Pretreatment Modified Glasgow Prognostic Score for Predicting Prognosis and Survival in Elderly Patients with Gastric Cancer Treated with Perioperative FLOT
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Datasets
2.2. Immunonutritional Indexes
- A score of 0 for CRP serum levels within the normal range (≤10 mg/L),
- A score of 1 for high CRP serum levels (>10 mg/L) and serum albumin levels within the normal range (≥3.5 g/dL),
- A score of 2 in the presence of both high CRP serum levels (>10 mg/L) and hypoalbuminemia (<3.5 g/dL) [22].
2.3. Patient Follow-Up
2.4. Ethical Approval
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, M.; George, R.; Sharma, A.; Graham, D.Y. Changing Trends in Stomach Cancer Throughout the World. Curr. Gastroenterol. Rep. 2017, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.H.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ychou, M.; Boige, V.; Pignon, J.P.; Conroy, T.; Bouché, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.M.; Saint-Aubert, B.; et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Kattan, M.W.; Karpeh, M.S.; Mazumdar, M.; Brennan, M.F. Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. J. Clin. Oncol. 2003, 21, 3647–3650. [Google Scholar] [CrossRef]
- Han, D.S.; Suh, Y.S.; Kong, S.H.; Lee, H.J.; Choi, Y.; Aikou, S.; Sano, T.; Park, B.J.; Kim, W.H.; Yang, H.K. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J. Clin. Oncol. 2012, 30, 3834–3840. [Google Scholar] [CrossRef]
- van den Ende, T.; Ter Veer, E.; Mali, R.M.A.; van Berge Henegouwen, M.I.; Hulshof, M.; van Oijen, M.G.H.; van Laarhoven, H.W.M. Prognostic and Predictive Factors for the Curative Treatment of Esophageal and Gastric Cancer in Randomized Controlled Trials: A Systematic Review and Meta-Analysis. Cancers 2019, 11, 530. [Google Scholar] [CrossRef]
- Li, X.; An, B.; Zhao, Q.; Qi, J.; Wang, W.; Zhang, D.; Li, Z.; Qin, C. Combined fibrinogen and neutrophil-lymphocyte ratio as a predictive factor in resectable colorectal adenocarcinoma. Cancer Manag. Res. 2018, 10, 6285–6294. [Google Scholar] [CrossRef]
- Han, C.L.; Meng, G.X.; Ding, Z.N.; Dong, Z.R.; Chen, Z.Q.; Hong, J.G.; Yan, L.J.; Liu, H.; Tian, B.W.; Yang, L.S.; et al. The Predictive Potential of the Baseline C-Reactive Protein Levels for the Efficiency of Immune Checkpoint Inhibitors in Cancer Patients: A Systematic Review and Meta-Analysis. Front. Immunol. 2022, 13, 827788. [Google Scholar] [CrossRef]
- Carr, B.I.; Ince, V.; Bag, H.G.; Usta, S.; Ersan, V.; Isik, B.; Yilmaz, S. CRP is a superior and prognostically significant inflammation biomarker for hepatocellular cancer patients treated by liver transplantation. Clin. Pract. 2021, 18, 1626–1632. [Google Scholar]
- Toyokawa, T.; Muguruma, K.; Yoshii, M.; Tamura, T.; Sakurai, K.; Kubo, N.; Tanaka, H.; Lee, S.; Yashiro, M.; Ohira, M. Clinical significance of prognostic inflammation-based and/or nutritional markers in patients with stage III gastric cancer. BMC Cancer 2020, 20, 517. [Google Scholar] [CrossRef] [PubMed]
- Ozveren, A.; Erdogan, A.P.; Ekinci, F. The inflammatory prognostic index as a potential predictor of prognosis in metastatic gastric cancer. Sci. Rep. 2023, 13, 7755. [Google Scholar] [CrossRef]
- Nogueiro, J.; Santos-Sousa, H.; Pereira, A.; Devezas, V.; Fernandes, C.; Sousa, F.; Fonseca, T.; Barbosa, E.; Barbosa, J.A. The impact of the prognostic nutritional index (PNI) in gastric cancer. Langenbeck’s Arch. Surg. 2022, 407, 2703–2714. [Google Scholar] [CrossRef]
- Aoyama, T.; Komori, K.; Nakazano, M.; Hara, K.; Tamagawa, H.; Kazama, K.; Hashimoto, I.; Yamada, T.; Maezawa, Y.; Segami, K.; et al. The Clinical Influence of the CONUT Score on Survival of Patients with Gastric Cancer Receiving Curative Treatment. In Vivo 2022, 36, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Saal, J.; Bald, T.; Eckstein, M.; Ralser, D.J.; Ritter, M.; Brossart, P.; Grünwald, V.; Hölzel, M.; Ellinger, J.; Klümper, N. Integrating On-Treatment Modified Glasgow Prognostic Score and Imaging to Predict Response and Outcomes in Metastatic Renal Cell Carcinoma. JAMA Oncol. 2023, 9, 1048–1055. [Google Scholar] [CrossRef]
- Nagashima, Y.; Funahashi, K.; Kagami, S.; Ushigome, M.; Kaneko, T.; Miura, Y.; Yoshida, K.; Koda, T.; Kurihara, A. Which preoperative immunonutritional index best predicts postoperative mortality after palliative surgery for malignant bowel obstruction in patients with late-stage cancer? A single-center study in Japan comparing the modified Glasgow prognostic score (mGPS), the prognostic nutritional index (PNI), and the controlling nutritional status (CONUT). Surg. Today 2023, 53, 22–30. [Google Scholar] [CrossRef]
- Yang, C.; Ren, G.; Yang, Q. Prognostic value of preoperative modified Glasgow prognostic score in surgical non-small cell lung cancer: A meta-analysis. Front. Surg. 2022, 9, 1094973. [Google Scholar] [CrossRef]
- Nozoe, T.; Iguchi, T.; Egashira, A.; Adachi, E.; Matsukuma, A.; Ezaki, T. Significance of modified Glasgow prognostic score as a useful indicator for prognosis of patients with gastric carcinoma. Am. J. Surg. 2011, 201, 186–191. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- McMillan, D.C.; Crozier, J.E.; Canna, K.; Angerson, W.J.; McArdle, C.S. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int. J. Colorectal Dis. 2007, 22, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.a.; Yang, P.; Sun, C.; Tian, Y.; Guo, H.; Liu, Y.; Li, Y.; Zhao, Q. Predictive Effect of Systemic Immune-Inflammation Index Combined with Prognostic Nutrition Index Score on Efficacy and Prognosis of Neoadjuvant Intraperitoneal and Systemic Paclitaxel Combined with Apatinib Conversion Therapy in Gastric Cancer Patients with Positive Peritoneal Lavage Cytology: A Prospective Study. Front. Oncol. 2022, 11, 791912. [Google Scholar] [CrossRef]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Yoshida, T.; Masuda, K.; Takeyasu, Y.; Shinno, Y.; Matsumoto, Y.; Okuma, Y.; Goto, Y.; Horinouchi, H.; Yamamoto, N.; et al. Prognostic role of modified Glasgow Prognostic score in elderly non-small cell lung cancer patients treated with anti-PD-1 antibodies. Respir. Investig. 2023, 61, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef]
- Kim, M.R.; Kim, A.S.; Choi, H.I.; Jung, J.H.; Park, J.Y.; Ko, H.J. Inflammatory markers for predicting overall survival in gastric cancer patients: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0236445. [Google Scholar] [CrossRef]
- Wu, D.; Wang, X.; Shi, G.; Sun, H.; Ge, G. Prognostic and clinical significance of modified glasgow prognostic score in pancreatic cancer: A meta-analysis of 4629 patients. Aging 2021, 13, 1410–1421. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Wu, Y.; Li, P.; Che, G. The prognostic value of modified Glasgow prognostic score in patients with esophageal squamous cell cancer: A Meta-analysis. Nutr. Cancer 2020, 72, 1146–1154. [Google Scholar] [CrossRef]
- Chen, H.; Hu, N.; Chang, P.; Kang, T.; Han, S.; Lu, Y.; Li, M. Modified Glasgow prognostic score might be a prognostic factor for hepatocellular carcinoma: A meta-analysis. Panminerva Med. 2017, 59, 302–307. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Yang, W.X.; Dou, W.C.; Shao, Y.X.; Li, X. Modified Glasgow prognostic score as a prognostic factor for renal cell carcinomas: A systematic review and meta-analysis. Cancer Manag. Res. 2019, 11, 6163–6173. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Chen, H.; Li, W. A comprehensive analysis of Glasgow Prognostic Score (GPS)/the modified Glasgow Prognostic Score (mGPS) on immune checkpoint inhibitor efficacy among patients with advanced cancer. Cancer Med. 2023, 12, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Demirelli, B.; Babacan, N.A.; Ercelep, Ö.; Öztürk, M.A.; Kaya, S.; Tanrıkulu, E.; Khalil, S.; Hasanov, R.; Alan, Ö.; Telli, T.A.; et al. Modified Glasgow Prognostic Score, Prognostic Nutritional Index and ECOG Performance Score Predicts Survival Better than Sarcopenia, Cachexia and Some Inflammatory Indices in Metastatic Gastric Cancer. Nutr. Cancer 2021, 73, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Díaz Del Arco, C.; Ortega Medina, L.; Estrada Muñoz, L.; García Gómez de Las Heras, S.; Fernández Aceñero, M.J. Pathologic Lymph Node Staging of Gastric Cancer. Am. J. Clin. Pathol. 2021, 156, 749–765. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.X.; Yang, L.P.; Wang, Z.X.; He, M.M.; Yun, J.P.; Zhang, D.S.; Wang, F.; Xu, R.H. Lymph node staging systems in patients with gastric cancer treated with D2 resection plus adjuvant chemotherapy. J. Cancer 2018, 9, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Zhou, W.; Li, J.; Ou, X.; Chen, C.; Cai, S.; He, W.; Xu, J.; He, Y. Association of Preoperative Serum Carcinoembryonic Antigen and Gastric Cancer Recurrence: A Large Cohort Study. J. Cancer 2021, 12, 397–403. [Google Scholar] [CrossRef]
- Shibata, C.; Nakano, T.; Yasumoto, A.; Mitamura, A.; Sawada, K.; Ogawa, H.; Miura, T.; Ise, I.; Takami, K.; Yamamoto, K.; et al. Comparison of CEA and CA19-9 as a predictive factor for recurrence after curative gastrectomy in gastric cancer. BMC Surg. 2022, 22, 213. [Google Scholar] [CrossRef]
- Wang, W.; Chen, X.L.; Zhao, S.Y.; Xu, Y.H.; Zhang, W.H.; Liu, K.; Chen, X.Z.; Yang, K.; Zhang, B.; Chen, Z.X.; et al. Prognostic significance of preoperative serum CA125, CA19-9 and CEA in gastric carcinoma. Oncotarget 2016, 7, 35423–35436. [Google Scholar] [CrossRef]
- Ye, X.-J.; Ji, Y.-B.; Ma, B.-W.; Huang, D.-D.; Chen, W.-Z.; Pan, Z.-Y.; Shen, X.; Zhuang, C.-L.; Yu, Z. Comparison of three common nutritional screening tools with the new European Society for Clinical Nutrition and Metabolism (ESPEN) criteria for malnutrition among patients with geriatric gastrointestinal cancer: A prospective study in China. BMJ Open 2018, 8, e019750. [Google Scholar] [CrossRef]
- Bullock, A.F.; Greenley, S.L.; McKenzie, G.A.G.; Paton, L.W.; Johnson, M.J. Relationship between markers of malnutrition and clinical outcomes in older adults with cancer: Systematic review, narrative synthesis and meta-analysis. Eur. J. Clin. Nutr. 2020, 74, 1519–1535. [Google Scholar] [CrossRef]
- White, J.V.; Guenter, P.; Jensen, G.; Malone, A.; Schofield, M. Consensus Statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: Characteristics Recommended for the Identification and Documentation of Adult Malnutrition (Undernutrition). J. Acad. Nutr. Diet. 2012, 112, 730–738. [Google Scholar] [CrossRef]
- Ji, T.; Li, Y.; Ma, L. Sarcopenic Obesity: An Emerging Public Health Problem. Aging Dis. 2022, 13, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, R.; Kusunoki, T.; Abe, M.; Kohara, K.; Miki, T. An association between body mass index and high-sensitivity C-reactive protein concentrations is influenced by age in community-dwelling persons. Ann. Clin. Biochem. 2013, 50, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Kushiyama, S.; Sakurai, K.; Kubo, N.; Tamamori, Y.; Nishii, T.; Tachimori, A.; Inoue, T.; Maeda, K. The Preoperative Geriatric Nutritional Risk Index Predicts Postoperative Complications in Elderly Patients with Gastric Cancer Undergoing Gastrectomy. In Vivo 2018, 32, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, H.; Yang, J.; Jiang, L.; Yang, J.; Wu, H.; Wen, T.; Yan, L. Geriatric nutritional risk index predicts prognosis after hepatectomy in elderly patients with hepatitis B virus-related hepatocellular carcinoma. Sci. Rep. 2018, 8, 12561. [Google Scholar] [CrossRef] [PubMed]
- Furuke, H.; Matsubara, D.; Kubota, T.; Kiuchi, J.; Kubo, H.; Ohashi, T.; Shimizu, H.; Arita, T.; Yamamoto, Y.; Konishi, H.; et al. Geriatric Nutritional Risk Index Predicts Poor Prognosis of Patients After Curative Surgery for Gastric Cancer. Cancer Diagn. Progn. 2021, 1, 43–52. [Google Scholar] [CrossRef]
- Yin, J.; Qu, J.; Liang, X.; Wang, M. Prognostic significance of controlling nutritional status score for patients with gastric cancer: A systematic review and meta-analysis. Exp. Ther. Med. 2023, 25, 202. [Google Scholar] [CrossRef]
- Manoglu, B.; Sokmen, S.; Bisgin, T.; Semiz, H.S.; Görken, I.B.; Ellidokuz, H. Inflammation-based prognostic scores in geriatric patients with rectal cancer. Tech. Coloproctol. 2023, 27, 397–405. [Google Scholar] [CrossRef]
- Mari, A.; Muto, G.; Di Maida, F.; Tellini, R.; Bossa, R.; Bisegna, C.; Campi, R.; Cocci, A.; Viola, L.; Grosso, A.; et al. Oncological impact of inflammatory biomarkers in elderly patients treated with radical cystectomy for urothelial bladder cancer. Arab. J. Urol. 2020, 19, 2–8. [Google Scholar] [CrossRef]
- Mahalingam, S.; Amaranathan, A.; Sathasivam, S.; Udayakumar, K.P. Correlation of Preoperative Anemia Subtypes with Tumor Characteristics, Systemic Inflammation and Immediate Postoperative Outcomes in Gastrointestinal Cancer Patients—A Prospective Observational Study. J. Gastrointest. Cancer 2023. [Google Scholar] [CrossRef]
- Sahakyan, M.A.; Brudvik, K.W.; Angelsen, J.-H.; Dille-Amdam, R.G.; Sandvik, O.M.; Edwin, B.; Nymo, L.S.; Lassen, K. Preoperative Inflammatory Markers in Liver Resection for Colorectal Liver Metastases: A National Registry-Based Study. World J. Surg. 2023, 47, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total (n = 71) | mGPS 0 (n = 41) | mGPS 1 (n = 15) | mGPS 2 (n = 15) | p-Value |
|---|---|---|---|---|---|
| Age (years) | 68.9 ± 4.16 | 69.7 ± 4.29 | 67.7 ± 4.18 | 68.0 ± 3.48 | 0.117 |
| Gender (male/female) | 50/21 | 29/12 | 11/4 | 10/5 | 0.982 |
| BMI (kg/m2) | 24.6 ± 3.82 | 23.7 ± 3.27 | 25.2 ± 3.34 | 26.4 ± 4.99 | 0.064 |
| ECOG-PS (0/1) | 60/11 | 33/8 | 13/2 | 14/1 | 0.525 |
| Tumor subtype | |||||
| Adenocarcinoma | 53 [74.6] | 31 [75.6] | 12 [80] | 10 [66.7] | 0.567 |
| Mucinous adenocarcinoma | 2 [2.8] | 2 [4.9] | 0 | 0 | |
| Signet-ring cell carcinoma | 16 [22.5] | 8 [19.5] | 3 [20] | 5 [33.3] | |
| Tumor location | |||||
| Cardia | 29 [40.8] | 16 [39.0] | 10 [66.7] | 3 [20] | 0.126 |
| Corpus | 14 [19.7] | 8 [19.5] | 1 [6.7] | 5 [33.4] | |
| Antrum | 21 [29.6] | 14 [34.1] | 3 [20] | 4 [26.7] | |
| Esophagogastric junction | 7 [9.9] | 3 [7.3] | 1 [6.7] | 3 [20] | |
| Lauren classification (Intestinal/Diffuse) | 63/8 | 35/6 | 15/0 | 13/2 | 0.250 |
| T Stage (T1/T2/T3/T4a/T4b) | 3/10/27/28/3 [4.2/14.1/38/39.5/4.2] | 2/8/17/12/2 [4.9/19.5/41.4/29.3/4.9] | 1/2/5/6/1 [6.7/13.3/33.3/40/6.7] | -/-/5/10/- [-/-/33.3/66.7/-] | 0.374 |
| N Stage (N0/N1/N2/N3a/N3b) | 7/21/22/18/3 [9.9/29.6/31/25.3/4.2] | 5/16/12/8/-[12.2/39/29.3/19.5/-] | 2/3/5/3/2 [13.3/20/33.3/20/13.3] | -/2/5/7/1 [-/13.3/33.3/46.7/6.7] | 0.038 |
| Radiologic response | |||||
| Complete response | 15 [21.1] | 12 [29.3] | 2 [13.3] | 1 [6.7] | 0.123 |
| Partial response | 30 [42.3] | 20 [48.8] | 5 [33.3] | 5 [33.3] | |
| Stable disease | 21 [29.6] | 9 [22.0] | 7 [46.7] | 5 [33.3] | |
| Progressive disease | 5 [7.0] | - | 1 [6.7] | 4 [26.7] | |
| Pathologic response | |||||
| Complete response | 24 [33.8] | 16 [39.0] | 4 [26.7] | 1 [6.7] | 0.486 |
| Residual disease | 47 [66.2] | 25 [61] | 11 [73.4] | 14 [93.3] | |
| Neutrophil count (/L) | 4.97 ± 1.89 | 4.70 ± 1.87 | 4.79 ± 1.78 | 4.97 ± 1.67 | 0.996 |
| Lymphocyte count (/L) | 1.94 ± 1.15 | 1.71 ± 0.49 | 1.79 ± 0.46 | 1.97 ± 0.95 | 0.807 |
| Hemoglobin (g/dL) | 12.1 ± 2.03 | 11.7 ± 1.87 | 12.1 ± 2.87 | 12.2 ± 1.52 | 0.730 |
| CRP (mg/dL) | 10.0 (0.34–156.0) | 4.5 (2.0–10.0) a | 15.0 (12.0–30.0) b | 31.0 (16.0–65.0) b | ˂0.001 |
| Albumin (g/dL) | 3.59 ± 0.47 | 3.68 ± 0.39 a | 3.83 ± 0.18 a | 2.97 ± 0.31 b | ˂0.001 |
| Preoperative CEA (ng/mL) | 4.0 (0.33–74.0) | 3.49 (0.51–9.30) a | 2.85 (0.33–74.0) a,b | 6.1 (1.59–42.0) b | 0.027 |
| Preoperative CA 19-9 (U/mL) | 11 (0.3–2307.0) | 5.20 (0.3–276.0) a | 13.5 (0.4–2307.0) a,b | 60.0 (0.5–1792.0) b | 0.004 |
| PNI | 36.2 ± 4.78 | 37.3 ± 3.99 a | 38.7 ± 2.87 a,b | 30.5 ± 4.07 b | ˂0.001 |
| GNRI | 52.0 ± 7.23 | 50.5 ± 6.32 | 53.5 ± 6.42 | 54.7 ± 9.47 | 0.139 |
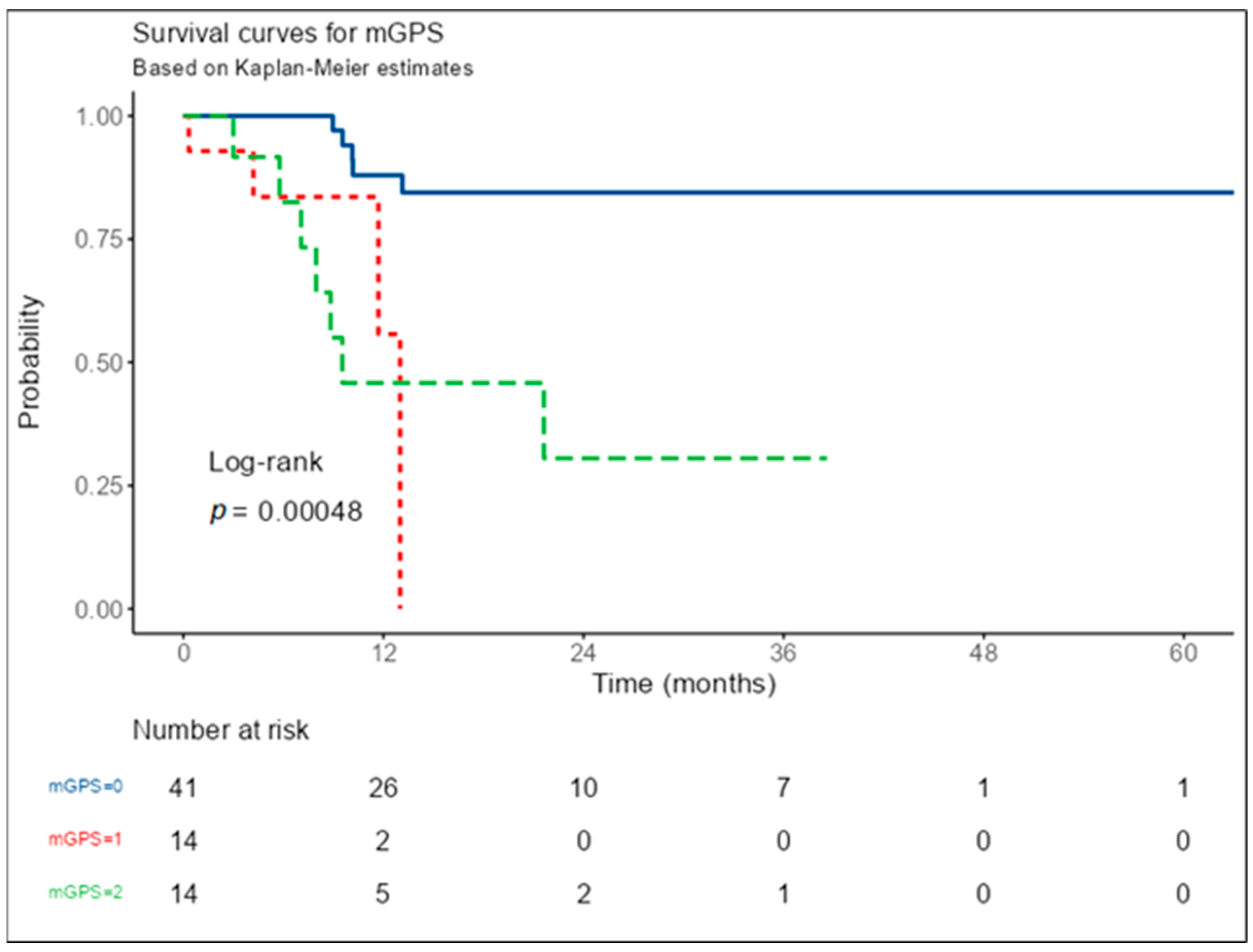
| Numbers of Event | Time (Months) | Cox Table-mGPS | Pairwise Comparisons mGPS | |||
|---|---|---|---|---|---|---|
| Levels | Records | Events | Median | HR (95% CI) | Level | p-Value |
| mGPS 0 | 41 | 5 | NA | - | 1-0 | 0.005 |
| mGPS 1 | 14 | 4 | 13.00 | 6.25 (1.61–24.28), p = 0.008 | 2-0 | <0.001 |
| mGPS 2 | 14 | 7 | 9.53 | 6.59 (2.08–20.85), p = 0.001 | 2-1 | 1.000 |
| Levels | Time (Months) | Number at Risk | Number of Events | Survival | Lower | Upper |
|---|---|---|---|---|---|---|
| mGPS 0 | 12 | 26 | 4 | 88.0% | 77.6% | 99.8% |
| 24 | 10 | 1 | 84.4% | 72.7% | 98.0% | |
| 36 | 7 | 0 | 84.4% | 72.7% | 98.0% | |
| 48 | 1 | 0 | 84.4% | 72.7% | 98.0% | |
| 60 | 1 | 0 | 84.4% | 72.7% | 98.0% | |
| mGPS 1 | 12 | 2 | 3 | 55.7% | 24.1% | 100.0% |
| mGPS 2 | 12 | 5 | 6 | 45.8% | 24.1% | 87.2% |
| 24 | 2 | 1 | 30.6% | 10.9% | 85.3% | |
| 36 | 1 | 0 | 30.6% | 10.9% | 85.3% |
| mGPS | Predictor | Estimate | SE | Z Score | p-Value | OR | 95% Cl |
|---|---|---|---|---|---|---|---|
| 1-0 | Intercept | −3.803 | 2.1284 | −1.79 | 0.074 | 0.02229 | 3.44–1.445 |
| BMI | 0.115 | 0.0854 | 1.34 | 0.179 | 1.12149 | 0.949–1.326 | |
| 2-0 | Intercept | −5.706 | 2.1962 | −2.60 | 0.009 | 0.00333 | 4.49–0.246 |
| BMI | 0.186 | 0.0859 | 2.16 | 0.030 | 1.20426 | 1.018–1.425 |
| mGPS 0 (n = 41) | mGPS 1 (n = 15) | mGPS 2 (n = 15) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| None | G1–2 | G3–4 | None | G1–2 | G3–4 | None | G1–2 | G3–4 | p-Value | |
| Dose deferral | 29 [70.7] | 12 [29.3] | - | 8 [53.3] | 7 [46.7] | - | 12 [80.0] | 3 [20.0] | - | 0.162 |
| G-CSF prophylaxis | 6 [14.6] | 35 [85.4] | - | 2 [13.3] | 13 [86.7] | - | - | 15 [100.0] | - | 0.320 |
| Grade 3/4 toxicity | 30 [73.2] | 11 [26.8] | - | 11 [73.3] | 4 [26.7] | - | 13 [86.7] | 2 [13.3] | - | 0.294 |
| Dose reduction | 26 [63.4] | 15 [36.6] | - | 10 [66.7] | 5 [33.7] | - | 12 [80.0] | 3 [20.0] | - | 0.295 |
| Fatigue | 9 [22] | 32 [78.0] | - | 5 [33.3] | 9 [60.0] | 1 [6.7] | 1 [6.7] | 14 [93.3] | - | 0.135 |
| Hand-foot syndrome | 29 [70.7] | 12 [29.3] | - | 9 [60.0] | 6 [40.0] | - | 13 [86.7] | 2 [13.3] | - | 0.306 |
| Neutropenia | 23 [56.1] | 14 [34.1] | 4 [9.8] | 7 [46.7] | 4 [26.7] | 4 [26.7] | 11 [73.3] | 4 [26.7] | - | 0.150 |
| Anemia | 19 [46.3] | 21 [51.2] | 1 [2.4] | 8 [53.3] | 7 [46. 7] | - | 4 [26.7] | 10 [66.6] | 1 [6.7] | 0.593 |
| Thrombocytopenia | 28 [68.3] | 13 [31.7] | - | 12 [80.0] | 3 [20.0] | - | 9 [60.0] | 6 [40.0] | - | 0.610 |
| Febrile neutropenia | 33 [80.5] | 8 [19.5] | - | 11 [73.3] | 4 [26.7] | - | 12 [80.0] | 3 [20.0] | - | 0.702 |
| Mucositis | 23 [56.1] | 17 [41.5] | 1 [2.4] | 8 [53.3] | 7 [46.7] | - | 12 [80.0] | 2 [13.3] | 1 [6.7] | 0.293 |
| Diarrhea | 13 [31.7] | 27 [65.9] | 1 [2.4] | 4 [26.7] | 11 [73.3] | - | 3 [20.0] | 11 [73.3] | 1 [6.7] | 0.846 |
| Neuropathy | 21 [51.2] | 19 [46.3] | 1 [2.4] | 8 [53.3] | 7 [46.7] | - | 8 [53.3] | 7 [46.7] | - | 0.937 |
| Nausea | 7 [17.1] | 33 [80.5] | 1 [2.4] | 3 [20.0] | 12 [80.8] | - | 1 [6.7] | 13 [86.6] | 1 [6.7] | 0.773 |
| Vomiting | 15 [36.6] | 25 [61.0] | 1 [2.4] | 6 [40.0] | 9 [60.0] | - | 7 [50.0] | 7 [50.0] | 1 [6.7] | 0.845 |
| Stomatitis | 29 [70.7] | 11 [26.8] | 1 [2.4] | 12 [80.0] | 3 [20.0] | - | 11 [73.3] | 4 [26.7] | - | 0.887 |
| Allergic complications | 36 [87.8] | 5 [12.2] | - | 12 [80.0] | 3 [20.0] | - | 14 [93.3] | 1 [6.7] | - | 0.223 |
| Thrombosis | 36 [87.7] | 5 [12.2] | - | 14 [93.3] | 1 [6.7] | - | 15 [100.0] | - | - | 0.355 |
| Renal toxicity | 36 [87.8] | 5 [12.2] | - | 15 [100.0] | - | - | 14 [93.3] | 1 [6.7] | - | 0.767 |
| Hepatic toxicity | 36 [87.8] | 5 [12.2] | - | 15 [100.0] | - | - | 14 [93.3] | 1 [6.7] | - | 0.355 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melekoglu, E.; Bayram, E.; Secmeler, S.; Mete, B.; Sahin, B. Pretreatment Modified Glasgow Prognostic Score for Predicting Prognosis and Survival in Elderly Patients with Gastric Cancer Treated with Perioperative FLOT. Nutrients 2023, 15, 4156. https://doi.org/10.3390/nu15194156
Melekoglu E, Bayram E, Secmeler S, Mete B, Sahin B. Pretreatment Modified Glasgow Prognostic Score for Predicting Prognosis and Survival in Elderly Patients with Gastric Cancer Treated with Perioperative FLOT. Nutrients. 2023; 15(19):4156. https://doi.org/10.3390/nu15194156
Chicago/Turabian StyleMelekoglu, Ebru, Ertugrul Bayram, Saban Secmeler, Burak Mete, and Berksoy Sahin. 2023. "Pretreatment Modified Glasgow Prognostic Score for Predicting Prognosis and Survival in Elderly Patients with Gastric Cancer Treated with Perioperative FLOT" Nutrients 15, no. 19: 4156. https://doi.org/10.3390/nu15194156
APA StyleMelekoglu, E., Bayram, E., Secmeler, S., Mete, B., & Sahin, B. (2023). Pretreatment Modified Glasgow Prognostic Score for Predicting Prognosis and Survival in Elderly Patients with Gastric Cancer Treated with Perioperative FLOT. Nutrients, 15(19), 4156. https://doi.org/10.3390/nu15194156






