Taxifolin Suppresses Inflammatory Responses of High-Glucose-Stimulated Mouse Microglia by Attenuating the TXNIP–NLRP3 Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Cytotoxicity Assays
2.3. ROS Measurement
2.4. Quantitative Reverse Transcription–Polymerase Chain Reaction (RT-PCR)
2.5. Western Blot Analyses
2.6. Cytokine Measurements
2.7. Statistical Analysis
3. Results
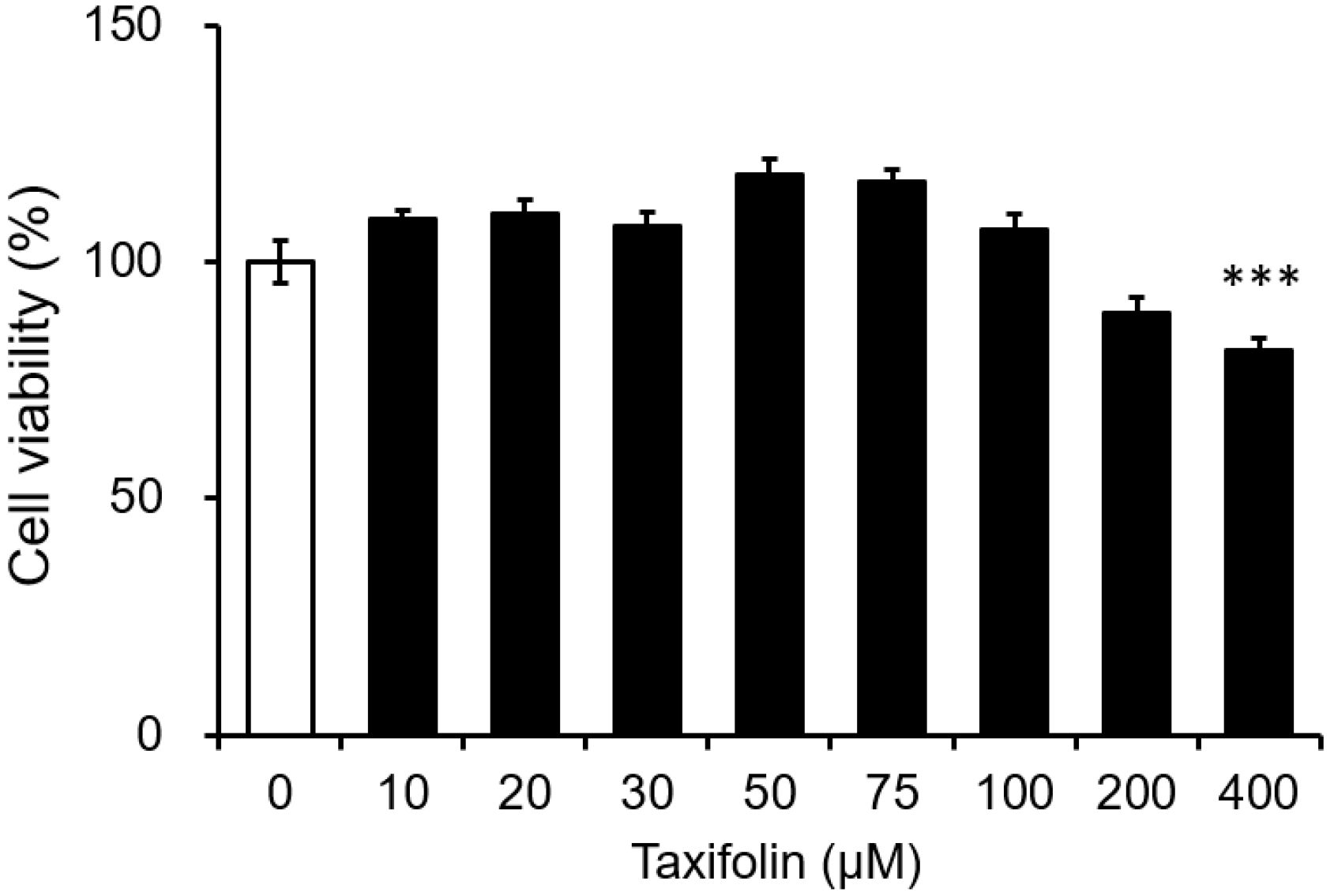
3.1. Cytotoxicity of Taxifolin on the Mouse Microglial Cell Line
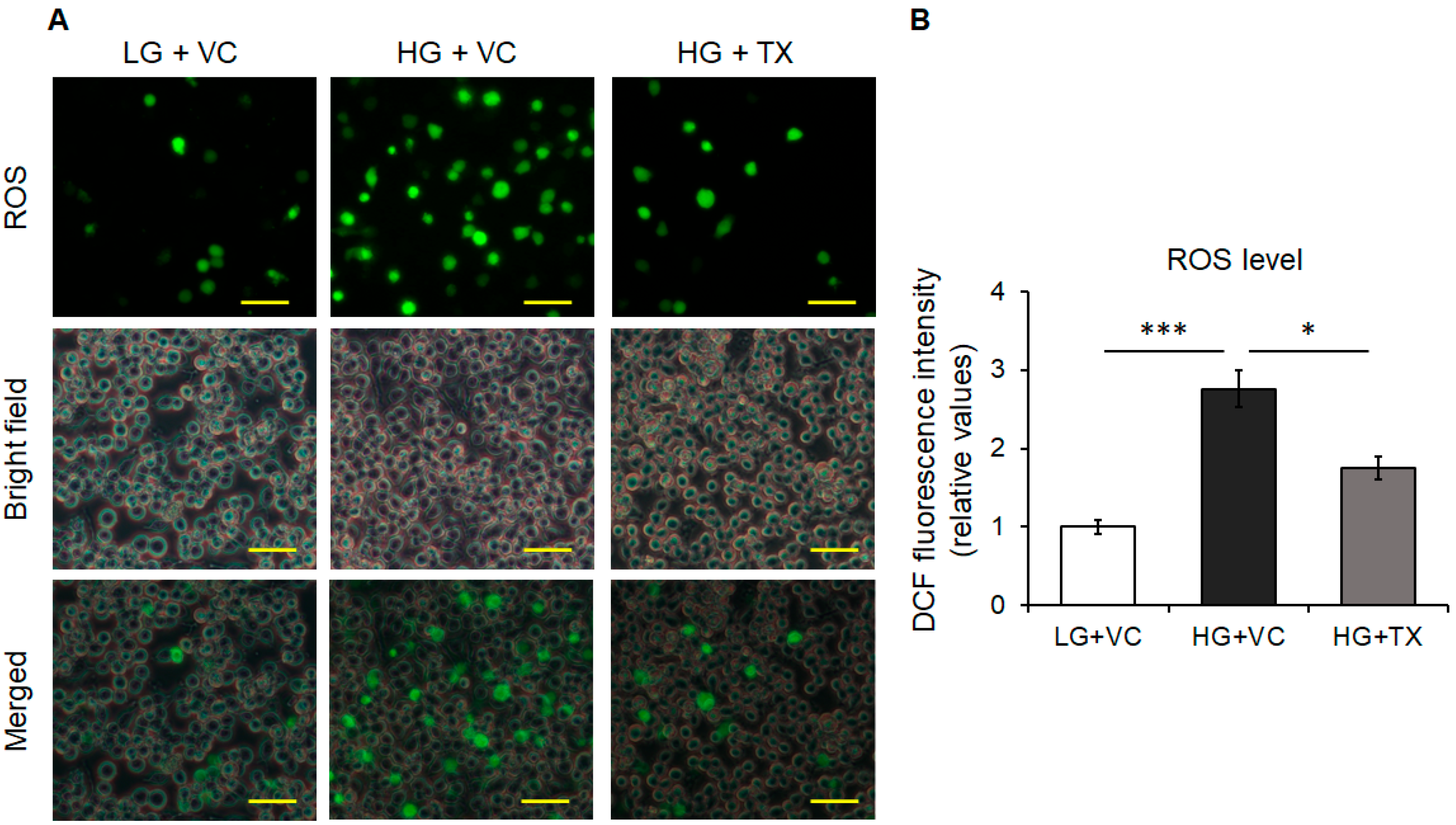
3.2. Effects of Taxifolin on the Intracellular ROS Levels Induced in an HG Environment
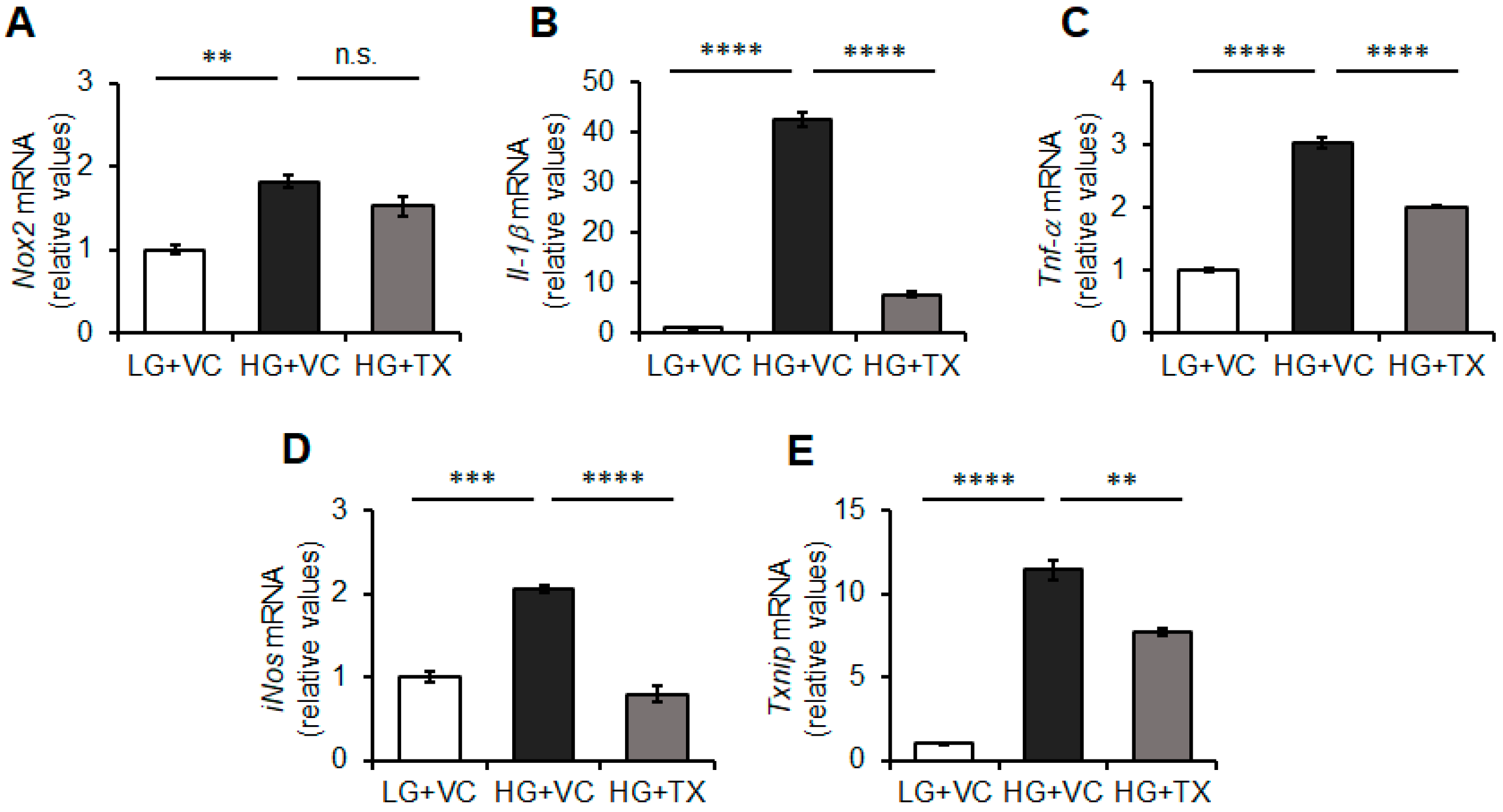
3.3. Effects of Taxifolin on the Inflammatory Responses of BV-2 Microglia Triggered by HG Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohara, T.; Doi, Y.; Ninomiya, T.; Hirakawa, Y.; Hata, J.; Iwaki, T.; Kanba, S.; Kiyohara, Y. Glucose tolerance status and risk of dementia in the community: The Hisayama study. Neurology 2011, 77, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Peters, S.A.; Woodward, M.; Mejia Arango, S.; Batty, G.D.; Beckett, N.; Beiser, A.; Borenstein, A.R.; Crane, P.K.; Haan, M.; et al. Type 2 Diabetes as a Risk Factor for Dementia in Women Compared with Men: A Pooled Analysis of 2.3 Million People Comprising More Than 100,000 Cases of Dementia. Diabetes Care 2016, 39, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E. Diabetes: The diabetic brain. Nat. Rev. Endocrinol. 2017, 13, 570–571. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Tan, C.C.; Xu, W.; Hu, H.; Cao, X.P.; Dong, Q.; Tan, L.; Yu, J.T. The Prevalence of Dementia: A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2020, 73, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.; Saeedi, P.; Kaundal, A.; Karuranga, S.; Malanda, B.; Williams, R. Diabetes and global ageing among 65–99-year-old adults: Findings from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2020, 162, 108078. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, N.; Hata, J.; Ohara, T.; Mukai, N.; Nagata, M.; Shibata, M.; Gotoh, S.; Furuta, Y.; Yamashita, F.; Yoshihara, K.; et al. Association Between Diabetes and Hippocampal Atrophy in Elderly Japanese: The Hisayama Study. Diabetes Care 2016, 39, 1543–1549. [Google Scholar] [CrossRef]
- Biessels, G.J.; Despa, F. Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications. Nat. Rev. Endocrinol. 2018, 14, 591–604. [Google Scholar] [CrossRef]
- Tumminia, A.; Vinciguerra, F.; Parisi, M.; Frittitta, L. Type 2 Diabetes Mellitus and Alzheimer’s Disease: Role of Insulin Signalling and Therapeutic Implications. Int. J. Mol. Sci. 2018, 19, 3306. [Google Scholar] [CrossRef]
- Berlanga-Acosta, J.; Guillen-Nieto, G.; Rodriguez-Rodriguez, N.; Bringas-Vega, M.L.; Garcia-Del-Barco-Herrera, D.; Berlanga-Saez, J.O.; Garcia-Ojalvo, A.; Valdes-Sosa, M.J.; Valdes-Sosa, P.A. Insulin Resistance at the Crossroad of Alzheimer Disease Pathology: A Review. Front. Endocrinol. 2020, 11, 560375. [Google Scholar] [CrossRef]
- Park, K.A.; Jin, Z.; Lee, J.Y.; An, H.S.; Choi, E.B.; Kim, K.E.; Shin, H.J.; Jeong, E.A.; Min, K.A.; Shin, M.C.; et al. Long-Lasting Exendin-4 Fusion Protein Improves Memory Deficits in High-Fat Diet/Streptozotocin-Induced Diabetic Mice. Pharmaceutics 2020, 12, 159. [Google Scholar] [CrossRef]
- Lin, M.H.; Cheng, P.C.; Hsiao, P.J.; Chen, S.C.; Hung, C.H.; Kuo, C.H.; Huang, S.K.; Clair Chiou, H.Y. The GLP-1 receptor agonist exenatide ameliorates neuroinflammation, locomotor activity, and anxiety-like behavior in mice with diet-induced obesity through the modulation of microglial M2 polarization and downregulation of SR-A4. Int. Immunopharmacol. 2023, 115, 109653. [Google Scholar] [CrossRef]
- Tanaka, M.; Yamakage, H.; Masuda, S.; Inoue, T.; Ohue-Kitano, R.; Araki, R.; Matoba, Y.; Saito, M.; Nagaoka, T.; Yonezawa, K.; et al. Serum soluble TREM2 is a potential novel biomarker of cognitive impairment in Japanese non-obese patients with diabetes. Diabetes Metab. 2019, 45, 86–89. [Google Scholar] [CrossRef]
- Tanaka, M.; Yamakage, H.; Muranaka, K.; Yamada, T.; Araki, R.; Ogo, A.; Matoba, Y.; Watanabe, T.; Saito, M.; Kurita, S.; et al. Higher Serum Soluble TREM2 as a Potential Indicative Biomarker for Cognitive Impairment in Inadequately Controlled Type 2 Diabetes Without Obesity: The DOR-KyotoJ-1. Front. Endocrinol. 2022, 13, 880148. [Google Scholar] [CrossRef]
- Satoh-Asahara, N.; Yamakage, H.; Tanaka, M.; Kawasaki, T.; Matsuura, S.; Tatebe, H.; Akiguchi, I.; Tokuda, T. Soluble TREM2 and Alzheimer-related biomarker trajectories in the blood of patients with diabetes based on their cognitive status. Diabetes Res. Clin. Pract. 2022, 193, 110121. [Google Scholar] [CrossRef]
- Michailidis, M.; Tata, D.A.; Moraitou, D.; Kavvadas, D.; Karachrysafi, S.; Papamitsou, T.; Vareltzis, P.; Papaliagkas, V. Antidiabetic Drugs in the Treatment of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 4641. [Google Scholar] [CrossRef]
- Vargas-Soria, M.; Garcia-Alloza, M.; Corraliza-Gomez, M. Effects of diabetes on microglial physiology: A systematic review of in vitro, preclinical and clinical studies. J. Neuroinflamm. 2023, 20, 57. [Google Scholar] [CrossRef]
- Quan, Y.; Jiang, C.T.; Xue, B.; Zhu, S.G.; Wang, X. High glucose stimulates TNFalpha and MCP-1 expression in rat microglia via ROS and NF-kappaB pathways. Acta Pharmacol. Sin. 2011, 32, 188–193. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Xiu, F. Sesamin suppresses high glucose-induced microglial inflammation in the retina in vitro and in vivo. J. Neurophysiol. 2022, 127, 405–411. [Google Scholar] [CrossRef]
- Kongtawelert, P.; Kaewmool, C.; Phitak, T.; Phimphilai, M.; Pothacharoen, P.; Shwe, T.H. Sesamin protects against neurotoxicity via inhibition of microglial activation under high glucose circumstances through modulating p38 and JNK signaling pathways. Sci. Rep. 2022, 12, 11296. [Google Scholar] [CrossRef]
- Li, Y.; Long, W.; Gao, M.; Jiao, F.; Chen, Z.; Liu, M.; Yu, L. TREM2 Regulates High Glucose-Induced Microglial Inflammation via the NLRP3 Signaling Pathway. Brain Sci 2021, 11, 896. [Google Scholar] [CrossRef]
- Tsubaki, H.; Tooyama, I.; Walker, D.G. Thioredoxin-Interacting Protein (TXNIP) with Focus on Brain and Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 9357. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xu, X.; Tang, C.; Gao, P.; Chen, X.; Xiong, X.; Yang, M.; Yang, S.; Zhu, X.; Yuan, S.; et al. Reactive oxygen species promote tubular injury in diabetic nephropathy: The role of the mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol. 2018, 16, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Lu, Q.; Chen, W.; Li, J.; Li, C.; Zheng, Z. Vitamin D3 Protects against Diabetic Retinopathy by Inhibiting High-Glucose-Induced Activation of the ROS/TXNIP/NLRP3 Inflammasome Pathway. J. Diabetes Res. 2018, 2018, 8193523. [Google Scholar] [CrossRef] [PubMed]
- Topal, F.; Nar, M.; Gocer, H.; Kalin, P.; Kocyigit, U.M.; Gulcin, I.; Alwasel, S.H. Antioxidant activity of taxifolin: An activity-structure relationship. J. Enzyme Inhib. Med. Chem. 2016, 31, 674–683. [Google Scholar] [CrossRef]
- Yang, P.; Xu, F.; Li, H.F.; Wang, Y.; Li, F.C.; Shang, M.Y.; Liu, G.X.; Wang, X.; Cai, S.Q. Detection of 191 Taxifolin Metabolites and Their Distribution in Rats Using HPLC-ESI-IT-TOF-MS(n). Molecules 2016, 21, 1209. [Google Scholar] [CrossRef]
- Tanaka, M.; Saito, S.; Inoue, T.; Satoh-Asahara, N.; Ihara, M. Novel Therapeutic Potentials of Taxifolin for Amyloid-β-associated Neurodegenerative Diseases and Other Diseases: Recent Advances and Future Perspectives. Int. J. Mol. Sci. 2019, 20, 2139. [Google Scholar] [CrossRef]
- Saito, S.; Tanaka, M.; Satoh-Asahara, N.; Carare, R.O.; Ihara, M. Taxifolin: A Potential Therapeutic Agent for Cerebral Amyloid Angiopathy. Front. Pharmacol. 2021, 12, 643357. [Google Scholar] [CrossRef]
- Saito, S.; Yamamoto, Y.; Maki, T.; Hattori, Y.; Ito, H.; Mizuno, K.; Harada-Shiba, M.; Kalaria, R.N.; Fukushima, M.; Takahashi, R.; et al. Taxifolin inhibits amyloid-β oligomer formation and fully restores vascular integrity and memory in cerebral amyloid angiopathy. Acta Neuropathol. Commun. 2017, 5, 26. [Google Scholar] [CrossRef]
- Inoue, T.; Saito, S.; Tanaka, M.; Yamakage, H.; Kusakabe, T.; Shimatsu, A.; Ihara, M.; Satoh-Asahara, N. Pleiotropic neuroprotective effects of taxifolin in cerebral amyloid angiopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 10031–10038. [Google Scholar] [CrossRef]
- Inoue, T.; Fu, B.; Nishio, M.; Tanaka, M.; Kato, H.; Tanaka, M.; Itoh, M.; Yamakage, H.; Ochi, K.; Ito, A.; et al. Novel Therapeutic Potentials of Taxifolin for Obesity-Induced Hepatic Steatosis, Fibrogenesis, and Tumorigenesis. Nutrients 2023, 15, 350. [Google Scholar] [CrossRef]
- Hattori, Y.; Saito, S.; Nakaoku, Y.; Ogata, S.; Hattori, M.; Nakatsuji, M.; Nishimura, K.; Ihara, M. Taxifolin for Cognitive Preservation in Patients with Mild Cognitive Impairment or Mild Dementia. J. Alzheimers Dis. 2023, 93, 743–754. [Google Scholar] [CrossRef]
- Kanno, K.; Sakaue, T.; Hamaguchi, M.; Namiguchi, K.; Nanba, D.; Aono, J.; Kurata, M.; Masumoto, J.; Higashiyama, S.; Izutani, H. Hypoxic Culture Maintains Cell Growth of the Primary Human Valve Interstitial Cells with Stemness. Int. J. Mol. Sci. 2021, 22, 534. [Google Scholar] [CrossRef]
- Helfinger, V.; Palfi, K.; Weigert, A.; Schroder, K. The NADPH Oxidase Nox4 Controls Macrophage Polarization in an NFkappaB-Dependent Manner. Oxid. Med. Cell Longev. 2019, 2019, 3264858. [Google Scholar] [CrossRef]
- Nakamura, A.; Kitamura, N.; Yokoyama, Y.; Uchida, S.; Kumadaki, K.; Tsubota, K.; Watanabe, M. Melon GliSODin((R)) Prevents Diet-Induced NASH Onset by Reducing Fat Synthesis and Improving Liver Function. Nutrients 2019, 11, 1779. [Google Scholar] [CrossRef]
- Nishikai-Yan Shen, T.; Kanazawa, S.; Kado, M.; Okada, K.; Luo, L.; Hayashi, A.; Mizuno, H.; Tanaka, R. Interleukin-6 stimulates Akt and p38 MAPK phosphorylation and fibroblast migration in non-diabetic but not diabetic mice. PLoS ONE 2017, 12, e0178232. [Google Scholar] [CrossRef]
- Wei, X.M.; Kim, H.S.; Kumar, R.K.; Heywood, G.J.; Hunt, J.E.; McNeil, H.P.; Thomas, P.S. Effects of cigarette smoke on degranulation and NO production by mast cells and epithelial cells. Respir. Res. 2005, 6, 108. [Google Scholar] [CrossRef]
- Kang, S.; Bennett, C.N.; Gerin, I.; Rapp, L.A.; Hankenson, K.D.; Macdougald, O.A. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 2007, 282, 14515–14524. [Google Scholar] [CrossRef]
- Kato, H.; Minamizato, H.; Ohno, H.; Ohira, Y.; Izawa, T. Exercise ameliorates high-fat diet-induced impairment of differentiation of adipose-derived stem cells into neuron-like cells in rats. J. Cell Physiol. 2019, 234, 1452–1460. [Google Scholar] [CrossRef]
- Wang, X.; Duan, C.; Li, Y.; Lu, H.; Guo, K.; Ge, X.; Chen, T.; Shang, Y.; Liu, H.; Zhang, D. Sodium butyrate reduces overnutrition-induced microglial activation and hypothalamic inflammation. Int. Immunopharmacol. 2022, 111, 109083. [Google Scholar] [CrossRef]
- Sierra, A.; Navascues, J.; Cuadros, M.A.; Calvente, R.; Martin-Oliva, D.; Ferrer-Martin, R.M.; Martin-Estebane, M.; Carrasco, M.C.; Marin-Teva, J.L. Expression of inducible nitric oxide synthase (iNOS) in microglia of the developing quail retina. PLoS ONE 2014, 9, e106048. [Google Scholar] [CrossRef]
- Najjar, R.S.; Mu, S.; Feresin, R.G. Blueberry Polyphenols Increase Nitric Oxide and Attenuate Angiotensin II-Induced Oxidative Stress and Inflammatory Signaling in Human Aortic Endothelial Cells. Antioxidants 2022, 11, 616. [Google Scholar] [CrossRef] [PubMed]
- So, S.W.; Fleming, K.M.; Duffy, C.M.; Nixon, J.P.; Bernlohr, D.A.; Butterick, T.A. Microglial FABP4-UCP2 Axis Modulates Neuroinflammation and Cognitive Decline in Obese Mice. Int. J. Mol. Sci. 2022, 23, 4354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, Y.; Zhang, J.; Zhao, Y.; Zhang, Y.; Fu, J. Astragaloside IV supplementation attenuates cognitive impairment by inhibiting neuroinflammation and oxidative stress in type 2 diabetic mice. Front. Aging Neurosci. 2022, 14, 1004557. [Google Scholar] [CrossRef] [PubMed]
- Henriquez-Olguin, C.; Knudsen, J.R.; Raun, S.H.; Li, Z.; Dalbram, E.; Treebak, J.T.; Sylow, L.; Holmdahl, R.; Richter, E.A.; Jaimovich, E.; et al. Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat. Commun. 2019, 10, 4623. [Google Scholar] [CrossRef]
- Hallakou-Bozec, S.; Vial, G.; Kergoat, M.; Fouqueray, P.; Bolze, S.; Borel, A.L.; Fontaine, E.; Moller, D.E. Mechanism of action of Imeglimin: A novel therapeutic agent for type 2 diabetes. Diabetes Obes. Metab. 2021, 23, 664–673. [Google Scholar] [CrossRef]
- Rizwan, H.; Pal, S.; Sabnam, S.; Pal, A. High glucose augments ROS generation regulates mitochondrial dysfunction and apoptosis via stress signalling cascades in keratinocytes. Life Sci. 2020, 241, 117148. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasa, M.; Kato, H.; Iwashita, K.; Yamakage, H.; Kato, S.; Saito, S.; Ihara, M.; Nishimura, H.; Kawamoto, A.; Suganami, T.; et al. Taxifolin Suppresses Inflammatory Responses of High-Glucose-Stimulated Mouse Microglia by Attenuating the TXNIP–NLRP3 Axis. Nutrients 2023, 15, 2738. https://doi.org/10.3390/nu15122738
Iwasa M, Kato H, Iwashita K, Yamakage H, Kato S, Saito S, Ihara M, Nishimura H, Kawamoto A, Suganami T, et al. Taxifolin Suppresses Inflammatory Responses of High-Glucose-Stimulated Mouse Microglia by Attenuating the TXNIP–NLRP3 Axis. Nutrients. 2023; 15(12):2738. https://doi.org/10.3390/nu15122738
Chicago/Turabian StyleIwasa, Masayo, Hisashi Kato, Kaori Iwashita, Hajime Yamakage, Sayaka Kato, Satoshi Saito, Masafumi Ihara, Hideo Nishimura, Atsuhiko Kawamoto, Takayoshi Suganami, and et al. 2023. "Taxifolin Suppresses Inflammatory Responses of High-Glucose-Stimulated Mouse Microglia by Attenuating the TXNIP–NLRP3 Axis" Nutrients 15, no. 12: 2738. https://doi.org/10.3390/nu15122738
APA StyleIwasa, M., Kato, H., Iwashita, K., Yamakage, H., Kato, S., Saito, S., Ihara, M., Nishimura, H., Kawamoto, A., Suganami, T., Tanaka, M., & Satoh-Asahara, N. (2023). Taxifolin Suppresses Inflammatory Responses of High-Glucose-Stimulated Mouse Microglia by Attenuating the TXNIP–NLRP3 Axis. Nutrients, 15(12), 2738. https://doi.org/10.3390/nu15122738









