Abstract
(1) Background: it is unclear whether serum vitamin B12 and circulating methylmalonic acid (MMA) are related with a poor prognosis among individuals with chronic kidney disease (CKD); (2) Methods: this prospective cohort study included 2589 individuals with CKD who participated in the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2004, and from 2011 to 2014, respectively. Hazard ratios (HRs) and 95% Cis for the associations of MMA and vitamin B12 levels with the risk of all-cause and cardiovascular disease (CVD) mortality were calculated using multivariable Cox proportional hazards regression models. Restricted cubic spline analyses were used to examine the non-linear association of MMA levels with all-cause and CVD mortality. (3) Results: among the 2589 participants, we identified 1192 all-cause deaths and 446 CVD deaths, respectively, with a median follow-up of 7.7 years. Compared with participants with MMA < 123 nmol/L, those with MMA ≥ 240 nmol/L had an increased all-cause and CVD mortality in the multivariable-adjusted model [HR (95% CI), 2.01 (1.54–2.62) and 1.76 (1.18–2.63), respectively]; (4) Conclusions: higher circulating MMA levels were found to be strongly associated with an elevated all-cause and CVD mortality among individuals with CKD, while serum vitamin B12 levels were not associated.
1. Introduction
Chronic kidney disease (CKD) is one of the leading noncommunicable causes of death worldwide, affecting more than 10% of the general population in the world, amounting to more than 800 million individuals [1].By 2024, the global burden of CKD has been predicted to increase rapidly and become the 5th leading cause of years of life lost [2,3]. CKD can lead to end-stage renal disease (ESRD), and is associated with an increased risk of cardiovascular morbidity and mortality [4]. Therefore, the identification of modifiable factors related to CKD is crucial to the prevention or delay of CKD-related complications and premature mortality.
Vitamin B12, also referred to as cobalamin, is an essential water-soluble vitamin that serves a significant function in metabolism, specifically in DNA synthesis, methylation, and mitochondrial metabolism [5]. Vitamin B12 is a cofactor for L-methylmalonyl-coenzyme A mutase, which is involved in the conversion of methylmalonyl-CoA to succinyl-CoA [6]. In vitamin B12 deficiency, this process could be impeded by the impaired activity of L-methylmalonyl-CoA mutase, resulting in the conversion of methylmalonyl-CoA to methylmalonic acid (MMA), and then causing the increase in the MMA concentrations in the blood [6]. MMA has been currently recognized as a functional metabolic marker for vitamin B12 deficiency [7]. Moreover, methylmalonyl-CoA is an intermediate in the metabolism of propionic acid (PA) [8,9]. In cases where there is no deficiency in vitamin B12 or renal dysfunction, elevated levels of MMA may be caused by an increase in the production of PA and its metabolites, or a decrease in their renal clearance [10]. MMA concentrations may be incorrectly increased during renal dysfunction [11,12]. Furthermore, there is increasing evidence in that MMA accumulation has an influence on mitochondrial dysfunction and oxidative stress by regulating the mitochondrial respiratory chain, and initiating the release of reactive oxygen species (ROS) [13,14], which could result in the development and progression of various kidney diseases [15].
The relationship between vitamin B12 and mortality has previously been explored in the elderly [16,17], hospitalized [18,19], hemodialysis patients [20], and general population [21]. And the relationship between MMA and mortality has previously been explored in patients with diabetes and the general population [10,22,23]. However, the relationship between MMA and vitamin B12 concentrations and mortality in patients with CKD has not been studied. Previous studies have shown that elevated MMA concentrations are associated with a vitamin B12 deficiency and renal dysfunction [11,12]. Thus, vitamin B12 and MMA levels are markers that may indicate different medical signs and conditions that should be carefully considered in different populations.
In order to fill in gaps in the current research, we conducted a population-based cohort study to investigate the potential association between circulating methylmalonic acid, serum vitamin B12 levels, and mortality rates due to all-cause and cardiovascular disease among individuals with chronic kidney disease.
2. Materials and Methods
2.1. Study Population
The National Health and Nutrition Examination Survey (NHANES) is a survey conducted by the Center for Disease Control and Prevention of the United States. It was designed to assess the health and nutritional status of the non-institutionalized civilian population of all ages in the U.S. The survey uses a stratified, multistage, and probability cluster design. Standardized questionnaires, physical measurements, and laboratory tests are all involved in this survey, and the study design and procedures have been previously published [24]. The National Center for Health Statistics Ethics Review Board approved the study protocol, and all participants provided their informed consent.
In our analyses, we used data from 51,507 participants in five NHANES circles (1999–2000, 2001–2002, 2003–2004, 2011–2012, and 2013–2014, respectively). MMA data were not available in NHANES 2005–2010. CKD was defined as an estimated glomerular filtration rate (eGFR) < 60 mL/min per 1.73 m2 or urinary albumin creatinine ratio (ACT) > 30 mg/g, and the eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [25]. CKD was classified into 5 stages according to the Kidney Disease: Improving Global Outcomes (KDIGO) guideline: stage 1 (eGFR ≥ 90 mL/min/1.73 m2 with ACT > 30 mg/g), stage 2 (eGFR > 60 and <90 with ACT > 30 mg/g), stage 3 (eGFR > 30 and <60), stage 4 (eGFR > 15 and <30), and stage 5 (eGFR < 15 mL/min/1.73 m2), respectively [26]. We excluded participants without CKD (n = 46,887), those <20 years (n = 962), those with pregnancy (n = 17), those with missing MMA values (n = 38), those with missing vitamin B12 values (n = 5), and those with a lack of covariates (n = 1009) including education, marital status, poverty-to-income ratio (PIR), body mass index (BMI), smoking, drinking, physical activity, Healthy Eating Index-2015 (HEI-2015), systolic pressure, and eGFR. In total, 2589 participants with CKD were included and available for analyses (Figure 1).
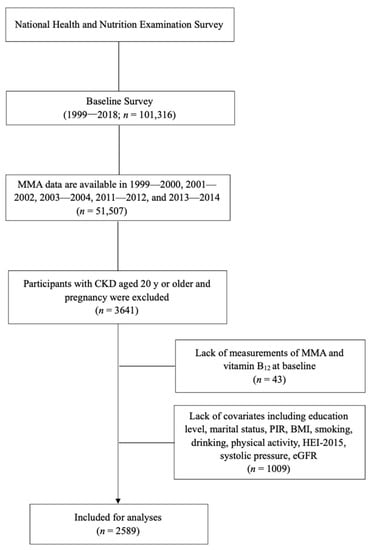
Figure 1.
Flowchart of the participants included in the analyses.
2.2. Measurements of MMA and Vitamin B12
MMA was measured in the plasma and/or serum, with plasma being preferred. MMA was measured in the NHANES from 1999 to 2004, respectively, using a gas chromatography-mass spectrometry (GC/MS) procedure that required 275 μL of the sample and had a low throughput (36 samples/run). In the NHANES 2011–2012 circle, MMA was measured using liquid chromatography-tandem mass spectrometry (LC-MS/MS) with multiple reaction monitoring. These two measurement methods had an excellent comparability and bias-free agreement [27]. The units used for MMA in this study were nmol/L.
In the NHANES III and 1999–2016 circles, the Bio-Rad Laboratories Quantaphase II radioimmunoassay was used to measure vitamin B12, while in the NHANES 2011–2014 the fully automated Roche electrochemiluminescence immunoassay was. But the weighted distributions of the 2005–2006 and 2011–2012 datasets were slightly different, resulting in the Deming regression equations [Roche vitamin B12 = 10 × (0.97 × log10 (Bio-Rad vitamin B12) + 0.14 pg/mL)] being recommended to use for our analyses [28]. The vitamin B12 in pg/mL was converted into pmol/L by multiplying it with 0.738. In our study, the vitamin B12 values were converted in the Roche assay to pmol/L.
2.3. Ascertainment of Mortality
The endpoint in our study was all-cause and CVD mortality. Death data were obtained by linking the public-use database from the National Death Index (NDI) up through 31 December 2019. Causes of death were determined using the International Classification of Diseases Tenth Revision code (ICD-10) codes. All-cause mortality was defined as any cause of death, and CVD mortality was coded as I00–I09, I11, I13, I20–I51, and I60–I69. Participants with no record of death during follow-up were considered to be alive.
2.4. Measurement of Covariates
Data regarding age, sex (male and female), race (Hispanic, non-Hispanic white, non-Hispanic black, and others), education (less than high school, high school, and college or higher), marital status (married, separated, and never married), PIR (≤1, 1–4, and ≥4) [29], smoking (never, former, and current), and drinking (none, moderate, and heavy), physical activity (low, moderate, and high) [30] were all collected from the baseline standardized questionnaires. BMI was calculated as the weight (kg) divided by the height squared (m2). HEI-2015 is a scoring system that ranges from 0 to 100 and is based on the 24 h dietary recalls which were conducted by trained interviewers. This system consists of 13 components, including eight food groups and five nutrients [31]. A higher score indicates a higher quality of diet. Systolic blood pressures were calculated as the mean of the readings obtained according to a standardized protocol.
2.5. Statistical Analysis
Statistical analyses were performed using R version 4.2.2, Stata version 15.1. All estimates were weighed with survey design, survey non-response, and post-stratification, which were considered in all analyses according to the recommendations from the NHANES Analytic and Reporting Guidelines Baseline [32]. Statistical tests were two-tailed, and the statistical significance was defined as p < 0.05.
In this study, the baseline characteristics of the total population and quartiles of the baseline MMA and vitamin B12 levels were presented. This comparison was performed using the Wald and F test for continuous variables, and the Rao–Scott chi-square test for categorical variables. The mean (standard error, SE) was used to express the continuous variables, while counts (percentage, %) were used to express the categorical variables
In this study, hazard ratios (HRs) and 95% confidence intervals (Cis) were calculated using multivariable Cox proportional hazards regression models to determine the associations between the MMA and vitamin B12 levels and the risks of all-cause and CVD mortality. Three multivariable-adjusted models were constructed stepwise to account for potential confounding. In Model 1, we adjusted for the NHANES circles (categorical variables for 1999–2000, 2001–2002, 2003–2004, 2011–2012, and 2013–2014, respectively), age, and sex. In Model 2, we additionally adjusted for race, education, marital status, PIR, BMI, smoking, drinking, physical activity, diet quality, systolic blood pressure, and eGFR. In Model 3, we further adjusted for the vitamin B12 and MMA levels.
To examine the non-linear association of the natural logarithm of the MMA levels with mortality, restricted cubic spline analyses with 4 knots (5th, 35th, 65th, and 95th percentiles, respectively) were conducted. These analyses were limited to values between the 5th and 95th percentiles to minimize the influence of potential outliers, with the 25th percentile as the reference point, respectively.
We further stratified these analyses by age (<65 and ≥65 years), sex (male and female), BMI (<30 and ≥30 kg/m2), smoking (never, former, and current), drinking (none, moderate and heavy), physical activity (low, moderate, and high), eGFR (≥60 and <60 mL/min/1.73 m2), and CKD stages (Stage 1, 2, and 3–5). The interaction (modification effect) between a stratifying variable and the composite MMA quartiles were estimated by adding interaction terms into the Cox proportional hazard regression and were assessed using the Wald test.
We performed three sensitivity analyses: (1) participants with a history of CVD and diabetes were further excluded from the main analyses. (2) Participants who died within 3 years of follow-up were excluded from the main analyses. (3) We further adjusted for several biomarkers, including homocysteine, HbA1c, and total cholesterol.
3. Results
3.1. Baseline Characteristics
Of the 2589 study participants aged 59.0 years (SE: 0.6), 1324 (46.8%) were men, and we identified 1192 all-cause deaths and 446 CVD deaths with a median follow-up of 7.7 years, and with a median follow-up of 14.5 years, 13.0 years, 12.2 years, 7.7 years, and 5.8 years, respectively, in five different circles (1999–2000, 2001–2002, 2003–2004, 2011–2012, and 2013–2014, respectively). For the analysis of MMA, participants with higher MMA levels were more likely to be older, non-Hispanic white, married, non-smokers, high physical activity, higher diet quality, higher systolic blood pressure, worsened CKD stage, lower vitamin B12 and eGFR (Table 1). For the analysis of vitamin B12, participants with higher vitamin B12 levels were more likely to be older, female, non-Hispanic white, lower BMI, higher diet quality, higher systolic blood pressure, lower eGFR, and lower MMA levels (Table S1).

Table 1.
Baseline characteristics of the study participants for CKD among U.S. adults by their baseline MMA levels.
3.2. MMA and Mortality
Restricted cubic splines presented no evidence for non-linear associations between the MMA levels and all-cause mortality (p for non-linearity = 0.385), nor with CVD mortality (p for non-linearity = 0.789) (Figure 2). After multivariable adjustment, the association showed a dose-response pattern, with a 30% increased risk of CVD mortality per unit increase in log-transformed MMA (HR, 1.30; 95% CI: 1.08, 1.57). When analyzing categorical MMA, compared with the reference group (first quartile, MMA < 123 nmol/L), the HRs (95% CI) of all-cause mortality with MMA 123–169 nmol/L, MMA 170–239 nmol/L, and MMA ≥ 240 nmol/L were 1.20 (0.91, 1.57), 1.28 (0.94, 1.76), and 2.01 (1.54, 2.62), respectively. Similarly, compared with the reference group (the first quartile), the HRs (95% CI) for CVD mortality were 1.29 (0.85, 1.97) for the second quartile, 1.04 (0.69, 1.58) for the third quartile, and 1.76 (1.18, 2.63) for the fourth quartile, respectively (Table 2).
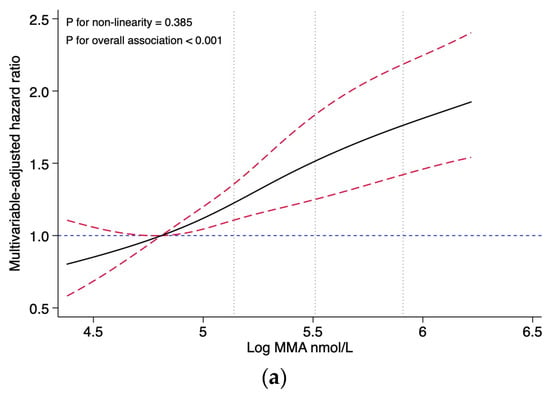
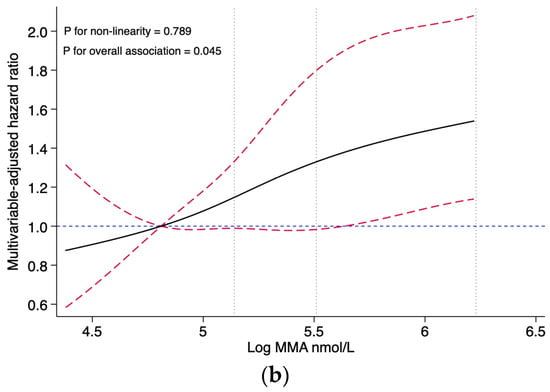
Figure 2.
Association of the MMA levels with all-cause and CVD mortality, evaluated using restricted cubic splines. (a) Association of the MMA levels with all-cause mortality. (b) Association of the MMA levels with CVD mortality. Abbreviations: MMA, methylmalonic acid. Hazard ratios and confidence intervals were estimated for MMA using four knots placed at specific percentiles of the natural logarithm of MMA. The reference value was set at the 25th percentile, and the dotted vertical lines represent the 50th, 75th, and 90th percentiles, respectively. The results are presented using solid black lines for the hazard ratios, red dashed lines (indicating the 95% confidence interval) for the confidence intervals and blue dashed lines for hazard ratios for reference lines of 1.0. The model was adjusted for the NHANES circles, age (continuous, year), sex (male and female), race (Hispanic, non-Hispanic white, non-Hispanic black, and others), education (less than high school, high school, and college or higher), marital status (married, separated, and never married), PIR (≤1, 1–4, and ≥4), BMI (continuous, kg/m2), smoking (never, former, and current), drinking (never, former, and current), physical activity (low, moderate, and high), diet quality (continuous, HEI-2015 score), systolic blood pressure (continuous, mmHg), eGFR (continuous, mL/min/1.73 m2), and vitamin B12 (continuous, pmol/L). No non-linear association was observed between MMA and all-cause mortality (p for non-linearity = 0.385), and no non-linear association was observed between MMA and CVD mortality (p for non-linearity = 0.789).

Table 2.
Association of the MMA levels with all-cause mortality and CVD mortality among U.S. adults with CKD.
3.3. Vitamin B12 and Mortality
Compared to those with vitamin B12 < 284.1 pmol/L, the HRs (95%) of all-cause mortality in participants with vitamin B12 284.1–397.1 pmol/L and 397.2–534.5 pmol/L, ≥534.6 pmol/L were 1.01 (0.78, 1.31), 0.93 (0.71, 1.22), and 1.22 (0.93, 1.61), respectively. Compared with the reference group (the first quartile), the HRs (95% CI) for CVD mortality were 1.02 (0.74, 1.39) for the second quartile, 1.02 (0.72, 1.44) for the third quartile, and 1.26 (0.94, 1.69) for the fourth quartile, respectively (Table S2).
3.4. Stratified and Sensitivity Analyses
In stratification analyses, the dose–response relationship between MMA and all-cause mortality was largely stratified by age (<65 and ≥65 years), sex (male and female), BMI (<30 and ≥30 kg/m2), smoking (never or former, and current), drinking (none, moderate, and heavy), physically active (low, moderate, and high), eGFR (<60 and ≥60 mL/min/1.73 m2), and CKD stages (stage1, 2, and 3–5) (all p ≥ 0.128 for interaction) (Table 3). Similarly, after correction for multiple testing, there were no significant interactions found between the levels of MMA and the risk of CVD mortality (Table S3).

Table 3.
Subgroup analyses for the HRs of the MMA levels and all-cause mortality among U.S. adults with CKD.
In general, the results were robust to sensitivity analyses when excluding the participants with a history of CVD and diabetes (Table S4), participants who died within 3 years of follow-up (Table S5), and adjusting for several biomarkers, including homocysteine, HbA1c, and total cholesterol (Table S6).
4. Discussion
This study is the first to analyze the levels of circulating MMA and vitamin B12 in individuals with CKD and their potential risks for all-cause and CVD mortality. No evidence of a non-linear association was found between the MMA levels and all-cause or CVD mortality in our study. It could be inferred that the relationship between the MMA levels and mortality is probably closer to a linear relationship. Our findings demonstrated that participants with MMA levels ≥ 240 nmol/L are at a higher risk for all-cause and CVD mortality compared to those with MMA levels < 123 nmol/L. Cox regression analysis of categorical MMA levels following the restricted cubic spline analysis of continuous MMA levels could reduce the effect of non-linear patterns in the data in the Cox regression model and improve the predictive power of the Cox regression model. Such associations remained robust after a series of sensitivity analyses. However, no significant association was found between serum vitamin B12 levels and the mortality risk.
The association between vitamin B12 levels and mortality has been controversial in epidemiological studies. A study conducted in the Netherlands indicated that higher levels of plasma vitamin B12 were associated with an increased risk of all-cause mortality, even after adjusting for age, sex, renal function, and other clinical and laboratory variables [33], but a NHANES study found that although there was a small but significant increase in CVD mortality in both low and high serum B12 groups, the overall association of the serum B12 concentration with mortality is U-shaped. These results do not support the suggestion that high serum B12 concentrations are inherently harmful or detrimental [21]. Studies on the association between vitamin B12 levels and mortality in older people have yielded conflicting results. The Newcastle 85+ study found that a higher concentration plasma vitamin B12 in women was linked to an increased risk of all-cause and CVD mortality [17], while an Australian study of people aged 55 years and older did not find any association between serum vitamin B12 and CHD and all-cause mortality [34]. Similarly, a community-dwelling Irish study showed that Vitamin B12 levels were not associated with death rates [35]. In populations with chronic diseases, studies have shown that both low and high serum levels of vitamin B12, as well as low serum levels of folate, are significantly associated with a higher risk of CVD mortality among individuals with T2D [36]. Additionally, vitamin B12 levels have been identified as an independent predicting factor for the three month mortality rate in AoCLF patients [37]. However, a cohort study of 1684 ICU patients found no significant associations between serum B12 levels and mortality after adjustment for liver function and liver disease [18]. While the evidence does not fully support considering vitamin B12 alterations as reliable risk markers for cardiovascular mortality in CKD and ESRD populations [38], there is little evidence available on the vitamin B12 status and long-term health outcomes.
Multiple epidemiological studies have found that MMA may be an independent risk factor for predicting mortality. A study conducted on the general population from the NHANES revealed that a high level of mitochondrial-derived MMA was strongly associated with an increased all-cause and cardiovascular mortality [23]. Another prospective study conducted on two cohorts demonstrated that elevated MMA levels were associated with a higher risk of acute myocardial infarction (AMI) and mortality in patients with a suspected or confirmed CHD [39]. MMA is a potential risk factor of CVD, diabetic complications, and dementias, and higher MMA levels have been associated with all-cause mortality, cancer mortality, and CVD mortality among individuals with diabetes [10,22]. Similar results were confirmed in our study, where there was a significant association found between the MMA levels and all-cause and cardiovascular mortality. It may suggest, therefore, that the levels of circulating MMA can predict long-term all-cause and cardiovascular mortality in patients with CKD.
MMA is currently best known for its use as a functional marker for vitamin B12 deficiency [7]. This deficiency can impair the activity of L-methylmalonyl-CoA mutase, resulting in the conversion of methylmalonyl-CoA to MMA instead of succinyl-CoA [6]. But vitamin B12 deficiency can only explain part of the increase in the MMA levels observed in adults [40,41]. Mitochondrial dysfunction [42,43] and impaired renal function [44,45,46] may account for proportions of the elevated MMA levels. Previous studies have suggested that MMA levels may be influenced by various factors, including catabolism, dietary components, and gut microbial production [10]. Mitochondrial dysfunction, characterized by impaired bioenergetics and a redox imbalance, is prevalent in patients with CKD [47] and many chronic diseases, such as CVD and diabetes [48]. MMA has been found to play a significant role in causing mitochondrial dysfunction and oxidative stress, both in vitro and in vivo, and does so by disrupting the mitochondrial respiratory chain and inducing reactive oxygen species generation [13,14]. This may then suggest that the underlying mechanism between the high levels of MMA and the risk of death may be related to mitochondrial dysfunction leading to disease progression, characterized by impaired bioenergetics and a redox imbalance in patients with CKD [23]. Patients with CKD may experience changes in the composition and metabolic activity of their gut microbiota due to their medications and diet. Elevated levels of MMA may indicate high levels of high PA bacterial abnormalities in the gut (MMA is an intermediate in the metabolism of PA), and the microbiota may impact the overall health by secreting metabolites associated with insulin resistance, obesity, endothelial dysfunction, and cardiovascular aging [49], all of which may also account for the increased risk of death in individuals through high levels of MMA by affecting catabolism. Furthermore, the association of MMA with all-cause and cardiovascular mortality risk remained significant even after adjusting for vitamin B12 and eGFR, indicating that this association is independent of the traditional risk factors.
There was no significant predictive effect observed between the serum vitamin B12 levels with CKD mortality. This could be because fewer outcome events occurred in our study, lacking the statistical power to detect associations between vitamin B12 levels and mortality. In addition, vitamin B12 exhibits a small effect on MMA levels, which may explain its non-significant association with mortality.
Thus, MMA is likely to be an underestimated biomarker that can be used to predict poor prognoses in individuals with CKD, and to clarify the role of MMA and vitamin B12 in long-term health, further mechanistic studies are needed.
As far as strengths of our study, we used a nationally large representative sample to assess the association of MMA and vitamin B12 levels with all-cause and CVD mortality among individuals with CKD, adjusting several potentially confounding factors, and assessing the robustness of our findings using several sensitivity analyses, which could facilitate the generalization of these findings. However, our study had several limitations that should be considered. First, MMA and vitamin B12 levels were determined based on a single measurement, which may not accurately reflect the long-term status of the participants. Second, both MMA and vitamin B12 were analyzed using different analytical methods at different stages in the NHANES. Although attempts have been made to statistically harmonize the values, the results cannot be as good as those obtained using the same laboratory methods at the same time. Third, we were not able to distinguish between the inborn and postnatal increases in MMA and vitamin B12. However, the prevalence of hereditary methylmalonic acidemia [50] and CblC deficiency [51] are pretty low, meaning they would not have as much of an impact on our conclusions. Fourth, due to the observational study design we utilized, our results are unable to completely eliminate the possibility of unascertained confounding factors. Thus, further studies are necessary to validate our findings. We cannot determine the causal relationship from this present study. Last, we categorized classified MMA and vitamin B12 levels according to the quartiles, meaning our results may not be consistent with those of other studies using different cut points.
5. Conclusions
Our study found that higher circulating levels of MMA were associated with an increased risk of all-cause and CVD mortality. However, we did not observe a significant association between serum vitamin B12 levels and mortality risk.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15132980/s1, Table S1. Baseline characteristics of study participants for CKD among U.S. adults by baseline vitamin B12 levels; Table S2. Association of vitamin B12 levels with all-cause mortality and CVD mortality among U.S. adults with CKD; Table S3. Subgroup analyses for the HRs of the MMA levels and CVD mortality among U.S. adults with CKD; Table S4. HRs (95% CIs) of all-cause mortality and CVD mortality according to the MMA levels after excluding CKD patients with cardiovascular disease and diabetes (n = 1335); Table S5. HRs (95% CIs) of all-cause mortality and CVD mortality according to the MMA levels after excluding CKD patients with less than three years of follow-up (n = 2349); Table S6. HRs (95% CIs) of all-cause mortality and CVD mortality according to the MMA levels among CKD patients with further adjustment for several biomarkers.
Author Contributions
Conceptualization, S.W. and W.C.; methodology, Z.X.; software, Z.X.; validation, B.Y., X.W. and W.C.; formal analysis, S.W.; investigation, B.Y.; data curation, Z.X. and W.C.; writing—original draft preparation, S.W.; writing—review and editing, C.Y.; visualization, X.W.; supervision, C.Y. and B.Y.; project administration, C.Y. and X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Our research utilizes the NHANES public database and does not require ethics committee approval.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found at https://www.cdc.gov/nchs/nhanes (accessed on 1 November 2021).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef] [PubMed]
- Li, P.K.; Garcia-Garcia, G.; Lui, S.F.; Andreoli, S.; Fung, W.W.; Hradsky, A.; Kumaraswami, L.; Liakopoulos, V.; Rakhimova, Z.; Saadi, G.; et al. Kidney Health for Everyone Everywhere-From Prevention to Detection and Equitable Access to Care. J. Ren. Care 2020, 46, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Kazancioglu, R. Risk factors for chronic kidney disease: An update. Kidney Int. Suppl. 2013, 3, 368–371. [Google Scholar] [CrossRef]
- Green, R.; Allen, L.H.; Bjorke-Monsen, A.L.; Brito, A.; Gueant, J.L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.H.; et al. Vitamin B(12) deficiency. Nat. Rev. Dis. Primers 2017, 3, 17040. [Google Scholar] [CrossRef]
- Allen, R.H.; Stabler, S.P.; Savage, D.G.; Lindenbaum, J. Metabolic abnormalities in cobalamin (vitamin B12) and folate deficiency. FASEB J. 1993, 7, 1344–1353. [Google Scholar] [CrossRef]
- Klee, G.G. Cobalamin and folate evaluation: Measurement of methylmalonic acid and homocysteine vs vitamin B(12) and folate. Clin. Chem. 2000, 46 Pt 2, 1277–1283. [Google Scholar] [CrossRef]
- Al-Lahham, S.H.; Peppelenbosch, M.P.; Roelofsen, H.; Vonk, R.J.; Venema, K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim. Biophys. Acta 2010, 1801, 1175–1183. [Google Scholar] [CrossRef]
- Hosseini, E.; Grootaert, C.; Verstraete, W.; Van de Wiele, T. Propionate as a health-promoting microbial metabolite in the human gut. Nutr. Rev. 2011, 69, 245–258. [Google Scholar] [CrossRef]
- Riphagen, I.J.; Minovic, I.; Groothof, D.; Post, A.; Eggersdorfer, M.L.; Kootstra-Ros, J.E.; de Borst, M.H.; Navis, G.; Muskiet, F.A.J.; Kema, I.P.; et al. Methylmalonic acid, vitamin B12, renal function, and risk of all-cause mortality in the general population: Results from the prospective Lifelines-MINUTHE study. BMC Med. 2020, 18, 380. [Google Scholar] [CrossRef]
- van Loon, S.L.; Wilbik, A.M.; Kaymak, U.; van den Heuvel, E.R.; Scharnhorst, V.; Boer, A.K. Improved testing for vitamin B(12) deficiency: Correcting MMA for eGFR reduces the number of patients classified as vitamin B(12) deficient. Ann. Clin. Biochem. 2018, 55, 685–692. [Google Scholar] [CrossRef]
- Herrmann, W.; Schorr, H.; Geisel, J.; Riegel, W. Homocysteine, cystathionine, methylmalonic acid and B-vitamins in patients with renal disease. Clin. Chem. Lab. Med. 2001, 39, 739–746. [Google Scholar] [CrossRef]
- Stepien, K.M.; Heaton, R.; Rankin, S.; Murphy, A.; Bentley, J.; Sexton, D.; Hargreaves, I.P. Evidence of Oxidative Stress and Secondary Mitochondrial Dysfunction in Metabolic and Non-Metabolic Disorders. J. Clin. Med. 2017, 6, 71. [Google Scholar] [CrossRef]
- Chandler, R.J.; Zerfas, P.M.; Shanske, S.; Sloan, J.; Hoffmann, V.; DiMauro, S.; Venditti, C.P. Mitochondrial dysfunction in mut methylmalonic acidemia. FASEB J. 2009, 23, 1252–1261. [Google Scholar] [CrossRef]
- Galvan, D.L.; Green, N.H.; Danesh, F.R. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int. 2017, 92, 1051–1057. [Google Scholar] [CrossRef]
- Huang, Y.C.; Lee, M.S.; Wahlqvist, M.L. Prediction of all-cause mortality by B group vitamin status in the elderly. Clin. Nutr. 2012, 31, 191–198. [Google Scholar] [CrossRef]
- Mendonca, N.; Jagger, C.; Granic, A.; Martin-Ruiz, C.; Mathers, J.C.; Seal, C.J.; Hill, T.R. Elevated Total Homocysteine in All Participants and Plasma Vitamin B12 Concentrations in Women Are Associated with All-Cause and Cardiovascular Mortality in the Very Old: The Newcastle 85+Study. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2018, 73, 1258–1264. [Google Scholar] [CrossRef]
- Callaghan, F.M.; Leishear, K.; Abhyankar, S.; Demner-Fushman, D.; McDonald, C.J. High vitamin B12 levels are not associated with increased mortality risk for ICU patients after adjusting for liver function: A cohort study. ESPEN J. 2014, 9, e76–e83. [Google Scholar] [CrossRef]
- Cappello, S.; Cereda, E.; Rondanelli, M.; Klersy, C.; Cameletti, B.; Albertini, R.; Magno, D.; Caraccia, M.; Turri, A.; Caccialanza, R. Elevated Plasma Vitamin B12 Concentrations Are Independent Predictors of In-Hospital Mortality in Adult Patients at Nutritional Risk. Nutrients 2017, 9, 1. [Google Scholar] [CrossRef]
- Soohoo, M.; Ahmadi, S.F.; Qader, H.; Streja, E.; Obi, Y.; Moradi, H.; Rhee, C.M.; Kim, T.H.; Kovesdy, C.P.; Kalantar-Zadeh, K. Association of serum vitamin B12 and folate with mortality in incident hemodialysis patients. Nephrol. Dial. Transplant. 2017, 32, 1024–1032. [Google Scholar] [CrossRef]
- Wolffenbuttel, B.H.R.; Heiner-Fokkema, M.R.; Green, R.; Gans, R.O.B. Relationship between serum B12 concentrations and mortality: Experience in NHANES. BMC Med. 2020, 18, 307. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, Y.; Liu, Y.; Cai, W.; Xu, J. Correlations between circulating methylmalonic acid levels and all-cause and cause-specific mortality among patients with diabetes. Front. Nutr. 2022, 9, 974938. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Y.; Liu, J.; Tian, W.; Zhang, X.; Cai, H.; Fang, S.; Yu, B. Mitochondria-derived methylmalonic acid, a surrogate biomarker of mitochondrial dysfunction and oxidative stress, predicts all-cause and cardiovascular mortality in the general population. Redox Biol. 2020, 37, 101741. [Google Scholar] [CrossRef] [PubMed]
- Statistics NCfH. NHANES Questionnaires, Datasets, and Related Documentation. 2020. Available online: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx (accessed on 13 December 2022).
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef]
- The Kidney Disease Improving Global Outcomes (KDIGO) Guideline. 2012. Available online: https://kdigo.org/guidelines/ckd-evaluation-and-management/ (accessed on 25 December 2022).
- Mineva, E.M.; Zhang, M.; Rabinowitz, D.J.; Phinney, K.W.; Pfeiffer, C.M. An LC-MS/MS method for serum methylmalonic acid suitable for monitoring vitamin B12 status in population surveys. Anal. Bioanal. Chem. 2015, 407, 2955–2964. [Google Scholar] [CrossRef]
- Yetley, E.A.; Pfeiffer, C.M.; Phinney, K.W.; Bailey, R.L.; Blackmore, S.; Bock, J.L.; Brody, L.C.; Carmel, R.; Curtin, L.R.; Durazo-Arvizu, R.A.; et al. Biomarkers of vitamin B-12 status in NHANES: A roundtable summary. Am. J. Clin. Nutr. 2011, 94, 313S–321S. [Google Scholar] [CrossRef]
- Odutayo, A.; Gill, P.; Shepherd, S.; Akingbade, A.; Hopewell, S.; Tennankore, K.; Hunn, B.H.; Emdin, C.A. Income Disparities in Absolute Cardiovascular Risk and Cardiovascular Risk Factors in the United States, 1999–2014. JAMA Cardiol. 2017, 2, 782–790. [Google Scholar] [CrossRef]
- Yang, X.; Xue, Q.; Wen, Y.; Huang, Y.; Wang, Y.; Mahai, G.; Yan, T.; Liu, Y.; Rong, T.; Wang, Y.; et al. Environmental polycyclic aromatic hydrocarbon exposure in relation to metabolic syndrome in US adults. Sci. Total Environ. 2022, 840, 156673. [Google Scholar] [CrossRef]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef]
- NHANES Survey Methods and Analytic Guidelines. 2022. Available online: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx (accessed on 29 December 2022).
- Flores-Guerrero, J.L.; Minovic, I.; Groothof, D.; Gruppen, E.G.; Riphagen, I.J.; Kootstra-Ros, J.; Muller Kobold, A.; Hak, E.; Navis, G.; Gansevoort, R.T.; et al. Association of Plasma Concentration of Vitamin B12 with All-Cause Mortality in the General Population in the Netherlands. JAMA Netw. Open 2020, 3, e1919274. [Google Scholar] [CrossRef]
- Gopinath, B.; Flood, V.M.; Rochtchina, E.; Thiagalingam, A.; Mitchell, P. Serum homocysteine and folate but not vitamin B12 are predictors of CHD mortality in older adults. Eur. J. Prev. Cardiol. 2012, 19, 1420–1429. [Google Scholar] [CrossRef]
- Robinson, D.J.; O’Luanaigh, C.; Tehee, E.; O’Connell, H.; Hamilton, F.; Chin, A.V.; Coen, R.; Molloy, A.M.; Scott, J.; Lawlor, B.A.; et al. Vitamin B12 status, homocysteine and mortality amongst community-dwelling Irish elders. Ir. J. Med. Sci. 2011, 180, 451–455. [Google Scholar] [CrossRef]
- Liu, Y.; Geng, T.; Wan, Z.; Lu, Q.; Zhang, X.; Qiu, Z.; Li, L.; Zhu, K.; Liu, L.; Pan, A.; et al. Associations of Serum Folate and Vitamin B12 Levels with Cardiovascular Disease Mortality Among Patients with Type 2 Diabetes. JAMA Netw. Open 2022, 5, e2146124. [Google Scholar] [CrossRef]
- Dou, J.; Xu, W.; Ye, B.; Zhang, Y.; Mao, W. Serum vitamin B12 levels as indicators of disease severity and mortality of patients with acute-on-chronic liver failure. Clin. Chim. Acta 2012, 413, 1809–1812. [Google Scholar] [CrossRef]
- Capelli, I.; Cianciolo, G.; Gasperoni, L.; Zappulo, F.; Tondolo, F.; Cappuccilli, M.; La Manna, G. Folic Acid and Vitamin B12 Administration in CKD, Why Not? Nutrients 2019, 11, 383. [Google Scholar] [CrossRef]
- Dhar, I.; Lysne, V.; Ulvik, A.; Svingen, G.F.T.; Pedersen, E.R.; Bjornestad, E.O.; Olsen, T.; Borsholm, R.; Laupsa-Borge, J.; Ueland, P.M.; et al. Plasma methylmalonic acid predicts risk of acute myocardial infarction and mortality in patients with coronary heart disease: A prospective 2-cohort study. J. Intern. Med. 2023, 293, 508–519. [Google Scholar] [CrossRef]
- Mineva, E.M.; Sternberg, M.R.; Zhang, M.; Aoki, Y.; Storandt, R.; Bailey, R.L.; Pfeiffer, C.M. Age-specific reference ranges are needed to interpret serum methylmalonic acid concentrations in the US population. Am. J. Clin. Nutr. 2019, 110, 158–168. [Google Scholar] [CrossRef]
- Vogiatzoglou, A.; Oulhaj, A.; Smith, A.D.; Nurk, E.; Drevon, C.A.; Ueland, P.M.; Vollset, S.E.; Tell, G.S.; Refsum, H. Determinants of plasma methylmalonic acid in a large population: Implications for assessment of vitamin B12 status. Clin. Chem. 2009, 55, 2198–2206. [Google Scholar] [CrossRef]
- Piquereau, J.; Moulin, M.; Zurlo, G.; Mateo, P.; Gressette, M.; Paul, J.L.; Lemaire, C.; Ventura-Clapier, R.; Veksler, V.; Garnier, A. Cobalamin and folate protect mitochondrial and contractile functions in a murine model of cardiac pressure overload. J. Mol. Cell Cardiol. 2017, 102, 34–44. [Google Scholar] [CrossRef]
- van de Lagemaat, E.E.; de Groot, L.; van den Heuvel, E. Vitamin B(12) in Relation to Oxidative Stress: A Systematic Review. Nutrients 2019, 11, 482. [Google Scholar] [CrossRef]
- Clarke, R.; Refsum, H.; Birks, J.; Evans, J.G.; Johnston, C.; Sherliker, P.; Ueland, P.M.; Schneede, J.; McPartlin, J.; Nexo, E.; et al. Screening for vitamin B-12 and folate deficiency in older persons. Am. J. Clin. Nutr. 2003, 77, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Valente, E.; Scott, J.M.; Ueland, P.M.; Cunningham, C.; Casey, M.; Molloy, A.M. Diagnostic accuracy of holotranscobalamin, methylmalonic acid, serum cobalamin, and other indicators of tissue vitamin B₁₂ status in the elderly. Clin. Chem. 2011, 57, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, A. Elevated serum methylmalonic acid. How much comes from cobalamin deficiency and how much comes from the kidneys? Scand. J. Clin. Lab. Investig. 2002, 62, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Fontecha-Barriuso, M.; Lopez-Diaz, A.M.; Guerrero-Mauvecin, J.; Miguel, V.; Ramos, A.M.; Sanchez-Niño, M.D.; Ruiz-Ortega, M.; Ortiz, A.; Sanz, A.B. Tubular Mitochondrial Dysfunction, Oxidative Stress, and Progression of Chronic Kidney Disease. Antioxidants 2022, 11, 1356. [Google Scholar] [CrossRef]
- Mc Guire, P.J.; Parikh, A.; Diaz, G.A. Profiling of oxidative stress in patients with inborn errors of metabolism. Mol. Genet. Metab. 2009, 98, 173–180. [Google Scholar] [CrossRef]
- Meijers, B.; Evenepoel, P.; Anders, H.J. Intestinal microbiome and fitness in kidney disease. Nat. Rev. Nephrol. 2019, 15, 531–545. [Google Scholar] [CrossRef]
- Melo, D.R.; Kowaltowski, A.J.; Wajner, M.; Castilho, R.F. Mitochondrial energy metabolism in neurodegeneration associated with methylmalonic acidemia. J. Bioenerg. Biomembr. 2011, 43, 39–46. [Google Scholar] [CrossRef]
- Weisfeld-Adams, J.D.; Morrissey, M.A.; Kirmse, B.M.; Salveson, B.R.; Wasserstein, M.P.; McGuire, P.J.; Sunny, S.; Cohen-Pfeffer, J.L.; Yu, C.; Caggana, M.; et al. Newborn screening and early biochemical follow-up in combined methylmalonic aciduria and homocystinuria, cblC type, and utility of methionine as a secondary screening analyte. Mol. Genet. Metab. 2010, 99, 116–123. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).