Capybara Oil Improves Renal Pathophysiology and Inflammation in Obese Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining Capybara Oil
2.2. Experimental Groups and Diets
2.3. Treatment with Capybara Oil and Experimental Groups
2.4. Body Mass and Systolic Blood Pressure
2.5. Assessment of Renal Function
2.6. Euthanasia
2.7. Biochemical Measurement
2.8. Morphological Analysis
2.9. Immunohistochemistry
2.10. Oxidative Stress Assay
2.11. Electron Microscopic Study
2.12. Statistical Analysis
3. Results
3.1. Physiological Analysis
3.2. Plasma Biochemical Analysis
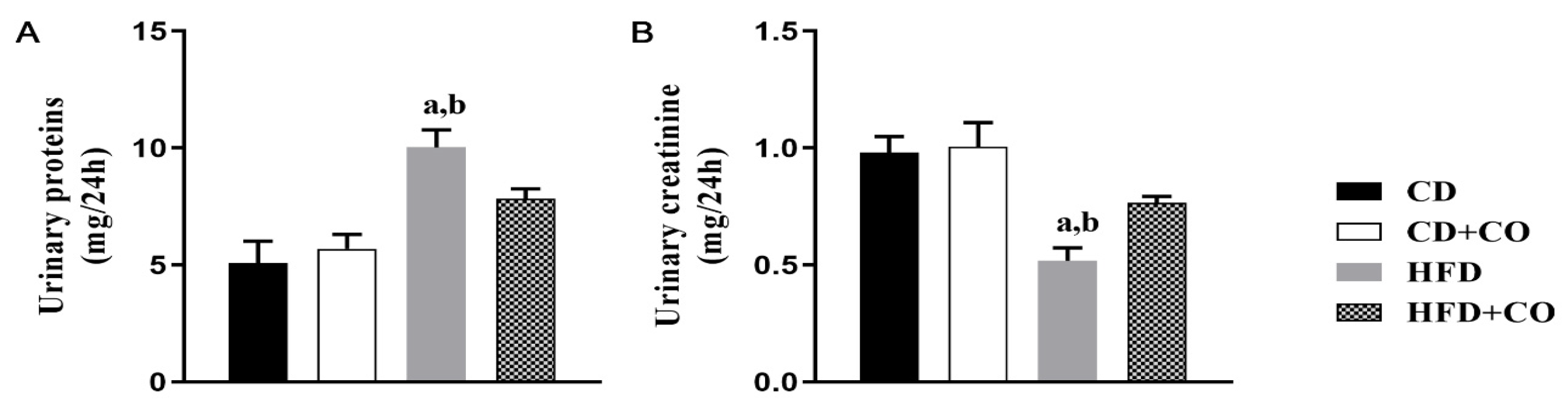
3.3. Assessment of Renal Function
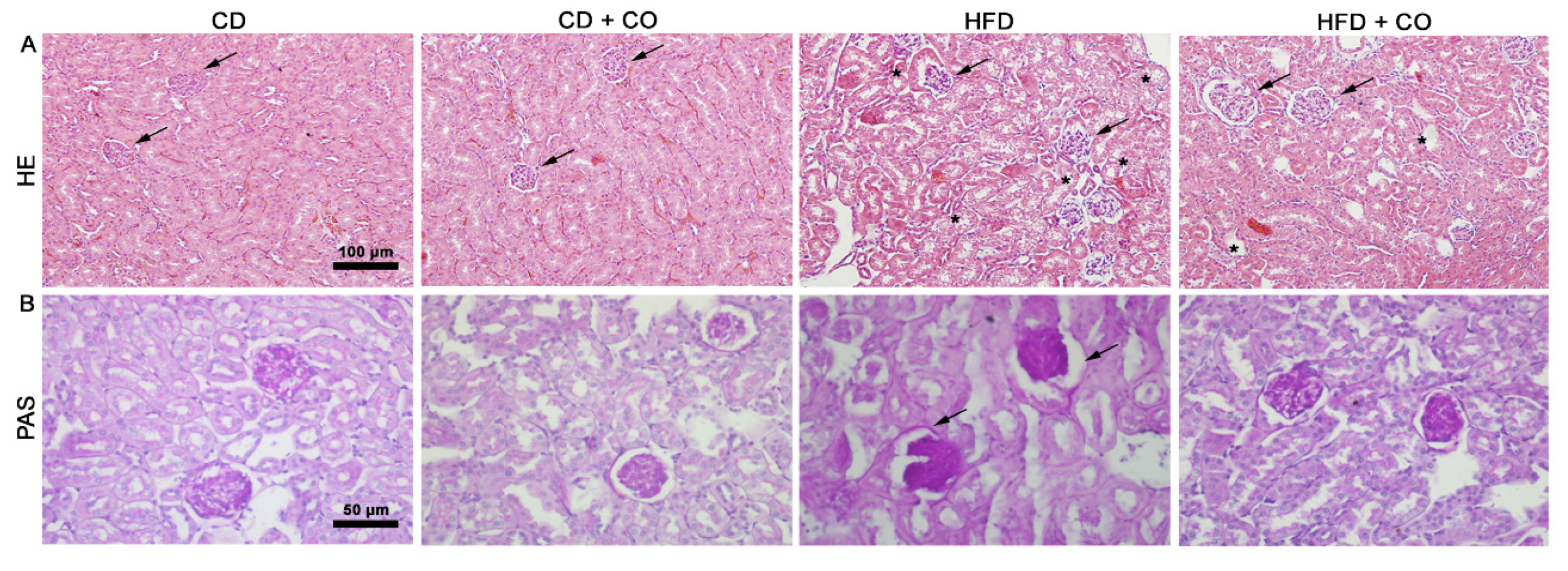
3.4. Histopathological Evaluation
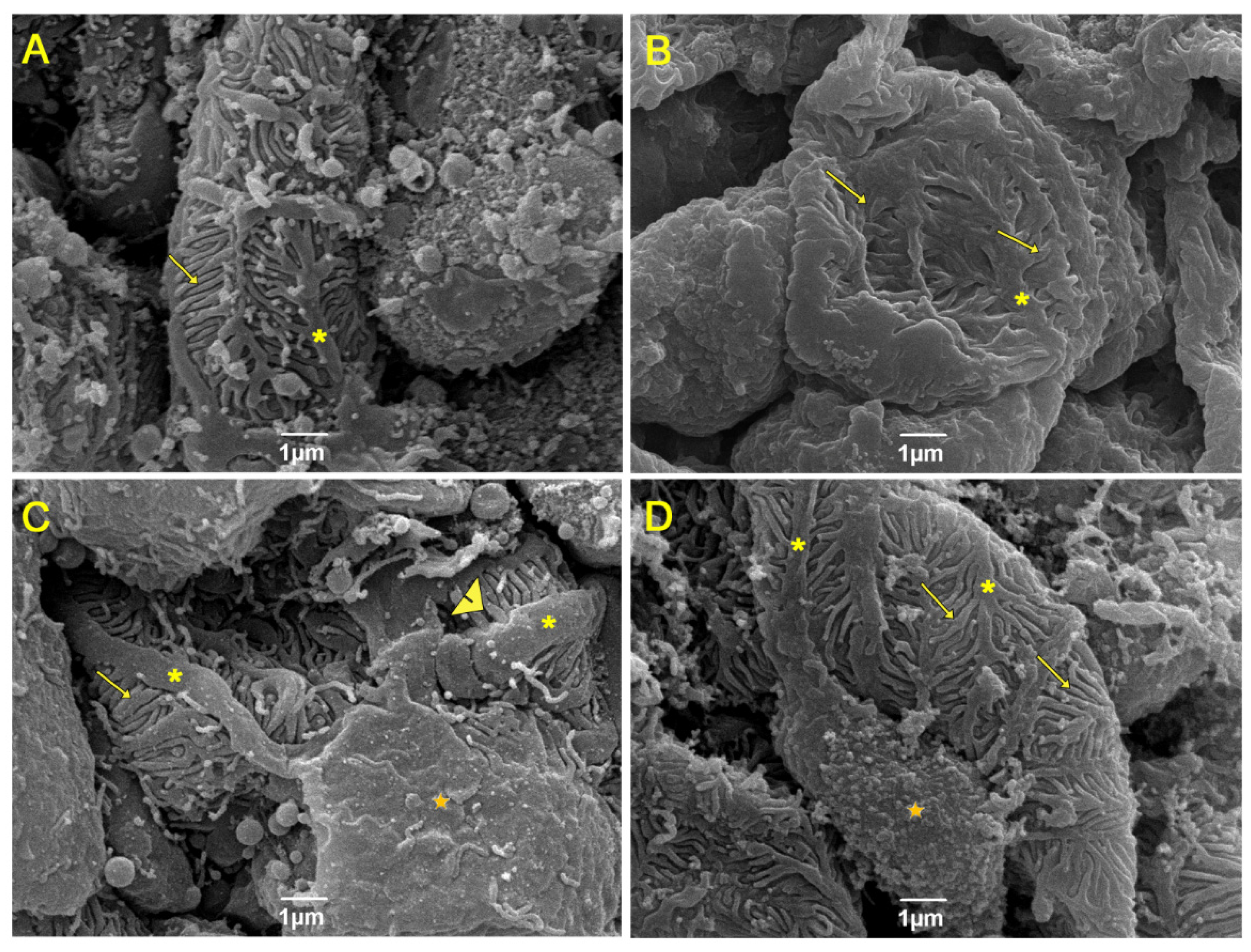
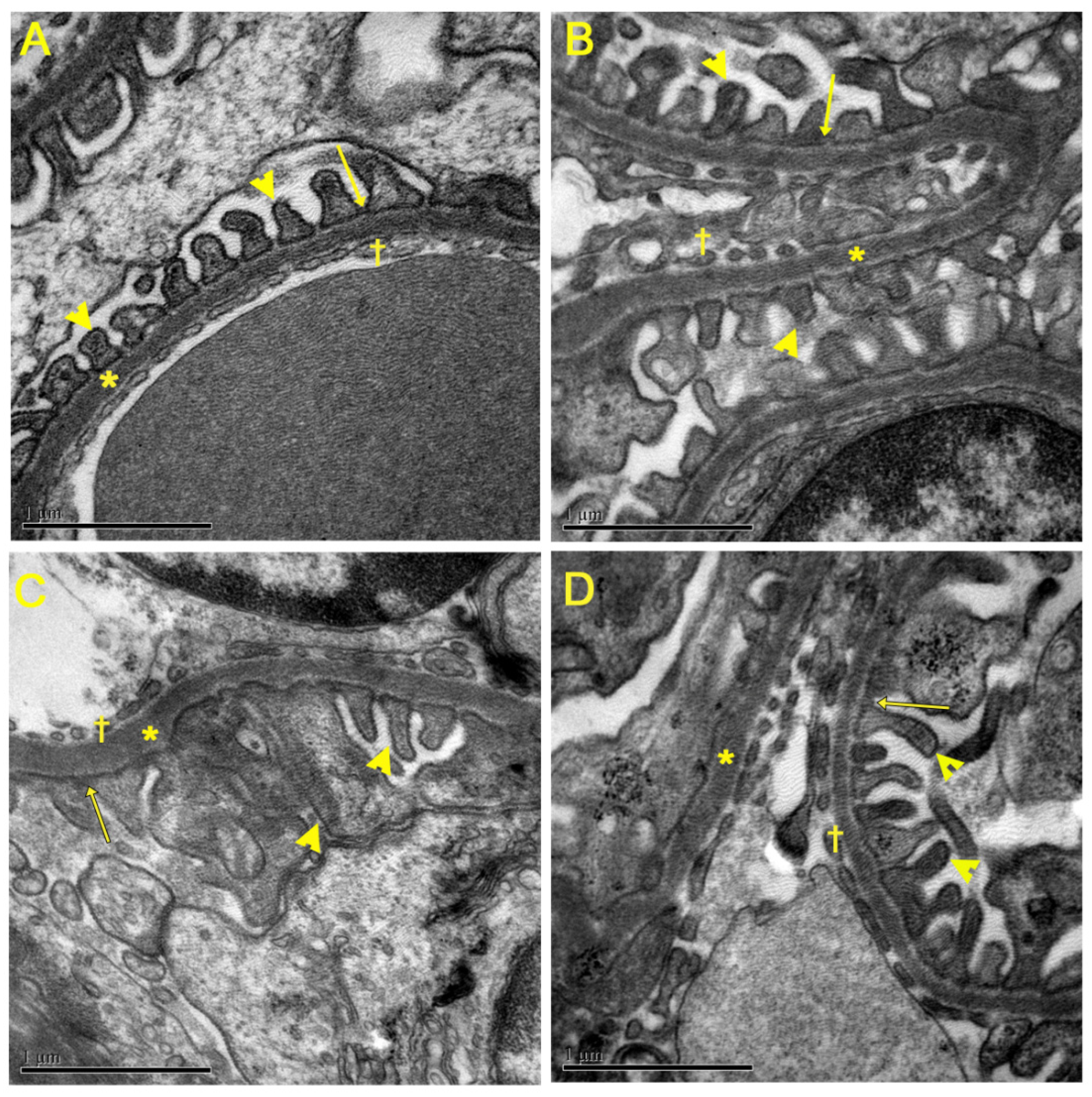
3.5. Evaluation of Renal Ultrastructure
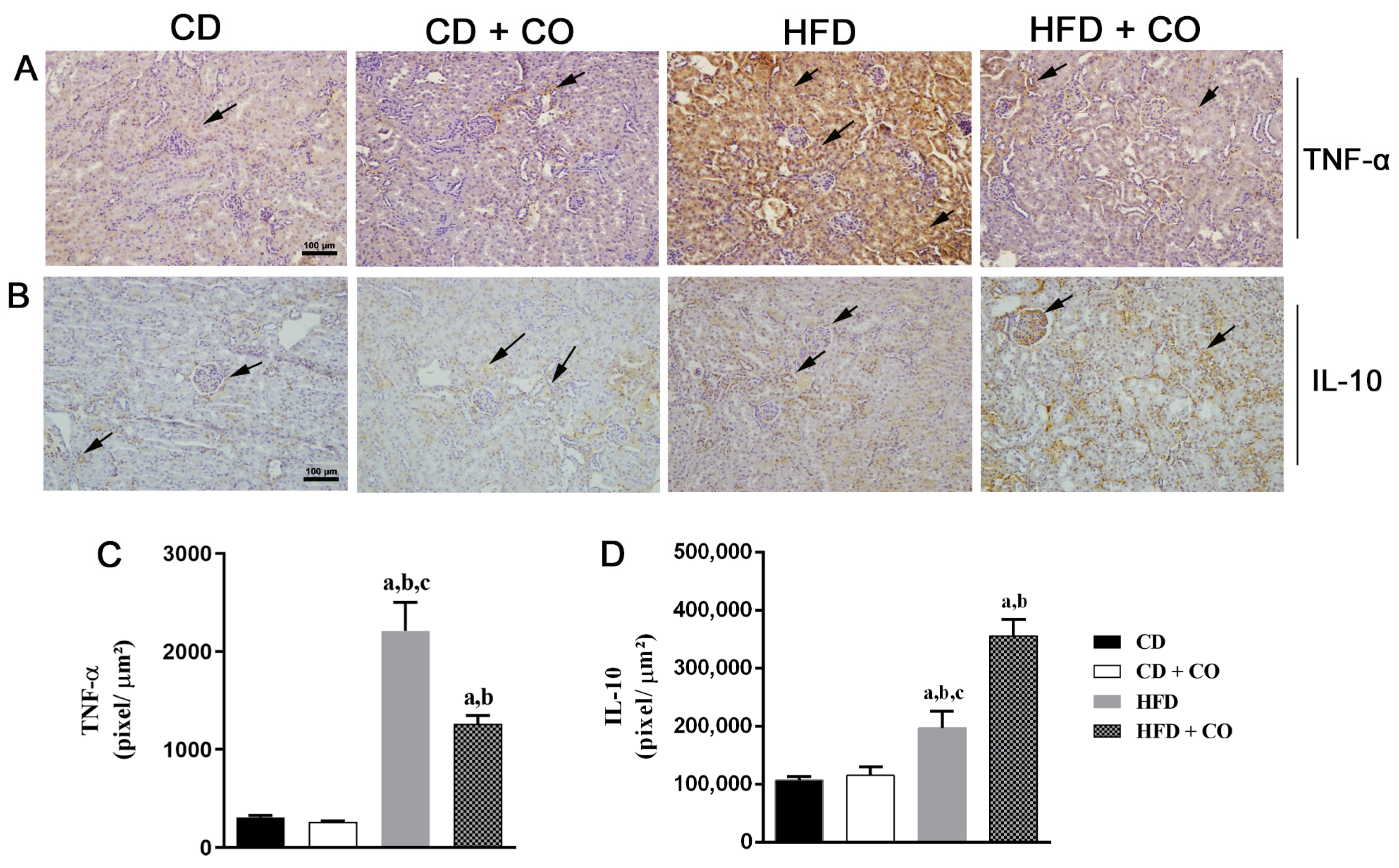
3.6. Renal Inflammatory Response Evaluation by Immunohistochemistry
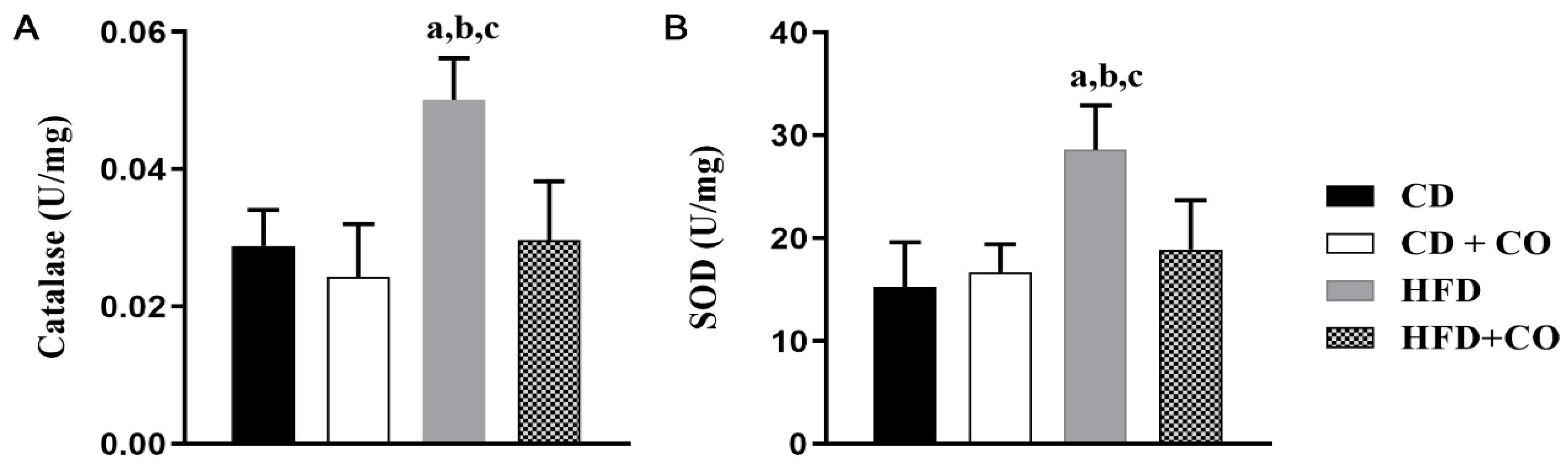
3.7. Analysis of the Activity of Enzymes Related to Oxidative Stress
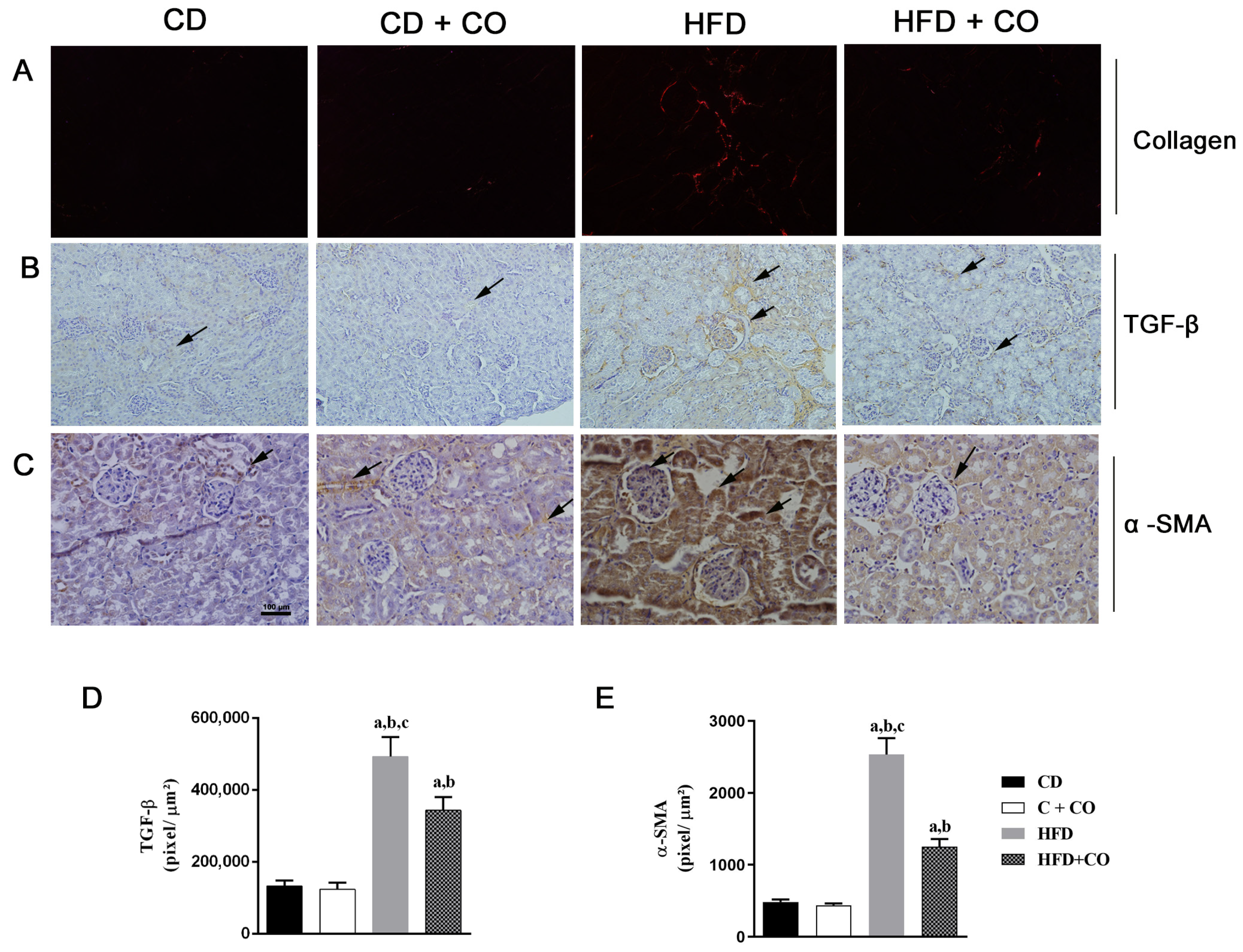
3.8. Renal Fibrosis Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Prospective Studies Collaboration; Whitlock, G.; Lewington, S.; Sherliker, P.; Clarke, R.; Emberson, J.; Halsey, J.; Qizilbash, N.; Collins, R.; Peto, R. Body-mass index and cause-specific mortality in 900,000 adults: Collaborative analyses of 57 prospective studies. Lancet 2009, 373, 1083–1096. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight; World Health Organization: Geneva, Switzerland, 2016. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 28 July 2017).
- World Health Organization. Obesity and Overweight; World Health Organization: Geneva, Switzerland, 2021. Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 8 June 2022).
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- Williams, E.P.; Mesidor, M.; Winters, K.; Dubbert, P.M.; Wyatt, S.B. Overweight and Obesity: Prevalence, Consequences, and Causes of a Growing Public Health Problem. Curr. Obes. Rep. 2015, 4, 363–370. [Google Scholar] [CrossRef]
- Szeto, H.H.; Liu, S.; Soong, Y.; Alam, N.; Prusky, G.T.; Seshan, S.V. Protection of mitochondria prevents high-fat diet-induced glomerulopathy and proximal tubular injury. Kidney Int. 2016, 90, 997–1011. [Google Scholar] [CrossRef]
- Eo, H.; Park, J.E.; Jeon, Y.J.; Lim, Y. Ameliorative Effect of Ecklonia cava Polyphenol Extract on Renal Inflammation Associated with Aberrant Energy Metabolism and Oxidative Stress in High Fat Diet-Induced Obese Mice. J. Agric. Food Chem. 2017, 65, 3811–3818. [Google Scholar] [CrossRef]
- Kume, S.; Uzu, T.; Araki, S.; Sugimoto, T.; Isshiki, K.; Chin-Kanasaki, M.; Sakaguchi, M.; Kubota, N.; Terauchi, Y.; Kadowaki, T.; et al. Role of altered renal lipid metabolism in the development of renal injury induced by a high-fat diet. J. Am. Soc. Nephrol. 2007, 18, 2715–2723. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Furth, S.L.; Zoccali, C.; World Kidney Day Steering Committee. Obesity and kidney disease: Hidden consequences of the epidemic. J. Nephrol. 2017, 30, 1–10. [Google Scholar] [CrossRef]
- Bobulescu, I.A. Renal lipid metabolism and lipotoxicity. Curr. Opin. Nephrol. Hypertens. 2010, 19, 393–402. [Google Scholar] [CrossRef]
- Rüster, C.; Wolf, G. The role of the renin-angiotensin-aldosterone system in obesity-related renal diseases. Semin. Nephrol. 2013, 33, 44–53. [Google Scholar] [CrossRef]
- Ruan, X.Z.; Varghese, Z.; Moorhead, J.F. An update on the lipid nephrotoxicity hypothesis. Nat. Rev. Nephrol. 2009, 5, 713–721. [Google Scholar] [CrossRef]
- Deji, N.; Kume, S.; Araki, S.; Soumura, M.; Sugimoto, T.; Isshiki, K.; Chin-Kanasaki, M.; Sakaguchi, M.; Koya, D.; Haneda, M.; et al. Structural and functional changes in the kidneys of high-fat diet-induced obese mice. Am. J. Physiol. Renal Physiol. 2009, 296, F118–F126. [Google Scholar] [CrossRef] [PubMed]
- Molfino, A.; Amabile, M.I.; Monti, M.; Muscaritoli, M. Omega-3 Polyunsaturated Fatty Acids in Critical Illness: Anti-Inflammatory, Proresolving, or Both? Oxidative Med. Cell. Longev. 2017, 2017, 5987082. [Google Scholar] [CrossRef] [PubMed]
- Borsonelo, E.C.; Galduróz, J.C. The role of polyunsaturated fatty acids (PUFAs) in development, aging and substance abuse disorders: Review and propositions. Prostaglandins Leukot. Essent. Fatty Acids 2008, 78, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Börgeson, E.; Johnson, A.M.; Lee, Y.S.; Till, A.; Syed, G.H.; Ali-Shah, S.T.; Guiry, P.J.; Dalli, J.; Colas, R.A.; Serhan, C.N.; et al. Lipoxin A4 Attenuates Obesity-Induced Adipose Inflammation and Associated Liver and Kidney Disease. Cell Metab. 2015, 22, 125–137. [Google Scholar] [CrossRef]
- Panahi, Y.; Dashti-Khavidaki, S.; Farnood, F.; Noshad, H.; Lotfi, M.; Gharekhani, A. Therapeutic Effects of Omega-3 Fatty Acids on Chronic Kidney Disease-Associated Pruritus: A Literature Review. Adv. Pharm. Bull. 2016, 6, 509–514. [Google Scholar] [CrossRef]
- Fukushima, M.; Takayama, Y.; Habaguchi, T.; Nakano, M. Comparative hypocholesterolemic effects of capybara (Hydrochoerus hydrochaeris dabbenei) oil, horse oil, and sardine oil in cholesterol-fed rats. Lipids 1997, 32, 391–395. [Google Scholar] [CrossRef]
- Pinheiro, M.S.; Silva, J.J.C.; Rodrigues, R.C. Utilização Sustentável e Domesticação da Capivara; Embrapa Clima Temperado: Pelotas, Brazil, 2001; Volume 31, p. 86. [Google Scholar]
- Marinho, P.C.; Neto-Ferreira, R.; José de Carvalho, J. Evaluation of therapeutic intervention with a natural product in cutaneous wound healing: The use of capybara oil. Evid.-Based Complement. Altern. Med. 2013, 2013, 217198. [Google Scholar] [CrossRef]
- Marinho, P.C.; Vieira, A.B.; Pereira, P.G.; Rabelo, K.; Ciambarella, B.T.; Nascimento, A.; Cortez, E.; Moura, A.S.; Guimarães, F.V.; Martins, M.A.; et al. Capybara Oil Improves Hepatic Mitochondrial Dysfunction, Steatosis, and Inflammation in a Murine Model of Nonalcoholic Fatty Liver Disease. Evid.-Based Complement. Altern. Med. 2018, 2018, 4956079. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Kennedy-Feitosa, E.; Okuro, R.T.; Pinho Ribeiro, V.; Lanzetti, M.; Barroso, M.V.; Zin, W.A.; Porto, L.C.; Brito-Gitirana, L.; Valenca, S.S. Eucalyptol attenuates cigarette smoke-induced acute lung inflammation and oxidative stress in the mouse. Pulm. Pharmacol. Ther. 2016, 41, 11–18. [Google Scholar] [CrossRef]
- Bellanti, F.; Villani, R.; Facciorusso, A.; Vendemiale, G.; Serviddio, G. Lipid oxidation products in the pathogenesis of non-alcoholic steatohepatitis. Free Radic. Biol. Med. 2017, 111, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.V.; Fernandes-Santos, C.; Faria, T.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Diets rich in saturated fat and/or salt differentially modulate atrial natriuretic peptide and renin expression in C57BL/6 mice. Eur. J. Nutr. 2012, 51, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Kambham, N.; Markowitz, G.S.; Valeri, A.M.; Lin, J.; D’Agati, V.D. Obesity-related glomerulopathy: An emerging epidemic. Kidney Int. 2001, 59, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Re, R.N. Obesity-related hypertension. Ochsner J. 2009, 9, 133–136. [Google Scholar] [PubMed]
- DeMarco, V.G.; Aroor, A.R.; Sowers, J.R. The pathophysiology of hypertension in patients with obesity. Nat. Rev. Endocrinol. 2014, 10, 364–376. [Google Scholar] [CrossRef]
- Katsoulieris, E.; Mabley, J.G.; Samai, M.; Sharpe, M.A.; Green, I.C.; Chatterjee, P.K. Lipotoxicity in renal proximal tubular cells: Relationship between endoplasmic reticulum stress and oxidative stress pathways. Free Radic. Biol. Med. 2010, 48, 1654–1662. [Google Scholar] [CrossRef]
- Böttinger, E.P. TGF-beta in renal injury and disease. Semin. Nephrol. 2007, 27, 309–320. [Google Scholar] [CrossRef]
- Nussberger, J.; Gradman, A.H.; Schmieder, R.E.; Lins, R.L.; Chiang, Y.; Prescott, M.F. Plasma renin and the antihypertensive effect of the orally active renin inhibitor aliskiren in clinical hypertension. Int. J. Clin. Pract. 2007, 61, 1461–1468. [Google Scholar] [CrossRef]
- Kelly, D.J.; Zhang, Y.; Moe, G.; Naik, G.; Gilbert, R.E. Aliskiren, a novel renin inhibitor, is renoprotective in a model of advanced diabetic nephropathy in rats. Diabetologia 2007, 50, 2398–2404. [Google Scholar] [CrossRef]
- Feldman, D.L.; Jin, L.; Xuan, H.; Contrepas, A.; Zhou, Y.; Webb, R.L.; Mueller, D.N.; Feldt, S.; Cumin, F.; Maniara, W.; et al. Effects of aliskiren on blood pressure, albuminuria, and (pro)renin receptor expression in diabetic TG(mRen-2)27 rats. Hypertension 2008, 52, 130–136. [Google Scholar] [CrossRef]
- Meier, M.; Menne, J.; Park, J.K.; Holtz, M.; Gueler, F.; Kirsch, T.; Schiffer, M.; Mengel, M.; Lindschau, C.; Leitges, M.; et al. Deletion of protein kinase C-epsilon signaling pathway induces glomerulosclerosis and tubulointerstitial fibrosis in vivo. J. Am. Soc. Nephrol. 2007, 18, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.H. Renal fibrosis. Korean J. Pediatr. 2010, 53, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Nagamoto, T.; Eguchi, G.; Beebe, D.C. Alpha-smooth muscle actin expression in cultured lens epithelial cells. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1122–1129. [Google Scholar]
- Barnes, J.L.; Glass Ii, W.F. Renal interstitial fibrosis: A critical evaluation of the origin of myofibroblasts. Contrib. Nephrol. 2011, 169, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J. Am. Soc. Nephrol. 2010, 21, 212–222. [Google Scholar] [CrossRef]
- Kriz, W.; Kaissling, B.; Le Hir, M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: Fact or fantasy? J. Clin. Investig. 2011, 121, 468–474. [Google Scholar] [CrossRef]
- Fujikawa, M.; Yamazaki, K.; Hamazaki, T.; Wakaki, K.; Koizumi, F.; Yano, S.; Kobayashi, M. Effect of eicosapentaenoic acid ethyl ester on albuminuria in streptozotocin-induced diabetic rats. J. Nutr. Sci. Vitaminol. 1994, 40, 49–61. [Google Scholar] [CrossRef]
- Sabry, A.; El-Husseini, A.; Sheashaa, H.; Abdel-Shafy, E.; El-Dahshan, K.; Abdel-Rahim, M.; Abdel-Kaleek, E.; Abo-Zena, H. Colchicine vs. omega-3 fatty acids for prevention of chronic cyclosporine nephrotoxicity in Sprague Dawley rats: An experimental animal model. Arch. Med. Res. 2006, 37, 933–940. [Google Scholar] [CrossRef]
- Barbosa, K.B.; Costa, N.M.; Alfenas, R.C.; Sergio, S.O.; Minim, V.P.; Bressan, J. Estresse oxidativo: Conceito, implicações e fatores modulatórios. Rev. Nutr. 2010, 23, 629–643. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Yu, B.P. Cellular defenses against damage from reactive oxygen species. Physiol. Rev. 1994, 74, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, M.H.; Nam, D.H.; Song, H.K.; Kang, Y.S.; Lee, J.E.; Kim, H.W.; Cha, J.J.; Hyun, Y.Y.; Han, S.Y.; et al. Celastrol, an NF-κB inhibitor, improves insulin resistance and attenuates renal injury in db/db mice. PLoS ONE 2013, 8, e62068. [Google Scholar] [CrossRef]
- Maric-Bilkan, C. Obesity and diabetic kidney disease. Med. Clin. N. Am. 2013, 97, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Kobori, H.; Ohashi, N.; Urushihara, M.; Nishiyama, A.; Mori, T.; Ishizuka, T.; Nako, K.; Ito, S. Angiotensin II Type 1 Receptor Blockers Reduce Urinary Angiotensinogen Excretion and the Levels of Urinary Markers of Oxidative Stress and Inflammation in Patients with Type 2 Diabetic Nephropathy. Biomark. Insights 2009, 4, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Choi, M.E. Urinary biomarkers for early diabetic nephropathy: Beyond albuminuria. Pediatr. Nephrol. 2015, 30, 1063–1075. [Google Scholar] [CrossRef]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Ruan, X.Z.; Navarrete, C.; Turner, D.; Reynard, M.; Sweny, P.; Hamilton, G.; Wheeler, D.C.; Powis, S.H.; Moorhead, J.F.; et al. Renovascular disease is associated with low producer genotypes of the anti-inflammatory cytokine interleukin-10. Tissue Antigens 2004, 63, 470–475. [Google Scholar] [CrossRef]
- Abensur, H. E-Book-Biomarcadores na Nefrologia; Associação Brasileira de Nefrologia: São Paulo, Brazil, 2011; Available online: https://arquivos.sbn.org.br/pdf/biomarcadores.pdf (accessed on 5 July 2022).
- Gorriz, J.L.; Martinez-Castelao, A. Proteinuria: Detection and role in native renal disease progression. Transplant. Rev. 2012, 26, 3–13. [Google Scholar] [CrossRef]
- Rund, K.M.; Peng, S.; Greite, R.; Claaßen, C.; Nolte, F.; Oger, C.; Galano, J.M.; Balas, L.; Durand, T.; Chen, R.; et al. Dietary omega-3 PUFA improved tubular function after ischemia induced acute kidney injury in mice but did not attenuate impairment of renal function. Prostaglandins Other Lipid Mediat. 2020, 146, 106386. [Google Scholar] [CrossRef]
- Jefferson, J.A.; Shankland, S.J.; Pichler, R.H. Proteinuria in diabetic kidney disease: A mechanistic viewpoint. Kidney Int. 2008, 74, 22–36. [Google Scholar] [CrossRef]
- Garg, P. A Review of Podocyte Biology. Am. J. Nephrol. 2008, 47 (Suppl. 1), 3–13. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, J.M. Lipotoxicity. Kidney Int. 2006, 70, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
- Go, R.E.; Hwang, K.A.; Park, G.T.; Lee, H.M.; Lee, G.A.; Kim, C.W.; Jeon, S.Y.; Seo, J.W.; Hong, W.K.; Choi, K.C. Effects of microalgal polyunsaturated fatty acid oil on body weight and lipid accumulation in the liver of C57BL/6 mice fed a high fat diet. J. Biomed. Res. 2016, 30, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Lanza, I.R.; Blachnio-Zabielska, A.; Johnson, M.L.; Schimke, J.M.; Jakaitis, D.R.; Lebrasseur, N.K.; Jensen, M.D.; Sreekumaran Nair, K.; Zabielski, P. Influence of fish oil on skeletal muscle mitochondrial energetics and lipid metabolites during high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1391–E1403. [Google Scholar] [CrossRef]
- Nascimento, F.A.; Barbosa-da-Silva, S.; Fernandes-Santos, C.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Adipose tissue, liver and pancreas structural alterations in C57BL/6 mice fed high-fat-high-sucrose diet supplemented with fish oil (n-3 fatty acid rich oil). Exp. Toxicol. Pathol. 2010, 62, 17–25. [Google Scholar] [CrossRef]
- Tishinsky, J.M.; Gulli, R.A.; Mullen, K.L.; Dyck, D.J.; Robinson, L.E. Fish oil prevents high-saturated fat diet-induced impairments in adiponectin and insulin response in rodent soleus muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R598–R605. [Google Scholar] [CrossRef]
- Braddock, R.; Simán, C.M.; Hamilton, K.; Devlin, H.; Garland, H.; Sibley, C.P. Gamma-Linoleic acid and ascorbic acid ameliorate the effects of experimental diabetes on electrolyte and bone homeostasis in pregnant rats. J. Endocrinol. 2002, 173, 273–284. [Google Scholar] [CrossRef]
- Aguila, M.B.; Pinheiro, A.R.; Aquino, J.C.; Gomes, A.P.; Mandarim-de-Lacerda, C.A. Different edible oil beneficial effects (canola oil, fish oil, palm oil, olive oil, and soybean oil) on spontaneously hypertensive rat glomerular enlargement and glomeruli number. Prostaglandins Other Lipid Mediat. 2005, 76, 74–85. [Google Scholar] [CrossRef]
- Diaz Encarnacion, M.M.; Warner, G.M.; Gray, C.E.; Cheng, J.; Keryakos, H.K.; Nath, K.A.; Grande, J.P. Signaling pathways modulated by fish oil in salt-sensitive hypertension. Am. J. Physiol. Renal Physiol. 2008, 294, F1323–F1335. [Google Scholar] [CrossRef]
- Kasiske, B.L.; O’Donnell, M.P.; Lee, H.; Kim, Y.; Keane, W.F. Impact of dietary fatty acid supplementation on renal injury in obese Zucker rats. Kidney Int. 1991, 39, 1125–1134. [Google Scholar] [CrossRef]

| Ingredients (g/Kg) | Control Diet | High-Fat Diet |
|---|---|---|
| Casein | 140 | 190 |
| Corn starch | 620.69 | 250.68 |
| Sucrose | 100 | 100 |
| Lard | 0 | 320 |
| Soy oil | 40 | 40 |
| Fiber | 50 | 50 |
| Vitamin mix | 10 | 10 |
| Mineral mix | 35 | 35 |
| Cysteine | 1.8 | 1.8 |
| Choline | 2.5 | 2.5 |
| Antioxidants | 0.008 | 0.016 |
| Total mass | 1000 | 1000 |
| Carbohydrates (% energy) | 76 | 26 |
| Protein (% energy) | 14 | 14 |
| Lipids (% energy) | 10 | 60 |
| Total energy (kcal/kg) | 3573 | 5404 |
| Parameters (mg/dL) | Groups | |||
|---|---|---|---|---|
| CD | CD + CO | HFD | HFD + CO | |
| Total cholesterol | 117 ± 19.5 | 133 ± 8.6 | 187.8 ± 15.5 a,b,c | 150.5 ± 23.9 |
| HDL | 75 ± 5.2 | 94.2 ± 4.3 | 76.8 ± 5.7 c | 98 ± 15.9 a |
| LDL | 36 ± 9.8 | 34.6 ± 7.9 | 80.9 ± 9 a,b,c | 63.1 ± 10.6 |
| VLDL | 13.6 ± 3 | 14 ± 2.2 | 23.3 ± 3.8 a,b,c | 15.8 ± 3.2 |
| Triglycerides | 62 ± 9.7 | 64 ± 10.5 | 107 ± 16.2 a,b | 81.5 ± 16.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, P.G.; Alves, L.L.; Ciambarella, B.T.; Rabelo, K.; Nascimento, A.L.R.; Moraes, A.C.N.; Bernardi, A.; Guimarães, F.V.; Carvalho, G.M.; da Silva, J.F.R.; et al. Capybara Oil Improves Renal Pathophysiology and Inflammation in Obese Mice. Nutrients 2023, 15, 2925. https://doi.org/10.3390/nu15132925
Pereira PG, Alves LL, Ciambarella BT, Rabelo K, Nascimento ALR, Moraes ACN, Bernardi A, Guimarães FV, Carvalho GM, da Silva JFR, et al. Capybara Oil Improves Renal Pathophysiology and Inflammation in Obese Mice. Nutrients. 2023; 15(13):2925. https://doi.org/10.3390/nu15132925
Chicago/Turabian StylePereira, Priscila G., Luciana L. Alves, Bianca T. Ciambarella, Kíssila Rabelo, Ana Lúcia R. Nascimento, Alan Cesar N. Moraes, Andressa Bernardi, Fernanda V. Guimarães, Gabriela M. Carvalho, Jemima F. R. da Silva, and et al. 2023. "Capybara Oil Improves Renal Pathophysiology and Inflammation in Obese Mice" Nutrients 15, no. 13: 2925. https://doi.org/10.3390/nu15132925
APA StylePereira, P. G., Alves, L. L., Ciambarella, B. T., Rabelo, K., Nascimento, A. L. R., Moraes, A. C. N., Bernardi, A., Guimarães, F. V., Carvalho, G. M., da Silva, J. F. R., & de Carvalho, J. J. (2023). Capybara Oil Improves Renal Pathophysiology and Inflammation in Obese Mice. Nutrients, 15(13), 2925. https://doi.org/10.3390/nu15132925








