Abstract
Multiple sclerosis (MS) is a complex neurological condition that involves both inflammatory demyelinating and neurodegenerative components. MS research and treatments have traditionally focused on immunomodulation, with less investigation of neuroprotection, and this holds true for the role of vitamin D in MS. Researchers have already established that vitamin D plays an anti-inflammatory role in modulating the immune system in MS. More recently, researchers have begun investigating the potential neuroprotective role of vitamin D in MS. The active form of vitamin D, 1,25(OH)2D3, has a range of neuroprotective properties, which may be important in remyelination and/or the prevention of demyelination. The most notable finding relevant to MS is that 1,25(OH)2D3 promotes stem cell proliferation and drives the differentiation of neural stem cells into oligodendrocytes, which carry out remyelination. In addition, 1,25(OH)2D3 counteracts neurodegeneration and oxidative stress by suppressing the activation of reactive astrocytes and M1 microglia. 1,25(OH)2D3 also promotes the expression of various neuroprotective factors, including neurotrophins and antioxidant enzymes. 1,25(OH)2D3 decreases blood–brain barrier permeability, reducing leukocyte recruitment into the central nervous system. These neuroprotective effects, stimulated by 1,25(OH)2D3, all enhance neuronal survival. This review summarizes and connects the current evidence supporting the vitamin D-mediated mechanisms of action for neuroprotection in MS.
1. Introduction
Multiple sclerosis (MS) is a demyelinating autoimmune disease of the central nervous system (CNS), characterized by an interplay of genetic and environmental factors [1]. Vitamin D deficiency during childhood and adolescence is a risk factor for the development of MS [2]. Vitamin D is obtained primarily via sun exposure (UVB, wavelengths ~295–315 nm) and/or taking vitamin D supplements, with limited intake from food in most populations. Higher MS prevalence and earlier onset are associated with geographical locations of increasing latitude (away from the equator) and/or with reduced annual sunlight exposure [3,4,5,6,7,8]. High consumption of fatty fish, a select dietary source of vitamin D, is believed to help protect against this latitude gradient MS association [9,10,11]. Furthermore, evidence has shown that HLA-DRB1*1501, the strongest genetic association in MS, is regulated by 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) via a vitamin D response element [12,13,14].
Arguably, the strongest evidence comes from large longitudinal population-based cohorts with banked serum samples from persons with MS (pwMS) at time points prior to their MS onset. Using samples from large prospective cohorts of nurses and military personnel in the US, Munger and colleagues found a significant reduction in MS risk (30–60%) associated with the highest quintile of serum 25-hydroxyvitamin D3 (25(OH)D3) [15,16]. Scandinavian registry cohorts observed similar results using banked maternal and newborn serum samples—lower circulating 25(OH)D3 was associated with significant increases in MS risk [17,18].
With regards to altering disease activity after MS diagnosis, some studies have shown that vitamin D supplementation can reduce relapses and MRI lesion activity in pwMS [19,20,21,22,23,24]. Randomized controlled trials are difficult to execute due to the requirements of large sample size and study length, disease heterogeneity, the sensitivity of detecting differences in clinical endpoints, retention rates, the influence of sun exposure, and vitamin D maintenance dose in the control arms, among other challenges. Several studies have shown promising results with respect to certain clinical endpoints with high-dose vitamin D supplementation compared to placebo groups [25,26,27,28,29]. However, consistent results as to the long-term clinical benefits of MS are lacking. It may be likely that RCTs with vitamin D supplementation will never be able to assess its potential benefits, especially given that a control arm of zero supplementation is deemed non-ethical [30].
Both the circulating and biologically active forms of vitamin D (25(OH)D3 and 1,25(OH)2D3, respectively) cross the blood–brain barrier (BBB) into the CNS, where they can act on various neuronal and glial cells [31,32]. Neurons, microglia, and astrocytes express 1α-hydroxylase (CYP27B1), the enzyme responsible for converting 25(OH)D3 into 1,25(OH)2D3 [33,34,35,36]. Along with oligodendrocytes, these cells also all express the vitamin D receptor (VDR) [33,34,35,36,37,38,39]. 1,25(OH)2D3 can thus be synthesized in the cytosol or diffuse through the plasma membrane of target cells and bind to the VDR in the cytoplasm [40]. The VDR-1,25(OH)2D3 complex then translocates to the nucleus where it couples with the retinoid X receptor (RXR) and binds to the vitamin D response elements of target genes to regulate their expression [40]. This leads to the biological actions of vitamin D, mediated through changes in the expression of a huge array of target genes, involved in diverse functions ranging from bone health to CNS development to immunomodulation.
Both inflammatory demyelinating and subsequent neurodegenerative processes contribute to the complex pathogenesis of MS [41]. The autoimmune component is driven by the T-cell-mediated attack of the myelin sheath surrounding the axons of CNS neurons, which prevents efficient impulse transmission, leading to neurological symptoms [42,43]. These abnormal immune responses in MS cause focal inflammatory demyelinated lesions in the CNS, and chronic myelin destruction enhances axonal damage and eventual neuronal loss [41,44,45,46]. Remyelination is important in preventing permanent disability, but the ability to restore myelin becomes increasingly impaired with progressive MS [47]. Neuroprotective treatment strategies—aimed at preventing initial loss or promoting remyelination—remain elusive in MS treatment [46,47]. Understanding the mechanisms by which vitamin D deficiency affects immune regulation, myelination, and neurodegeneration is important to potentially altering MS risk and disease activity.
The mechanism of vitamin D-mediated immunological activity, including reducing inflammation, is much more established in the literature than its proposed neuroprotective benefits [48,49,50]. Evidence that vitamin D sufficiency is important in combatting axonal degeneration, as well as both glial and neuronal loss in MS, has been rather limited in the past [48,50]. However, the number of studies investigating the neuroprotective mechanisms of vitamin D is growing. As such, the intent of this review is to examine the current state of research in order to summarize and clarify the latest findings regarding the mechanistic role of vitamin D-mediated neuroprotection in MS. We map out its actions via the following mechanisms: enhancing oligodendrocyte lineage differentiation, enhancing neurotrophin expression, attenuating aberrant microglial and reactive astrocyte activation, stabilizing the BBB, and reducing oxidative stress.
2. Promoting Oligodendrocyte Proliferation and Differentiation
Oligodendrocyte dystrophy and apoptosis are significant pathological features in the demyelinating lesions of MS [51]. Oligodendrocytes are myelin-producing glial cells that support neurons in the CNS. During periods of tissue injury, mature oligodendrocytes have the ability to remyelinate CNS neuronal axons to maintain saltatory conduction, which is a prerequisite for proper brain functioning [52]. Remyelination by oligodendrocytes can be robust and restorative, especially in early MS, but declines during later stages of the disease [52,53]. The ability to regenerate the oligodendrocyte population depends on the availability of neural stem cells (NSCs) and oligodendrocyte progenitor cells (OPCs) [52]. Oligodendrogenesis is the process by which NSCs commit to an oligodendrocyte lineage and differentiate into OPCs, which ultimately differentiate into oligodendrocytes [54]. However, in MS, especially during the progressive stage, the regenerative capacity of NSCs and OPCs to give rise to oligodendrocytes is considerably diminished, contributing to neuronal degeneration and impaired axonal conduction [6,53,55].
It has previously been shown that OPCs and oligodendrocytes express VDR [34]. VDR-RXR heterodimerization is present in OPCs and is necessary for OPC differentiation [37,56]. The capacity of 1,25(OH)2D3 to promote OPC differentiation is diminished in the presence of a VDR antagonist in a dose-dependent manner [37]. By blocking VDR, it becomes apparent that 1,25(OH)2D3 is exerting its proposed neuroprotective effect on oligodendrocyte lineage cells via VDR-RXR signalling [37]. In 2015, it was demonstrated that VDR is constitutively expressed in NSCs [57]. Increasing vitamin D in vitro upregulates VDR expression in NSCs in a dose-dependent manner [57].
Increased 1,25(OH)2D3 exposure stimulates an increase in NSC proliferation and, importantly, increases the proportion of NSCs that can differentiate into oligodendrocyte lineage cells [57,58] (Figure 1). In a study utilizing a lysolecithin-induced model in the corpus callosum of male rats, the group that received oral 1,25(OH)2D3 had a higher concentration of OPCs at lesion sites compared to sham and control groups [58]. Furthermore, 1,25(OH)2D3 administration increases the proportion of mature oligodendrocytes in a cuprizone-induced demyelination model as well as in NSC and OPC cultures [37,57,59]. Consistent results were found in a murine experimental autoimmune encephalomyelitis (EAE) model, where 1,25(OH)2D3 administration increased the concentration of NSCs, OPCs, and oligodendrocytes [60]. EAE models are often considered more reflective of MS pathogenesis than demyelination models as they exhibit both immune-mediated inflammation and demyelination [61]. Additionally, 1,25(OH)2D3 administration upregulates the expression of myelin basic protein and proteolipid protein, which are markers of myelin content [37,58,59,60]. The upregulation of these markers may suggest that demyelination is reduced and/or remyelination is increased in response to 1,25(OH)2D3 [37,58,59,60].
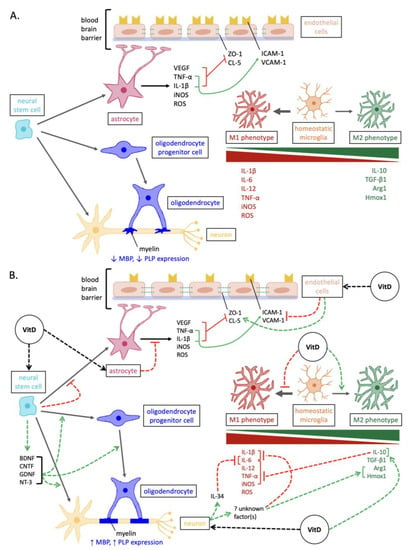
Figure 1.
Overview of the mechanisms involved in neurodegeneration versus vitamin D-mediated neuroprotection in MS. (A) Pathways of neurodegenerative pathogenesis in MS. In addition to the depicted, the expression of neurotrophins and antioxidant enzymes is reduced in neurons and glia. (B) Neuroprotective pathways elicited by vitamin D in MS. Abbreviations: Arg1, arginase 1; BDNF, brain-derived neurotrophic factor; CNTF, ciliary neurotrophic factor; CL-5, claudin-5; GDNF, glial cell line-derived neurotrophic factor; Hmox1, heme oxygenase 1; ICAM-1, intercellular cell adhesion molecule-1; iNOS, inducible nitric oxide synthase; IL, interleukins; MBP, myelin basic protein; NT-3, neurotrophin-3; PLP, proteolipid protein; ROS, reactive oxygen species; VCAM-1, vascular cell adhesion molecule-1; TGF, transforming growth factor; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor; ZO-1, zonula occluden-1.
3. Enhancing Neurotrophin Expression
Reduced neurotrophin secretion is another factor that contributes to inadequate neuroprotection in neurodegenerative disorders such as MS [62,63]. Neurotrophins are a family of proteins that elicit protective and regenerative effects by stimulating the proliferation and differentiation of NSCs, as well as the growth, survival, and proper functioning of neuronal and glial cells [62,64,65]. Neurotrophins are secreted by multiple cell types, some of which include NSCs, neurons, oligodendrocytes, astrocytes, and M2 microglia [57,65,66,67,68]. Key neurotrophins include NT-3, BDNF, CNTF, GDNF, and NGF [64].
Vitamin D has previously been demonstrated to increase neurotrophin expression [69,70,71]. When mouse NSCs were cultured with 1,25(OH)2D3, the expression of NT-3, BDNF, CNTF, and GDNF was upregulated [57]. Consistent results were observed in rodent models showing the upregulation of NGF and BDNF in CNS tissue following 25(OH)D3 supplementation [71,72,73]. In addition, 1,25(OH)2D3 exposure stimulated oligodendrogenesis and neurogenesis in mouse NSCs [57]. This effect may be mediated by the induction of these neurotrophins, whereby 1,25(OH)2D3 enhances NSC proliferation and differentiation into neurons and oligodendrocytes [57]. These neurotrophins have all been previously associated with enhanced oligodendrogenesis and neurogenesis [74,75,76,77,78,79,80]. Overall, increased neurotrophin secretion in response to vitamin D may tip the balance towards a less neurotoxic environment in which CNS cells can more effectively contribute to repair and regeneration (Figure 1).
4. Attenuating the Activation of Pro-Inflammatory/Neurodegenerative Microglia
Microglia play a central role in normal CNS development and maintenance, including resident immune surveillance. In MS, activated microglia are abundant in the focal plaques of demyelination and contribute to disease progression [81,82]. The polarization of activated microglia has typically been classified into two opposing phenotypes, which likely exist on a continuum: M1 microglia, which are considered pro-inflammatory and neurotoxic, or M2 microglia, which are considered anti-inflammatory and neuroregenerative [66,81,83]. The classically activated M1 phenotype contributes to neurodegeneration via the release of reactive oxygen species (ROS) and pro-inflammatory cytokines, as well as enhancing antigen presentation to CD4+ T cells [66,67,81,83,84,85].
There are various lines of evidence supporting the attenuation of the M1 phenotype with increased vitamin D exposure [35,39,58,59,86,87,88,89]. Firstly, the release of pro-inflammatory cytokines TNF-α, IL-1β, IL-6, and IL-12, as well as inducible nitric oxide synthase (iNOS), ROS formation, and CD86 (a marker of the M1 phenotype) are reduced in cultured microglia exposed to 1,25(OH)2D3 and in vitamin D-supplemented mouse models of CNS disease [35,86,88,89,90,91,92]. A reduction in these M1 cytokines and ROS then contributes to oligodendrocyte and neuronal survival [67,93,94,95,96,97]. The vitamin D-mediated phenotypic shift also downregulates MHC II expression by M1 microglia, reducing their antigen-presenting capacity to CD4+ T cells [39,84,88,96], which fosters an environment that permits tissue repair.
Vitamin D helps mediate a microglial shift towards the M2 phenotype [98]. The alternative M2 phenotype is neuroprotective in nature, given its association with the increased secretion of anti-inflammatory cytokines as well as the upregulation of neurotrophins and ROS-regulating enzymes arginase 1 and heme oxygenase 1 [91,99]. In a mouse model of Parkinson’s disease, vitamin D has been shown to increase the expression of CD163, CD206, and CD204, which are all markers of M2 microglia [90]. Furthermore, several studies have demonstrated that vitamin D is associated with increased concentrations of M2-associated cytokines, IL-4, IL-10, and TGF-β1, in both EAE mice and in serum from pwMS [100,101,102,103]. These cytokines are implicated in regenerative CNS functions, such as oligodendrogenesis and neurogenesis [104,105,106]. In addition, M2 microglia have a stronger phagocytic capacity to engulf myelin debris, which is a prerequisite for myelin repair in CNS disorders (due to the inflammatory and neurotoxic nature of myelin debris) [39,66,67,83,107]. Given that 1,25(OH)2D3 helps to promote a shift towards the M2 microglial phenotype [88,108,109], this could potentially also enhance the clearance of myelin debris.
The vitamin D-induced release of IL-10 from microglia is one mechanism mediating the M1 to M2 phenotypic shift [35]. Boontanrart et al. (2016) exposed cultured microglia to LPS, IFN-γ, and Theiler’s murine encephalomyelitis virus to induce M1 polarization [35]. In these M1 microglial cultures, they found that 1,25(OH)2D3 stimulated microglia to release IL-10, which binds to the microglial IL-10 receptor in an autocrine and paracrine manner to upregulate SOCS3 [35]. SOCS3 then acts via a negative feedback loop to downregulate TNF-α, IL-6, IL-12, and iNOS [35].
Another mechanism by which vitamin D is hypothesized to shift microglial phenotypes is via its stimulation of neuronal factors such as IL-34 [39]. In vitro experiments were conducted on neurons and LPS-activated M1 microglia, revealing that 1,25(OH)2D3 stimulates neurons to release factor(s) that act on microglia to influence a transition from M1 to M2 polarization, as evidenced by downregulated IL-6, IL-1β, and MHC II, as well as upregulated heme oxygenase 1 and arginase 1 [39]. Neuronal IL-34, in particular, is a survival factor, contains a VDRE in its promoter region, and its expression is slightly increased when stimulated by 1,25(OH)2D3 [39,97]. Under the influence of 1,25(OH)2D3, neuronal IL-34 inhibited the expression of IL-6 in M1 microglia, partially contributing to the phenotypic transition from M1 to M2 polarization [39]. This indicates that other unknown neuronal factor(s) are likely involved in facilitating the microglial shift towards the M2 phenotype under the influence of 1,25(OH)2D3 [39]. Overall, it is hypothesized that vitamin D promotes a shift away from M1 and towards the M2 microglial phenotype, thus reducing damage to neurons and oligodendrocytes, promoting greater potential for repair and recovery (Figure 1).
5. Reducing Reactive Astrogliosis
Astrocytes comprise a large portion of the glial cell population in the CNS [110]. They elicit essential functions, such as metabolically assisting neuronal growth, signalling immune cell entry into the CNS, and forming a critical component of the BBB [110]. When CNS injury occurs, astrocytes become reactive and divide rapidly, also termed astrogliosis, which has both positive and negative consequences [110,111]. Reactive astrocytes aid in recovery by encompassing the site of demyelination, resulting in the construction of a glial scar, which prevents the injury from expanding [112]. However, after a certain point, the abnormal increase in the number of reactive astrocytes is detrimental, as it contributes to the development of MS lesions [110,113,114]. Reactive astrocytes release a number of pro-inflammatory cytokines and ROS, which can be neurotoxic to OPCs, oligodendrocytes, and neurons [115].
Reducing the activation and abundance of astrocytes may make the neurodegenerative microenvironment more conducive to repair processes [67,116]. MS plaques with fewer reactive astrocytes exhibit elevated OPC content and greater remyelination [116]. The expression of VDR and CYP27B1 were upregulated in the astrocytes of LPS-stimulated rats, supporting a potential response via vitamin D [36]. In rodent models of cuprizone-induced demyelination and LPS injection, it was shown that the concentration and activation of astrocytes were decreased in mice that were administered intraperitoneal injections of 25(OH)D3 and 1,25(OH)2D3 [36,59]. Findings from other rodent CNS disease models have similarly supported a decrease in GFAP expression and astrocyte activation upon supplementation with oral or injected vitamin D [36,71]. More specifically, 25(OH)D3 and 1,25(OH)2D3 downregulated iNOS, TLR4, TNF-α, and IL-1β in cultured astrocytes and EAE [36,117,118] (Figure 1). Additionally, the in vitro exposure of mouse NSCs to 1,25(OH)2D3 reduces NSC differentiation into astrocytes [57] (Figure 1). This is interesting, as vitamin D has the opposite effect of increasing NSC differentiation into oligodendrocytes and neurons (discussed above) [57], consistent with its role in neuroprotection.
6. Stabilizing the Blood–Brain Barrier
The BBB regulates the movement of blood-borne molecules, ions, and cells into the CNS, leading to the stabilization and protection of the neuronal microenvironment [119,120,121,122]. Breakdown of the BBB and consequent hyperpermeability occurs early in MS [123]. When stimulated by pro-inflammatory cytokines from various immune cells, endothelial cells of the BBB downregulate tight junctions and upregulate cell-adhesion molecules, which destabilizes the BBB and increases leukocyte recruitment into the CNS, respectively [124]. Reactive astrocytes also play a role in BBB instability [120,125,126]. Pro-inflammatory cytokines, including TNF-α and IL-1β, secreted from reactive astrocytes stimulate the endothelial cells to downregulate tight junctions and upregulate cell-adhesion molecules [120,125,126]. The reactive astrocytes also detach their endfeet processes from the capillary endothelium, making the BBB more permeable [127]. Interestingly, in a neurodegenerative environment, reactive astrocytes release vascular endothelial growth factor (VEGF), which signals endothelial cells to lower tight junction expression, which destabilizes the BBB [128]. As a result of BBB hyperpermeability, CD4+ Th1 and Th17 cells are able to translocate into the CNS, where their secreted cytokines prompt the degeneration of oligodendrocytes and myelinated axons [123].
Vitamin D is thought to counteract BBB hyperpermeability through multiple mechanisms (Figure 1). In a study using human brain endothelial cells, the effects of 1,25(OH)2D3 exposure were examined following exposure to TNF-α and exposure to sera derived from MS patients [129]. It was found that 1,25(OH)2D3 can act directly on endothelial cells to upregulate tight junction proteins (zonula occluden-1 and claudin-5) and downregulate cell adhesion molecules (ICAM-1 and VCAM-1) [129]. These two outcomes both contribute to BBB stabilization [129]. More recently, de Oliveira et al. (2020) reported similar findings, observing that 1,25(OH)2D3 supplementation in EAE mice upregulated zonula occluden-1 and lowered BBB permeability, alongside symptom improvement [88]. They also analyzed immune cell entry into the CNS and axonal loss; 1,25(OH)2D3-stimulated BBB stabilization was associated with the limited migration of immune cells into the CNS and reduced demyelination scores [88]. Additionally, reactive astrocytes exposed to 25(OH)D3 demonstrate decreased expression of TNF-α, IL-1β, and VEGF, which may help further stabilize the BBB [36,120,125,126,128] to promote neuroprotection.
In addition to reducing BBB permeability by upregulating tight junction proteins and downregulating cell-adhesion molecules, 1,25(OH)2D3 has been shown to lower the expression of matrix metalloproteinase-9 (MMP-9) in mouse-brain endothelial cells and in a rat model of ischemic stroke [130,131]. Various cell types, including endothelial cells, CNS cells, and leukocytes, release MMPs [132]. MMPs are responsible for breaking down extracellular matrix components (such as collagen, fibronectin, and laminin) and tight junction proteins, thereby contributing to BBB instability [132,133,134,135]. As such, reducing MMP-9 expression may be another underlying mechanism by which vitamin D promotes BBB stabilization [98]. It has also been demonstrated that 1,25(OH)2D3 reduces the apoptosis of human endothelial cells exposed to MS sera, which may indicate a further protective effect of vitamin D on the BBB [98,136].
7. Reducing Oxidative Stress
Another significant contributor to the pathogenesis of MS is oxidative stress [137]. Normally, ROS, such as hydrogen peroxide, nitric oxide, and superoxide, are generated during cellular respiration when a small quantity of electrons “leak out” of the electron transport chain and react with oxygen [137,138]. Under physiological conditions, ROS that are produced in low amounts are neutralized by antioxidant enzymes present in cells [137]. However, when ROS production exceeds neutralization, the excess ROS can oxidize nucleic acids, proteins, lipids, and carbohydrates, contributing to cell injury and death [137,139]. In MS, the inflammatory environment continually activates a number of cells, including peripheral immune cells, microglia, and astrocytes, that release ROS and pro-inflammatory cytokines, further enhancing inflammation in a positive feedback loop [137]. Microglia and peripheral macrophages are the greatest generators of ROS in MS [96,137]. While ROS production is increased in MS, ROS neutralization is decreased due to lower antioxidant enzyme expression [140,141]. In the CNS microenvironment, excessive ROS are particularly damaging to oligodendrocyte lineage cells and neurons, as these cell types do not have sufficient antioxidant enzymes to neutralize the ROS [96,97,142]. Injuring OPCs and neurons can impair remyelination, leading to neurodegeneration [143].
Vitamin D has been shown to mitigate oxidative stress in the CNS tissue of EAE mice [88]. 1,25(OH)2D3 treatment reduced the biomarkers of oxidative stress (lipid hydroxides and protein carbonyls), while the expression of antioxidant enzymes (glutathione peroxidase, superoxide dismutase, and catalase) was restored to normal levels [85]. Similarly, vitamin D sufficiency and supplementation have been associated with decreased oxidative stress and increased antioxidant biomarkers in both animal models [144,145,146,147] and human studies [148,149,150,151,152] of other health conditions, including Type II Diabetes. In addition, as discussed, vitamin D helps decrease the M1 microglial population, a potent contributor to elevated nitric oxide and reactive oxygen intermediates in MS. 1,25(OH)2D3 can downregulate iNOS in activated microglia in culture and in reactive astrocytes from EAE mice [35,118]. In contrast, 1,25(OH)2D3 may promote the ability to neutralize ROS in microglia by upregulating the expression of heme oxygenase 1 and arginase 1 [39].
Additionally, Nrf2 is an intracellular factor that helps protect cells against oxidative stress by inducing the transcription of various antioxidant enzymes [50,153]. It has been found that the induction of EAE in a mouse model progresses more quickly and more severely in Nrf2 knockout mice compared to wild-type mice [154]. iNOS levels were also significantly increased in the Nrf2 knockout mice versus the wild-type mice [154]. Nrf2 expression is high in the active MS lesions, especially in the neurons and glia, of post-mortem brain tissue in pwMS [155]. 1,25(OH)2D3 has been demonstrated to increase the expression of Nrf2 alongside heme oxygenase-1 and NAD(P)H quinone oxidoreductase-1 in the CNS tissue of a neurodegenerative mouse model [153]. It has been hypothesized that 1,25(OH)2D3 simulates the activation of Nrf2, after which Nrf2 translocates into the nucleus to bind to the antioxidant response element to upregulate antioxidant enzyme expression [50,153]. In summary, vitamin D-mediated antioxidant synthesis and reduced ROS production provide a path by which CNS cells reduce oxidative stress to promote neuroprotection (Figure 1).
8. Conclusions
MS continues to have a large impact worldwide, with an estimated 2.9 million people living with MS [156]. There are two key intertwined facets of MS pathophysiology: inflammation and neurodegeneration. The bulk of MS research and therapeutic targets focus primarily on immunomodulation, while research on how to combat the neurodegenerative components of MS remains much more limited. Neuroprotective strategies continue to represent a promising area of research that could yield strategies to directly target specific cell types and components of the nervous system. Intentional neuroprotective strategies, either on their own or in conjunction with immunomodulatory therapies, may be effective in attenuating the neurodegenerative processes in MS [2,45].
Vitamin D is a proposed neuroprotective agent in MS. We summarized a number of recent studies that investigated the neuroprotective effects of vitamin D, reporting favourable results [36,37,39,57,58,59,60,85,89]. Overall, vitamin D supports the neuronal population in various ways. Under the influence of vitamin D, NSCs express various neurotrophins, namely NT-3, BDNF, CNTF, and GDNF [57]. These neurotrophins then stimulate neurogenesis and protect neurons from degeneration and apoptosis [57,64]. In addition to increasing neurotrophin expression, vitamin D helps promote a shift in the microglial population towards the M2-like phenotype, which is associated with the secretion of anti-inflammatory cytokines to help counteract neuronal damage [35,100,101,102,103]. Vitamin D also promotes neuronal survival by suppressing M1 microglia and reactive astrocytes, thus decreasing the secretion of pro-inflammatory cytokines and ROS, sparing neurons from harm [35,36,39,58,59,87,88,89]. In addition, vitamin D helps preserve the integrity and promotes the stabilization of the BBB, decreasing the entry of autoreactive T-cells with the potential to target neurons. Collectively, we see that evidence supports that vitamin D acts via various pathways, implicating a variety of CNS cell types, to promote neuronal integrity and survival.
Combined, these neuroprotective effects elicited by vitamin D promote a more stable microenvironment in which CNS glial cells can more easily participate in repair and recovery processes to help restore the structure and functioning of neurons. One limitation of the proposed mechanisms is that much of the supporting evidence comes from animal models, and potential differences in neuroprotective pathways in humans remain to be discovered [46,82,157]. Future directions for research include extending this work in cultured human neuronal and glial cells, with a potential for some of the findings to be studied in post-mortem brain tissue. With respect to prospective studies employing vitamin D supplementation, assessing endpoints that better capture neuroprotective benefits would be a useful avenue to explore.
This review has connected and mapped out current evidence to summarize the proposed mechanisms of vitamin D neuroprotection in MS. The aim is to promote a better understanding of the potential interactions between CNS cell types stimulated by vitamin D, neuroprotection in MS, and overall outcomes. The combined evidence further suggests that vitamin D supplementation and promoting vitamin D sufficiency at a population level, alongside the development of new neuroprotective agents, remains a worthwhile pursuit in the fight against MS.
Author Contributions
Conceptualization, A.S., M.Q., G.P. and S.-M.O.; literature search and investigation, A.S., M.Q. and S.-M.O.; selection of content A.S., M.Q. and S.-M.O.; writing—original draft preparation, A.S. and M.Q.; writing—review and editing, A.S., M.Q., G.P. and S.-M.O.; visualization, A.S. and M.Q.; supervision, G.P. and S.-M.O.; funding acquisition, A.S., G.P. and S.-M.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Alberta MS Network Summer Studentship funding (A.S.). The APC was funded by the Mount Royal University Library Open Access Fund (S.-M.O.).
Institutional Review Board Statement
Ethical review and approval were not required for this study due to it being a literature review.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Compston, A.; Coles, A. Multiple Sclerosis. Lancet 2002, 359, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Giovannoni, G. Multiple Sclerosis—A Review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef]
- Kurland, L.T. The Frequency and Geographic Distribution of Multiple Sclerosis as Indicated by Mortality Statistics and Morbidity Surveys in the United States and Canada. Am. J. Epidemiol. 1952, 55, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Acheson, E.D. Multiple Sclerosis in British Commonwealth Countries in the Southern Hemisphere. Br. J. Prev. Soc. Med. 1961, 15, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Orton, S.-M.; Wald, L.; Confavreux, C.; Vukusic, S.; Krohn, J.P.; Ramagopalan, S.V.; Herrera, B.M.; Sadovnick, A.D.; Ebers, G.C. Association of UV Radiation with Multiple Sclerosis Prevalence and Sex Ratio in France. Neurology 2011, 76, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.; Imitola, J.; Ayuso-Sacido, A.; Wang, Y.; Starossom, S.C.; Kivisäkk, P.; Zhu, B.; Meyer, M.; Bronson, R.T.; Garcia-Verdugo, J.M.; et al. Reversible Neural Stem Cell Niche Dysfunction in a Model of Multiple Sclerosis. Ann. Neurol. 2011, 69, 878–891. [Google Scholar] [CrossRef]
- Tao, C.; Simpson, S.; van der Mei, I.; Blizzard, L.; Havrdova, E.; Horakova, D.; Shaygannejad, V.; Lugaresi, A.; Izquierdo, G.; Trojano, M.; et al. Higher Latitude Is Significantly Associated with an Earlier Age of Disease Onset in Multiple Sclerosis. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1343–1349. [Google Scholar] [CrossRef]
- Tremlett, H.; Zhu, F.; Ascherio, A.; Munger, K.L. Sun Exposure over the Life Course and Associations with Multiple Sclerosis. Neurology 2018, 90, e1191–e1199. [Google Scholar] [CrossRef]
- Bäärnhielm, M.; Olsson, T.; Alfredsson, L. Fatty Fish Intake Is Associated with Decreased Occurrence of Multiple Sclerosis. Mult. Scler. 2014, 20, 726–732. [Google Scholar] [CrossRef]
- Kampman, M.T.; Brustad, M. Vitamin D: A Candidate for the Environmental Effect in Multiple Sclerosis–Observations from Norway. Neuroepidemiology 2008, 30, 140–146. [Google Scholar] [CrossRef]
- Jelinek, G.A.; Hadgkiss, E.J.; Weiland, T.J.; Pereira, N.G.; Marck, C.H.; van der Meer, D.M. Association of Fish Consumption and Omega 3 Supplementation with Quality of Life, Disability and Disease Activity in an International Cohort of People with Multiple Sclerosis. Int. J. Neurosci. 2013, 123, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Ramagopalan, S.V.; Maugeri, N.J.; Handunnetthi, L.; Lincoln, M.R.; Orton, S.M.; Dyment, D.A.; DeLuca, G.C.; Herrera, B.M.; Chao, M.J.; Sadovnick, A.D.; et al. Expression of the Multiple Sclerosis-Associated MHC Class II Allele HLA-DRB1*1501 Is Regulated by Vitamin D. PLoS Genet. 2009, 5, e1000369. [Google Scholar] [CrossRef] [PubMed]
- Cocco, E.; Meloni, A.; Murru, M.R.; Corongiu, D.; Tranquilli, S.; Fadda, E.; Murru, R.; Schirru, L.; Secci, M.A.; Costa, G.; et al. Vitamin D Responsive Elements within the HLA-DRB1 Promoter Region in Sardinian Multiple Sclerosis Associated Alleles. PLoS ONE 2012, 7, e41678. [Google Scholar] [CrossRef]
- Kular, L.; Liu, Y.; Ruhrmann, S.; Zheleznyakova, G.; Marabita, F.; Gomez-Cabrero, D.; James, T.; Ewing, E.; Lindén, M.; Górnikiewicz, B.; et al. DNA Methylation as a Mediator of HLA-DRB1*15:01 and a Protective Variant in Multiple Sclerosis. Nat. Commun. 2018, 9, 2397. [Google Scholar] [CrossRef] [PubMed]
- Munger, K.L.; Zhang, S.M.; O’Reilly, E.; Hernán, M.A.; Olek, M.J.; Willett, W.C.; Ascherio, A. Vitamin D Intake and Incidence of Multiple Sclerosis. Neurology 2004, 62, 60–65. [Google Scholar] [CrossRef]
- Munger, K.L.; Levin, L.I.; Hollis, B.W.; Howard, N.S.; Ascherio, A. Serum 25-Hydroxyvitamin D Levels and Risk of Multiple Sclerosis. JAMA 2006, 296, 2832–2838. [Google Scholar] [CrossRef]
- Nielsen, N.M.; Munger, K.L.; Koch-Henriksen, N.; Hougaard, D.M.; Magyari, M.; Jørgensen, K.T.; Lundqvist, M.; Simonsen, J.; Jess, T.; Cohen, A.; et al. Neonatal Vitamin D Status and Risk of Multiple Sclerosis: A Population-Based Case-Control Study. Neurology 2017, 88, 44–51. [Google Scholar] [CrossRef]
- Munger, K.L.; Hongell, K.; Åivo, J.; Soilu-Hänninen, M.; Surcel, H.-M.; Ascherio, A. 25-Hydroxyvitamin D Deficiency and Risk of MS among Women in the Finnish Maternity Cohort. Neurology 2017, 89, 1578–1583. [Google Scholar] [CrossRef]
- Burton, J.M.; Kimball, S.; Vieth, R.; Bar-Or, A.; Dosch, H.-M.; Cheung, R.; Gagne, D.; D’Souza, C.; Ursell, M.; O’Connor, P. A Phase I/II Dose-Escalation Trial of Vitamin D3 and Calcium in Multiple Sclerosis. Neurology 2010, 74, 1852–1859. [Google Scholar] [CrossRef]
- Laursen, J.H.; Søndergaard, H.B.; Sørensen, P.S.; Sellebjerg, F.; Oturai, A.B. Vitamin D Supplementation Reduces Relapse Rate in Relapsing-Remitting Multiple Sclerosis Patients Treated with Natalizumab. Mult. Scler. Relat. Disord. 2016, 10, 169–173. [Google Scholar] [CrossRef]
- Goldberg, P.; Fleming, M.C.; Picard, E.H. Multiple Sclerosis: Decreased Relapse Rate through Dietary Supplementation with Calcium, Magnesium and Vitamin D. Med. Hypotheses 1986, 21, 193–200. [Google Scholar] [CrossRef]
- Achiron, A.; Givon, U.; Magalashvili, D.; Dolev, M.; Liraz Zaltzman, S.; Kalron, A.; Stern, Y.; Mazor, Z.; Ladkani, D.; Barak, Y. Effect of Alfacalcidol on Multiple Sclerosis-Related Fatigue: A Randomized, Double-Blind Placebo-Controlled Study. Mult. Scler. 2015, 21, 767–775. [Google Scholar] [CrossRef]
- Ascherio, A.; Munger, K.L.; White, R.; Köchert, K.; Simon, K.C.; Polman, C.H.; Freedman, M.S.; Hartung, H.-P.; Miller, D.H.; Montalbán, X.; et al. Vitamin D as an Early Predictor of Multiple Sclerosis Activity and Progression. JAMA Neurol. 2014, 71, 306–314. [Google Scholar] [CrossRef]
- Mowry, E.M.; Waubant, E.; McCulloch, C.E.; Okuda, D.T.; Evangelista, A.A.; Lincoln, R.R.; Gourraud, P.-A.; Brenneman, D.; Owen, M.C.; Qualley, P.; et al. Vitamin D Status Predicts New Brain Magnetic Resonance Imaging Activity in Multiple Sclerosis. Ann. Neurol. 2012, 72, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Soilu-Hänninen, M.; Åivo, J.; Lindström, B.M.; Elovaara, I.; Sumelahti, M.L.; Färkkilä, M.; Tienari, P.; Atula, S.; Sarasoja, T.; Herrala, L.; et al. A Randomised, Double Blind, Placebo Controlled Trial with Vitamin D3 as an Add on Treatment to Interferon β-1b in Patients with Multiple Sclerosis. J. Neurol. Neurosurg. Psychiatry 2012, 83, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Hupperts, R.; Smolders, J.; Vieth, R.; Holmøy, T.; Marhardt, K.; Schluep, M.; Killestein, J.; Barkhof, F.; Beelke, M.; Grimaldi, L.M.E. Randomized Trial of Daily High-Dose Vitamin D3 in Patients with RRMS Receiving Subcutaneous Interferon β-1a. Neurology 2019, 93, E1906–E1916. [Google Scholar] [CrossRef] [PubMed]
- Camu, W.; Lehert, P.; Pierrot-Deseilligny, C.; Hautecoeur, P.; Besserve, A.; Deleglise, A.S.J.; Payet, M.; Thouvenot, E.; Souberbielle, J.C. Cholecalciferol in Relapsing-Remitting MS: A Randomized Clinical Trial (CHOLINE). Neurol.-Neuroimmunol. Neuroinflamm. 2019, 6, e597. [Google Scholar] [CrossRef]
- Kampman, M.T.; Steffensen, L.H.; Mellgren, S.I.; Jørgensen, L. Effect of Vitamin D3 Supplementation on Relapses, Disease Progression, and Measures of Function in Persons with Multiple Sclerosis: Exploratory Outcomes from a Double-Blind Randomised Controlled Trial. Mult. Scler. 2012, 18, 1144–1151. [Google Scholar] [CrossRef]
- Golan, D.; Halhal, B.; Glass-Marmor, L.; Staun-Ram, E.; Rozenberg, O.; Lavi, I.; Dishon, S.; Barak, M.; Ish-Shalom, S.; Miller, A. Vitamin D Supplementation for Patients with Multiple Sclerosis Treated with Interferon-Beta: A Randomized Controlled Trial Assessing the Effect on Flu-like Symptoms and Immunomodulatory Properties. BMC Neurol. 2013, 13, 60. [Google Scholar] [CrossRef]
- Scragg, R. Limitations of Vitamin D Supplementation Trials: Why Observational Studies Will Continue to Help Determine the Role of Vitamin D in Health. J. Steroid Biochem. Mol. Biol. 2018, 177, 6–9. [Google Scholar] [CrossRef]
- Pardridge, W.M.; Sakiyama, R.; Coty, W.A. Restricted Transport of Vitamin D and A Derivatives through the Rat Blood-Brain Barrier. J. Neurochem. 1985, 44, 1138–1141. [Google Scholar] [CrossRef]
- Yu, J.; Gattoni-Celli, M.; Zhu, H.; Bhat, N.R.; Sambamurti, K.; Gattoni-Celli, S.; Kindy, M.S. Vitamin D3-Enriched Diet Correlates with a Decrease of Amyloid Plaques in the Brain of AβPP Transgenic Mice. J. Alzheimers Dis. 2011, 25, 295–307. [Google Scholar] [CrossRef]
- Smolders, J.; Schuurman, K.G.; van Strien, M.E.; Melief, J.; Hendrickx, D.; Hol, E.M.; van Eden, C.; Luchetti, S.; Huitinga, I. Expression of Vitamin D Receptor and Metabolizing Enzymes in Multiple Sclerosis-Affected Brain Tissue. J. Neuropathol. Exp. Neurol. 2013, 72, 91–105. [Google Scholar] [CrossRef]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the Vitamin D Receptor and 1 Alpha-Hydroxylase in Human Brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef]
- Boontanrart, M.; Hall, S.D.; Spanier, J.A.; Hayes, C.E.; Olson, J.K. Vitamin D3 Alters Microglia Immune Activation by an IL-10 Dependent SOCS3 Mechanism. J. Neuroimmunol. 2016, 292, 126–136. [Google Scholar] [CrossRef]
- Jiao, K.-P.; Li, S.-M.; Lv, W.-Y.; Jv, M.-L.; He, H.-Y. Vitamin D3 Repressed Astrocyte Activation Following Lipopolysaccharide Stimulation in Vitro and in Neonatal Rats. Neuroreport 2017, 28, 492–497. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, A.G.; Errea, O.; van Wijngaarden, P.; Gonzalez, G.A.; Kerninon, C.; Jarjour, A.A.; Lewis, H.J.; Jones, C.A.; Nait-Oumesmar, B.; Zhao, C.; et al. Vitamin D Receptor-Retinoid X Receptor Heterodimer Signaling Regulates Oligodendrocyte Progenitor Cell Differentiation. J. Cell Biol. 2015, 211, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Sloka, S.; Zhornitsky, S.; Silva, C.; Metz, L.M.; Yong, V.W. 1,25-Dihydroxyvitamin D3 Protects against Immune-Mediated Killing of Neurons in Culture and in Experimental Autoimmune Encephalomyelitis. PLoS ONE 2015, 10, e0144084. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.W.; Selhorst, A.; Lampe, S.G.; Liu, Y.; Yang, Y.; Lovett-Racke, A.E. Neuron-Specific Vitamin D Signaling Attenuates Microglia Activation and CNS Autoimmunity. Front. Neurol. 2020, 11, 19. [Google Scholar] [CrossRef]
- Nurminen, V.; Seuter, S.; Carlberg, C. Primary Vitamin D Target Genes of Human Monocytes. Front. Physiol. 2019, 10, 194. [Google Scholar] [CrossRef]
- Van Schependom, J.; Guldolf, K.; D’hooghe, M.B.; Nagels, G.; D’haeseleer, M. Detecting Neurodegenerative Pathology in Multiple Sclerosis before Irreversible Brain Tissue Loss Sets In. Transl. Neurodegener. 2019, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Goverman, J. Autoimmune T Cell Responses in the Central Nervous System. Nat. Rev. Immunol. 2009, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Høglund, R.A.; Maghazachi, A.A. Multiple Sclerosis and the Role of Immune Cells. World J. Exp. Med. 2014, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Coggan, J.S.; Bittner, S.; Stiefel, K.M.; Meuth, S.G.; Prescott, S.A. Physiological Dynamics in Demyelinating Diseases: Unraveling Complex Relationships through Computer Modeling. Int. J. Mol. Sci. 2015, 16, 21215–21236. [Google Scholar] [CrossRef] [PubMed]
- Allanach, J.R.; Farrell, J.W.; Mésidor, M.; Karimi-Abdolrezaee, S. Current Status of Neuroprotective and Neuroregenerative Strategies in Multiple Sclerosis: A Systematic Review. Mult. Scler. J. 2022, 28, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Antel, J.; Kuhlmann, T. Inflammation in Multiple Sclerosis: Consequences for Remyelination and Disease Progression. Nat. Rev. Neurol. 2023, 19, 305–320. [Google Scholar] [CrossRef]
- Stangel, M.; Kuhlmann, T.; Matthews, P.M.; Kilpatrick, T.J. Achievements and Obstacles of Remyelinating Therapies in Multiple Sclerosis. Nat. Rev. Neurol. 2017, 13, 742–754. [Google Scholar] [CrossRef]
- Cadden, M.H.; Koven, N.S.; Ross, M.K. Neuroprotective Effects of Vitamin D in Multiple Sclerosis. Neurosci. Med. 2011, 2, 198–207. [Google Scholar] [CrossRef]
- Gandhi, F.; Jhaveri, S.; Avanthika, C.; Singh, A.; Jain, N.; Gulraiz, A.; Shah, P.; Nasir, F. Impact of Vitamin D Supplementation on Multiple Sclerosis. Cureus 2021, 13, e18487. [Google Scholar] [CrossRef]
- Gombash, S.E.; Lee, P.W.; Sawdai, E.; Lovett-Racke, A.E. Vitamin D as a Risk Factor for Multiple Sclerosis: Immunoregulatory or Neuroprotective? Front. Neurol. 2022, 13, 796933. [Google Scholar] [CrossRef]
- Cudrici, C.; Niculescu, T.; Niculescu, F.; Shin, M.L.; Rus, H. Oligodendrocyte Cell Death in Pathogenesis of Multiple Sclerosis: Protection of Oligodendrocytes from Apoptosis by Complement. J. Rehabil. Res. Dev. 2006, 43, 123–132. [Google Scholar] [CrossRef]
- Chamberlain, K.A.; Nanescu, S.E.; Psachoulia, K.; Huang, J.K. Oligodendrocyte Regeneration: Its Significance in Myelin Replacement and Neuroprotection in Multiple Sclerosis. Neuropharmacology 2016, 110, 633–643. [Google Scholar] [CrossRef]
- Voskuhl, R.R.; Itoh, N.; Tassoni, A.; Matsukawa, M.A.; Ren, E.; Tse, V.; Jang, E.; Suen, T.T.; Itoh, Y. Gene Expression in Oligodendrocytes during Remyelination Reveals Cholesterol Homeostasis as a Therapeutic Target in Multiple Sclerosis. Proc. Natl. Acad. Sci. USA 2019, 116, 10130–10139. [Google Scholar] [CrossRef] [PubMed]
- Armada-Moreira, A.; Ribeiro, F.F.; Sebastião, A.M.; Xapelli, S. Neuroinflammatory Modulators of Oligodendrogenesis. Neuroimmunol. Neuroinflamm. 2015, 2, 263–273. [Google Scholar] [CrossRef]
- Brousse, B.; Magalon, K.; Durbec, P.; Cayre, M. Region and Dynamic Specificities of Adult Neural Stem Cells and Oligodendrocyte Precursors in Myelin Regeneration in the Mouse Brain. Biol. Open 2015, 4, 980–992. [Google Scholar] [CrossRef]
- Huang, J.K.; Jarjour, A.A.; Oumesmar, B.N.; Kerninon, C.; Williams, A.; Krezel, W.; Kagechika, H.; Bauer, J.; Zhao, C.; Evercooren, A.B.V.; et al. Retinoid X Receptor Gamma Signaling Accelerates CNS Remyelination. Nat. Neurosci. 2011, 14, 45–55. [Google Scholar] [CrossRef]
- Shirazi, H.A.; Rasouli, J.; Ciric, B.; Rostami, A.; Zhang, G.-X. 1,25-Dihydroxyvitamin D3 Enhances Neural Stem Cell Proliferation and Oligodendrocyte Differentiation. Exp. Mol. Pathol. 2015, 98, 240–245. [Google Scholar] [CrossRef]
- Gomez-Pinedo, U.; Cuevas, J.A.; Benito-Martín, M.S.; Moreno-Jiménez, L.; Esteban-Garcia, N.; Torre-Fuentes, L.; Matías-Guiu, J.A.; Pytel, V.; Montero, P.; Matías-Guiu, J. Vitamin D Increases Remyelination by Promoting Oligodendrocyte Lineage Differentiation. Brain Behav. 2019, 10, e01498. [Google Scholar] [CrossRef] [PubMed]
- Nystad, A.E.; Wergeland, S.; Aksnes, L.; Myhr, K.-M.; Bø, L.; Torkildsen, Ø. Effect of High-Dose 1.25 Dihydroxyvitamin D3 on Remyelination in the Cuprizone Model. Apmis 2014, 122, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, H.A.; Rasouli, J.; Ciric, B.; Wei, D.; Rostami, A.; Zhang, G.-X. 1,25-Dihydroxyvitamin D3 Suppressed Experimental Autoimmune Encephalomyelitis through Both Immunomodulation and Oligodendrocyte Maturation. Exp. Mol. Pathol. 2017, 102, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Denic, A.; Johnson, A.J.; Bieber, A.J.; Warrington, A.E.; Rodriguez, M.; Pirko, I. The Relevance of Animal Models in Multiple Sclerosis Research. Pathophysiology 2011, 18, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska-Lyszczarz, A.; Losy, J. The Role of Neurotrophins in Multiple Sclerosis-Pathological and Clinical Implications. Int. J. Mol. Sci. 2012, 13, 13713–13725. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.; Nazem, G.; Mardani, M.; Esfandiari, E.; Salehi, H.; Esfahani, S.H.Z. Neurotrophic Factors and Their Effects in the Treatment of Multiple Sclerosis. Adv. Biomed. Res. 2015, 4, 53. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Lu, P.; Jones, L.L.; Snyder, E.Y.; Tuszynski, M.H. Neural Stem Cells Constitutively Secrete Neurotrophic Factors and Promote Extensive Host Axonal Growth after Spinal Cord Injury. Exp. Neurol. 2003, 181, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Grajchen, E.; Hendriks, J.J.A.; Bogie, J.F.J. The Physiology of Foamy Phagocytes in Multiple Sclerosis. Acta Neuropathol. Commun. 2018, 6, 124. [Google Scholar] [CrossRef]
- Traiffort, E.; Kassoussi, A.; Zahaf, A.; Laouarem, Y. Astrocytes and Microglia as Major Players of Myelin Production in Normal and Pathological Conditions. Front. Cell. Neurosci. 2020, 14, 79. [Google Scholar] [CrossRef]
- Xiao, J.; Kilpatrick, T.J.; Murray, S.S. The Role of Neurotrophins in the Regulation of Myelin Development. Neurosignals 2009, 17, 265–276. [Google Scholar] [CrossRef]
- Brown, J.; Bianco, J.I.; McGrath, J.J.; Eyles, D.W. 1,25-Dihydroxyvitamin D3 Induces Nerve Growth Factor, Promotes Neurite Outgrowth and Inhibits Mitosis in Embryonic Rat Hippocampal Neurons. Neurosci. Lett. 2003, 343, 139–143. [Google Scholar] [CrossRef]
- Naveilhan, P.; Neveu, I.; Wion, D.; Brachet, P. 1,25-Dihydroxyvitamin D3, an Inducer of Glial Cell Line-Derived Neurotrophic Factor. Neuroreport 1996, 7, 2171–2175. [Google Scholar] [CrossRef]
- Zou, Y.; Mu, M.; Zhang, S.; Li, C.; Tian, K.; Li, Z.; Li, B.; Wang, W.; Cao, H.; Sun, Q.; et al. Vitamin D3 Suppresses Astrocyte Activation and Ameliorates Coal Dust-Induced Mood Disorders in Mice. J. Affect. Disord. 2022, 303, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Khairy, E.Y.; Attia, M.M. Protective Effects of Vitamin D on Neurophysiologic Alterations in Brain Aging: Role of Brain-Derived Neurotrophic Factor (BDNF). Nutr. Neurosci. 2021, 24, 650–659. [Google Scholar] [CrossRef]
- Gezen-Ak, D.; Dursun, E.; Yilmazer, S. The Effect of Vitamin D Treatment on Nerve Growth Factor (NGF) Release from Hippocampal Neurons. Noro Psikiyatr. Ars. 2014, 51, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Sun, C.; Liu, W. Effects of Neurotrophin-3 on the Differentiation of Neural Stem Cells into Neurons and Oligodendrocytes. Neural Regen. Res. 2012, 7, 1483–1487. [Google Scholar] [CrossRef]
- Barres, B.A.; Burne, J.F.; Holtmann, B.; Thoenen, H.; Sendtner, M.; Raff, M.C. Ciliary Neurotrophic Factor Enhances the Rate of Oligodendrocyte Generation. Mol. Cell. Neurosci. 1996, 8, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Pansri, P.; Phanthong, P.; Suthprasertporn, N.; Kitiyanant, Y.; Tubsuwan, A.; Dinnyes, A.; Kobolak, J.; Kitiyanant, N. Brain-Derived Neurotrophic Factor Increases Cell Number of Neural Progenitor Cells Derived from Human Induced Pluripotent Stem Cells. PeerJ 2021, 9, e11388. [Google Scholar] [CrossRef] [PubMed]
- Kokoeva, M.V.; Yin, H.; Flier, J.S. Neurogenesis in the Hypothalamus of Adult Mice: Potential Role in Energy Balance. Science 2005, 310, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Deng, L.; Wang, Y.; Yin, L.; Yang, C.; Du, J.; Yuan, Q. GDNF Enhances Therapeutic Efficiency of Neural Stem Cells-Based Therapy in Chronic Experimental Allergic Encephalomyelitis in Rat. Stem Cells Int. 2016, 2016, 1431349. [Google Scholar] [CrossRef]
- Linker, R.A.; Mäurer, M.; Gaupp, S.; Martini, R.; Holtmann, B.; Giess, R.; Rieckmann, P.; Lassmann, H.; Toyka, K.V.; Sendtner, M.; et al. CNTF Is a Major Protective Factor in Demyelinating CNS Disease: A Neurotrophic Cytokine as Modulator in Neuroinflammation. Nat. Med. 2002, 8, 620–624. [Google Scholar] [CrossRef]
- Langhnoja, J.; Buch, L.; Pillai, P. Potential Role of NGF, BDNF, and Their Receptors in Oligodendrocytes Differentiation from Neural Stem Cell: An in Vitro Study. Cell Biol. Int. 2021, 45, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Zrzavy, T.; Hametner, S.; Wimmer, I.; Butovsky, O.; Weiner, H.L.; Lassmann, H. Loss of ‘Homeostatic’ Microglia and Patterns of Their Activation in Active Multiple Sclerosis. Brain 2017, 140, 1900–1913. [Google Scholar] [CrossRef]
- Lassmann, H. Pathology of Inflammatory Diseases of the Nervous System: Human Disease versus Animal Models. Glia 2020, 68, 830–844. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, E.; Madore, C.; Lassmann, H.; Butovsky, O. Microglial Phenotypes and Functions in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a028993. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization from M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef]
- Haider, L.; Fischer, M.T.; Frischer, J.M.; Bauer, J.; Hoftberger, R.; Botond, G.; Esterbauer, H.; Binder, C.J.; Witztum, J.L.; Lassmann, H. Oxidative Damage in Multiple Sclerosis Lesions. Brain 2011, 134, 1914–1924. [Google Scholar] [CrossRef]
- Cui, C.; Xu, P.; Li, G.; Qiao, Y.; Han, W.; Geng, C.; Liao, D.; Yang, M.; Chen, D.; Jiang, P. Vitamin D Receptor Activation Regulates Microglia Polarization and Oxidative Stress in Spontaneously Hypertensive Rats and Angiotensin II-Exposed Microglial Cells: Role of Renin-Angiotensin System. Redox Biol. 2019, 26, 101295. [Google Scholar] [CrossRef]
- Wergeland, S.; Torkildsen, Ø.; Myhr, K.M.; Aksnes, L.; Mørk, S.J.; Bø, L. Dietary Vitamin D3 Supplements Reduce Demyelination in the Cuprizone Model. PLoS ONE 2011, 6, e26262. [Google Scholar] [CrossRef]
- de Oliveira, L.R.C.; Mimura, L.A.N.; Fraga-Silva, T.F.d.C.; Ishikawa, L.L.W.; Fernandes, A.A.H.; Zorzella-Pezavento, S.F.G.; Sartori, A. Calcitriol Prevents Neuroinflammation and Reduces Blood-Brain Barrier Disruption and Local Macrophage/Microglia Activation. Front. Pharm. 2020, 11, 161. [Google Scholar] [CrossRef]
- Verma, R.; Kim, J.Y. 1,25-Dihydroxyvitamin D_3 Facilitates M2 Polarization and Upregulates TLR10 Expression on Human Microglial Cells. Neuroimmunomodulation 2016, 23, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Calvello, R.; Cianciulli, A.; Nicolardi, G.; De Nuccio, F.; Giannotti, L.; Salvatore, R.; Porro, C.; Trotta, T.; Panaro, M.A.; Lofrumento, D.D. Vitamin D Treatment Attenuates Neuroinflammation and Dopaminergic Neurodegeneration in an Animal Model of Parkinson’s Disease, Shifting M1 to M2 Microglia Responses. J. Neuroimmune Pharmacol. 2017, 12, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 Microglia: The Good, the Bad, and the Inflamed. J. Neuroinflammation 2014, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Huang, Z.; Sun, X.; Zhu, X.; Zhou, L.; Li, M.; Cheng, B.; Liu, X.; He, C. Microglia Polarization with M1/M2 Phenotype Changes in Rd1 Mouse Model of Retinal Degeneration. Front. Neuroanat. 2017, 11, 282782. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; De Marchi, E.; Patergnani, S.; Suski, J.M.; Celsi, F.; Bononi, A.; Giorgi, C.; Marchi, S.; Rimessi, A.; Duszyński, J.; et al. Tumor Necrosis Factor-α Impairs Oligodendroglial Differentiation through a Mitochondria-Dependent Process. Cell Death Differ. 2014, 21, 1198–1208. [Google Scholar] [CrossRef]
- Vladić, A.; Horvat, G.; Vukadin, S.; Sučić, Z.; Šimaga, Š. Cerebrospinal Fluid and Serum Protein Levels of Tumour Necrosis Factor-Alpha (TNF-Alpha) Interleukin-6 (IL-6) and Soluble Interleukin-6 Receptor (SIL-6R Gp80) in Multiple Sclerosis Patients. Cytokine 2002, 20, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Sierra, A.; Abiega, O.; Shahraz, A.; Neumann, H. Janus-Faced Microglia: Beneficial and Detrimental Consequences of Microglial Phagocytosis. Front. Cell. Neurosci. 2013, 7, 6. [Google Scholar] [CrossRef]
- Luo, C.; Jian, C.; Liao, Y.; Huang, Q.; Wu, Y.; Liu, X.; Zou, D.; Wu, Y. The Role of Microglia in Multiple Sclerosis. Neuropsychiatr. Dis. Treat. 2017, 13, 1661–1667. [Google Scholar] [CrossRef]
- Baxter, P.S.; Hardingham, G.E. Adaptive Regulation of the Brain’s Antioxidant Defences by Neurons and Astrocytes. Free Radic. Biol. Med. 2016, 100, 147–152. [Google Scholar] [CrossRef]
- Galoppin, M.; Kari, S.; Soldati, S.; Pal, A.; Rival, M.; Engelhardt, B.; Astier, A.; Thouvenot, E. Full Spectrum of Vitamin D Immunomodulation in Multiple Sclerosis: Mechanisms and Therapeutic Implications. Brain Commun. 2022, 4, fcac171. [Google Scholar] [CrossRef]
- Zhang, D.; Li, M.; Dong, Y.; Zhang, X.; Liu, X.; Chen, Z.; Zhu, Y.; Wang, H.; Liu, X.; Zhu, J.; et al. 1α,25-Dihydroxyvitamin D3 up-Regulates IL-34 Expression in SH-SY5Y Neural Cells. Innate Immun. 2017, 23, 584–591. [Google Scholar] [CrossRef]
- Ashtari, F.; Toghianifar, N.; Zarkesh-Esfahani, S.H.; Mansourian, M. Short-Term Effect of High-Dose Vitamin D on the Level of Interleukin 10 in Patients with Multiple Sclerosis: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Neuroimmunomodulation 2015, 22, 400–404. [Google Scholar] [CrossRef]
- Hashemi, R.; Hosseini-Asl, S.S.; Arefhosseini, S.R.; Morshedi, M. The Impact of Vitamin D3 Intake on Inflammatory Markers in Multiple Sclerosis Patients and Their First-Degree Relatives. PLoS ONE 2020, 15, e0231145. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Humpal-Winter, J.; DeLuca, H.F. In Vivo Upregulation of Interleukin-4 Is One Mechanism Underlying the Immunoregulatory Effects of 1,25-Dihydroxyvitamin D(3). Arch. Biochem. Biophys. 2000, 377, 135–138. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Woodward, W.D.; Hayes, C.E.; DeLuca, H.F. 1,25-Dihydroxyvitamin D3 Is a Positive Regulator for the Two Anti-Encephalitogenic Cytokines TGF-Beta 1 and IL-4. J. Immunol. 1998, 160, 5314–5319. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Cusimano, M.; Zambito, M.; Finardi, A.; Capotondo, A.; Garcia-Manteiga, J.M.; Comi, G.; Furlan, R.; Martino, G.; Muzio, L. Interleukin 4 Modulates Microglia Homeostasis and Attenuates the Early Slowly Progressive Phase of Amyotrophic Lateral Sclerosis. Cell Death Dis. 2018, 9, 250. [Google Scholar] [CrossRef] [PubMed]
- Butovsky, O.; Ziv, Y.; Schwartz, A.; Landa, G.; Talpalar, A.E.; Pluchino, S.; Martino, G.; Schwartz, M. Microglia Activated by IL-4 or IFN-Gamma Differentially Induce Neurogenesis and Oligodendrogenesis from Adult Stem/Progenitor Cells. Mol. Cell. Neurosci. 2006, 31, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jiang, Z.; Fitzgerald, D.C.; Ma, C.; Yu, S.; Li, H.; Zhao, Z.; Li, Y.; Ciric, B.; Curtis, M.; et al. Adult Neural Stem Cells Expressing IL-10 Confer Potent Immunomodulation and Remyelination in Experimental Autoimmune Encephalitis. J. Clin. Investig. 2009, 119, 3678–3691. [Google Scholar] [CrossRef] [PubMed]
- Hammel, G.; Zivkovic, S.; Ayazi, M.; Ren, Y. Consequences and Mechanisms of Myelin Debris Uptake and Processing by Cells in the Central Nervous System. Cell. Immunol. 2022, 380, 104591. [Google Scholar] [CrossRef]
- Matías-Guíu, J.; Oreja-Guevara, C.; Matias-Guiu, J.A.; Gomez-Pinedo, U. Vitamin D and Remyelination in Multiple Sclerosis. Neurologia 2018, 33, 177–186. [Google Scholar] [CrossRef]
- Moretti, R.; Morelli, M.E.; Caruso, P. Vitamin D in Neurological Diseases: A Rationale for a Pathogenic Impact. Int. J. Mol. Sci. 2018, 19, 2245. [Google Scholar] [CrossRef]
- Colombo, E.; Farina, C. Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol. 2016, 37, 608–620. [Google Scholar] [CrossRef]
- Chiareli, R.A.; Carvalho, G.A.; Marques, B.L.; Mota, L.S.; Oliveira-Lima, O.C.; Gomes, R.M.; Birbrair, A.; Gomez, R.S.; Simão, F.; Klempin, F.; et al. The Role of Astrocytes in the Neurorepair Process. Front. Cell Dev. Biol. 2021, 9, 665795. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Farez, M.F. The Role of Astrocytes in Multiple Sclerosis Progression. Front. Neurol. 2015, 6, 180. [Google Scholar] [CrossRef]
- Holley, J.E.; Gveric, D.; Newcombe, J.; Cuzner, M.L.; Gutowski, N.J. Astrocyte Characterization in the Multiple Sclerosis Glial Scar. Neuropathol. Appl. Neurobiol. 2003, 29, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Rosenthal, H.; Cymring, B.; Gratch, D.; Pagano, B.; Xie, B.; Sadiq, S.A. Progressive Multiple Sclerosis Cerebrospinal Fluid Induces Inflammatory Demyelination, Axonal Loss, and Astrogliosis in Mice. Exp. Neurol. 2014, 261, 620–632. [Google Scholar] [CrossRef]
- Ding, Z.-B.; Song, L.-J.; Wang, Q.; Kumar, G.; Yan, Y.-Q.; Ma, C.-G. Astrocytes: A Double-Edged Sword in Neurodegenerative Diseases. Neural Regen. Res. 2021, 16, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Staugaitis, S.M.; Dutta, R.; Batt, C.E.; Easley, K.E.; Chomyk, A.M.; Yong, V.W.; Fox, R.J.; Kidd, G.J.; Trapp, B.D. Cortical Remyelination: A New Target for Repair Therapies in Multiple Sclerosis. Ann. Neurol. 2012, 72, 918–926. [Google Scholar] [CrossRef]
- Molinari, C.; Morsanuto, V.; Ghirlanda, S.; Ruga, S.; Notte, F.; Gaetano, L.; Uberti, F. Role of Combined Lipoic Acid and Vitamin D3 on Astrocytes as a Way to Prevent Brain Ageing by Induced Oxidative Stress and Iron Accumulation. Oxid. Med. Cell. Longev. 2019, 2019, 2843121. [Google Scholar] [CrossRef]
- Garcion, E.; Nataf, S.; Berod, A.; Darcy, F.; Brachet, P. 1,25-Dihydroxyvitamin D3 Inhibits the Expression of Inducible Nitric Oxide Synthase in Rat Central Nervous System during Experimental Allergic Encephalomyelitis. Mol. Brain Res. 1997, 45, 255–267. [Google Scholar] [CrossRef]
- Jiang, S.; Xia, R.; Jiang, Y.; Wang, L.; Gao, F. Vascular Endothelial Growth Factors Enhance the Permeability of the Mouse Blood-Brain Barrier. PLoS ONE 2014, 9, e86407. [Google Scholar] [CrossRef]
- Elahy, M.; Jackaman, C.; Mamo, J.C.; Lam, V.; Dhaliwal, S.S.; Giles, C.; Nelson, D.; Takechi, R. Blood-Brain Barrier Dysfunction Developed during Normal Aging Is Associated with Inflammation and Loss of Tight Junctions but Not with Leukocyte Recruitment. Immun. Ageing 2015, 12, 2. [Google Scholar] [CrossRef]
- Minagar, A.; Alexander, J.S. Blood-Brain Barrier Disruption in Multiple Sclerosis. Mult. Scler. J. 2003, 9, 540–549. [Google Scholar] [CrossRef]
- Luissint, A.-C.; Artus, C.; Glacial, F.; Ganeshamoorthy, K.; Couraud, P.-O. Tight Junctions at the Blood Brain Barrier: Physiological Architecture and Disease-Associated Dysregulation. Fluids Barriers CNS 2012, 9, 23. [Google Scholar] [CrossRef]
- Lutz, S.E.; Smith, J.R.; Kim, D.H.; Olson, C.V.L.; Ellefsen, K.; Bates, J.M.; Gandhi, S.P.; Agalliu, D. Caveolin1 Is Required for Th1 Cell Infiltration, but Not Tight Junction Remodeling, at the Blood-Brain Barrier in Autoimmune Neuroinflammation. Cell Rep. 2017, 21, 2104–2117. [Google Scholar] [CrossRef] [PubMed]
- Larochelle, C.; Alvarez, J.I.; Prat, A. How Do Immune Cells Overcome the Blood-Brain Barrier in Multiple Sclerosis? FEBS Lett. 2011, 585, 3770–3780. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.S.; Min, M.; Cummings, E.E.; Chen, X.; Sadowska, G.B.; Sharma, S.; Stonestreet, B.S. Effects of Interleukin-6 on the Expression of Tight Junction Proteins in Isolated Cerebral Microvessels from Yearling and Adult Sheep. Neuroimmunomodulation 2013, 20, 264–273. [Google Scholar] [CrossRef]
- Kebir, H.; Kreymborg, K.; Ifergan, I.; Dodelet-Devillers, A.; Cayrol, R.; Bernard, M.; Giuliani, F.; Arbour, N.; Becher, B.; Prat, A. Human TH17 Lymphocytes Promote Blood-Brain Barrier Disruption and Central Nervous System Inflammation. Nat. Med. 2007, 13, 1173–1175. [Google Scholar] [CrossRef] [PubMed]
- Aharoni, R.; Eilam, R.; Arnon, R. Astrocytes in Multiple Sclerosis-Essential Constituents with Diverse Multifaceted Functions. Int. J. Mol. Sci. 2021, 22, 5904. [Google Scholar] [CrossRef] [PubMed]
- Argaw, A.T.; Asp, L.; Zhang, J.; Navrazhina, K.; Pham, T.; Mariani, J.N.; Mahase, S.; Dutta, D.J.; Seto, J.; Kramer, E.G.; et al. Astrocyte-Derived VEGF-A Drives Blood-Brain Barrier Disruption in CNS Inflammatory Disease. J. Clin. Investig. 2012, 122, 2454–2468. [Google Scholar] [CrossRef]
- Takahashi, S.; Maeda, T.; Sano, Y.; Nishihara, H.; Takeshita, Y.; Shimizu, F.; Kanda, T. Active Form of Vitamin D Directly Protects the Blood–Brain Barrier in Multiple Sclerosis. Clin. Exp. Neuroimmunol. 2017, 8, 244–254. [Google Scholar] [CrossRef]
- Sayeed, I.; Turan, N.; Stein, D.G.; Wali, B. Vitamin D Deficiency Increases Blood-Brain Barrier Dysfunction after Ischemic Stroke in Male Rats. Exp. Neurol. 2019, 312, 63–71. [Google Scholar] [CrossRef]
- Won, S.; Sayeed, I.; Peterson, B.L.; Wali, B.; Kahn, J.S.; Stein, D.G. Vitamin D Prevents Hypoxia/Reoxygenation-Induced Blood-Brain Barrier Disruption via Vitamin D Receptor-Mediated NF-KB Signaling Pathways. PLoS ONE 2015, 10, e0122821. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, G.A. Matrix Metalloproteinases Biomarkers in Multiple Sclerosis. Lancet 2005, 365, 1291–1293. [Google Scholar] [CrossRef] [PubMed]
- Yabluchanskiy, A.; Ma, Y.; Iyer, R.P.; Hall, M.E.; Lindsey, M.L. Matrix Metalloproteinase-9: Many Shades of Function in Cardiovascular Disease. Physiology 2013, 28, 391. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Estrada, E.Y.; Thompson, J.F.; Liu, W.; Rosenberg, G.A. Matrix Metalloproteinase-Mediated Disruption of Tight Junction Proteins in Cerebral Vessels Is Reversed by Synthetic Matrix Metalloproteinase Inhibitor in Focal Ischemia in Rat. J. Cereb. Blood Flow Metab. 2007, 27, 697–709. [Google Scholar] [CrossRef]
- Baeten, K.M.; Akassoglou, K. Extracellular Matrix and Matrix Receptors in Blood-Brain Barrier Formation and Stroke. Dev. Neurobiol. 2011, 71, 1018. [Google Scholar] [CrossRef]
- Dehghani, L.; Meamar, R.; Etemadifar, M.; Sheshde, Z.D.; Shaygannejad, V.; Sharifkhah, M.; Tahani, S. Can Vitamin D Suppress Endothelial Cells Apoptosis in Multiple Sclerosis Patients? Int. J. Prev. Med. 2013, 4, S211. [Google Scholar]
- Ohl, K.; Tenbrock, K.; Kipp, M. Oxidative Stress in Multiple Sclerosis: Central and Peripheral Mode of Action. Exp. Neurol. 2016, 277, 58–67. [Google Scholar] [CrossRef]
- Zhao, R.-Z.; Jiang, S.; Zhang, L.; Yu, Z.-B. Mitochondrial Electron Transport Chain, ROS Generation and Uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Della Nera, G.; Sabatino, L.; Gaggini, M.; Gorini, F.; Vassalle, C. Vitamin D Determinants, Status, and Antioxidant/Anti-Inflammatory-Related Effects in Cardiovascular Risk and Disease: Not the Last Word in the Controversy. Antioxidants 2023, 12, 948. [Google Scholar] [CrossRef]
- Carvalho, A.N.; Lim, J.L.; Nijland, P.G.; Witte, M.E.; Van Horssen, J. Glutathione in Multiple Sclerosis: More than Just an Antioxidant? Mult. Scler. 2014, 20, 1425–1431. [Google Scholar] [CrossRef]
- Witherick, J.; Wilkins, A.; Scolding, N.; Kemp, K. Mechanisms of Oxidative Damage in Multiple Sclerosis and a Cell Therapy Approach to Treatment. Autoimmune Dis. 2010, 2011, 164608. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.M.; Pennypacker, K.R. Targeting Antioxidant Enzyme Expression as a Therapeutic Strategy for Ischemic Stroke. Neurochem. Int. 2017, 107, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Lassmann, H. The Role of Nitric Oxide in Multiple Sclerosis. Lancet Neurol. 2002, 1, 232–241. [Google Scholar] [CrossRef]
- Al-Sroji, R.Y.; Al-Laham, S.; Almandili, A. Protective Effects of Vitamin D3 (Cholecalciferol) on Vancomycin-Induced Oxidative Nephrotoxic Damage in Rats. Pharm. Biol. 2023, 61, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Fujii, H.; Kono, K.; Goto, S.; Kitazawa, R.; Kitazawa, S.; Hirata, M.; Shinohara, M.; Fukagawa, M.; Nishi, S. Vitamin D Activates the Nrf2-Keap1 Antioxidant Pathway and Ameliorates Nephropathy in Diabetic Rats. Am. J. Hypertens. 2014, 27, 586–595. [Google Scholar] [CrossRef]
- Ali, A.; Shah, S.A.; Zaman, N.; Uddin, M.N.; Khan, W.; Ali, A.; Riaz, M.; Kamil, A. Vitamin D Exerts Neuroprotection via SIRT1/Nrf-2/NF-KB Signaling Pathways against D-Galactose-Induced Memory Impairment in Adult Mice. Neurochem. Int. 2021, 142, 104893. [Google Scholar] [CrossRef] [PubMed]
- Gezen-Ak, D.; Dursun, E.; Yilmazer, S. The Effects of Vitamin D Receptor Silencing on the Expression of LVSCC-A1C and LVSCC-A1D and the Release of NGF in Cortical Neurons. PLoS ONE 2011, 6, e17553. [Google Scholar] [CrossRef] [PubMed]
- Codoñer-Franch, P.; Tavárez-Alonso, S.; Simó-Jordá, R.; Laporta-Martín, P.; Carratalá-Calvo, A.; Alonso-Iglesias, E. Vitamin D Status Is Linked to Biomarkers of Oxidative Stress, Inflammation, and Endothelial Activation in Obese Children. J. Pediatr. 2012, 161, 848–854. [Google Scholar] [CrossRef]
- Bhat, M.; Ismail, A. Vitamin D Treatment Protects against and Reverses Oxidative Stress Induced Muscle Proteolysis. J. Steroid Biochem. Mol. Biol. 2015, 152, 171–179. [Google Scholar] [CrossRef]
- Sepidarkish, M.; Farsi, F.; Akbari-Fakhrabadi, M.; Namazi, N.; Almasi-Hashiani, A.; Maleki Hagiagha, A.; Heshmati, J. The Effect of Vitamin D Supplementation on Oxidative Stress Parameters: A Systematic Review and Meta-Analysis of Clinical Trials. Pharm. Res. 2019, 139, 141–152. [Google Scholar] [CrossRef]
- Cojic, M.; Kocic, R.; Klisic, A.; Kocic, G. The Effects of Vitamin D Supplementation on Metabolic and Oxidative Stress Markers in Patients with Type 2 Diabetes: A 6-Month Follow Up Randomized Controlled Study. Front. Endocrinol. 2021, 12, 610893. [Google Scholar] [CrossRef] [PubMed]
- Moslemi, E.; Musazadeh, V.; Kavyani, Z.; Naghsh, N.; Shoura, S.M.S.; Dehghan, P. Efficacy of Vitamin D Supplementation as an Adjunct Therapy for Improving Inflammatory and Oxidative Stress Biomarkers: An Umbrella Meta-Analysis. Pharm. Res. 2022, 186, 106484. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Niu, Z.; Xue, Y.; Gao, J.; Zhang, M.; Li, M.; Peng, Y.; Zhang, S.; Li, W.; Zhang, Q.; et al. Chronic Vitamin D3 Supplementation Alleviates Cognition Impairment via Inhibition of Oxidative Stress Regulated by PI3K/AKT/Nrf2 in APP/PS1 Transgenic Mice. Neurosci. Lett. 2022, 783, 136725. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.A.; Amirahmadi, S.; Ward, C.; Fabry, Z.; Johnson, J.A. The Absence of the Pro-Antioxidant Transcription Factor Nrf2 Exacerbates Experimental Autoimmune Encephalomyelitis. Toxicol. Sci. 2010, 114, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Licht-Mayer, S.; Wimmer, I.; Traffehn, S.; Metz, I.; Brück, W.; Bauer, J.; Bradl, M.; Lassmann, H. Cell Type-Specific Nrf2 Expression in Multiple Sclerosis Lesions. Acta Neuropathol. 2015, 130, 263–277. [Google Scholar] [CrossRef]
- Number of People with MS|Atlas of MS. Available online: https://www.atlasofms.org/map/global/epidemiology/number-of-people-with-ms (accessed on 29 May 2023).
- Wimmer, I.; Zrzavy, T.; Lassmann, H. Neuroinflammatory Responses in Experimental and Human Stroke Lesions. J. Neuroimmunol. 2018, 323, 10–18. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).