Binge-Eating Precursors in Children and Adolescents: Neurodevelopment, and the Potential Contribution of Ultra-Processed Foods
Abstract
1. Introduction
Review Scope
2. Eating Brain Circuitry
3. Neurodevelopment of Emotional Self-Regulation
4. sBED-Related Brain Vulnerability Markers and BED
4.1. Response and Behavioral Inhibition Deficits
4.2. Reward-Based Deficits
4.3. Beyond Inhibition and Reward
4.4. Relevant Considerations
5. Ultra-Processed Food and Drinks and BED?
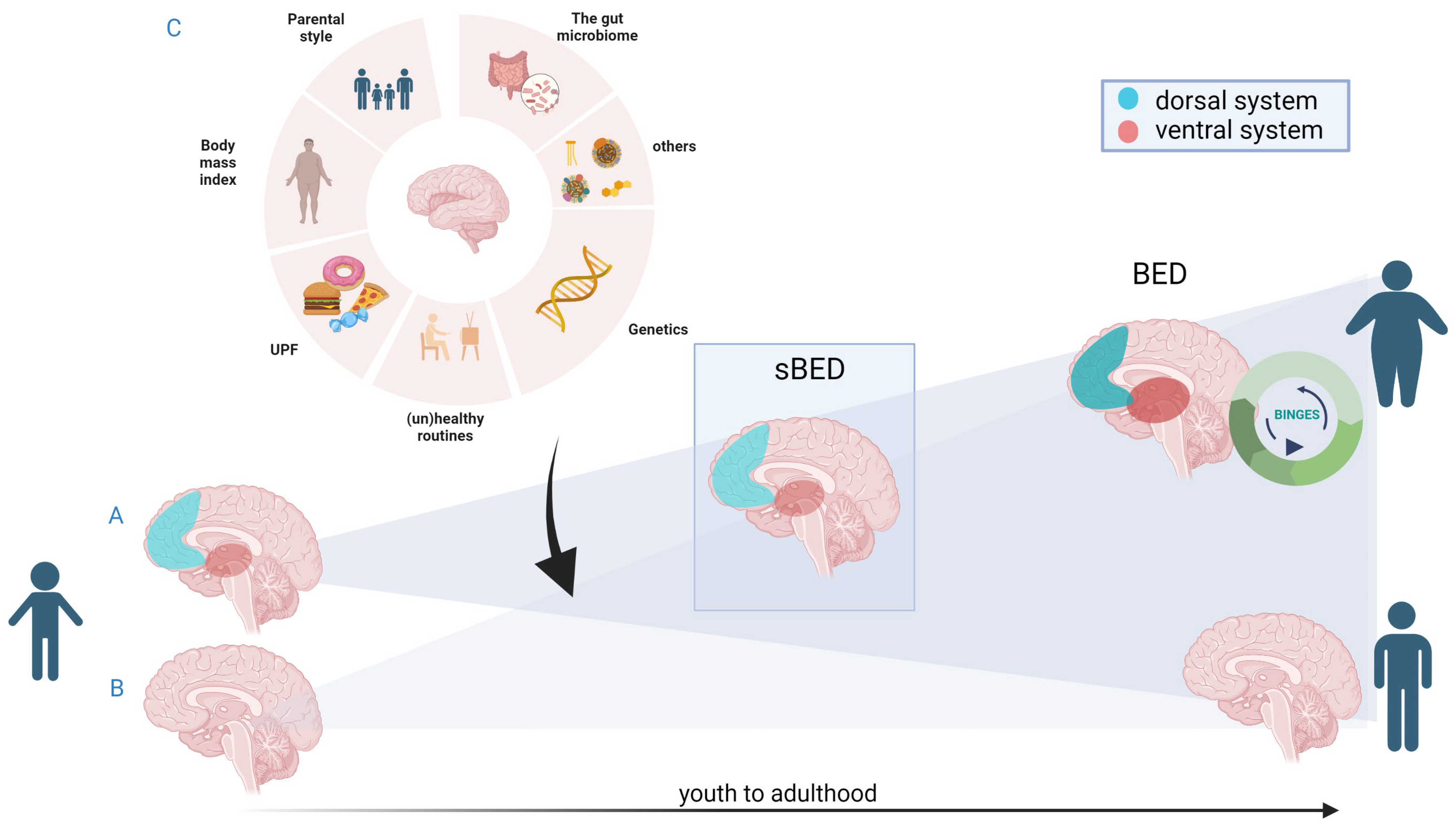
6. Conclusions: Future
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA; Arlington, VA, USA, 2013; ISBN 9780890425541. [Google Scholar]
- Santomauro, D.F.; Melen, S.; Mitchison, D.; Vos, T.; Whiteford, H.; Ferrari, A.J. The hidden burden of eating disorders: An extension of estimates from the Global Burden of Disease Study 2019. Lancet Psychiatry 2021, 8, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Smink, F.R.E.E.; Van Hoeken, D.; Oldehinkel, A.J.; Hoek, H.W. Prevalence and severity of DSM-5 eating disorders in a community cohort of adolescents. Int. J. Eat. Disord. 2014, 47, 610–619. [Google Scholar] [CrossRef]
- Kjeldbjerg, M.L.; Clausen, L. Prevalence of binge-eating disorder among children and adolescents: A systematic review and meta-analysis. Eur. Child Adolesc. Psychiatry 2021, 32, 549–574. [Google Scholar] [CrossRef] [PubMed]
- Tanofsky-Kraff, M.; Marcus, M.D.; Yanovski, S.Z.; Yanovski, J.A. Loss of control eating disorder in children age 12 years and younger: Proposed research criteria. Eat. Behav. 2008, 9, 360–365. [Google Scholar] [CrossRef]
- Decaluwé, V.; Braet, C. Prevalence of binge-eating disorder in obese children and adolescents seeking weight-loss treatment. Int. J. Obes. 2003, 27, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Marzilli, E.; Cerniglia, L.; Cimino, S. A narrative review of binge eating disorder in adolescence: Prevalence, impact, and psychological treatment strategies. Adolesc. Health Med. Ther. 2018, 9, 17–30. [Google Scholar] [CrossRef]
- Ágh, T.; Kovács, G.; Supina, D.; Pawaskar, M.; Herman, B.K.; Vokó, Z.; Sheehan, D.V. A systematic review of the health-related quality of life and economic burdens of anorexia nervosa, bulimia nervosa, and binge eating disorder. Eat. Weight Disord. 2016, 21, 353–364. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 11 June 2023).
- Guerdjikova, A.I.; Mori, N.; Casuto, L.S.; McElroy, S.L. Update on Binge Eating Disorder. Med. Clin. N. Am. 2019, 103, 669–680. [Google Scholar] [CrossRef]
- Kessler, R.C.; Berglund, P.A.; Chiu, W.T.; Deitz, A.C.; Hudson, J.I.; Shahly, V.; Aguilar-Gaxiola, S.; Alonso, J.; Angermeyer, M.C.; Benjet, C.; et al. The prevalence and correlates of binge eating disorder in the World Health Organization World Mental Health Surveys. Biol. Psychiatry 2013, 73, 904–914. [Google Scholar] [CrossRef]
- Neumark-Sztainer, D.; Wall, M.; Guo, J.; Story, M.; Haines, J.; Eisenberg, M. Obesity, disordered eating, and eating disorders in a longitudinal study of adolescents: How do dieters fare 5 years later? J. Am. Diet. Assoc. 2006, 106, 559–568. [Google Scholar] [CrossRef]
- Smith, K.E.; Luo, S.; Mason, T.B. A systematic review of neural correlates of dysregulated eating associated with obesity risk in youth. Neurosci. Biobehav. Rev. 2021, 124, 245–266. [Google Scholar] [CrossRef]
- Vainik, U.; Neseliler, S.; Konstabel, K.; Fellows, L.K.; Dagher, A. Eating traits questionnaires as a continuum of a single concept. Uncontrolled eating. Appetite 2015, 90, 229–239. [Google Scholar] [CrossRef]
- Hebebrand, J.; Gearhardt, A.N. The concept of “food addiction” helps inform the understanding of overeating and obesity: NO. Am. J. Clin. Nutr. 2021, 113, 268–273. [Google Scholar] [CrossRef]
- Eldredge, K.L.; Agras, W.S. Weight and shape overconcern and emotional eating in binge eating disorder. Int. J. Eat. Disord. 1996, 19, 73–82. [Google Scholar] [CrossRef]
- Nakamura, Y.; Koike, S. Association of Disinhibited Eating and Trait of Impulsivity with Insula and Amygdala Responses to Palatable Liquid Consumption. Front. Syst. Neurosci. 2021, 15, 647143. [Google Scholar] [CrossRef]
- Tanofsky-Kraff, M.; Shomaker, L.B.; Olsen, C.; Roza, C.A.; Wolkoff, L.E.; Columbo, K.M.; Raciti, G.; Zocca, J.M.; Wilfley, D.E.; Yanovski, S.Z.; et al. A Prospective study of pediatric loss of control eating and psychological outcomes. J. Abnorm. Psychol. 2011, 120, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, A.; Nelson, E.E.; Bongiorno, D.M.; Pine, D.S.; Yanovski, J.A.; Tanofsky-Kraff, M. Behavioral and neurodevelopmental precursors to binge-type eating disorders: Support for the role of negative valence systems. Psychol. Med. 2015, 45, 2921–2936. [Google Scholar] [CrossRef]
- Davis, C.; Carter, J.C. Compulsive overeating as an addiction disorder. A review of theory and evidence. Appetite 2009, 53, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Von Deneen, K.M.; Liu, Y. Obesity as an addiction: Why do the obese eat more? Maturitas 2011, 68, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Hauck, C.; Cook, B.; Ellrott, T. Food addiction, eating addiction and eating disorders. Proc. Nutr. Soc. 2020, 79, 103–112. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Corbin, W.R.; Brownell, K.D. Preliminary validation of the Yale Food Addiction Scale. Appetite 2009, 52, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Imperatori, C.; Fabbricatore, M.; Vumbaca, V.; Innamorati, M.; Contardi, A.; Farina, B. Food Addiction: Definition, measurement and prevalence in healthy subjects and in patients with eating disorders. Riv. Psichiatr. 2016, 51, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Cebolla, A.; Perpiñá, C.; Lurbe, E.; Alvarez-Pitti, J.; Botella, C. Prevalencia del trastorno por atracón en una muestra clínica de obesos. An. Pediatría 2012, 77, 98–102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shapiro, J.R.; Woolson, S.L.; Hamer, R.M.; Kalarchian, M.A.; Marcus, M.D.; Bulik, C.M. Evaluating binge eating disorder in children: Development of the children’s binge eating disorder scale (C-BEDS). Int. J. Eat. Disord. 2007, 40, 82–89. [Google Scholar] [CrossRef]
- Marcus, M.D.; Kalarchian, M.A. Binge eating in children and adolescents. Int. J. Eat. Disord. 2003, 34, S47–S57. [Google Scholar] [CrossRef]
- Chamay-Weber, C.; Combescure, C.; Lanza, L.; Carrard, I.; Haller, D.M. Screening Obese Adolescents for Binge Eating Disorder in Primary Care: The Adolescent Binge Eating Scale. J. Pediatr. 2017, 185, 68–72.e1. [Google Scholar] [CrossRef]
- Balantekin, K.N.; Birch, L.L.; Savage, J.S. Eating in the absence of hunger during childhood predicts self-reported binge eating in adolescence. Eat. Behav. 2017, 24, 7–10. [Google Scholar] [CrossRef]
- Herle, M.; Stavola, B.; De Hübel, C.; Abdulkadir, M.; Ferreira, D.S.; Loos, R.J.F.; Bryant-Waugh, R.; Bulik, C.M.; Micali, N. A longitudinal study of eating behaviours in childhood and later eating disorder behaviours and diagnoses. Br. J. Psychiatry 2020, 216, 113–119. [Google Scholar] [CrossRef]
- Ashcroft, J.; Semmler, C.; Carnell, S.; Van Jaarsveld, C.; Wardle, J. Continuity and stability of eating behaviour traits in children. Eur. J. Clin. Nutr. 2008, 62, 985–990. [Google Scholar] [CrossRef]
- Brewerton, T.D.; Rance, S.J.; Dansky, B.S.; O’Neil, P.M.; Kilpatrick, D.G. A comparison of women with child-adolescent versus adult onset binge eating: Results from the National Women’s Study. Int. J. Eat. Disord. 2014, 47, 836–843. [Google Scholar] [CrossRef]
- Stice, E.; Nathan Marti, C.; Rohde, P. Prevalence, incidence, impairment, and course of the proposed DSM-5 eating disorder diagnoses in an 8-year prospective community study of young women. J. Abnorm. Psychol. 2013, 122, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Brockmeyer, T.; Skunde, M.; Wu, M.; Bresslein, E.; Rudofsky, G.; Herzog, W.; Friederich, H.C. Difficulties in emotion regulation across the spectrum of eating disorders. Compr. Psychiatry 2014, 55, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Favieri, F.; Marini, A.; Casagrande, M. Emotional regulation and overeating behaviors in children and adolescents: A systematic review. Behav. Sci. 2021, 11, 11. [Google Scholar] [CrossRef]
- Mischel, W.; Shoda, Y.; Rodriguez, M.L. Delay of Gratification in Children. Science. 1989, 244, 933–938. [Google Scholar] [CrossRef]
- Stein, R.I.; Kenardy, J.; Wiseman, C.V.; Dounchis, J.Z.; Arnow, B.A.; Wilfley, D.E. What’s driving the binge in binge eating disorder?: A prospective examination of precursors and consequences. Int. J. Eat. Disord. 2007, 40, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Penzenstadler, L.; Soares, C.; Karila, L.; Khazaal, Y. Systematic Review of Food Addiction as Measured with the Yale Food Addiction Scale: Implications for the Food Addiction Construct. Curr. Neuropharmacol. 2019, 17, 526–538. [Google Scholar] [CrossRef]
- Bleck, J.R.; DeBate, R.D.; Olivardia, R. The Comorbidity of ADHD and Eating Disorders in a Nationally Representative Sample. J. Behav. Health Serv. Res. 2015, 42, 437–451. [Google Scholar] [CrossRef]
- Goldschmidt, A.B.; Lavender, J.M.; Hipwell, A.E.; Stepp, S.D.; Keenan, K. Emotion Regulation and Loss of Control Eating in Community-Based Adolescents. J. Abnorm. Child Psychol. 2017, 45, 183–191. [Google Scholar] [CrossRef]
- Levin, R.L.; Rawana, J.S. Attention-deficit/hyperactivity disorder and eating disorders across the lifespan: A systematic review of the literature. Clin. Psychol. Rev. 2016, 50, 22–36. [Google Scholar] [CrossRef]
- Kalarchian, M.A.; Marcus, M.D. Psychiatric comorbidity of childhood obesity. Int. Rev. Psychiatry 2012, 24, 241–246. [Google Scholar] [CrossRef]
- Lavagnino, L.; Arnone, D.; Cao, B.; Soares, J.C.; Selvaraj, S. Inhibitory control in obesity and binge eating disorder: A systematic review and meta-analysis of neurocognitive and neuroimaging studies. Neurosci. Biobehav. Rev. 2016, 68, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Stice, E.; Davis, K.; Miller, N.P.; Marti, C.N. Fasting Increases Risk for Onset of Binge Eating and Bulimic Pathology: A 5-Year Prospective Study. J. Abnorm. Psychol. 2008, 117, 941. [Google Scholar] [CrossRef] [PubMed]
- Da Luz, F.Q.; Hay, P.; Gibson, A.A.; Touyz, S.W.; Swinbourne, J.M.; Roekenes, J.A.; Sainsbury, A. Does severe dietary energy restriction increase binge eating in overweight or obese individuals? A systematic review. Obes. Rev. 2015, 16, 652–665. [Google Scholar] [CrossRef]
- Elran-Barak, R.; Sztainer, M.; Goldschmidt, A.B.; Crow, S.J.; Peterson, C.B.; Hill, L.L.; Crosby, R.D.; Powers, P.; Mitchell, J.E.; Le Grange, D. Dietary Restriction Behaviors and Binge Eating in Anorexia Nervosa, Bulimia Nervosa and Binge Eating Disorder: Trans-diagnostic Examination of the Restraint Model. Eat. Behav. 2015, 18, 192–196. [Google Scholar] [CrossRef]
- Racine, S.E.; Burt, S.A.; Iacono, W.G.; McGue, M.; Klump, K.L. Dietary Restraint Moderates Genetic Risk for Binge Eating. J. Abnorm. Psychol. 2011, 120, 119. [Google Scholar] [CrossRef]
- Conceição, E.M.; Moreira, C.S.; de Lourdes, M.; Ramalho, S.; Vaz, A.R. Exploring Correlates of Loss of Control Eating in a Nonclinical Sample. Front. Psychol. 2022, 12, 787558. [Google Scholar] [CrossRef] [PubMed]
- Fairburn, C.G. Overcoming Binge Eating: The Proven Program to Learn Why You Binge and How You Can Stop; Barnes & Noble, Ed.; Guilford Publications Inc.: New York, NY, USA, 2013; ISBN 1572305614. [Google Scholar]
- Ricciardelli, L.A.; McCabe, M.P. Dietary restraint and negative affect as mediators of body dissatisfaction and bulimic behavior in adolescent girls and boys. Behav. Res. Ther. 2001, 39, 1317–1328. [Google Scholar] [CrossRef]
- Ayton, A.; Ibrahim, A.; Dugan, J.; Galvin, E.; Wright, O.W. Ultra-processed foods and binge eating: A retrospective observational study. Nutrition 2021, 84, 111023. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, N.; Kose, J.; Srour, B.; Julia, C.; Kesse-Guyot, E.; Péneau, S.; Allès, B.; Paz Graniel, I.; Chazelas, E.; Deschasaux-Tanguy, M.; et al. Ultra-processed food intake and eating disorders: Cross-sectional associations among French adults. J. Behav. Addict. 2022, 11, 588–599. [Google Scholar] [CrossRef]
- Wang, L.; Martínez Steele, E.; Du, M.; Pomeranz, J.L.; O’Connor, L.E.; Herrick, K.A.; Luo, H.; Zhang, X.; Mozaffarian, D.; Zhang, F.F. Trends in Consumption of Ultraprocessed Foods Among US Youths Aged 2–19 Years, 1999–2018. JAMA 2021, 326, 519–530. [Google Scholar] [CrossRef]
- Marino, M.; Puppo, F.; Del Bo’, C.; Vinelli, V.; Riso, P.; Porrini, M.; Martini, D. A systematic review of worldwide consumption of ultra-processed foods: Findings and criticisms. Nutrients 2021, 13, 2778. [Google Scholar] [CrossRef] [PubMed]
- Latasa, P.; Louzada, M.L.D.C.; Martinez Steele, E.; Monteiro, C.A. Added sugars and ultra-processed foods in Spanish households (1990–2010). Eur. J. Clin. Nutr. 2018, 72, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Pagliai, G.; Dinu, M.; Madarena, M.P.; Bonaccio, M.; Iacoviello, L.; Sofi, F. Consumption of ultra-processed foods and health status: A systematic review and meta-analysis. Br. J. Nutr. 2021, 125, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Mitchison, D.; Touyz, S.; González-Chica, D.A.; Stocks, N.; Hay, P. How abnormal is binge eating? 18-Year time trends in population prevalence and burden. Acta Psychiatr. Scand. 2017, 136, 147–155. [Google Scholar] [CrossRef]
- Bulik, C.M.; Sullivan, P.F.; Kendler, K.S. Genetic and environmental contributions to obesity and binge eating. Int. J. Eat. Disord. 2003, 33, 293–298. [Google Scholar] [CrossRef]
- Frank, G.K.W. The Perfect Storm—A Bio-Psycho-Social Risk Model for Developing and Maintaining Eating Disorders. Front. Behav. Neurosci. 2016, 10, 44. [Google Scholar] [CrossRef]
- Costa, A.; Oliveira, A. Parental Feeding Practices and Children’s Eating Behaviours: An Overview of Their Complex Relationship. Healthcare 2023, 11, 400. [Google Scholar] [CrossRef]
- Paroche, M.M.; Caton, S.J.; Vereijken, C.M.J.L.; Weenen, H.; Houston-Price, C. How Infants and Young Children Learn about Food: A Systematic Review. Front. Psychol. 2017, 8, 1046. [Google Scholar] [CrossRef]
- Vaughn, A.E.; Ward, D.S.; Fisher, J.O.; Faith, M.S.; Hughes, S.O.; Kremers, S.P.J.; Musher-Eizenman, D.R.; O’Connor, T.M.; Patrick, H.; Power, T.G. Fundamental constructs in food parenting practices: A content map to guide future research. Nutr. Rev. 2016, 74, 98–117. [Google Scholar] [CrossRef]
- Giel, K.E.; Bulik, C.M.; Fernandez-Aranda, F.; Hay, P.; Keski-Rahkonen, A.; Schag, K.; Schmidt, U.; Zipfel, S. Binge eating disorder. Nat. Rev. Dis. Prim. 2022, 8, 16. [Google Scholar] [CrossRef]
- Kaye, W.H.; Fudge, J.L.; Paulus, M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat. Rev. Neurosci. 2009, 10, 573–584. [Google Scholar] [CrossRef]
- Tepper, B.J.; Barbarossa, I.T. Taste, Nutrition, and Health. Nutrients 2020, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M.; Prescott, J. Odor/taste integration and the perception of flavor. Exp. Brain Res. 2005, 166, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. Taste, olfactory and food texture reward processing in the brain and the control of appetite. Proc. Nutr. Soc. 2012, 71, 488–501. [Google Scholar] [CrossRef]
- Shepherd, G.M. Smell images and the flavour system in the human brain. Nature 2006, 444, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M. Taste representation in the human insula. Brain Struct. Funct. 2010, 214, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Chikazoe, J.; Lee, D.H.; Kriegeskorte, N.; Anderson, A.K. Distinct representations of basic taste qualities in human gustatory cortex. Nat. Commun. 2019, 10, 1048. [Google Scholar] [CrossRef]
- Seabrook, L.T.; Borgland, S.L. The orbitofrontal cortex, food intake and obesity. J. Psychiatry Neurosci. 2020, 45, 304–312. [Google Scholar] [CrossRef]
- Dagher, A.; Neseliler, S.; Han, J.E. Appetite as Motivated Choice: Hormonal and Environmental Influences. Decis. Neurosci. Integr. Perspect. 2017, 397–409. [Google Scholar] [CrossRef]
- Kaye, W.H.; Wagner, A.; Fudge, J.L.; Paulus, M. Neurocircuity of eating disorders. Curr. Top. Behav. Neurosci. 2011, 6, 37–57. [Google Scholar] [CrossRef]
- Shapiro, A.L.B.; Johnson, S.L.; Sutton, B.; Legget, K.T.; Dabelea, D.; Tregellas, J.R. Eating in the absence of hunger in young children is related to brain reward network hyperactivity and reduced functional connectivity in executive control networks. Pediatr. Obes. 2019, 14, e12502. [Google Scholar] [CrossRef] [PubMed]
- Leenaerts, N.; Jongen, D.; Ceccarini, J.; Van Oudenhove, L.; Vrieze, E. The neurobiological reward system and binge eating: A critical systematic review of neuroimaging studies. Int. J. Eat. Disord. 2022, 55, 1421–1458. [Google Scholar] [CrossRef] [PubMed]
- Monosov, I.E. Anterior cingulate is a source of valence-specific information about value and uncertainty. Nat. Commun. 2017, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Weafer, J.; Crane, N.A.; Gorka, S.M.; Phan, K.L.; de Wit, H. Neural Correlates of Inhibition and Reward are Negatively Associated. Neuroimage 2019, 196, 188. [Google Scholar] [CrossRef]
- Kelley, A.E.; Baldo, B.A.; Pratt, W.E.; Will, M.J. Corticostriatal-hypothalamic circuitry and food motivation: Integration of energy, action and reward. Physiol. Behav. 2005, 86, 773–795. [Google Scholar] [CrossRef]
- Berridge, K.C. Food reward: Brain substrates of wanting and liking. Neurosci. Biobehav. Rev. 1996, 20, 1–25. [Google Scholar] [CrossRef]
- O’Doherty, J.P.; Deichmann, R.; Critchley, H.D.; Dolan, R.J. Neural responses during anticipation of a primary taste reward. Neuron 2002, 33, 815–826. [Google Scholar] [CrossRef]
- Small, D.M.; Jones-Gotman, M.; Dagher, A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage 2003, 19, 1709–1715. [Google Scholar] [CrossRef]
- Craig, A.D. How do you feel--now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009, 10, 59–70. [Google Scholar] [CrossRef]
- Craig, A.D. Interoception: The sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003, 13, 500–505. [Google Scholar] [CrossRef]
- Li, J.; An, R.; Zhang, Y.; Li, X.; Wang, S. Correlations of macronutrient-induced functional magnetic resonance imaging signal changes in human brain and gut hormone responses. Am. J. Clin. Nutr. 2012, 96, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Simmons, W.K.; Rapuano, K.M.; Kallman, S.J.; Ingeholm, J.E.; Miller, B.; Gotts, S.J.; Avery, J.A.; Hall, K.D.; Martin, A. Category-specific integration of homeostatic signals in caudal but not rostral human insula. Nat. Neurosci. 2013, 16, 1551–1552. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Kullmann, S.; Veit, R. Food related processes in the insular cortex. Front. Hum. Neurosci. 2013, 7, 499. [Google Scholar] [CrossRef]
- Romei, A.; Voigt, K.; Verdejo-Garcia, A. A Perspective on Candidate Neural Underpinnings of Binge Eating Disorder: Reward and Homeostatic Systems. Curr. Pharm. Des. 2020, 26, 2327–2333. [Google Scholar] [CrossRef]
- Wang, G.J.; Volkow, N.D.; Telang, F.; Jayne, M.; Ma, J.; Rao, M.; Zhu, W.; Wong, C.T.; Pappas, N.R.; Geliebter, A.; et al. Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage 2004, 21, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Pelchat, M.L.; Johnson, A.; Chan, R.; Valdez, J.; Ragland, J.D. Images of desire: Food-craving activation during fMRI. Neuroimage 2004, 23, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Rodríguez, O.; Cano, M.; Vilar-López, R.; Rio-Valle, J.S.; Verdejo-Román, J.; Navas, J.F.; Martín-Pérez, C.; Fernández-Aranda, F.; Menchón, J.M.; Soriano-Mas, C.; et al. Visceral adiposity and insular networks: Associations with food craving. Int. J. Obes. 2019, 43, 503–511. [Google Scholar] [CrossRef]
- Wonderlich, J.A.; Bershad, M.; Steinglass, J.E. Exploring Neural Mechanisms Related to Cognitive Control, Reward, and Affect in Eating Disorders: A Narrative Review of FMRI Studies. Neuropsychiatr. Dis. Treat. 2021, 17, 2053–2062. [Google Scholar] [CrossRef]
- Contreras-Rodriguez, O.; Escorihuela, R.M. Dissecting ultra—Processed foods and drinks: Do they have a potential to impact the brain? Rev. Endocr. Metab. Disord. 2022, 23, 697–717. [Google Scholar] [CrossRef]
- Berthoud, H.R. Neural control of appetite: Cross-talk between homeostatic and non-homeostatic systems. Appetite 2004, 43, 315–317. [Google Scholar] [CrossRef]
- Berthoud, H.R. Homeostatic and non-homeostatic pathways involved in the control of food intake and energy balance. Obesity 2006, 14 (Suppl. 5), 197S–200S. [Google Scholar] [CrossRef] [PubMed]
- Bodell, L.P.; Wildes, J.E.; Goldschmidt, A.B.; Lepage, R.; Keenan, K.E.; Guyer, A.E.; Hipwell, A.E.; Stepp, S.D.; Forbes, E.E. Associations between Neural Reward Processing and Binge Eating among Adolescent Girls. J. Adolesc. Health 2018, 62, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.J. The Extended Process Model of Emotion Regulation: Elaborations, Applications, and Future Directions. Psychol. Inq. 2015, 26, 130–137. [Google Scholar] [CrossRef]
- Somerville, L.; Casey, B. Developmental neurobiology of cognitive control and motivational systems. Curr. Opin. Neurobiol. 2010, 20, 236–241. [Google Scholar] [CrossRef]
- Willner, C.J.; Hoffmann, J.D.; Bailey, C.S.; Harrison, A.P.; Garcia, B.; Ng, Z.J.; Cipriano, C.; Brackett, M.A. The Development of Cognitive Reappraisal from Early Childhood through Adolescence: A Systematic Review and Methodological Recommendations. Front. Psychol. 2022, 13, 875964. [Google Scholar] [CrossRef]
- Berner, L.A.; Marsh, R. Frontostriatal circuits and the development of bulimia nervosa. Front. Behav. Neurosci. 2014, 8, 395. [Google Scholar] [CrossRef]
- Ochsner, K.N.; Silvers, J.A.; Buhle, J.T. Review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci. 2012, 1251, E1–E24. [Google Scholar] [CrossRef]
- McRae, K.; Gross, J.J.; Weber, J.; Robertson, E.R.; Sokol-Hessner, P.; Ray, R.D.; Gabrieli, J.D.E.; Ochsner, K.N. The development of emotion regulation: An fMRI study of cognitive reappraisal in children, adolescents and young adults. Soc. Cogn. Affect. Neurosci. 2012, 7, 11–22. [Google Scholar] [CrossRef]
- Morris, A.S.; Criss, M.M.; Silk, J.S.; Houltberg, B.J. The Impact of Parenting on Emotion Regulation during Childhood and Adolescence. Child Dev. Perspect. 2017, 11, 233–238. [Google Scholar] [CrossRef]
- Cimino, S.; Marzilli, E.; Tafà, M.; Cerniglia, L. Emotional-Behavioral Regulation, Temperament and Parent–Child Interactions Are Associated with Dopamine Transporter Allelic Polymorphism in Early Childhood: A Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 8564. [Google Scholar] [CrossRef]
- Zalewski, M.; Lengua, L.J.; Wilson, A.C.; Trancik, A.; Bazinet, A. Emotion Regulation Profiles, Temperament, and Adjustment Problems in Preadolescents. Child Dev. 2011, 82, 951. [Google Scholar] [CrossRef] [PubMed]
- Barzman, D.; Geise, C.; Lin, P.-I. Review of the genetic basis of emotion dysregulation in children and adolescents. World J. Psychiatry 2015, 5, 112. [Google Scholar] [CrossRef]
- Zeman, J.; Cassano, M.; Perry-Parrish, C.; Stegall, S. Emotion regulation in children and adolescents. J. Dev. Behav. Pediatr. 2006, 27, 155–168. [Google Scholar] [CrossRef]
- Bartholdy, S.; O’Daly, O.G.; Campbell, I.C.; Banaschewski, T.; Barker, G.; Bokde, A.L.W.; Bromberg, U.; Büchel, C.; Burke Quinlan, E.; Desrivieres, S.; et al. Neural Correlates of Failed Inhibitory Control as an Early Marker of Disordered Eating in Adolescents. Biol. Psychiatry 2019, 85, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Olsavsky, A.K.; Shott, M.E.; Deguzman, M.C.; Frank, G.K.W.W. Neural correlates of taste reward value across eating disorders. Psychiatry Res. Neuroimaging 2019, 288, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Treasure, J.; Duarte, T.A.; Schmidt, U.; Antunes Duarte, T.; Schmidt, U. Eating disorders. Lancet 2020, 395, 899–911. [Google Scholar] [CrossRef]
- Hardee, J.E.; Phaneuf, C.; Cope, L.; Zucker, R.; Gearhardt, A.; Heitzeg, M. Neural correlates of inhibitory control in youth with symptoms of food addiction. Appetite 2020, 148, 104578. [Google Scholar] [CrossRef]
- Jarcho, J.M.; Tanofsky-Kraff, M.; Nelson, E.E.; Engel, S.G.; Vannucci, A.; Field, S.E.; Romer, A.L.; Hannallah, L.; Brady, S.M.; Demidowich, A.P.; et al. Neural activation during anticipated peer evaluation and laboratory meal intake in overweight girls with and without loss of control eating. Neuroimage 2015, 108, 343–353. [Google Scholar] [CrossRef]
- Goldschmidt, A.B.; Dickstein, D.P.; MacNamara, A.E.; Phan, K.L.; O’Brien, S.; Le Grange, D.; Fisher, J.O.; Keedy, S. A pilot study of neural correlates of loss of control eating in children with overweight/obesity: Probing intermittent access to food as a means of eliciting disinhibited eating. J. Pediatr. Psychol. 2018, 43, 846–855. [Google Scholar] [CrossRef]
- ABCD Study. US Department of Health & Human Services (HHS) ABCD Study. Available online: https://abcdstudy.org/ (accessed on 12 April 2023).
- Schumann, G.; Loth, E.; Banaschewski, T.; Barbot, A.; Barker, G.; Büchel, C.; Conrod, P.J.; Dalley, J.W.; Flor, H.; Gallinat, J.; et al. The IMAGEN study: Reinforcement-related behaviour in normal brain function and psychopathology. Mol. Psychiatry 2010, 15, 1128–1139. [Google Scholar] [CrossRef]
- Frank, G.K.W.; Shott, M.E.; DeGuzman, M.C. The Neurobiology of Eating Disorders. Child Adolesc. Psychiatr. Clin. N. Am. 2019, 28, 629–640. [Google Scholar] [CrossRef]
- Balodis, I.M.; Molina, N.D.; Kober, H.; Worhunsky, P.D.; White, M.A.; Sinha, R.; Grilo, C.M.; Potenza, M.N. Divergent neural substrates of inhibitory control in binge eating disorder relative to other manifestations of obesity. Obesity 2013, 21, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Hege, M.A.; Stingl, K.T.; Kullmann, S.; Schag, K.; Giel, K.E.; Zipfel, S.; Preissl, H. Attentional impulsivity in binge eating disorder modulates response inhibition performance and frontal brain networks. Int. J. Obes. 2015, 39, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Stice, E.; Burger, K. Neural vulnerability factors for obesity. Clin. Psychol. Rev. 2019, 68, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Frank, G.K.W. Altered brain reward circuits in eating disorders: Chicken or egg? Curr. Psychiatry Rep. 2013, 15, 396. [Google Scholar] [CrossRef]
- Hartmann, A.S.; Czaja, J.; Rief, W.; Hilbert, A. Personality and psychopathology in children with and without loss of control over eating. Compr. Psychiatry 2010, 51, 572–578. [Google Scholar] [CrossRef]
- Giel, K.E.; Teufel, M.; Junne, F.; Zipfel, S.; Schag, K. Food-Related Impulsivity in Obesity and Binge Eating Disorder-A Systematic Update of the Evidence. Nutrients 2017, 9, 1170. [Google Scholar] [CrossRef]
- Rapuano, K.M.; Laurent, J.S.; Hagler, D.J.; Hatton, S.N.; Thompson, W.K.; Jernigan, T.L.; Dale, A.M.; Casey, B.J.; Watts, R. Nucleus accumbens cytoarchitecture predicts weight gain in children. Proc. Natl. Acad. Sci. USA 2020, 117, 26977–26984. [Google Scholar] [CrossRef]
- Assari, S.; Boyce, S.; Bazargan, M. Nucleus accumbens functional connectivity with the frontoparietal network predicts subsequent change in body mass index for American children. Brain Sci. 2020, 10, 703. [Google Scholar] [CrossRef]
- Balodis, I.M.; Kober, H.; Worhunsky, P.D.; White, M.A.; Stevens, M.C.; Pearlson, G.D.; Sinha, R.; Grilo, C.M.; Potenza, M.N. Monetary reward processing in obese individuals with and without binge eating disorder. Biol. Psychiatry 2013, 73, 877–886. [Google Scholar] [CrossRef]
- Frank, G.K.W.; Shott, M.E.; Stoddard, J.; Swindle, S.; Pryor, T.L. Association of Brain Reward Response with Body Mass Index and Ventral Striatal-Hypothalamic Circuitry among Young Women with Eating Disorders. JAMA Psychiatry 2021, 78, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Hartogsveld, B.; Quaedflieg, C.W.E.M.; van Ruitenbeek, P.; Smeets, T. Decreased putamen activation in balancing goal-directed and habitual behavior in binge eating disorder. Psychoneuroendocrinology 2022, 136, 105596. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Olivos, R.; Steward, T.; Martínez-Zalacaín, I.; Mestre-Bach, G.; Juaneda-Seguí, A.; Jiménez-Murcia, S.; Fernández-Formoso, J.A.; Vilarrasa, N.; Veciana de las Heras, M.; Custal, N.; et al. The neural correlates of delay discounting in obesity and binge eating disorder. J. Behav. Addict. 2021, 10, 498–507. [Google Scholar] [CrossRef]
- Schienle, A.; Schäfer, A.; Hermann, A.; Vaitl, D. Binge-eating disorder: Reward sensitivity and brain activation to images of food. Biol. Psychiatry 2009, 65, 654–661. [Google Scholar] [CrossRef]
- Reiter, A.M.F.; Heinze, H.J.; Schlagenhauf, F.; Deserno, L. Impaired Flexible Reward-Based Decision-Making in Binge Eating Disorder: Evidence from Computational Modeling and Functional Neuroimaging. Neuropsychopharmacology 2017, 42, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Voon, V.; Joutsa, J.; Majuri, J.; Baek, K.; Nord, C.L.; Arponen, E.; Forsback, S.; Kaasinen, V. The neurochemical substrates of habitual and goal-directed control. Transl. Psychiatry 2020, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Voon, V.; Derbyshire, K.; Rück, C.; Irvine, M.A.; Worbe, Y.; Enander, J.; Schreiber, L.R.N.; Gillan, C.; Fineberg, N.A.; Sahakian, B.J.; et al. Disorders of compulsivity: A common bias towards learning habits. Mol. Psychiatry 2015, 20, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Skandali, N.; Majuri, J.; Joutsa, J.; Baek, K.; Arponen, E.; Forsback, S.; Kaasinen, V.; Voon, V. The neural substrates of risky rewards and losses in healthy volunteers and patient groups: A PET imaging study. Psychol. Med. 2022, 52, 3280–3288. [Google Scholar] [CrossRef]
- Murray, S.B.; Alba, C.; Duval, C.J.; Nagata, J.M.; Cabeen, R.P.; Lee, D.J.; Toga, A.W.; Siegel, S.J.; Jann, K. Aberrant functional connectivity between reward and inhibitory control networks in pre-adolescent binge eating disorder. Psychol. Med. 2022, 1–10. [Google Scholar] [CrossRef]
- Simon, J.J.; Skunde, M.; Walther, S.; Bendszus, M.; Herzog, W.; Friederich, H.C. Neural signature of food reward processing in bulimic-type eating disorders. Soc. Cogn. Affect. Neurosci. 2016, 11, 1393–1401. [Google Scholar] [CrossRef]
- Critchley, H.D.; Rolls, E.T. Hunger and satiety modify the responses of olfactory and visual neurons in the primate orbitofrontal cortex. J. Neurophysiol. 1996, 75, 1673–1686. [Google Scholar] [CrossRef] [PubMed]
- Ely, A.V.; Wierenga, C.E.; Bischoff-Grethe, A.; Bailer, U.F.; Berner, L.A.; Fudge, J.L.; Paulus, M.P.; Kaye, W.H. Response in taste circuitry is not modulated by hunger and satiety in women remitted from bulimia nervosa. J. Abnorm. Psychol. 2017, 126, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.Z.; Volkow, N.D. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry 2002, 159, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Rodriguez, L.; Ruiz de Azua, I.; Dominguez, E.; Senabre, E.; Serra, I.; Kummer, S.; Navandar, M.; Baddenhausen, S.; Hofmann, C.; Andero, R.; et al. A specific prelimbic-nucleus accumbens pathway controls resilience versus vulnerability to food addiction. Nat. Commun. 2020, 11, 782. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.R.; Morrison, C.D.; Münzberg, H. The obesity epidemic in the face of homeostatic body weight regulation: What went wrong and how can it be fixed? Physiol. Behav. 2020, 222, 112959. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.X.; Kroemer, N.B.; Veldhuizen, M.G.; Babbs, A.E.; De Araujo, I.E.; Gitelman, D.R.; Sherwin, R.S.; Sinha, R.; Small, D.M. Basolateral Amygdala Response to Food Cues in the Absence of Hunger Is Associated with Weight Gain Susceptibility. J. Neurosci. 2015, 35, 7964–7976. [Google Scholar] [CrossRef]
- Martín-Pérez, C.; Contreras-Rodríguez, O.; Vilar-López, R.; Verdejo-García, A. Hypothalamic Networks in Adolescents with Excess Weight: Stress-Related Connectivity and Associations with Emotional Eating. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 211–220.e5. [Google Scholar] [CrossRef]
- Naumann, E.; Svaldi, J.; Wyschka, T.; Heinrichs, M.; von Dawans, B. Stress-induced body dissatisfaction in women with binge eating disorder. J. Abnorm. Psychol. 2018, 127, 548–558. [Google Scholar] [CrossRef]
- Phelps, E.A.; Lempert, K.M.; Sokol-Hessner, P. Emotion and Decision Making: Multiple Modulatory Neural Circuits. Annu. Rev. Neurosci. 2014, 37, 263–287. [Google Scholar] [CrossRef]
- Lyu, Z.; Jackson, T. Acute Stressors Reduce Neural Inhibition to Food Cues and Increase Eating among Binge Eating Disorder Symptomatic Women. Front. Behav. Neurosci. 2016, 10, 188. [Google Scholar] [CrossRef]
- Villarejo, C.; Fernández-Aranda, F.; Jiménez-Murcia, S.; Peñas-Lledó, E.; Granero, R.; Penelo, E.; Tinahones, F.J.; Sancho, C.; Vilarrasa, N.; Montserrat-Gil De Bernabé, M.; et al. Lifetime obesity in patients with eating disorders: Increasing prevalence, clinical and personality correlates. Eur. Eat. Disord. Rev. 2012, 20, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Rapuano, K.M.; Zieselman, A.L.; Kelley, W.M.; Sargent, J.D.; Heatherton, T.F.; Gilbert-Diamond, D. Genetic risk for obesity predicts nucleus accumbens size and responsivity to real-world food cues. Proc. Natl. Acad. Sci. USA 2017, 114, 160–165. [Google Scholar] [CrossRef]
- Tomasi, D.; Volkow, N.D. Striatocortical pathway dysfunction in addiction and obesity: Differences and similarities. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 1–19. [Google Scholar] [CrossRef]
- Boutelle, K.N.; Wierenga, C.E.; Bischoff-Grethe, A.; Melrose, A.J.; Grenesko-Stevens, E.; Paulus, M.P.; Kaye, W.H. Increased brain response to appetitive tastes in the insula and amygdala in obese compared with healthy weight children when sated. Int. J. Obes. 2015, 39, 620–628. [Google Scholar] [CrossRef]
- Herbert, B.M.; Pollatos, O. Attenuated interoceptive sensitivity in overweight and obese individuals. Eat. Behav. 2014, 15, 445–448. [Google Scholar] [CrossRef]
- Mata, F.; Verdejo-Roman, J.; Soriano-Mas, C.; Verdejo-Garcia, A. Insula tuning towards external eating versus interoceptive input in adolescents with overweight and obesity. Appetite 2015, 93, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Hawk, T.; Aggarwal, A.; Drewnowski, A. Characterizing ultra-processed foods by energy density, nutrient density, and cost. Front. Nutr. 2019, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Cannon, G.; Levy, R.; Moubarac, J.-C. The Food System. NOVA. The star shines bright. Public Health 2016, 7, 28–38. [Google Scholar]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.C.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Martínez Steele, E.; Khandpur, N.; da Costa Louzada, M.L.; Monteiro, C.A. Association between dietary contribution of ultra-processed foods and urinary concentrations of phthalates and bisphenol in a nationally representative sample of the US population aged 6 years and older. PLoS ONE 2020, 15, e0236738. [Google Scholar] [CrossRef]
- Moodie, R.; Stuckler, D.; Monteiro, C.; Sheron, N.; Neal, B.; Thamarangsi, T.; Lincoln, P.; Casswell, S. Profits and pandemics: Prevention of harmful effects of tobacco, alcohol, and ultra-processed food and drink industries. Lancet 2013, 381, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Elizabeth, L.; Machado, P.; Zinöcker, M.; Baker, P.; Lawrence, M. Ultra-processed foods and health outcomes: A narrative review. Nutrients 2020, 12, 1955. [Google Scholar] [CrossRef] [PubMed]
- De Amicis, R.; Mambrini, S.P.; Pellizzari, M.; Foppiani, A.; Bertoli, S.; Battezzati, A.; Leone, A. Ultra-processed foods and obesity and adiposity parameters among children and adolescents: A systematic review. Eur. J. Nutr. 2022, 61, 2297–2311. [Google Scholar] [CrossRef] [PubMed]
- Neri, D.; Steele, E.M.; Khandpur, N.; Cediel, G.; Zapata, M.E.; Rauber, F.; Marrón-Ponce, J.A.; Machado, P.; da Costa Louzada, M.L.; Andrade, G.C.; et al. Ultraprocessed food consumption and dietary nutrient profiles associated with obesity: A multicountry study of children and adolescents. Obes. Rev. 2022, 23, e13387. [Google Scholar] [CrossRef] [PubMed]
- Schulte, E.; Avena, N.; Gerhardt, A. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS ONE 2015, 10, e0117959. [Google Scholar]
- Filgueiras, A.R.; Pires de Almeida, V.B.; Koch Nogueira, P.C.; Alvares Domene, S.M.; Eduardo da Silva, C.; Sesso, R.; Sawaya, A.L. Exploring the consumption of ultra-processed foods and its association with food addiction in overweight children. Appetite 2019, 135, 137–145. [Google Scholar] [CrossRef]
- Pursey, K.M.; Davis, C.; Burrows, T.L. Nutritional Aspects of Food Addiction. Curr. Addict. Rep. 2017, 4, 142–150. [Google Scholar] [CrossRef]
- Faisal-Cury, A.; Leite, M.A.; Loureiro Escuder, M.M.; Levy, R.B.; Fernanda, M.; Peres, T. The relationship between ultra-processed food consumption and internalising symptoms among adolescents from São Paulo city, Southeast Brazil. Public Health Nutr. 2022, 25, 2498–2506. [Google Scholar] [CrossRef]
- Werneck, A.O.; Vancampfort, D.; Oyeyemi, A.L.; Stubbs, B.; Silva, D.R. Joint association of ultra-processed food and sedentary behavior with anxiety-induced sleep disturbance among Brazilian adolescents. J. Affect. Disord. 2020, 266, 135–142. [Google Scholar] [CrossRef]
- Werneck, A.O.; Hoare, E.; Silva, D.R. Do TV viewing and frequency of ultra-processed food consumption share mediators in relation to adolescent anxiety-induced sleep disturbance? Public Health Nutr. 2021, 24, 5491–5497. [Google Scholar] [CrossRef]
- Swartz, J.; Monk, C. The Role of Corticolimbic Circuitry in the Development of Anxiety Disorders in Children and Adolescents. Curr. Top. Behav. Neurosci. 2014, 16, 133–148. [Google Scholar] [CrossRef]
- Bruce, A.S.; Bruce, J.M.; Black, W.R.; Lepping, R.J.; Henry, J.M.; Cherry, J.B.C.; Martin, L.E.; Papa, V.B.; Davis, A.M.; Brooks, W.M.; et al. Branding and a child’s brain: An fMRI study of neural responses to logos. Soc. Cogn. Affect. Neurosci. 2014, 9, 118. [Google Scholar] [CrossRef]
- Adise, S.; Geier, C.F.; Roberts, N.J.; White, C.N.; Keller, K.L. Is brain response to food rewards related to overeating? A test of the reward surfeit model of overeating in children. Appetite 2018, 128, 167–179. [Google Scholar] [CrossRef] [PubMed]
- May, C.E.; Dus, M. Confection Confusion: Interplay between Diet, Taste, and Nutrition. Trends Endocrinol. Metab. 2021, 32, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Archer, N.; Duesing, K.; Hannan, G.; Keast, R. Mechanism of fat taste perception: Association with diet and obesity. Prog. Lipid Res. 2016, 63, 41–49. [Google Scholar] [CrossRef]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2020, 32, 690. [Google Scholar] [CrossRef] [PubMed]
- Puig-Vallverdú, J.; Romaguera, D.; Fernández-Barrés, S.; Gignac, F.; Ibarluzea, J.; Santa-Maria, L.; Llop, S.; Gonzalez, S.; Vioque, J.; Riaño-Galán, I.; et al. The association between maternal ultra-processed food consumption during pregnancy and child neuropsychological development: A population-based birth cohort study. Clin. Nutr. 2022, 41, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, S.; Suksasilp, C.; Lucas, L.; Sebastian, C.L.; Norbury, C. Relationship between early language competence and cognitive emotion regulation in adolescence. R. Soc. Open Sci. 2021, 8, 210742. [Google Scholar] [CrossRef]
- Contreras-Rodriguez, O.; Rales-Moreno, M.; Fernández-Barrès, S.; Cimpean, A.; Arnoriaga-Rodríguez, M.; Puig, J.; Biarnés, C.; Motger-Albertí, A.; Cano, M.; José, M.F.-R. Consumption of ultra-processed foods is associated with depression, mesocorticolimbic volume, and inflammation. J. Affect. Disord. 2023, 335, 340–348. [Google Scholar] [CrossRef]
- Yunker, A.G.; Patel, R.; Page, K.A. Effects of Non-nutritive Sweeteners on Sweet Taste Processing and Neuroendocrine Regulation of Eating Behavior. Curr. Nutr. Rep. 2020, 9, 278–289. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Wong, N.S.M. How does our brain process sugars and non-nutritive sweeteners differently: A systematic review on functional magnetic resonance imaging studies. Nutrients 2020, 12, 3010. [Google Scholar] [CrossRef] [PubMed]
- Pepino, M.Y. Physiology & behavior metabolic effects of non-nutritive sweeteners. Physiol. Behav. 2015, 152, 450–455. [Google Scholar] [PubMed]
- De Graaf, C.; Kok, F.J. Slow food, fast food and the control of food intake. Nat. Rev. Endocrinol. 2010, 6, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Smeets, P.A.M.; De Graaf, C.; Stafleu, A.; Van Osch, M.J.P.; Van Der Grond, J. Functional magnetic resonance imaging of human hypothalamic responses to sweet taste and calories. Am. J. Clin. Nutr. 2005, 82, 1011–1016. [Google Scholar] [CrossRef]
- Van Opstal, A.M.; Kaal, I.; van den Berg-Huysmans, A.A.; Hoeksma, M.; Blonk, C.; Pijl, H.; Rombouts, S.A.R.B.; van der Grond, J. Dietary sugars and non-caloric sweeteners elicit different homeostatic and hedonic responses in the brain. Nutrition 2019, 60, 80–86. [Google Scholar] [CrossRef]
- Van Opstal, A.M.; Hafkemeijer, A.; van den Berg-Huysmans, A.A.; Hoeksma, M.; Mulder, T.P.J.; Pijl, H.; Rombouts, S.A.R.B.; van der Grond, J. Brain activity and connectivity changes in response to nutritive natural sugars, non-nutritive natural sugar replacements and artificial sweeteners. Nutr. Neurosci. 2021, 24, 395–405. [Google Scholar] [CrossRef]
- Crézé, C.; Candal, L.; Cros, J.; Knebel, J.F.; Seyssel, K.; Stefanoni, N.; Schneiter, P.; Murray, M.M.; Tappy, L.; Toepel, U. The impact of caloric and non-caloric sweeteners on food intake and brain responses to food: A randomized crossover controlled trial in healthy humans. Nutrients 2018, 10, 615. [Google Scholar] [CrossRef]
- Bolhuis, D.P.; Forde, C.G.; Cheng, Y.; Xu, H.; Martin, N.; De Graaf, C. Slow food: Sustained impact of harder foods on the reduction in energy intake over the course of the day. PLoS ONE 2014, 9, e93370. [Google Scholar] [CrossRef]
- Krop, E.M.; Hetherington, M.M.; Nekitsing, C.; Miquel, S.; Postelnicu, L.; Sarkar, A. Influence of oral processing on appetite and food intake—A systematic review and meta-analysis. Appetite 2018, 125, 253–269. [Google Scholar] [CrossRef]
- Viskaal-van Dongen, M.; Kok, F.J.; de Graaf, C. Eating rate of commonly consumed foods promotes food and energy intake. Appetite 2011, 56, 25–31. [Google Scholar] [CrossRef]
- Bian, X.; Chi, L.; Gao, B.; Tu, P.; Ru, H.; Lu, K. Gut microbiome response to sucralose and its potential role in inducing liver inflammation in mice. Front. Physiol. 2017, 8, 487. [Google Scholar] [CrossRef] [PubMed]
- Medina-Reyes, E.I.; Rodríguez-Ibarra, C.; Déciga-Alcaraz, A.; Díaz-Urbina, D.; Chirino, Y.I.; Pedraza-Chaverri, J. Food additives containing nanoparticles induce gastrotoxicity, hepatotoxicity and alterations in animal behavior: The unknown role of oxidative stress. Food Chem. Toxicol. 2020, 146, 111814. [Google Scholar] [CrossRef]
- Laster, J.; Frame, L.A. Beyond the Calories—Is the Problem in the Processing? Curr. Treat. Options Gastroenterol. 2019, 17, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Edalati, S.; Bagherzadeh, F.; Asghari Jafarabadi, M.; Ebrahimi-Mamaghani, M. Higher ultra-processed food intake is associated with higher DNA damage in healthy adolescents. Br. J. Nutr. 2021, 125, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Pase, C.S.; Metz, V.G.; Roversi, K.; Roversi, K.; Vey, L.T.; Dias, V.T.; Schons, C.F.; de David Antoniazzi, C.T.; Duarte, T.; Duarte, M.; et al. Trans fat intake during pregnancy or lactation increases anxiety-like behavior and alters proinflammatory cytokines and glucocorticoid receptor levels in the hippocampus of adult offspring. Brain Res. Bull. 2021, 166, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Trevizol, F.; Roversi, K.; Dias, V.T.; Roversi, K.; Pase, C.S.; Barcelos, R.C.S.; Benvegnu, D.M.; Kuhn, F.T.; Dolci, G.S.; Ross, D.H.; et al. Influence of lifelong dietary fats on the brain fatty acids and amphetamine-induced behavioral responses in adult rat. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2013, 45, 215–222. [Google Scholar] [CrossRef]
- Trevizol, F.; Roversi, K.R.; Dias, V.T.; Roversi, K.; Barcelos, R.C.S.; Kuhn, F.T.; Pase, C.S.; Golombieski, R.; Veit, J.C.; Piccolo, J.; et al. Cross-generational trans fat intake facilitates mania-like behavior: Oxidative and molecular markers in brain cortex. Neuroscience 2015, 286, 353–363. [Google Scholar] [CrossRef]
- D’cunha, N.M.; Sergi, D.; Lane, M.M.; Naumovski, N.; Gamage, E.; Rajendran, A.; Kouvari, M.; Gauci, S.; Dissanayka, T.; Marx, W.; et al. The Effects of Dietary Advanced Glycation End-Products on Neurocognitive and Mental Disorders. Nutrients 2022, 14, 2421. [Google Scholar] [CrossRef]
- Butler, M.J.; Perrini, A.A.; Eckel, L.A. The role of the gut microbiome, immunity, and neuroinflammation in the pathophysiology of eating disorders. Nutrients 2021, 13, 500. [Google Scholar] [CrossRef]
- Heidari, Z.; Mohammadipour, A.; Haeri, P.; Ebrahimzadeh-Bideskan, A. The effect of titanium dioxide nanoparticles on mice midbrain substantia nigra. Iran. J. Basic Med. Sci. 2019, 22, 745–751. [Google Scholar] [CrossRef]
- Hu, R.; Gong, X.; Duan, Y.; Li, N.; Che, Y.; Cui, Y.; Zhou, M.; Liu, C.; Wang, H.; Hong, F. Neurotoxicological effects and the impairment of spatial recognition memory in mice caused by exposure to TiO2 nanoparticles. Biomaterials 2010, 31, 8043–8050. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Ze, Y.; Wang, L.; Yu, X.; Hong, J.; Zhao, X.; Ze, X.; Liu, D.; Xu, B.; Zhu, Y.; et al. Mechanisms of TiO2 nanoparticle-induced neuronal apoptosis in rat primary cultured hippocampal neurons. J. Biomed. Mater. Res. 2015, 103, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Ze, Y.; Sheng, L.; Zhao, X.; Hong, J.; Ze, X.; Yu, X.; Pan, X.; Lin, A.; Zhao, Y.; Zhang, C.; et al. TiO2 nanoparticles induced hippocampal neuroinflammation in mice. PLoS ONE 2014, 9, e92230. [Google Scholar] [CrossRef] [PubMed]
- Węsierska, M.; Dziendzikowska, K.; Gromadzka-Ostrowska, J.; Dudek, J.; Polkowska-Motrenko, H.; Audinot, J.N.; Gutleb, A.C.; Lankoff, A.; Kruszewski, M. Silver ions are responsible for memory impairment induced by oral administration of silver nanoparticles. Toxicol. Lett. 2018, 290, 133–144. [Google Scholar] [CrossRef]
- Wierenga, C.E.; Ely, A.; Bischoff-Grethe, A.; Bailer, U.F.; Simmons, A.N.; Kaye, W.H. Are Extremes of Consumption in Eating Disorders Related to an Altered Balance between Reward and Inhibition? Front. Behav. Neurosci. 2014, 8, 410. [Google Scholar] [CrossRef]
- Rajjo, T.; Mohammed, K.; Alsawas, M.; Ahmed, A.T.; Farah, W.; Asi, N.; Almasri, J.; Prokop, L.J.; Murad, M.H. Treatment of Pediatric Obesity: An Umbrella Systematic Review. J. Clin. Endocrinol. Metab. 2017, 102, 763–775. [Google Scholar] [CrossRef]
- Fullana, M.A.; Abramovitch, A.; Via, E.; López-Sola, C.; Goldberg, X.; Reina, N.; Fortea, L.; Solanes, A.; Buckley, M.J.; Ramella-Cravaro, V.; et al. Diagnostic biomarkers for obsessive-compulsive disorder: A reasonable quest or ignis fatuus? Neurosci. Biobehav. Rev. 2020, 118, 504–513. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Via, E.; Contreras-Rodríguez, O. Binge-Eating Precursors in Children and Adolescents: Neurodevelopment, and the Potential Contribution of Ultra-Processed Foods. Nutrients 2023, 15, 2994. https://doi.org/10.3390/nu15132994
Via E, Contreras-Rodríguez O. Binge-Eating Precursors in Children and Adolescents: Neurodevelopment, and the Potential Contribution of Ultra-Processed Foods. Nutrients. 2023; 15(13):2994. https://doi.org/10.3390/nu15132994
Chicago/Turabian StyleVia, Esther, and Oren Contreras-Rodríguez. 2023. "Binge-Eating Precursors in Children and Adolescents: Neurodevelopment, and the Potential Contribution of Ultra-Processed Foods" Nutrients 15, no. 13: 2994. https://doi.org/10.3390/nu15132994
APA StyleVia, E., & Contreras-Rodríguez, O. (2023). Binge-Eating Precursors in Children and Adolescents: Neurodevelopment, and the Potential Contribution of Ultra-Processed Foods. Nutrients, 15(13), 2994. https://doi.org/10.3390/nu15132994




