Developmental Changes in Hypothalamic and Serum Oxytocin Levels in Prenatally Normally Nourished and Undernourished Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experiment
2.3. Hormone Assay
2.4. Quantitative Real-Time Polymerase Chain Reaction (PCR)
2.5. Statistical Analysis
3. Results
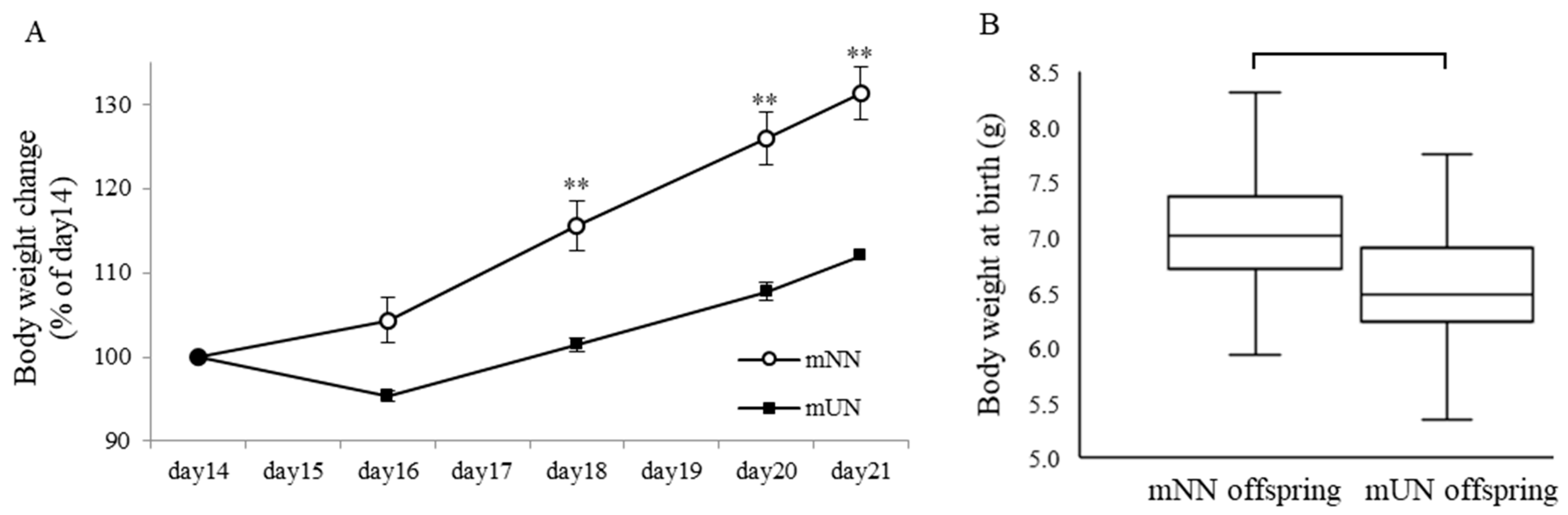
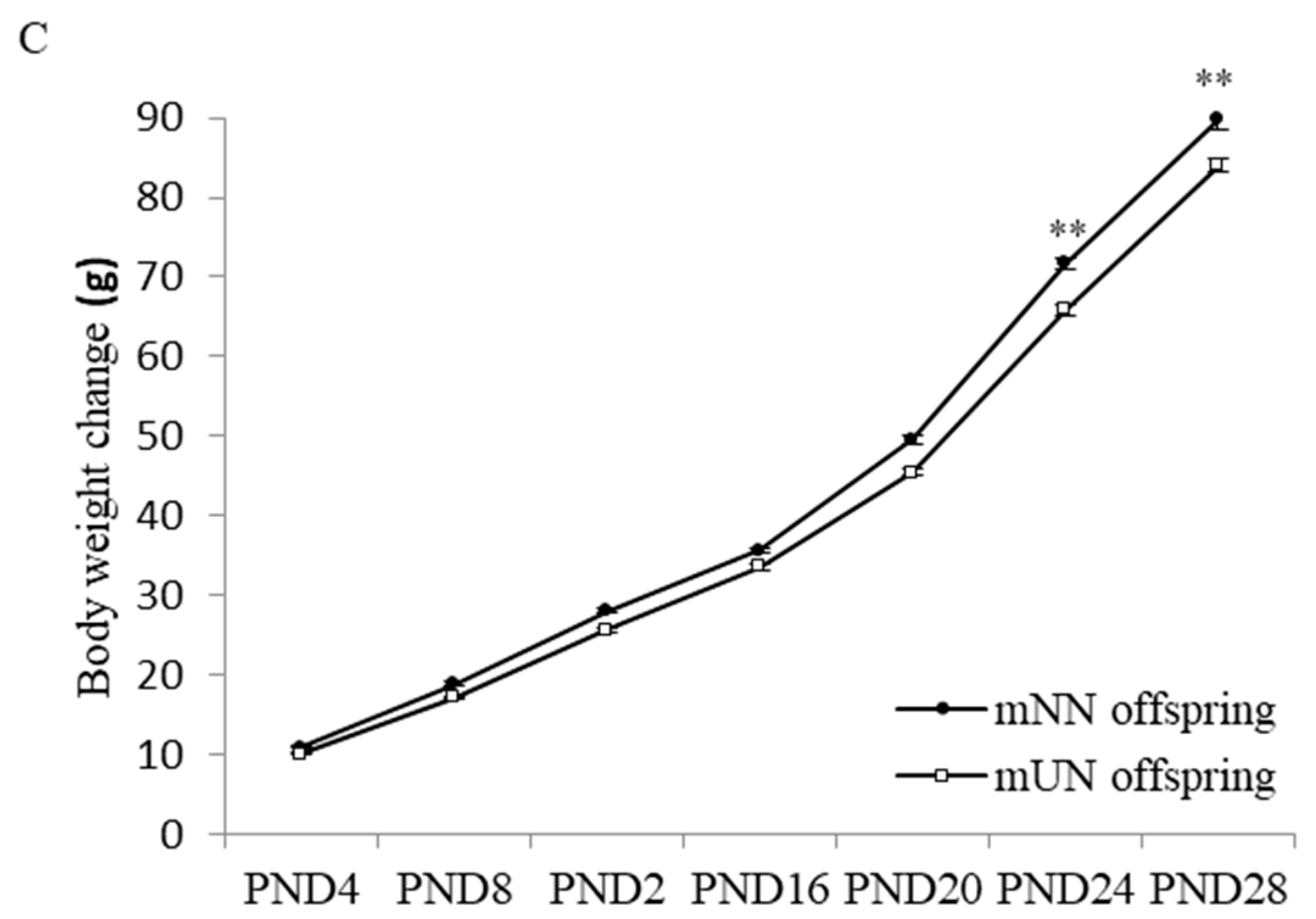
3.1. Effects of Prenatal Undernutrition on Maternal and Offspring Body Weight
3.2. Changes in Serum OT Concentrations of Offspring
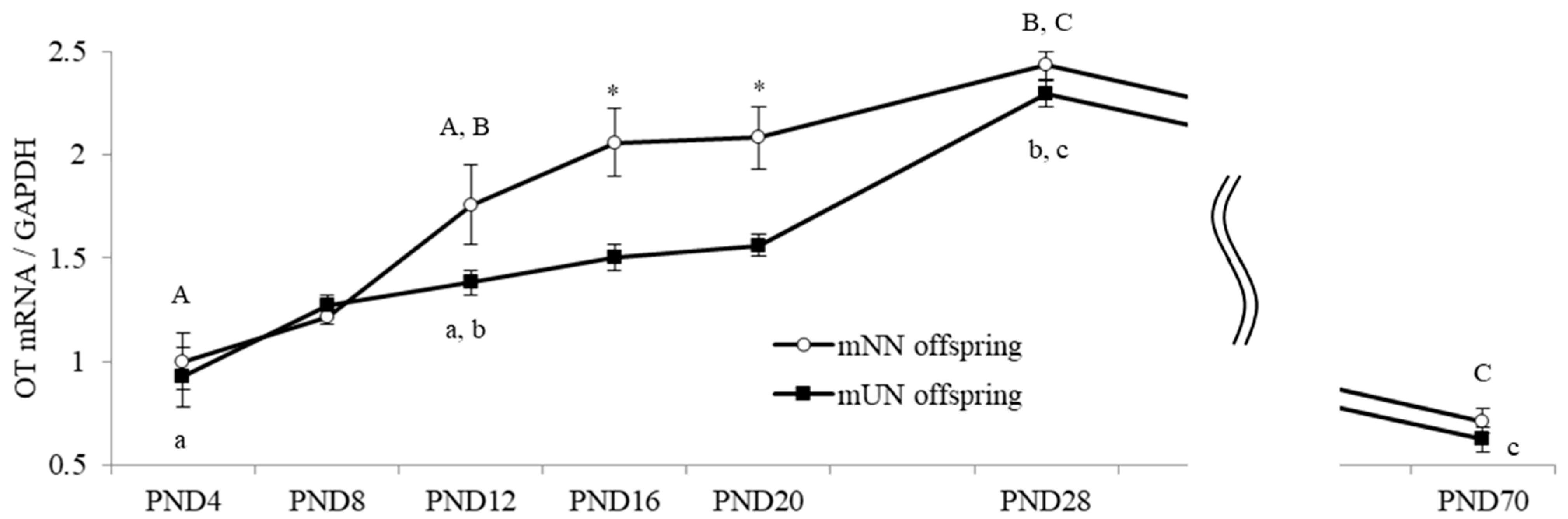
3.3. Changes in Hypothalamic OT and OTR mRNA Levels of Offspring
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morisaki, N.; Kawachi, I.; Oken, E.; Fujiwara, T. Social and anthropometric factors explaining racial/ethnical differences in birth weight in the United States. Sci. Rep. 2017, 7, 46657. [Google Scholar] [CrossRef] [PubMed]
- Bianco-Miotto, T.; Craig, J.M.; Gasser, Y.P.; Van Dijk, S.J.; Ozanne, S.E. Epigenetics and DOHaD: From basics to birth and beyond. J. Dev. Orig. Health Dis. 2017, 8, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Yura, S.; Itoh, H.; Sagawa, N.; Yamamoto, H.; Masuzaki, H.; Nakao, K.; Kawamura, M.; Takemura, M.; Kakui, K.; Ogawa, Y.; et al. Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab. 2005, 1, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging Roles for Serotonin in Regulating Metabolism: New Implications for an Ancient Molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef]
- Castañeda, T.R.; Tong, J.; Datta, R.; Culler, M.D.; Tschöp, M.H. Ghrelin in the regulation of body weight and metabolism. Front. Neuroendocrinol. 2010, 31, 44–60. [Google Scholar] [CrossRef]
- Blevins, J.E.; Schwartz, M.W.; Baskin, D.G. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am. J. Physiol. Integr. Comp. Physiol. 2004, 287, R87–R96. [Google Scholar] [CrossRef]
- Perello, M.; Raingo, J. Leptin Activates Oxytocin Neurons of the Hypothalamic Paraventricular Nucleus in Both Control and Diet-Induced Obese Rodents. PLoS ONE 2013, 8, e59625. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Woods, S.C.; Porte, D., Jr.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef]
- Cornejo, M.P.; Hentges, S.T.; Maliqueo, M.; Coirini, H.; Becu-Villalobos, D.; Elias, C.F. Neuroendocrine Regulation of Metabolism. J. Neuroendocr. 2016, 28. [Google Scholar] [CrossRef]
- Ludwig, M.; Leng, G. Dendritic peptide release and peptide-dependent behaviours. Nat. Rev. Neurosci. 2006, 7, 126–136. [Google Scholar] [CrossRef]
- Leithead, A.B.; Tasker, J.G.; Harony-Nicolas, H. The interplay between glutamatergic circuits and oxytocin neurons in the hypothalamus and its relevance to neurodevelopmental disorders. J. Neuroendocr. 2021, 33, e13061. [Google Scholar] [CrossRef] [PubMed]
- Lawson, E.A.; Marengi, D.A.; DeSanti, R.L.; Holmes, T.M.; Schoenfeld, D.A.; Tolley, C.J. Oxytocin reduces caloric intake in men. Obesity 2015, 23, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, C.; Chen, Q.; Chen, X.; Xu, Z.; Wu, J.; Cai, D. Treatment of Obesity and Diabetes Using Oxytocin or Analogs in Patients and Mouse Models. PLoS ONE 2013, 8, e61477. [Google Scholar] [CrossRef]
- Maejima, Y.; Rita, R.S.; Santoso, P.; Aoyama, M.; Hiraoka, Y.; Nishimori, K.; Gantulga, D.; Shimomura, K.; Yada, T. Nasal Oxytocin Administration Reduces Food Intake without Affecting Locomotor Activity and Glycemia with c-Fos Induction in Limited Brain Areas. Neuroendocrinology 2015, 101, 35–44. [Google Scholar] [CrossRef]
- Takayanagi, Y.; Kasahara, Y.; Onaka, T.; Takahashi, N.; Kawada, T.; Nishimori, K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport 2008, 19, 951–955. [Google Scholar] [CrossRef]
- Arletti, R.; Benelli, A.; Bertolini, A. Influence of oxytocin on feeding behavior in the rat. Peptides 1989, 10, 89–93. [Google Scholar] [CrossRef] [PubMed]
- McCormack, S.E.; Blevins, J.E.; Lawson, E.A. Metabolic Effects of Oxytocin. Endocr. Rev. 1989, 41, 121–145. [Google Scholar] [CrossRef]
- Quintana, D.S.; Rokicki, J.; van der Meer, D.; Alnæs, D.; Kaufmann, T.; Córdova-Palomera, A.; Dieset, I.; Andreassen, O.A.; Westlye, L.T. Oxytocin pathway gene networks in the human brain. Nat. Commun. 2019, 10, 668. [Google Scholar] [CrossRef]
- Elabd, C.; Cousin, W.; Upadhyayula, P.; Chen, R.Y.; Chooljian, M.S.; Li, J.; Kung, S.; Jiang, K.P.; Conboy, I.M. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat. Commun. 2014, 5, 4082. [Google Scholar] [CrossRef]
- Kosfeld, M.; Heinrichs, M.; Zak, P.J.; Fischbacher, U.; Fehr, E. Oxytocin increases trust in humans. Nature 2005, 435, 673–676. [Google Scholar] [CrossRef]
- Elabd, S.K.; Sabry, I.; Hassan, W.B.; Nour, H.; Zaky, K. Possible neuroendocrine role for oxytocin in bone remodeling. Endocr. Regul. 2007, 41, 131. [Google Scholar] [PubMed]
- Tungalagsuvd, A.; Matsuzaki, T.; Iwasa, T.; Munkhzaya, M.; Yiliyasi, M.; Kawami, T.; Kato, T.; Kuwahara, A.; Irahara, M. The expression of orexigenic and anorexigenic factors in middle-aged female rats that had been subjected to prenatal undernutrition. Int. J. Dev. Neurosci. 2016, 49, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, T.; Matsuzaki, T.; Murakami, M.; Fujisawa, S.; Kinouchi, R.; Gereltsetseg, G.; Kuwahara, A.; Yasui, T.; Irahara, M. Effects of intrauterine undernutrition on hypothalamic Kiss1 expression and the timing of puberty in female rats. J. Physiol. 2010, 588, 821–829. [Google Scholar] [CrossRef]
- Altirriba, J.; Poher, A.L.; Rohner-Jeanrenaud, F. Chronic Oxytocin Administration as a Treatment Against Impaired Leptin Signaling or Leptin Resistance in Obesity. Front. Endocrinol. 2015, 6, 119. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.S.; Kenkel, W.M.; MacLean, E.L.; Wilson, S.R.; Perkeybile, A.M.; Yee, J.R.; Ferris, C.F.; Nazarloo, H.P.; Porges, S.W.; Davis, J.M.; et al. Is Oxytocin “Nature’s Medicine”? Pharmacol. Rev. 2020, 72, 829–861. [Google Scholar] [CrossRef]
- McKay, E.C.; Counts, S.E. Oxytocin Receptor Signaling in Vascular Function and Stroke. Front. Neurosci. 2020, 14, 574499. [Google Scholar] [CrossRef]
- Jurek, B.; Neumann, I.D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef]
- Arletti, R.; Benelli, A.; Bertolini, A. Oxytocin inhibits food and fluid intake in rats. Physiol. Behav. 2019, 48, 825–830. [Google Scholar] [CrossRef]
- Zhang, G.; Cai, D.; Santoso, P.; Nakata, M.; Ueta, Y.; Yada, T.; Jurek, B.; Neumann, I.D.; Roberts, Z.S.; Wolden-Hanson, T.; et al. Circadian intervention of obesity development via resting-stage feeding manipulation or oxytocin treatment. Am. J. Physiol. Metab. 2011, 301, E1004–E1012. [Google Scholar] [CrossRef]
- Deblon, N.; Veyrat-Durebex, C.; Bourgoin, L.; Caillon, A.; Bussier, A.-L.; Petrosino, S.; Piscitelli, F.; Legros, J.-J.; Geenen, V.; Foti, M.; et al. Mechanisms of the Anti-Obesity Effects of Oxytocin in Diet-Induced Obese Rats. PLoS ONE 2011, 6, e25565. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, T.; Matsuzaki, T.; Mayila, Y.; Yanagihara, R.; Yamamoto, Y.; Kawakita, T.; Kuwahara, A.; Irahara, M. Oxytocin treatment reduced food intake and body fat and ameliorated obesity in ovariectomized female rats. Neuropeptides 2019, 75, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Noguchi, H.; Takeda, A.; Arakaki, R.; Uchishiba, M.; Imaizumi, J.; Minato, S.; Kamada, S.; Kagawa, T.; Yoshida, A.; et al. Changes in Endogenous Oxytocin Levels and the Effects of Exogenous Oxytocin Administration on Body Weight Changes and Food Intake in Polycystic Ovary Syndrome Model Rats. Int. J. Mol. Sci. 2022, 23, 8207. [Google Scholar] [CrossRef] [PubMed]
- Bouret, S.G.; Draper, S.J.; Simerly, R.B. Trophic Action of Leptin on Hypothalamic Neurons That Regulate Feeding. Science 2004, 304, 108–110. [Google Scholar] [CrossRef]
- Kuiri-Hänninen, T.; Sankilampi, U.; Dunkel, L. Activation of the Hypothalamic-Pituitary-Gonadal Axis in Infancy: Minipuberty. Horm. Res. Paediatr. 2014, 82, 73–80. [Google Scholar] [CrossRef]
- Delahaye, F.; Breton, C.; Risold, P.-Y.; Enache, M.; Dutriez-Casteloot, I.; Laborie, C.; Lesage, J.; Vieau, D. Maternal Perinatal Undernutrition Drastically Reduces Postnatal Leptin Surge and Affects the Development of Arcuate Nucleus Proopiomelanocortin Neurons in Neonatal Male Rat Pups. Endocrinology 2008, 149, 470–475. [Google Scholar] [CrossRef]
- Kerem, L.; Lawson, E.A. The Effects of Oxytocin on Appetite Regulation, Food Intake and Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 7737. [Google Scholar] [CrossRef]
- Phaneuf, S.; Asbóth, G.; Carrasco, M.P.; Europe-Finner, G.N.; Saji, F.; Kimura, T.; Harris, A.; Bernal, A.L. The desensitization of oxytocin receptors in human myometrial cells is accompanied by down-regulation of oxytocin receptor messenger RNA. J. Endocrinol. 1997, 154, 7–18. [Google Scholar] [CrossRef]


| Primer | Sequence | Annealing T (°C) |
|---|---|---|
| OT forward | GAACACAACGCCATGGCTGCCC | 62 |
| OT reverse | TCGGTGCGGCAGCCATCCGGGCTA | |
| OTR forward | CGATTGCTGGGCGGTCTT | 67 |
| OTR reverse | CCGCCGCTGCCGTCTTGA | |
| GAPDH forward | ATGGCACAGTCAAGGCTGAGA | 70 |
| GAPDH reverse | CGCTCGTGGAAGATGGTGAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imaizumi, J.; Kamada, S.; Taniguchi, M.; Sugimoto, T.; Maeda, T.; Arakaki, R.; Yamamoto, S.; Shirakawa, A.; Mineda, A.; Yoshida, A.; et al. Developmental Changes in Hypothalamic and Serum Oxytocin Levels in Prenatally Normally Nourished and Undernourished Rats. Nutrients 2023, 15, 2768. https://doi.org/10.3390/nu15122768
Imaizumi J, Kamada S, Taniguchi M, Sugimoto T, Maeda T, Arakaki R, Yamamoto S, Shirakawa A, Mineda A, Yoshida A, et al. Developmental Changes in Hypothalamic and Serum Oxytocin Levels in Prenatally Normally Nourished and Undernourished Rats. Nutrients. 2023; 15(12):2768. https://doi.org/10.3390/nu15122768
Chicago/Turabian StyleImaizumi, Junki, Shuhei Kamada, Miyu Taniguchi, Tatsuro Sugimoto, Takaaki Maeda, Ryosuke Arakaki, Shota Yamamoto, Aya Shirakawa, Ayuka Mineda, Atsuko Yoshida, and et al. 2023. "Developmental Changes in Hypothalamic and Serum Oxytocin Levels in Prenatally Normally Nourished and Undernourished Rats" Nutrients 15, no. 12: 2768. https://doi.org/10.3390/nu15122768
APA StyleImaizumi, J., Kamada, S., Taniguchi, M., Sugimoto, T., Maeda, T., Arakaki, R., Yamamoto, S., Shirakawa, A., Mineda, A., Yoshida, A., Iwasa, T., & Kaji, T. (2023). Developmental Changes in Hypothalamic and Serum Oxytocin Levels in Prenatally Normally Nourished and Undernourished Rats. Nutrients, 15(12), 2768. https://doi.org/10.3390/nu15122768






