Evaluating Bidirectional Predictive Pathways between Dietary Restraint and Food Addiction in Adolescents
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.3. Data Analytic Plan
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gearhardt, A.N.; Corbin, W.R.; Brownell, K.D. Preliminary validation of the Yale Food Addiction Scale. Appetite 2009, 52, 430–436. [Google Scholar] [CrossRef]
- Maxwell, A.L.; Gardiner, E.; Loxton, N.J. Investigating the relationship between reward sensitivity, impulsivity, and food addiction: A systematic review. Eur. Eat. Disord. Rev. 2020, 28, 368–384. [Google Scholar] [CrossRef] [PubMed]
- Wiss, D.; Brewerton, T. Separating the Signal from the Noise: How Psychiatric Diagnoses Can Help Discern Food Addiction from Dietary Restraint. Nutrients 2020, 12, 2937. [Google Scholar] [CrossRef]
- Herman, C.P.; Polivy, J. Anxiety, restraint, and eating behavior. J. Abnorm. Psychol. 1975, 84, 666–672. [Google Scholar] [CrossRef]
- Lowe, M.R. The effects of dieting on eating behavior: A three-factor model. Psychol. Bull. 1993, 114, 100–121. [Google Scholar] [CrossRef]
- Stice, E.; Fisher, M.; Lowe, M.R. Are Dietary Restraint Scales Valid Measures of Acute Dietary Restriction? Unobtrusive Observational Data Suggest Not. Psychol. Assess. 2004, 16, 51–59. [Google Scholar] [CrossRef]
- Lowe, M.R.; Annunziato, R.A.; Markowitz, J.T.; Didie, E.; Bellace, D.L.; Riddell, L.; Maille, C.; McKinney, S.; Stice, E. Multiple types of dieting prospectively predict weight gain during the freshman year of college. Appetite 2006, 47, 83–90. [Google Scholar] [CrossRef]
- Polivy, J.; Herman, C.P.; Mills, J.S. What is restrained eating and how do we identify it? Appetite 2020, 155, 104820. [Google Scholar] [CrossRef]
- Herman, C.; Polivy, J. From dietary restraint to binge eating: Attaching causes to effects. Appetite 1990, 14, 123–125. [Google Scholar] [CrossRef]
- Telch, C.F.; Agras, W.S. The effects of a very low calorie diet on binge eating. Behav. Ther. 1993, 24, 177–193. [Google Scholar] [CrossRef]
- Kirkley, B.G.; Burge, J.C.; Ammerman, A. Dietary restraint, binge eating, and dietary behavior patterns. Int. J. Eat. Disord. 1988, 7, 771–778. [Google Scholar] [CrossRef]
- Schulte, E.M.; Grilo, C.M.; Gearhardt, A.N. Shared and unique mechanisms underlying binge eating disorder and addictive disorders. Clin. Psychol. Rev. 2016, 44, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Ziauddeen, H.; Fletcher, P.C. Is food addiction a valid and useful concept? Obes. Rev. 2012, 14, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Gearhardt, A.N.; Grilo, C.M.; Dileone, R.J.; Brownell, K.D.; Potenza, M.N. Can food be addictive? Public health and policy implications. Addiction 2011, 106, 1208–1212. [Google Scholar] [CrossRef]
- Carter, J.C.; Van Wijk, M.; Rowsell, M. Symptoms of ‘food addiction’ in binge eating disorder using the Yale Food Addiction Scale version 2.0. Appetite 2019, 133, 362–369. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Corbin, W.R.; Brownell, K.D. Development of the Yale Food Addiction Scale Version 2.0. Psychol. Addict. Behav. 2016, 30, 113–121. [Google Scholar] [CrossRef]
- Rios, J.M.; Miller, A.L.; Lumeng, J.C.; Rosenblum, K.; Appugliese, D.P.; Gearhardt, A.N. Associations of maternal food addiction, dietary restraint, and pre-pregnancy BMI with infant eating behaviors and risk for overweight. Appetite 2023, 184, 106516. [Google Scholar] [CrossRef]
- Imperatori, C.; Fabbricatore, M.; Lester, D.; Manzoni, G.M.; Gianluca, C.; Raimondi, G.; Innamorati, M. Psychometric properties of the modified Yale Food Addiction Scale Version 2.0 in an Italian non-clinical sample. Eat. Weight. Disord. Stud. Anorex. Bulim. Obes. 2018, 24, 37–45. [Google Scholar] [CrossRef]
- Legendre, M.; Bégin, C. French validation of the addiction-like eating behavior scale and its clinical implication. Eat. Weight. Disord. Stud. Anorex. Bulim. Obes. 2020, 26, 1893–1902. [Google Scholar] [CrossRef]
- Crews, F.; He, J.; Hodge, C. Adolescent cortical development: A critical period of vulnerability for addiction. Pharmacol. Biochem. Behav. 2007, 86, 189–199. [Google Scholar] [CrossRef]
- Stice, E.; Mazotti, L.; Krebs, M.; Martin, S. Predictors of adolescent dieting behaviors: A longitudinal study. Psychol. Addict. Behav. 1998, 12, 195–205. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; White, M.; Masheb, R.M.; Grilo, C.M. An examination of food addiction in a racially diverse sample of obese patients with binge eating disorder in primary care settings. Compr. Psychiatry 2013, 54, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Schiestl, E.T.; Gearhardt, A.N. Preliminary validation of the Yale Food Addiction Scale for Children 2.0: A dimensional approach to scoring. Eur. Eat. Disord. Rev. 2018, 26, 605–617. [Google Scholar] [CrossRef]
- Alim, N.; Gokustun, K.; Caliskan, G.; Besler, Z. Do Disordered Eating Behaviors Have an Effect on Food Addiction? Health Behav. Policy Rev. 2021, 8, 319–330. [Google Scholar] [CrossRef]
- Kuczmarski, R.J.; Ogden, C.L.; Guo, S.S.; Grummer-Strawn, L.M.; Flegal, K.M.; Mei, Z.; Wei, R.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. 2000 CDC Growth Charts for the United States: Methods and Development. Vital Health Stat. 2002, 11, 1–190. [Google Scholar]
- Schulte, E.M.; Avena, N.M.; Gearhardt, A.N. Which Foods May Be Addictive? The Roles of Processing, Fat Content, and Glycemic Load. PLoS ONE 2015, 10, e0117959. [Google Scholar] [CrossRef] [PubMed]
- Lui, C.K.; Sterling, S.A.; Chi, F.W.; Lu, Y.; Campbell, C.I. Socioeconomic Differences in Adolescent Substance Abuse Treatment Participation and Long-Term Outcomes. Addict. Behav. 2017, 68, 45–51. [Google Scholar] [CrossRef]
- van Strien, T.; Frijters, J.E.; Bergers, G.P.; Defares, P.B. The Dutch Eating Behavior Questionnaire (DEBQ) for Assessment of Restrained, Emotional, and External Eating Behavior. Eat. Disord. 1986, 5, 295–315. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1002/1098-108X%28198602%295%3A2%3C295%3A%3AAID-EAT2260050209%3E3.0.CO%3B2-T (accessed on 6 June 2023). [CrossRef]
- van Strien, T.; Herman, C.P.; Anschutz, D. The predictive validity of the DEBQ-external eating scale for eating in response to food commercials while watching television. Int. J. Eat. Disord. 2011, 45, 257–262. [Google Scholar] [CrossRef]
- Banasiak, S.J.; Wertheim, E.H.; Koerner, J.; Voudouris, N.J. Test-retest reliability and internal consistency of a variety of measures of dietary restraint and body concerns in a sample of adolescent girls. Int. J. Eat. Disord. 2001, 29, 85–89. [Google Scholar] [CrossRef]
- Kenny, D.A.; Harackiewicz, J.M. Cross-lagged panel correlation: Practice and promise. J. Appl. Psychol. 1979, 64, 372–379. [Google Scholar] [CrossRef]
- Rosseel, Y. lavaan: AnRPackage for Structural Equation Modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics Title: Using Multivariate Statistics. 2019. Available online: https://lccn.loc.gov/2017040173 (accessed on 6 June 2023).
- Wilcox, R. Trimming and Winsorization. In Encyclopedia of Biostatistics; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Snoek, H.M.; van Strien, T.; Janssens, J.M.A.M.; Engels, R.C.M.E. Restrained Eating and BMI: A Longitudinal Study Among Adolescents. Health Psychol. 2008, 27, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, A.A.; Carr, M.M.; Ivezaj, V.; Barnes, R.D. Examining the construct validity of food addiction severity specifiers. Eat. Weight. Disord. Stud. Anorex. Bulim. Obes. 2020, 26, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.A.; Rhemtulla, M. Power Analysis for Parameter Estimation in Structural Equation Modeling: A Discussion and Tutorial. Adv. Methods Pract. Psychol. Sci. 2021, 4, 1–17. [Google Scholar] [CrossRef]
- Wiss, D.; Avena, N.M. Food Addiction, Binge Eating, and the Role of Dietary Restraint: Converging Evidence from Animal and Human Studies. In Binge Eating: A Transdiagnostic Psychopathology; Springer: Cham, Switzerland, 2020; pp. 193–209. [Google Scholar] [CrossRef]
- Grilo, C.; Masheb, R. Onset of dieting vs. binge eating in outpatients with binge eating disorder. Int. J. Obes. 2000, 24, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Hilbert, A.; Pike, K.M.; Goldschmidt, A.B.; Wilfley, D.E.; Fairburn, C.G.; Dohm, F.-A.; Walsh, B.T.; Weissman, R.S. Risk factors across the eating disorders. Psychiatry Res. 2014, 220, 500–506. [Google Scholar] [CrossRef]
- Reas, D.L.; Grilo, C.M. Timing and sequence of the onset of overweight, dieting, and binge eating in overweight patients with binge eating disorder. Int. J. Eat. Disord. 2006, 40, 165–170. [Google Scholar] [CrossRef]
- Brewerton, T.; Brewerton, T.D.; Dansky, B.S.; Kilpatrick, D.G.; O’neil, P.M. Which Comes First in the Pathogenesis of Bulimia Nervosa: Dieting or Bingeing? Int. J. Eat. Disord. 2000, 28, 259–2640. [Google Scholar] [CrossRef]
- Tanofsky-Kraff, M.; Faden, D.; Yanovski, S.Z.; Wilfley, D.E.; Yanovski, J.A. The Perceived Onset of Dieting and Loss of Control Eating Behaviors in Overweight Children. Int. J. Eat. Disord. 2005, 38, 112. [Google Scholar] [CrossRef]
- Lowe, M.R. Dieting: Proxy or Cause of Future Weight Gain? Obes. Rev. 2015, 16, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Lowe, M.R.; Doshi, S.D.; Katterman, S.N.; Feig, E.H. Dieting and Restrained Eating as Prospective Predictors of Weight Gain. Front. Psychol. 2013, 4, 577. [Google Scholar] [CrossRef] [PubMed]
- Thanarajah, S.E.; DiFeliceantonio, A.G.; Albus, K.; Br€, J.C.; Tittgemeyer, M.; Small, D.M. Habitual daily intake of a sweet and fatty snack modulates reward processing in humans. Cell Metab. 2023, 35, 571–584.e6. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Curtis, C.; Levitan, R.D.; Carter, J.C.; Kaplan, A.S.; Kennedy, J.L. Evidence That ‘Food Addiction’ Is a Valid Phenotype of Obesity. Appetite 2011, 57, 711–717. [Google Scholar] [CrossRef]
- Loxton, N.J.; Tipman, R.J. Reward sensitivity and food addiction in women. Appetite 2017, 115, 28–35. [Google Scholar] [CrossRef]
- Hoover, L.V.; Yu, H.P.; Cummings, J.R.; Ferguson, S.G.; Gearhardt, A.N. Co-occurrence of food addiction, obesity, problematic substance use, and parental history of problematic alcohol use. Psychol. Addict. Behav. J. Soc. Psychol. Addict. Behav. 2022. [Google Scholar] [CrossRef]
- Lee, A.; Ho, M.; Keung, V. Healthy School as an Ecological Model for Prevention of Childhood Obesity. Res. Sports Med. 2010, 18, 49–61. [Google Scholar] [CrossRef]
- Cassin, S.; Sockalingam, S. The Clinical Utility of Food Addiction and Eating Addiction; MDPI: Basel, Switzerland, 2021. [Google Scholar] [CrossRef]
- Harris, J.L.; Yokum, S.; Fleming-Milici, F. Hooked on Junk: Emerging Evidence on How Food Marketing Affects Adolescents’ Diets and Long-Term Health. Curr. Addict. Rep. 2021, 8, 19–27. [Google Scholar] [CrossRef]
- Brown, A.; Flint, S.W.; Batterham, R.L. Pervasiveness, impact and implications of weight stigma. Eclinicalmedicine 2022, 47, 101408. [Google Scholar] [CrossRef]
- Westenhoefer, J.; Stunkard, A.J.; Pudel, V. Validation of the Flexible and Rigid Control Dimensions of Dietary Restraint. Int. J. Eat. Disord. 1999, 26, 53–64. [Google Scholar] [CrossRef]
- Schembre, S.; Greene, G.; Melanson, K. Development and validation of a weight-related eating questionnaire. Eat. Behav. 2009, 10, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Mussell, M.P.; Mitchell, J.E.; Weller, C.L.; Raymond, N.C.; Crow, S.J.; Crosby, R.D. Onset of binge eating, dieting, obesity, and mood disorders among subjects seeking treatment for binge eating disorder. Int. J. Eat. Disord. 1995, 17, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Gearhardt, A.N.; Miller, A.L.; Sturza, J.; Epstein, L.H.; Kaciroti, N.; Lumeng, J.C. Behavioral Associations with Overweight in Low-Income Children. Obesity 2017, 25, 2123–2127. [Google Scholar] [CrossRef] [PubMed]
| Total (n) | Percent (%) | |
|---|---|---|
| Gender | ||
| Male | 61 | 48.0 |
| Female | 66 | 52.0 |
| Race | ||
| American Indian/Alaska Native | 3 | 2.4 |
| Black/African American | 19 | 15.0 |
| White | 91 | 71.7 |
| Other | 1 | 0.8 |
| Mixed | 8 | 6.3 |
| Unknown | 5 | 3.9 |
| Parental Education | ||
| Less than High School | 15 | 11.8 |
| High School Degree | 5 | 3.9 |
| Some College | 19 | 15 |
| Associates Degree | 11 | 8.7 |
| Bachelor’s Degree | 35 | 27.6 |
| Advanced Degree | 42 | 33.1 |
| Sample Size at Each Wave | ||
| Time 1 | 127 | 100.0 |
| Time 2 | 92 | 72.4 |
| Time 3 | 88 | 69.3 |
| Mean (SD) | Range (min, max) | |
| Age (months) at Time 1 | 177.3 (12.4) | (156.0, 202.5) |
| BMI z-score | 0.95 (0.9) | (−1.2, 2.7) |
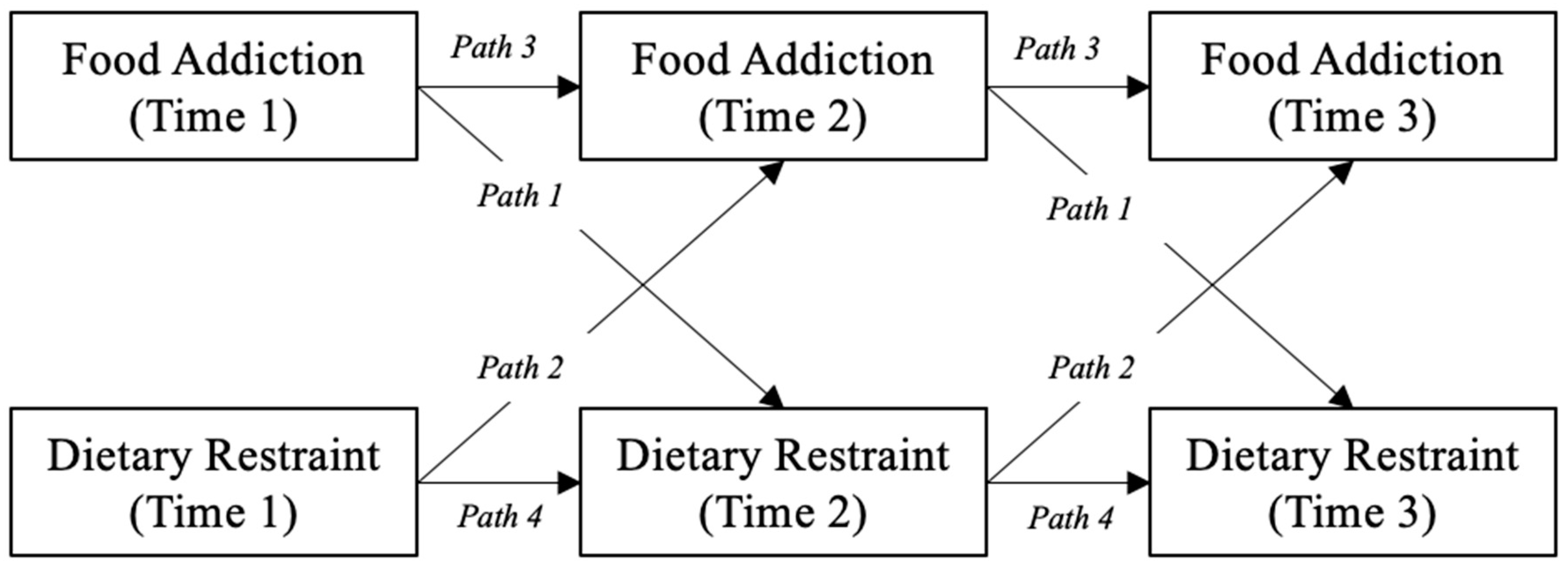
| CI (95%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Path | Predictor | Outcome | b | SE | z | p | Lower | Upper |
| 1 | Food addiction | Dietary restraint | 0.25 | 0.06 | 4.51 | <0.001 | 0.14 | 0.37 |
| 2 | Dietary restraint | Food addiction | 0.06 | 0.05 | 1.24 | 0.21 | −0.04 | 0.16 |
| 3 | Food addiction | Food addiction | 0.74 | 0.05 | 15.32 | <0.001 | 0.64 | 0.83 |
| 4 | Dietary restraint | Dietary restraint | 0.58 | 0.06 | 10.48 | <0.001 | 0.47 | 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rios, J.M.; Berg, M.K.; Gearhardt, A.N. Evaluating Bidirectional Predictive Pathways between Dietary Restraint and Food Addiction in Adolescents. Nutrients 2023, 15, 2977. https://doi.org/10.3390/nu15132977
Rios JM, Berg MK, Gearhardt AN. Evaluating Bidirectional Predictive Pathways between Dietary Restraint and Food Addiction in Adolescents. Nutrients. 2023; 15(13):2977. https://doi.org/10.3390/nu15132977
Chicago/Turabian StyleRios, Julia M., Martha K. Berg, and Ashley N. Gearhardt. 2023. "Evaluating Bidirectional Predictive Pathways between Dietary Restraint and Food Addiction in Adolescents" Nutrients 15, no. 13: 2977. https://doi.org/10.3390/nu15132977
APA StyleRios, J. M., Berg, M. K., & Gearhardt, A. N. (2023). Evaluating Bidirectional Predictive Pathways between Dietary Restraint and Food Addiction in Adolescents. Nutrients, 15(13), 2977. https://doi.org/10.3390/nu15132977






