Hainque, M.B.; Maloum, K. A Novel Parameter for the Diagnosis of Iron Deficiency: The Transferrin/Albumin Ratio. Blood 2006, 108, 3731. [Google Scholar] [CrossRef]
- Indrasari, Y.N.; Hapsari, S.N.; Fuadi, M.R. Potential Marker for Diagnosis and Screening of Iron Deficiency Anemia in Children; Intech: London, UK, 2022. [Google Scholar] [CrossRef]
- Choudhary, M.; Sharma, D.; Shekhawat, D.S.; Dabi, D. Significance of Red Cell Distribution Width in the Diagnosis of Iron Deficiency Anemia: An Observational Study from India. J. Pediatr. Neonatal Care 2015, 2, 102–106. [Google Scholar] [CrossRef]
- Peyssonnaux, C.; Zinkernagel, A.S.; Schuepbach, R.A.; Rankin, E.; Vaulont, S.; Haase, V.H.; Nizet, V.; Johnson, R.S. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs). J. Clin. Investig. 2007, 117, 1926–1932. [Google Scholar] [CrossRef]
- Xing, J.; Lu, J. HIF-1α Activation Attenuates IL-6 and TNF-α Pathways in Hippocampus of Rats Following Transient Global Ischemia. Cell. Physiol. Biochem. 2016, 39, 511–520. [Google Scholar] [CrossRef]
- Gao, X.; Li, Y.; Wang, H.; Li, C.; Ding, J. Inhibition of HIF-1α decreases expression of pro-inflammatory IL-6 and TNF-α in diabetic retinopathy. Acta Ophthalmol. 2017, 95, e746–e750. [Google Scholar] [CrossRef]
- Dritsoula, A.; Dowsett, L.; Pilotti, C.; O’connor, M.N.; Moss, S.E.; Greenwood, J. Angiopathic activity of LRG1 is induced by the IL-6/STAT3 pathway. Sci. Rep. 2022, 12, 4867. [Google Scholar] [CrossRef]
- Yang, F.-J.; Hsieh, C.-Y.; Shu, K.-H.; Chen, I.-Y.; Pan, S.-Y.; Chuang, Y.-F.; Chiu, Y.-L.; Yang, W.-S. Plasma Leucine-Rich α-2-Glycoprotein 1 Predicts Cardiovascular Disease Risk in End-Stage Renal Disease. Sci. Rep. 2020, 10, 5988. [Google Scholar] [CrossRef]
- Stenvinkel, P. Inflammation in end-stage renal failure: Could it be treated? Nephrol. Dial. Transplant. 2002, 17, 33–38. [Google Scholar] [CrossRef]
| Characteristic | N = 431 1 |
|---|---|
| Sex | |
| Female | 234 (54%) |
| Male | 197 (46%) |
| WBC (×109 cells/L) | 6.70 (5.50, 8.19) |
| Hb (g/dL) | 132 (126, 139) |
| Hct (L/L) | 0.404 (0.390, 0.419) |
| RBC (×1012 cells/L) | 5.02 (4.79, 5.26) |
| Gluc (mmol/L) | 4.80 (4.40, 5.30) |
| Characteristic | LRG, N = 408 1 | p-Value 2 |
|---|---|---|
| Iron (µg/dL) | <0.001 | |
| 1–10 | 31 (25, 41) | |
| 10–15 | 29 (23, 34) | |
| 15–29 | 25 (21, 32) | |
| Transferrin saturation (in %) | <0.001 | |
| 1.0–13.5 | 30 (24, 40) | |
| 13.5–21.3 | 29 (24, 36) | |
| 21.3–42.5 | 25 (21, 33) | |
| Albumin g/L | <0.001 | |
| 35.4–40.6 | 31 (25, 38) | |
| 40.6–42.4 | 29 (23, 37) | |
| 42.4–49.2 | 25 (21, 32) | |
| RDW (fL) | <0.001 | |
| 11.2–13.2 | 27 (22, 33) | |
| 13.2–14.0 | 27 (22, 35) | |
| 14.0–20.0 | 31 (26, 40) | |
| Ferritin (µg/L) | <0.001 | |
| 1.7–17.1 | 31 (25, 39) | |
| 17.1–28.5 | 25 (21, 31) | |
| 28.5–189.5 | 30 (24, 37) | |
| WBC (109/L) | 0.004 | |
| 3.1–5.9 | 27 (22, 34) | |
| 5.9–7.7 | 29 (23, 35) | |
| 7.7–12.8 | 31 (25, 38) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhammad, R.; Abu-Farha, M.; Rahman, A.; Thanaraj, T.A.; Shaban, L.; Alsabah, R.; Hamad, S.; Hammad, M.M.; Channanath, A.; Al-Mulla, F.; et al. LRG1 Associates with Iron Deficiency Anemia Markers in Adolescents. Nutrients 2023, 15, 3100. https://doi.org/10.3390/nu15143100
Alhammad R, Abu-Farha M, Rahman A, Thanaraj TA, Shaban L, Alsabah R, Hamad S, Hammad MM, Channanath A, Al-Mulla F, et al. LRG1 Associates with Iron Deficiency Anemia Markers in Adolescents. Nutrients. 2023; 15(14):3100. https://doi.org/10.3390/nu15143100
Chicago/Turabian StyleAlhammad, Rashed, Mohamed Abu-Farha, Abdur Rahman, Thangavel Alphonse Thanaraj, Lemia Shaban, Reem Alsabah, Samar Hamad, Maha M. Hammad, Arshad Channanath, Fahd Al-Mulla, and et al. 2023. "LRG1 Associates with Iron Deficiency Anemia Markers in Adolescents" Nutrients 15, no. 14: 3100. https://doi.org/10.3390/nu15143100
APA StyleAlhammad, R., Abu-Farha, M., Rahman, A., Thanaraj, T. A., Shaban, L., Alsabah, R., Hamad, S., Hammad, M. M., Channanath, A., Al-Mulla, F., & Abubaker, J. (2023). LRG1 Associates with Iron Deficiency Anemia Markers in Adolescents. Nutrients, 15(14), 3100. https://doi.org/10.3390/nu15143100










