Abstract
18F-FDG PET-CT is routinely performed as part of the initial staging of numerous cancers. Other than having descriptive, predictive and prognostic values for tumors, 18F-FDG PET-CT provides full-body data, which could inform on concurrent pathophysiological processes such as malnutrition. To test this hypothesis, we measured the 18F-FDG uptake in several organs and evaluated their association with weight loss in patients at diagnosis of esophageal cancer. Forty-eight patients were included in this retrospective monocentric study. 18F-FDG uptake quantification was performed in the brain, the liver, the spleen, bone marrow, muscle and the esophageal tumor itself and was compared between patients with different amounts of weight loss. We found that Total Lesion Glycolysis (TLG) and peak Standardized Uptake Values (SUVpeak) measured in the brain correlated with the amount of weight loss: TLG was, on average, higher in patients who had lost more than 5% of their usual weight, whereas brain SUVpeak were, on average, lower in patients who had lost more than 10% of their weight. Higher TLG and lower brain SUVpeak were associated with worse OS in the univariate analysis. This study reports a new and significant association between 18F-FDG uptake in the brain and initial weight loss in patients with esophageal cancer.
1. Introduction
In oncology, 2-deoxy-2-(18F)-fluoro-D-glucose positron emission tomography coupled to computed tomography (18F-FDG PET-CT) is widely used in order to detect regional and distant tumor spread as part of the initial tumor staging or treatment evaluation [1]. Specific metrics based on 18F-FDG uptake at the tumor level are also recognized as independent prognostic factors in several cancers, including esophageal cancer [2,3,4]. However, 18F-FDG uptake by neoplastic or non-neoplastic tissues, though measurable, has only rarely been used to explore concurrent pathophysiological processes, such as malnutrition [5,6]. Cancer-associated malnutrition is a severe systemic metabolic condition, with a high incidence in patients with esophageal cancers [7,8]. It is defined by involuntary WL, low body mass index (BMI) or reduced muscle mass in the context of reduced food intake, reduced nutrient absorption or active disease [9]. It impairs quality of life, associates with worse tolerance to treatment and negatively impacts overall survival (OS) [10,11,12]. How malnutrition impacts 18F-FDG uptake of tumors and healthy organs is largely unknown [13,14]. Likewise, to what extent 18F-FDG uptake could inform the pathophysiological changes occurring in malnourished patients is also unknown.
In this study, using data from routinely performed 18F-FDG PET-CT at the initial staging of esophageal cancer, we retrospectively and systematically assessed the association of 18F-FDG uptake values in the brain, the liver, the spleen, bone marrow, muscle and the esophageal tumor itself with weight loss.
2. Methods
2.1. Patient Selection
Patients aged 18 years old or above diagnosed with esophageal cancer (squamous cell carcinoma or adenocarcinoma) that underwent an 18F-FDG PET-CT scan for initial staging (before any treatment) between January 2014 and June 2019 were eligible for inclusion. The study was approved by the Institutional Review Board (ART-2021-02). Patients were excluded if anti-diabetic medications were listed in their medical files, if their capillary blood glucose concentration at the time of the 18F-FDG injection was below 65 mg/mL or above 135 mg/mL, if the time between tracer injection and imaging was under 55 min or above 75 min, if their brain had not been scanned, if they presented with brain, liver or spleen metastases or if CT and PET images were misaligned at visual inspection.
2.2. Imaging Data Acquisition and Processing
Patients were asked not to ingest anything other than plain water and to avoid intense physical activity for 6 h before the injection of 18F-FDG (3.5 MBq/kg). Their venous blood glucose level was measured before injection using a glucometer. Image acquisition from skull to mid-thigh was performed on the same Discovery PET/CT 690 scanner (GE Healthcare, Waukesha, WI, USA). Non-contrast CT scans of patients in the supine position were acquired, followed by 3D PET imaging. Data were corrected for geometrical response and detector efficiency, dead time, random coincidences, scatter and attenuation, as recommended in [15], and reconstructed into matrices of 256 × 256 pixels. Our PET/CT imaging facility was accredited for tumor imaging by the European Association of Nuclear Medicine Research Ltd.
2.3. Quantification of 18F-FDG Uptake
The quantification of 18F-FDG uptake was retrospectively performed. Spherical volumes of interest (VOI) were manually positioned over relevant organs using CT images and OsiriX MD software (version 7.5): over the right lobe of the liver (19.2 cm3), over the spleen (5.2 cm3), inside the brain (centered on the putamen) and inside the left iliac tuberosity in order to measure tracer uptake in the bone marrow. The putamen is an easily recognizable brain structure, which we used to reproducibly center the brain VOI. This warranted consistent measurements. SUVpeak were computed within these VOI using OsiriX MD. SUVpeak correspond to the average value within a 1 cm3 sphere positioned around the highest voxel value (SUVmax) [15,16,17]. SUVpeak were proposed to be more robust than SUVmax, especially in low-count conditions, as was the case for most organs in this study [18]. The esophageal tumor was circumscribed within a large spherical VOI. The Metabolic Tumor Volume (MTV) was defined as the volume inside the 3D isocontour at 41% of the maximum pixel value (as recommended in [15]) and the Total Lesion Glycolysis (TLG) as MTV multiplied by mean voxel SUV (SUVmean) within the MTV. For skeletal muscle, the mean SUV (SUVmean) of a 2D, manually drawn region of interest (ROI) delineating the cross-sectional area of skeletal muscle at the third lumbar vertebra was chosen. This region has been shown to be representative of whole-body muscle mass [19]. When indicated, SUVs were normalized to lean body mass (LBM) according to James’ and Janmahasatian’s predictive equations [20,21,22]) and referred to as SULJames or SULJanma, respectively. Similarly, when indicated, brain SUVpeak were also normalized to blood glucose concentrations at the time of the 18F-FDG injection as SUVglu = SUVpeak × (blood glucose in mg/dL)/100) [23].
2.4. Clinical Data Collection and Nutritional Assessment
Clinical parameters and imaging conditions were obtained from patients’ medical records, PET/CT reports and associated DICOM files. The reference weight (weight[ref]) was defined as the patient-reported usual stable weight. Weight loss (WL) was defined as: WL = ( with weight[PET] defined as the weight on the day of PET/CT. WL was categorized according to two thresholds: WL ≥ 5% and WL ≥ 10%. The reference weight was obtained from nutritional reports systematically filed for all patients by dieticians or physicians during consultation.
2.5. Statistical Analysis
Categorical variables are expressed as numbers in the indicated category and (%), with continuous variables as median and (range). Group differences between quantitative variables were tested using the non-parametric Kruskal-Wallis test by ranks or Pearson’s chi-square test for categorical variables. In order to examine the optimal cut-off values for SUVs, Receiver Operating Characteristic (ROC) curves were assessed with WL ≥ 10% as the reference. The cut-off value corresponding to the highest predictive value, which maximized the Youden index, was chosen. Overall survival (OS) was defined as the time between diagnosis and death or last follow-up (censored data). OS was estimated using the Kaplan-Meier estimator. The log-rank test was performed to assess differences between groups. Patients alive without event were censored at the last news date. The median follow-up was estimated according to «reverse Kaplan-Meier method» and presented with 95% confidence intervals (CIs). Multivariate analyses were carried out using logistic regressions or Cox’s proportional hazards regressions, with a stepwise selection procedure on covariables with p < 0.1 (dichotomized at median value) in univariate analyses. We added 3 more variables of interest, i.e., Glycemia, TLG and MTV, which were not automatically selected as categorical variables for the multivariate logistic regression, but were associated with WL ≥ 10% (p < 0.1) as continuous variables. Odds ratio (OR) and hazard ratios (HR) are presented with 95% CIs. All p values reported were two-sided and the significance level was set to 5% (p < 0.05) and indicated by *. Statistical analysis was performed using the STATA 16.1 software (Stata Corporation, College Station, TX, USA).
3. Results
3.1. Demographic and Nutritional Characteristics of Patients
Two hundred and eighteen patients with esophageal cancer underwent an initial 18F-FDG PET-CT scan in our institute between January 2014 and June 2019. One hundred and fifty-three patients were excluded because the PET-CT scans did not encompass their brain or showed improper alignment between the PET and CT images in the brain area; thirteen patients were excluded because of known diabetes or blood glucose outside of the 65–135 mg/L range; four patients were excluded because the time between the 18F-FDG injection and imaging was outside the predefined range. Forty-eight patients were selected for the study. The median usual BMI was 27.2 kg·m−2 before the onset of initial symptoms. In comparison to the usual weight, the median WL was 7% on the PET scan day. Thirty-two persons (67%) lost 5% or more of their usual weight and eighteen (37.5%) lost 10% or more of their usual weight. Values of BMI and glycemia before PET imaging were significantly different between patients who lost 10% or more of their initial weight compared to the rest of the cohort (Table 1).

Table 1.
Distribution of patient characteristics according to weight loss. Fractional weight difference is between the usual weight, stable weight and the weight measured just before PET imaging. Median and (range) are indicated. p is according to Kruskal-Wallis test by rank for continuous variables and to Pearson’s chi-square test for categorical variables.
3.2. TLG and Brain SUVpeak Associated with WL ≥ 10%
The SUVpeak measured in the brain, the liver, the spleen, bone marrow, muscle and primary tumor were compared between patients presenting with WL ≥ 5% versus <5% on one hand, and WL ≥ 10% versus <10% on the other hand. When a cut-off of 5% WL was chosen, no significant difference was observed between SUVpeak from any organs (Table 2, columns 3–5 and Figure 1A). Yet, when a cut-off value of 10% WL was used, the brain SUVpeak were significantly lower (p < 0.001, Kruskal-Wallis test) in patients who lost 10% or more of their usual weight compared to other patients (Table 2, columns 6–8 and Figure 1D). The Spearman correlation coefficient between the brain SUVpeak and weight difference was −0.44, p = 0.0015 (Supplementary Figure S1A). Representative 18F-FDG PET-CT images from two patients presenting with different levels of brain 18F-FDG uptake are shown (Figure 2).

Table 2.
Distribution of 18F-FDG uptake values in specified organs according to weight loss (WL). MTV, Metabolic Tumor Volume; TLG, Tumor Lesion Glycolysis. Median and (range) are indicated. p is according to Kruskal-Wallis test by rank.
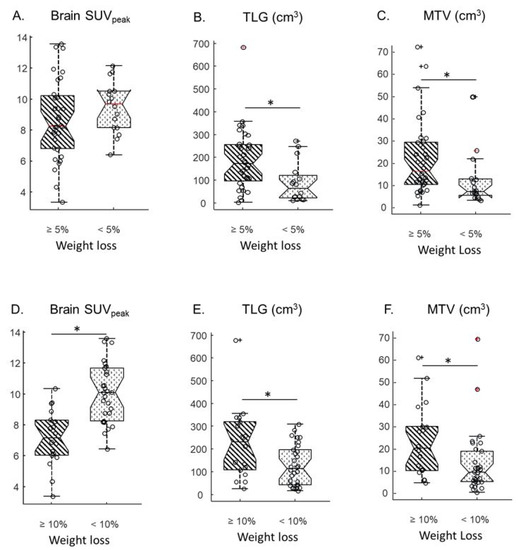
Figure 1.
Comparison of brain SUVpeak (A), Total Lesion Glycolysis (TLG) (B) and Metabolic Tumour Volume (MTV) (C) from patients who lost 5% or more of their initial weight (diagonal lines) versus patients who lost less than 10% of their initial weight (dots). Comparison of brain SUVpeak (D), TLG (E) and MTV (F) from patients who lost 10% or more of their initial weight (diagonal lines) versus patients who lost less than 10% of their initial weight (dots). Circles represent data points. Central horizontal marks correspond to medians; bottom and top edges of the box indicate the 25th and 75th percentiles, respectively; notches correspond to limits of 95% CI. Whiskers extend to the most extreme data value that is not beyond +/−2.7σ. Crosses correspond to data points beyond whiskers. * indicates p < 0.05.
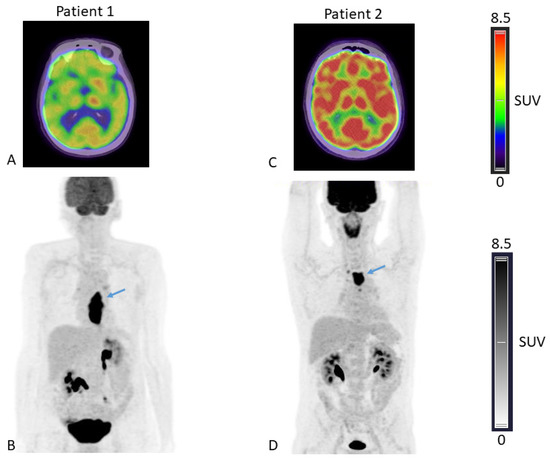
Figure 2.
18F-FDG-fused PET/CT axial slices passing through the brain (A,C) and Maximal Intensity Projection, anterior view (B,D), of patient 1 presenting with low brain 18F-FDG uptake (brain SUVpeak = 5.89) and a body weight loss of 10% compared to usual weight and patient 2 presenting with higher brain 18F-FDG uptake (brain SUVpeak= 10.17) and a body weight loss of 8% compared to usual weight. Arrows depicting oesophageal tumors in both patients.
No significant difference was observed in SUVpeak for the spleen, bone marrow muscle or primary tumor using a WL cut-off of 10% (Table 2). For primary tumors, the median MTV and TLG were both significantly higher in patients that met either the 5% WL cut-off or the 10% WL (Figure 1B,C,E,F). The Spearman correlation coefficient between TLG and weight difference was −0.48, p < 0.001 (Supplementary Figure S1B). There was no significant correlation between TLG and brain SUVpeak (Spearman correlation coefficient of −0.23, p > 0.1, Supplementary Figure S1C).
Patient BMI and glycemia were potential confounding factors as both are known to influence SUVs [21,23,24,25,26,27] and distributions of both parameters were significantly different between patients who lost 10% or more of their usual weight and the others (Table 1). However, in a multivariate logistic regression model to predict WL ≥ 10%, adjusted for BMI, glycemia, brain SUVpeak, MTV and TLG, only brain SUVpeak and TLG remained significant predictors of WL ≥ 10% (Table 3). Moreover, using brain SUVpeak normalized by lean body weight (i.e., SUL) or by glycemia (i.e., SUVglu) did not change the results: brain SUL or brain SUVglu were lower in patients who lost 10% or more of their usual weight (Supplementary Table S1).

Table 3.
Multivariate logistic regression predicting weight loss ≥ 10%. Covariables with p < 0.1 in univariate analysis were chosen as adjustment variables, i.e., age at diagnosis (categorical), BMI on TEP scan day (categorical), Brain SUVpeak (categorical), Spleen SUVpeak (categorical), Liver SUVpeak (categorical), Glycemia before PET (mg/dL) (continuous), TLG (continuous), MTV (continuous).
A cut-off value of 7.32 for brain SUVpeak determined with the analysis of the ROC curve (AUC 0.863) was able to predict WL ≥ 10% with a high specificity of 0.97, but with a low sensitivity 0.53. Similar trends were observed with ROC analysis of TLG but AUC was lower; i.e., 0.743 (Table 4).

Table 4.
ROC analysis of PET variables associated with weight loss. AUC, area under the ROC curve.
3.3. TLG and Brain SUVpeak Associated with Survival
The median follow-up period was 28.7 months. Using Kaplan Meier analysis and groups split at the median value, none of the PET variables significantly affected OS. Only the presence of distant metastasis, BMI on the day of the PET scan and WL ≥ 10% were prognostic factors (Table 5, Figure 3A). When cut-off values determined by ROC analysis to predict WL were used (Table 5), both brain SUVpeak and TLG were significant prognostic factors (Table 5, Figure 3B,C).

Table 5.
Univariate Kaplan Meier analysis of overall survival. Cut-offs defining groups of patients are median unless otherwise specified; for variables predictive of 10%WL in multivariate logistic regression, cut-offs were determined by ROC analysis. Median are indicated in Table 1, column 2. HR: Hazard Ratio; CI: Confidence Interval.
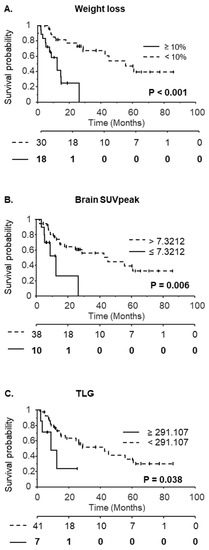
Figure 3.
Kaplan-Meier curves with respect to weight loss ≥ 10% (A), brain SUVpeak (B) or Total Lesion Glycolysis (TLG) (C). The number at risk is indicated below the x-axis. For brain SUVpeak and TLG, groups of patients were defined according to cut-off values determined by the ROC analysis.
In a cox multivariate model (Log likelihood = −67.31) including all variables with p < 0.1 in cox univariate analysis (i.e., distant metastasis, BMI on the day of the PET scan, WL ≥ 10%, brain SUVpeak, MTV and TLG), only the presence of distant metastasis and WL ≥ 10% were associated with overall survival (HR = 2.97, p = 0.046 and HR = 4.35, p = 0.015 respectively), indicating that brain SUVpeak, and TLG are not independent prognostic factors.
4. Discussion
Using data obtained from routine 18F-FDG PET-CT, we showed that, in patients at diagnosis of esophageal cancer, WL correlated with high TLG but also with low brain SUVpeak. In addition, in the univariate analysis, both TLG and brain SUVpeak were pre-therapeutic prognostic factors in these patients, possibly in connection with weight loss. In this group of patients, weight loss did not associate with SUVpeak measured in the liver, the spleen, bone marrow or muscle. This specificity of the brain results cannot be simply explained by the higher amplitude of the signal observed in the brain, as tumor SUVs are similarly high and do not differ between WL categories.
WL ≥ 5% within the past 6 months or WL ≥ 10% beyond 6 months defines malnutrition in the context of cancer [9]. Lower brain SUVs are specifically associated with more pronounced WL, i.e., ≥10%, whereas higher TLG is associated with both high and more moderate WL; i.e., ≥5%. A recent study has shown a significant association between esophageal tumor SUVmax and weight loss. Although we found a similar association between TLG and weight loss, SUVpeak were not significantly associated with weight loss in our group of patients [13]. To our knowledge, this is the first clinical report of the association of a routine 18F-FDG uptake measurement in the brain with malnutrition and survival in patients just diagnosed with esophageal cancer. It was made possible because of the unique and systematic survey and filing of patients’ weight history by dedicated dieticians in our clinical center [24]. In a cachexia-inducing murine model of adenocarcinoma, brain uptake was significantly higher in cachexic mice compared to the group of non-cachexic mice; the reason for this discrepancy with our results is unclear, but it may be explained by the inherent limitations of the preclinical model when compared to the patients [25].
The association of weight loss with high TLG may be explained by the higher metabolic burden of a high-volume tumor, as well as by the larger hindrance of such a tumor on the esophagus, hence limiting food intake. It is more difficult to explain the correlation between weight loss and brain SUVpeak. The SUV of a given organ is key as the brain depends on its intrinsic metabolic properties, but also on several other parameters [26]. Systemic changes in body composition, especially changes in the fraction of fat mass, may indeed affect the biodistribution of the tracer. For instance, liver, blood and spleen SUVs are overestimated in obese persons compared to non-obese persons [22,27,28]. The normalization of SUVs by lean mass was introduced in order to circumvent this effect. The opposite phenomenon, i.e., the underestimation of tissue SUVs, may explain our results in undernourished patients who might have lost more fat than lean mass. However, lower SUVs were specifically observed in the brains of undernourished persons and not in other tissues. After normalization to lean mass estimated by predictive equations [20,21], brain SUVs were still lower in patients who lost ≥ 10% of their initial weight (Supplementary Table S1). One cannot exclude that 18F-FDG uptake in voluminous tumors may reduce the amount of 18F-FDG available for uptake in the brain, but, in this case, a similar trend should have been observed in other tissues [29]. Moreover, we did not find any correlation between TLG and brain SUVpeak (Supplementary Figure S1C).
The blood glucose concentration may also affect SUVs, as endogenous glucose competes with the tracer and brain SUVs are known to be highly sensitive to glycemia [30,31,32]. In our group of patients, the blood glucose concentration was slightly higher in patients who lost 10% or more of their weight compared to patients who lost less than 10% of their weight (Table 1), indicating glycemia to be a possible confounding factor. However, brain SUVs reduction persisted in multivariate analysis after adjustment for glycemia, sex or age. In addition, when corrected for blood glucose [22], brain SUVglu were still significantly lower in patients who lost 10% or more of their weight (Supplementary Table S1).
The pathophysiology underlying the reduced 18F-FDG uptake in the brain of undernourished patients is unknown. The brain relies almost exclusively on glucose as an energy source and reduced cerebral glucose metabolism may be an adaptive mechanism to reduced nutrient availability. In agreement with this hypothesis, starvation was shown to be associated with decreased glucose consumption, specifically in the brain [33,34,35], and glycolytic flux and phosphofructokinase activity were significantly reduced in the neurons of starved mice [36]. Instead of glucose, neurons have been proposed to use ketone bodies as complementary fuel, which may decrease brain glucose uptake [36,37,38,39,40].
A decrease in brain SUVpeak was only observed with WLs ≥ 10%, which corresponds to stage 2/severe malnutrition [9]. Severe malnutrition may indeed correspond to extreme metabolic states, e.g., starvation and ketogenesis (cf above), which are associated with brain hypometabolism. A lesser weight loss may not trigger such a metabolic switch.
Several medical conditions, especially in neurology and psychiatry, have been shown to be associated with changes in 18F-FDG uptake in the brain. Alzheimer’s disease is associated with low 18F-FDG uptake in specific regions of the brain depending on the severity and the duration of the disease [41]. 18F-FDG uptake is also lower in the frontal cortex of schizophrenia patients [42] or in the thalami of patients with delirium [43]. It is unknown whether and how these observations relate to the lower 18F-FDG uptake described here, but the prevalence of mood disorders is high among patients with esophageal cancer, impacting their quality of life and pain perception [44,45].
As shown by others and confirmed in this study, WL is a strong prognostic factor in esophageal cancer. In our population, brain SUVpeak and TLG were also pre-therapeutic prognostic factors when cut-off values predictive of WL were used in the univariate analysis. TLG has already been identified as a prognostic factor, but not brain SUVpeak [46]. Their prognostic value is lost in multivariate Cox models, suggesting that brain SUVpeak or TLG are not independent prognostic factors and affect survival because of their association with other factors; e.g., WL.
This study has several limitations: it was retrospective, performed at a single clinical center and, above all, included a small number of patients, mostly as a consequence of the exclusion criteria requiring the inclusion of the brain in the full body scan. Additional work on a larger cohort will be necessary to confirm our results. Moreover, though statistically significant, data supporting the prognostic value of brain SUVpeak and TLG relied on a small number of patients, especially within the group with the lowest OS (Figure 2), and must be confirmed with a larger group of patients. The clinical significance of our findings is not yet clear. Although brain SUVpeak are indicative of severe weight loss, they are obviously not a substitute for the clinical approach; i.e., taking the patient’s actual weight and history. However, low brain SUVpeak could trigger nutritional assessment if it has not been carried out at the time of the PET scan.
This work revealed a so far unnoticed association between malnutrition and routine 18F-FDG uptake measurements in the tumors and, more surprisingly, brains of patients diagnosed with esophageal cancer. It may open up new avenues of research aimed at understanding the systemic consequences of malnutrition, especially on the central nervous system and its cognitive and behavioral functions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15133042/s1, Figure S1: Correlation between brain SUVpeak and fractional weight difference (A). Correlation between Total Lesion Glycolysis (TLG) and fractional weight difference (B). Correlations beween brain SUVpeak and TLG (C). Spearman correlation coefficient and p-value of observing the null hypothesis. Table S1: Brain 18FDG uptake values normalized to lean body mass or blood glucose according to weight loss.
Author Contributions
Project supervision: E.D.; project conceptualization: E.D. and T.G.; Expertise and guidance on nutritional data collection and analysis: P.S. and N.F.; images acquisition and collection: E.D., M.-C.E., S.G., A.D.I., P.-O.K. and L.S.; Provision of technical resources: P.-O.K.; Data collection and curation from patient files (biometric and imaging data): I.B. and T.G.; Data analysis: T.G. and S.T.; Supervision and validation of statistical analysis: S.T.; Writing (original draft): T.G.; Writing (review and editing): all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institut du Cancer de Montpellier (ART-2021-02).
Informed Consent Statement
Patient consent was waived due to because of the retrospective nature of the study and the analysis of anonymous clinical data.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank all the dieticians who collected biometric data: Stéphanie Arnac, Bérénice Clavie, Anne Fallières, Laure Francioni, Arnaud Vaillé and Sophie Zaessinger. We thank also Heloïse Lecornu for support and advice regarding nutrional data interpretation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Salaün, P.-Y.; Abgral, R.; Malard, O.; Querellou-Lefranc, S.; Quere, G.; Wartski, M.; Coriat, R.; Hindie, E.; Taieb, D.; Tabarin, A.; et al. Good Clinical Practice Recommendations for the Use of PET/CT in Oncology. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 28–50. [Google Scholar] [CrossRef] [PubMed]
- Butof, R.; Hofheinz, F.; Zophel, K.; Stadelmann, T.; Schmollack, J.; Jentsch, C.; Lock, S.; Kotzerke, J.; Baumann, M.; van den Hoff, J. Prognostic Value of Pretherapeutic Tumor-to-Blood Standardized Uptake Ratio in Patients with Esophageal Carcinoma. J. Nucl. Med. 2015, 56, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Hofheinz, F.; Li, Y.; Steffen, I.G.; Lin, Q.; Lili, C.; Hua, W.; van den Hoff, J.; Zschaeck, S. Confirmation of the Prognostic Value of Pretherapeutic Tumor SUR and MTV in Patients with Esophageal Squamous Cell Carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Lemarignier, C.; Di Fiore, F.; Marre, C.; Hapdey, S.; Modzelewski, R.; Gouel, P.; Michel, P.; Dubray, B.; Vera, P. Pretreatment Metabolic Tumour Volume Is Predictive of Disease-Free Survival and Overall Survival in Patients with Oesophageal Squamous Cell Carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2008–2016. [Google Scholar] [CrossRef] [PubMed]
- Otomi, Y.; Otsuka, H.; Terazawa, K.; Kondo, M.; Arai, Y.; Yamanaka, M.; Otomo, M.; Abe, T.; Shinya, T.; Harada, M. A Reduced Liver 18F-FDG Uptake May Be Related to Hypoalbuminemia in Patients with Malnutrition. Ann. Nucl. Med. 2019, 33, 689–696. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, H.; Zhao, Y.; Cui, Y.; Dai, J.; Huang, S.; Li, C.; Mao, H.; Ju, S.; Peng, X.-G. Abnormal [18F]FDG Uptake in Liver and Adipose Tissue: A Potential Imaging Biomarker for Cancer-Associated Cachexia. Eur. Radiol. 2023, 33, 2561–2573. [Google Scholar] [CrossRef]
- Anandavadivelan, P.; Lagergren, P. Cachexia in Patients with Oesophageal Cancer. Nat. Rev. Clin. Oncol. 2016, 13, 185–198. [Google Scholar] [CrossRef]
- Bozzetti, F. SCRINIO Working Group Screening the Nutritional Status in Oncology: A Preliminary Report on 1,000 Outpatients. Support. Care Cancer 2009, 17, 279–284. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM Criteria for the Diagnosis of Malnutrition—A Consensus Report from the Global Clinical Nutrition Community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef]
- Dewys, W.D.; Begg, C.; Lavin, P.T.; Band, P.R.; Bennett, J.M.; Bertino, J.R.; Cohen, M.H.; Douglass, H.O.; Engstrom, P.F.; Ezdinli, E.Z.; et al. Prognostic Effect of Weight Loss Prior Tochemotherapy in Cancer Patients. Am. J. Med. 1980, 69, 491–497. [Google Scholar] [CrossRef]
- Pan, Y.-P.; Kuo, H.-C.; Hsu, T.-Y.; Lin, J.-Y.; Chou, W.-C.; Lai, C.-H.; Chang, P.-H.; Yeh, K.-Y. Body Mass Index-Adjusted Body Weight Loss Grading Predicts Overall Survival in Esophageal Squamous Cell Carcinoma Patients. Nutr. Cancer 2020, 73, 1130–1137. [Google Scholar] [CrossRef]
- Di Fiore, F.; Lecleire, S.; Pop, D.; Rigal, O.; Hamidou, H.; Paillot, B.; Ducrotté, P.; Lerebours, E.; Michel, P. Baseline Nutritional Status Is Predictive of Response to Treatment and Survival in Patients Treated by Definitive Chemoradiotherapy for a Locally Advanced Esophageal Cancer. Am. J. Gastroenterol. 2007, 102, 2557–2563. [Google Scholar] [CrossRef]
- Olaechea, S.; Gannavarapu, B.S.; Gilmore, A.; Alvarez, C.; Iyengar, P.; Infante, R. The Influence of Tumour Fluorodeoxyglucose Avidity and Cachexia Development on Patient Survival in Oesophageal or Gastroesophageal Junction Cancer. JCSM Clin. Rep. 2021, 6, 128–136. [Google Scholar] [CrossRef]
- Nakamoto, R.; Okuyama, C.; Ishizu, K.; Higashi, T.; Takahashi, M.; Kusano, K.; Kagawa, S.; Yamauchi, H. Diffusely Decreased Liver Uptake on FDG PET and Cancer-Associated Cachexia With Reduced Survival. Clin. Nucl. Med. 2019, 44, 634–642. [Google Scholar] [CrossRef]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM Procedure Guidelines for Tumour Imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET Response Criteria in Solid Tumors. J. Nucl. Med. 2009, 50, 122S–150S. [Google Scholar] [CrossRef]
- O, J.H.; Lodge, M.A.; Wahl, R.L. Practical PERCIST: A Simplified Guide to PET Response Criteria in Solid Tumors 1.0. Radiology 2016, 280, 576–584. [Google Scholar] [CrossRef]
- Lodge, M.A.; Chaudhry, M.A.; Wahl, R.L. Noise Considerations for PET Quantification Using Maximum and Peak Standardized Uptake Value. J. Nucl. Med. 2012, 53, 1041–1047. [Google Scholar] [CrossRef]
- Mourtzakis, M.; Prado, C.M.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A Practical and Precise Approach to Quantification of Body Composition in Cancer Patients Using Computed Tomography Images Acquired during Routine Care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- James, W.P.T. Research on Obesity. Nutr. Bull. 1977, 4, 187–190. [Google Scholar] [CrossRef]
- Janmahasatian, S.; Duffull, S.B.; Ash, S.; Ward, L.C.; Byrne, N.M.; Green, B. Quantification of Lean Bodyweight. Clin. Pharm. 2005, 44, 1051–1065. [Google Scholar] [CrossRef] [PubMed]
- Tahari, A.K.; Chien, D.; Azadi, J.R.; Wahl, R.L. Optimum Lean Body Formulation for Correction of Standardized Uptake Value in PET Imaging. J. Nucl. Med. 2014, 55, 1481–1484. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, P.; Minn, H.; Joensuu, H. FDG Uptake in Cancer A PET Study. J. Nucl. Med. 1993, 34, 1–6. [Google Scholar] [PubMed]
- Zasadny, K.R.; Wahl, R.L. Standardized Uptake Values of Normal Tissues at PET with 2-[Fluorine-18]-Fluoro-2-Deoxy-D-Glucose: Variations with Body Weight and a Method for Correction. Radiology 1993, 189, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Claeys, J.; Mertens, K.; D’Asseler, Y.; Goethals, I. Normoglycemic Plasma Glucose Levels Affect F-18 FDG Uptake in the Brain. Ann. Nucl. Med. 2010, 24, 501–505. [Google Scholar] [CrossRef]
- Viglianti, B.L.; Wong, K.K.; Wimer, S.M.; Parameswaran, A.; Nan, B.; Ky, C.; Townsend, D.M.; Rubello, D.; Frey, K.A.; Gross, M.D. Effect of Hyperglycemia on Brain and Liver 18 F-FDG Standardized Uptake Value (FDG SUV) Measured by Quantitative Positron Emission Tomography (PET) Imaging. Biomed. Pharmacother. 2017, 88, 1038–1045. [Google Scholar] [CrossRef]
- Sprinz, C.; Zanon, M.; Altmayer, S.; Watte, G.; Irion, K.; Marchiori, E.; Hochhegger, B. Effects of Blood Glucose Level on 18F Fluorodeoxyglucose (18F-FDG) Uptake for PET/CT in Normal Organs: An Analysis on 5623 Patients. Sci. Rep. 2018, 8, 2126–2131. [Google Scholar] [CrossRef]
- Sarikaya, I.; Albatineh, A.; Sarikaya, A. Re-Visiting SUV-Weight and SUV-Lean Body Mass in FDG PET Studies. J. Nucl. Med. Technol. 2019, 48, 233–249. [Google Scholar] [CrossRef]
- Senesse, P.; Isambert, A.; Janiszewski, C.; Fiore, S.; Flori, N.; Poujol, S.; Arroyo, E.; Courraud, J.; Guillaumon, V.; Mathieu-Daudé, H.; et al. Management of Cancer Cachexia and Guidelines Implementation in a Comprehensive Cancer Center: A Physician-Led Cancer Nutrition Program Adapted to the Practices of a Country. J. Pain Symptom Manag. 2017, 54, 387–393.e3. [Google Scholar] [CrossRef]
- Penet, M.-F.; Gadiya, M.M.; Krishnamachary, B.; Nimmagadda, S.; Pomper, M.G.; Artemov, D.; Bhujwalla, Z.M. Metabolic Signatures Imaged in Cancer-Induced Cachexia. Cancer Res. 2011, 71, 6948–6956. [Google Scholar] [CrossRef]
- Huang, S.-C. Anatomy of SUV. Nucl. Med. Biol. 2000, 27, 643–646. [Google Scholar] [CrossRef]
- Viglianti, B.L.; Wale, D.J.; Wong, K.K.; Johnson, T.D.; Ky, C.; Frey, K.A.; Gross, M.D. Effects of Tumor Burden on Reference Tissue Standardized Uptake for PET Imaging: Modification of PERCIST Criteria. Radiology 2018, 287, 993–1002. [Google Scholar] [CrossRef]
- Hasselbalch, S.G.; Knudsen, G.M.; Jakobsen, J.; Hageman, L.P.; Holm, S.; Paulson, O.B. Brain Metabolism during Short-Term Starvation in Humans. J. Cereb. Blood Flow Metab. 1994, 14, 125–131. [Google Scholar] [CrossRef]
- Owen, O.E.; Morgan, A.P.; Kemp, H.G.; Sullivan, J.M.; Herrera, M.G.; Cahill, G.F. Brain Metabolism during Fasting. J. Clin. Investig. 1967, 46, 1589–1595. [Google Scholar] [CrossRef]
- Redies, C.; Hoffer, L.J.; Beil, C.; Marliss, E.B.; Evans, A.C.; Lariviere, F.; Marrett, S.; Meyer, E.; Diksic, M.; Gjedde, A.; et al. Generalized Decrease in Brain Glucose Metabolism during Fasting in Humans Studied by PET. Am. J. Physiol.-Endocrinol. Metab. 1989, 256, E805–E810. [Google Scholar] [CrossRef]
- Buschiazzo, A.; Cossu, V.; Bauckneht, M.; Orengo, A.; Piccioli, P.; Emionite, L.; Bianchi, G.; Grillo, F.; Rocchi, A.; Di Giulio, F.; et al. Effect of Starvation on Brain Glucose Metabolism and 18F-2-Fluoro-2-Deoxyglucose Uptake: An Experimental in-Vivo and Ex-Vivo Study. EJNMMI Res. 2018, 8, 44. [Google Scholar] [CrossRef]
- Courchesne-Loyer, A.; Croteau, E.; Castellano, C.-A.; St-Pierre, V.; Hennebelle, M.; Cunnane, S.C. Inverse Relationship between Brain Glucose and Ketone Metabolism in Adults during Short-Term Moderate Dietary Ketosis: A Dual Tracer Quantitative Positron Emission Tomography Study. J. Cereb. Blood Flow Metab. 2017, 37, 2485–2493. [Google Scholar] [CrossRef]
- LaManna, J.C.; Salem, N.; Puchowicz, M.; Erokwu, B.; Koppaka, S.; Flask, C.; Lee, Z. Ketones Suppress Brain Glucose Consumption. Adv. Exp. Med. Biol. 2009, 645, 301–306. [Google Scholar] [CrossRef]
- Zhang, Y.; Kuang, Y.; Xu, K.; Harris, D.; Lee, Z.; LaManna, J.; Puchowicz, M.A. Ketosis Proportionately Spares Glucose Utilization in Brain. J. Cereb. Blood Flow Metab. 2013, 33, 1307–1311. [Google Scholar] [CrossRef]
- Svart, M.; Gormsen, L.C.; Hansen, J.; Zeidler, D.; Gejl, M.; Vang, K.; Aanerud, J.; Moeller, N. Regional Cerebral Effects of Ketone Body Infusion with 3-Hydroxybutyrate in Humans: Reduced Glucose Uptake, Unchanged Oxygen Consumption and Increased Blood Flow by Positron Emission Tomography. A Randomized, Controlled Trial. PLoS ONE 2018, 13, e0190556. [Google Scholar] [CrossRef]
- Mosconi, L.; McHugh, P.F. FDG- and Amyloid-PET in Alzheimer’s Disease: Is the Whole Greater than the Sum of the Parts? Q. J. Nucl. Med. Mol. Imaging 2011, 55, 250–264. [Google Scholar] [PubMed]
- Townsend, L.; Pillinger, T.; Selvaggi, P.; Veronese, M.; Turkheimer, F.; Howes, O. Brain Glucose Metabolism in Schizophrenia: A Systematic Review and Meta-Analysis of 18FDG-PET Studies in Schizophrenia. Psychol. Med. 2022, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Nitchingham, A.; Pereira, J.V.-B.; Wegner, E.A.; Oxenham, V.; Close, J.; Caplan, G.A. Regional Cerebral Hypometabolism on 18F-FDG PET/CT Scan in Delirium Is Independent of Acute Illness and Dementia. Alzheimer’s Dement. 2023, 19, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, H.; Ruth, M.; Hammerlid, E. Psychiatric Morbidity among Patients with Cancer of the Esophagus or the Gastro-Esophageal Junction: A Prospective, Longitudinal Evaluation. Dis. Esophagus. 2007, 20, 523–529. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, Q.-D.; Hu, L.-J.; Sun, Z.-Q.; Sun, S.-P.; Yun, W.-W.; Yuan, Y.-G. Theory of Mind Deficits in Patients with Esophageal Cancer Combined with Depression. World J. Gastroenterol. 2013, 19, 2969–2973. [Google Scholar] [CrossRef]
- Han, S.; Kim, Y.J.; Woo, S.; Suh, C.H.; Lee, J.J. Prognostic Value of Volumetric Parameters of Pretreatment 18F-FDG PET/CT in Esophageal Cancer: A Systematic Review and Meta-Analysis. Clin. Nucl. Med. 2018, 43, 887–894. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).