Vitamin D Intake, Serum 25-Hydroxyvitamin-D (25(OH)D) Levels, and Cancer Risk: A Comprehensive Meta-Meta-Analysis Including Meta-Analyses of Randomized Controlled Trials and Observational Epidemiological Studies
Abstract
1. Introduction
2. Methods
- Population: “Patients with cancer and individuals without cancer”
- Intervention: “Vit-D intake and serum 25(OH)D levels”
- Comparison: (i) “Low and high Vit-D intake”; (ii) “low and high serum 25(OH)D levels”
- Outcomes: (i) “Cancer risk”; (ii) “Mortality”
- Study: “Systematic reviews with meta-analysis or meta-analysis alone”
2.1. Search Strategy and Data Extraction
2.2. Selection Criteria
2.3. Methodological Quality Assessment
2.4. Assessment of Risk of Bias
2.5. Data Appraisal, Synthesis, and Statistical Analysis
2.6. Sensitivity Analysis
2.7. Mapping
3. Results
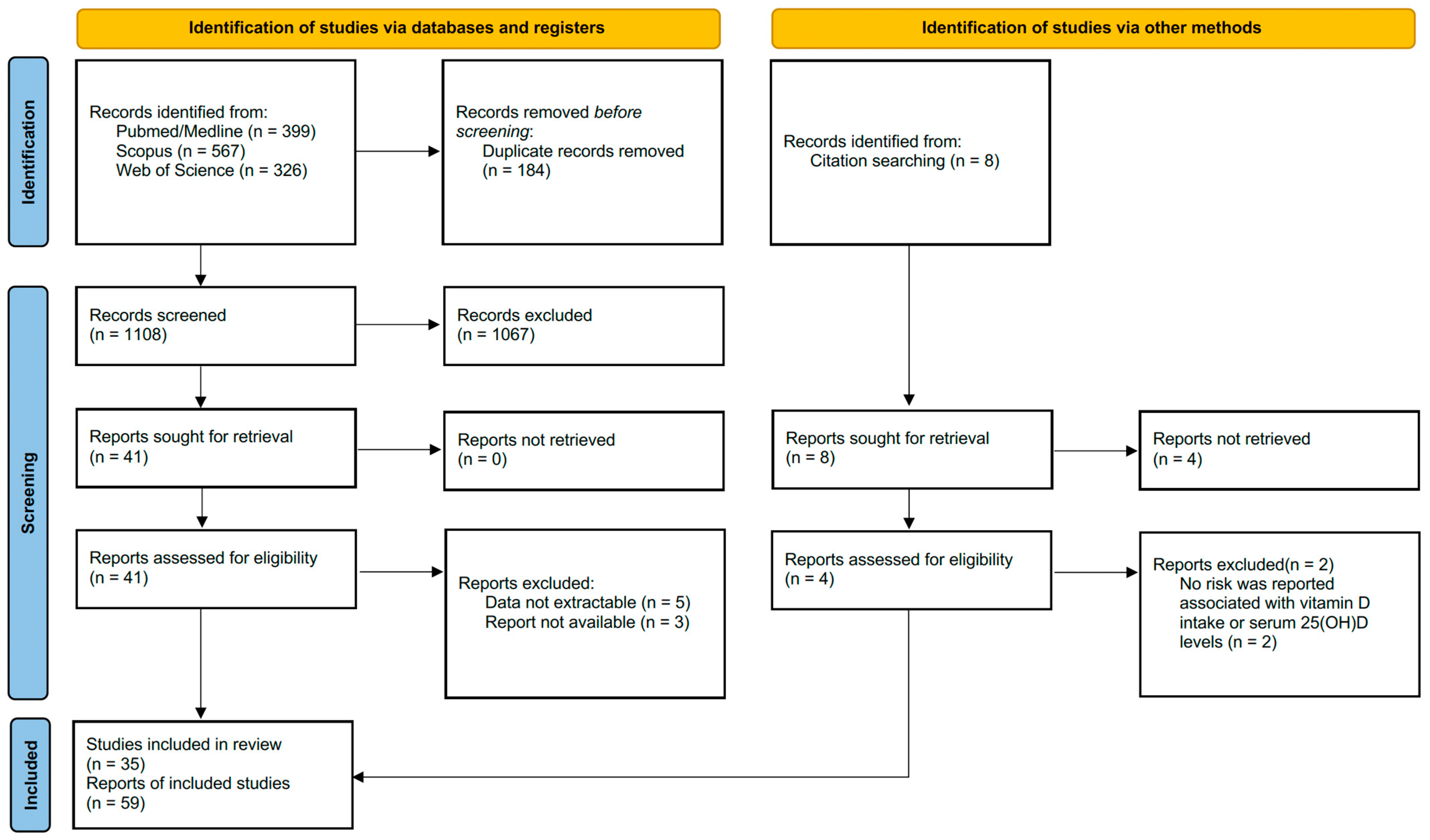
3.1. Search Results
3.2. Baseline Characteristics of the Meta-Analyses
3.3. Outcomes
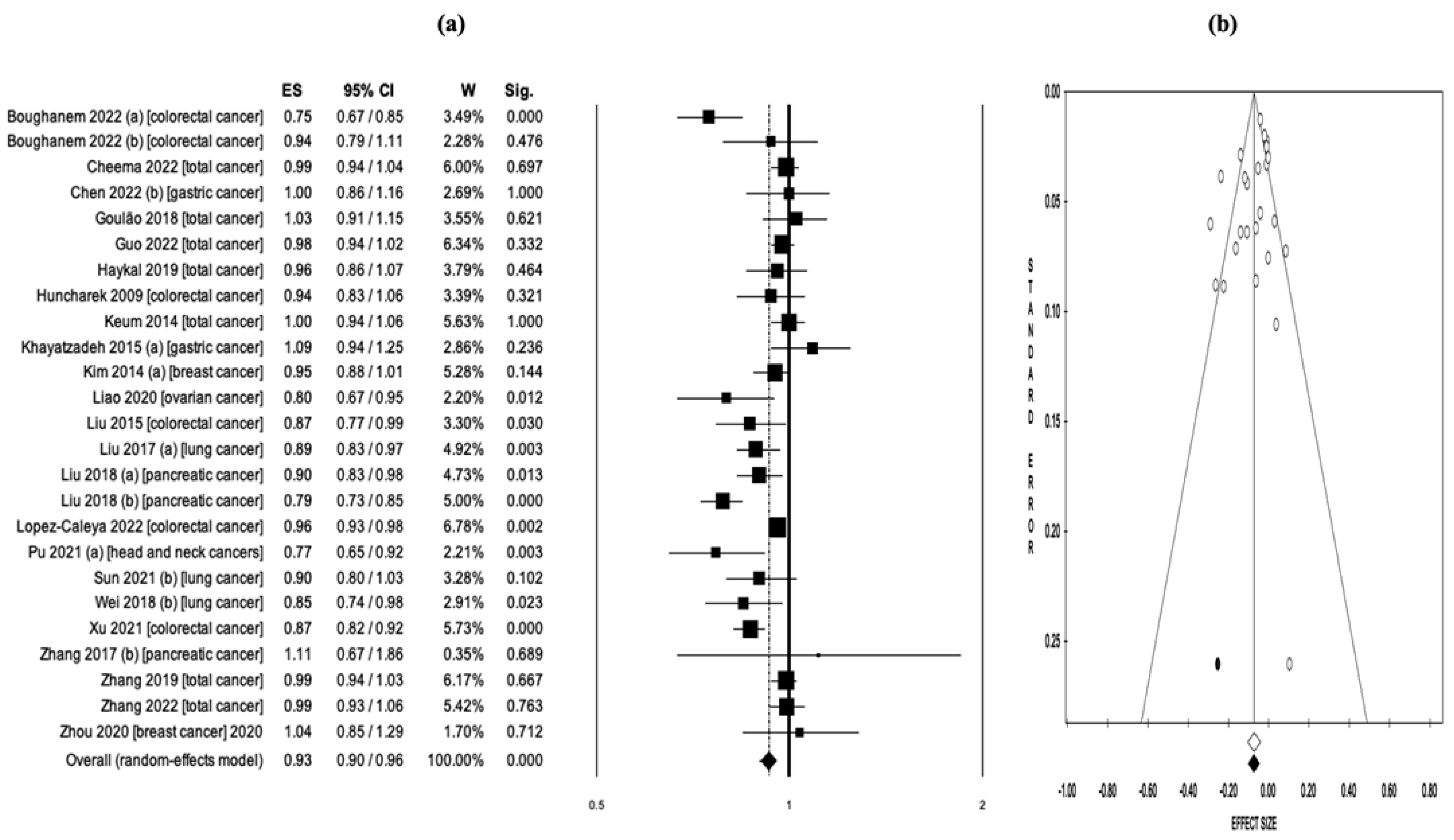
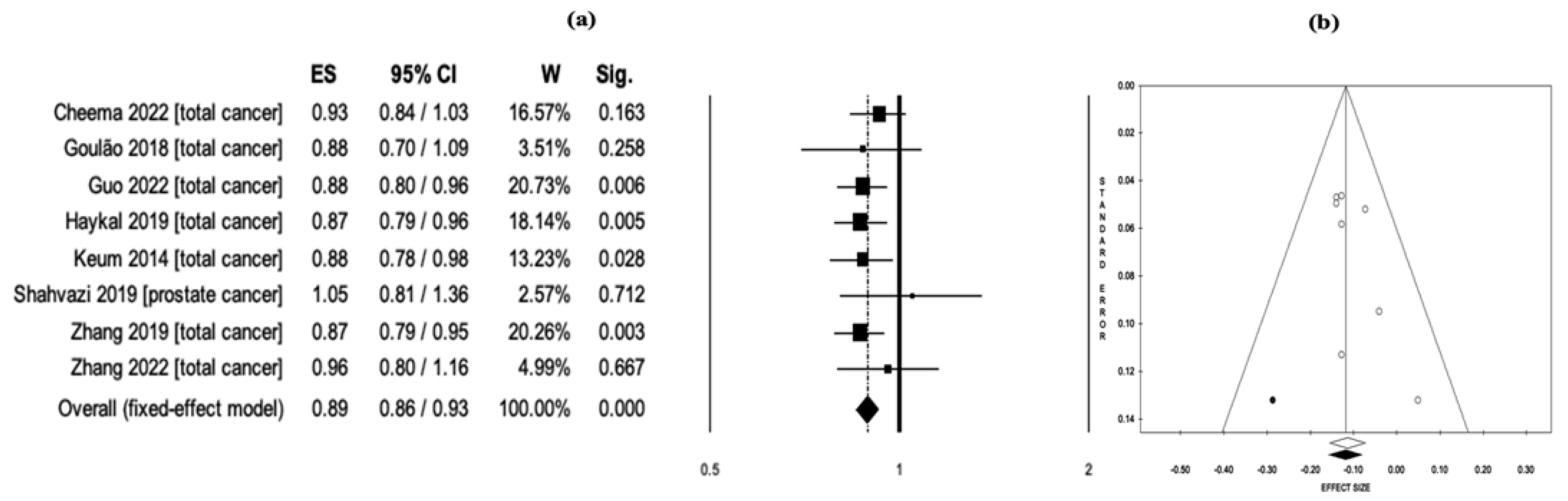
3.4. Vitamin D Intake and Cancer Risk/Mortality
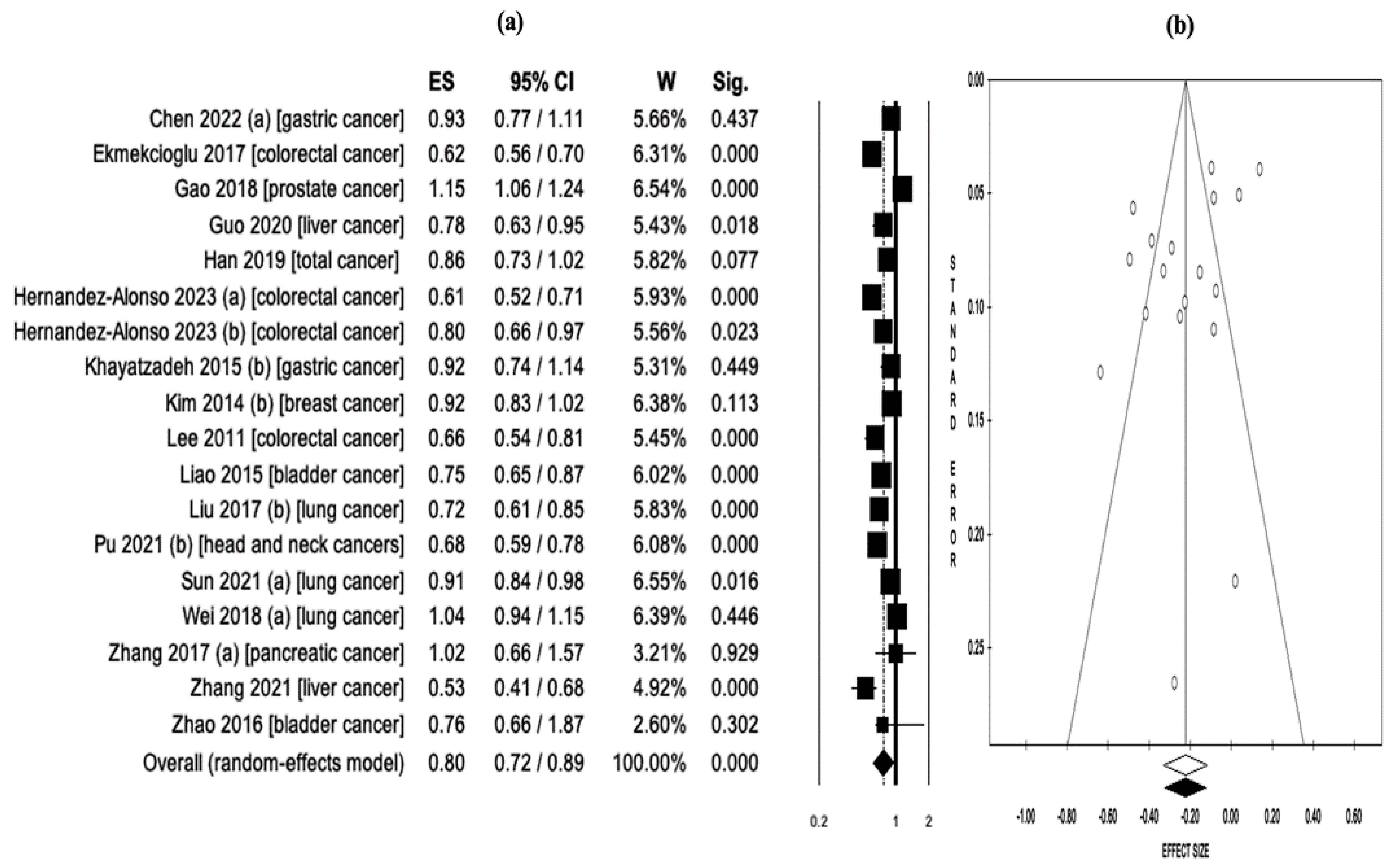
3.5. Serum 25-Hidroxyvitamin-D Levels and Cancer Risk/Mortality
3.6. Subgroup Analysis
3.7. Mapping
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Arayici, M.E.; Basbinar, Y.; Ellidokuz, H. The impact of cancer on the severity of disease in patients affected with COVID-19: An umbrella review and meta-meta-analysis of systematic reviews and meta-analyses involving 1,064,476 participants. Clin. Exp. Med. 2022, 1–9. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2019. WHO; 2020. Available online: who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 11 March 2023).
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef]
- Raiten, D.J.; Picciano, M.F. Vitamin D and health in the 21st century: Bone and beyond. Executive summary. Am. J. Clin. Nutr. 2004, 80 (Suppl. S6), 1673S–1677S. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, A.; Grant, W.B. Vitamin D and Cancer: An Historical Overview of the Epidemiology and Mechanisms. Nutrients 2022, 14, 1448. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M.; Shin, E. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2019, 50, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, P.; Li, J.; Chu, R.; Xie, D.; Wang, H. Review: The impacts of circulating 25-hydroxyvitamin D levels on cancer patient outcomes: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 2327–2336. [Google Scholar] [CrossRef]
- Ordóñez Mena, J.M.; Brenner, H. Vitamin D and cancer: An overview on epidemiological studies. Adv. Exp. Med. Biol. 2014, 810, 17–32. [Google Scholar]
- Ekmekcioglu, C.; Haluza, D.; Kundi, M. 25-Hydroxyvitamin D Status and Risk for Colorectal Cancer and Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Epidemiological Studies. Int. J. Environ. Res. Public Health 2017, 14, 127. [Google Scholar] [CrossRef]
- Guo, X.F.; Zhao, T.; Han, J.M.; Li, S.; Li, D. Vitamin D and liver cancer risk: A meta-analysis of prospective studies. Asia Pac. J. Clin. Nutr. 2020, 29, 175–182. [Google Scholar] [CrossRef]
- Hernández-Alonso, P.; Boughanem, H.; Canudas, S.; Becerra-Tomás, N.; Fernández de la Puente, M.; Babio, N.; Macias-Gonzalez, M.; Salas-Salvadó, J. Circulating vitamin D levels and colorectal cancer risk: A meta-analysis and systematic review of case-control and prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 1–17. [Google Scholar] [CrossRef]
- Chowdhury, R.; Kunutsor, S.; Vitezova, A.; Oliver-Williams, C.; Chowdhury, S.; Kiefte-de-Jong, J.C.; Khan, H.; Baena, C.P.; Prabhakaran, D.; Hoshen, M.B.; et al. Vitamin D and risk of cause specific death: Systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ 2014, 348, g1903. [Google Scholar] [CrossRef]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef]
- Giovannucci, E. The epidemiology of vitamin D and cancer incidence and mortality: A review (United States). Cancer Causes Control. 2005, 16, 83–95. [Google Scholar] [CrossRef]
- Goulão, B.; Stewart, F.; Ford, J.A.; MacLennan, G.; Avenell, A. Cancer and vitamin D supplementation: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 107, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Cheema, H.A.; Fatima, M.; Shahid, A.; Bouaddi, O.; Elgenidy, A.; Rehman, A.U.; Kacimi, S.E.O.; Hasan, M.M.; Lee, K.Y. Vitamin D supplementation for the prevention of total cancer incidence and mortality: An updated systematic review and meta-analysis. Heliyon 2022, 8, e11290. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Huang, M.; Fan, D.; Hong, Y.; Zhao, M.; Ding, R.; Cheng, Y.; Duan, S. Association between vitamin D supplementation and cancer incidence and mortality: A trial sequential meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Boughanem, H.; Canudas, S.; Hernandez-Alonso, P.; Becerra-Tomás, N.; Babio, N.; Salas-Salvadó, J.; Macias-Gonzalez, M. Vitamin D Intake and the Risk of Colorectal Cancer: An Updated Meta-Analysis and Systematic Review of Case-Control and Prospective Cohort Studies. Cancers 2021, 13, 2814. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.Q.; Gao, X.P.; Yu, X.X.; Zeng, Y.F.; Li, S.N.; Naicker, N.; Joseph, T.; Cao, W.-T.; Liu, Y.-H.; Zhu, S.; et al. Effects of dairy products, calcium and vitamin D on ovarian cancer risk: A meta-analysis of twenty-nine epidemiological studies. Br. J. Nutr. 2020, 124, 1001–1012. [Google Scholar] [CrossRef]
- Liu, J.; Dong, Y.; Lu, C.; Wang, Y.; Peng, L.; Jiang, M.; Tang, Y.; Zhao, Q. Meta-analysis of the correlation between vitamin D and lung cancer risk and outcomes. Oncotarget 2017, 8, 81040–81051. [Google Scholar] [CrossRef]
- Sun, K.; Zuo, M.; Zhang, Q.; Wang, K.; Huang, D.; Zhang, H. Anti-Tumor Effect of Vitamin D Combined with Calcium on Lung Cancer: A Systematic Review and Meta-Analysis. Nutr. Cancer 2021, 73, 2633–2642. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Brooke, B.S.; Schwartz, T.A.; Pawlik, T.M. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg. 2021, 156, 787–788. [Google Scholar] [CrossRef]
- Furlan, A.D.; Pennick, V.; Bombardier, C.; van Tulder, M. Editorial Board, Cochrane Back Review Group. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine 2009, 34, 1929–1941. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Whiting, P.; Savović, J.; Higgins, J.P.; Caldwell, D.M.; Reeves, B.C.; Shea, B.; Davies, P.; Kleijnen, J.; Churchill, R. ROBIS group. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J. Clin. Epidemiol. 2016, 69, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Tweedie, R. A nonparametric “Trim and Fill” method of accounting for Publication Bias in Meta-Analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A.; Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0; Wiley-Blackwell: Hoboken, NJ, USA, 2008. [Google Scholar]
- ProMeta-3 Professional Statistical Software for Conducting Meta-Analysis. It Is Based on ProMeta 2.1 Deployed by Internovi in 2015. Available online: https://idostatistics.com/prometa3/ (accessed on 26 April 2023).
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Liang, Y.; Huang, T.; Zhang, H.; Fan, S.; Sun, W.; Wang, Y. Relationship of vitamin D intake, serum 25(OH) D, and solar ultraviolet-B radiation with the risk of gastric cancer: A meta-analysis. J. Cancer Res. Ther. 2022, 18, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wei, W.; Wang, G.; Zhou, H.; Fu, Y.; Liu, N. Circulating vitamin D concentration and risk of prostate cancer: A dose-response meta-analysis of prospective studies. Ther. Clin. Risk Manag. 2018, 14, 95–104. [Google Scholar] [CrossRef]
- Han, J.; Guo, X.; Yu, X.; Liu, S.; Cui, X.; Zhang, B.; Liang, H. 25-Hydroxyvitamin D and Total Cancer Incidence and Mortality: A meta-analysis of prospective cohort studies. Nutrients 2019, 11, 2295. [Google Scholar] [CrossRef]
- Haykal, T.; Samji, V.; Zayed, Y.; Gakhal, I.; Dhillon, H.; Kheiri, B.; Kerbage, J.; Veerapaneni, V.; Obeid, M.; Danish, R.; et al. The role of vitamin D supplementation for primary prevention of cancer: Meta-analysis of randomized controlled trials. J. Community Hosp. Intern. Med. Perspect. 2019, 9, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Huncharek, M.; Muscat, J.; Kupelnick, B. Colorectal cancer risk and dietary intake of calcium, vitamin, D.; and dairy products: A meta-analysis of 26,335 cases from 60 observational studies. Nutr. Cancer 2009, 61, 47–69. [Google Scholar] [CrossRef]
- Keum, N.; Giovannucci, E. Vitamin D supplements and cancer incidence and mortality: A meta-analysis. Br. J. Cancer 2014, 111, 976–980. [Google Scholar] [CrossRef]
- Khayatzadeh, S.; Feizi, A.; Saneei, P.; Esmaillzadeh, A. Vitamin D intake, serum Vitamin D levels, and risk of gastric cancer: A systematic review and meta-analysis. J. Res. Med. Sci. 2015, 20, 790–796. [Google Scholar] [CrossRef]
- Kim, Y.; Je, Y. Vitamin D intake, blood 25(OH)D levels, and breast cancer risk or mortality: A meta-analysis. Br. J. Cancer 2014, 110, 2772–2784. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Li, H.; Chan, A.T.; Hollis, B.W.; Lee, I.M.; Stampfer, M.J.; Wu, K.; Giovannucci, E.; Ma, J. Circulating levels of vitamin D and colon and rectal cancer: The Physicians′ Health Study and a meta-analysis of prospective studies. Cancer Prev. Res. 2011, 4, 735–743. [Google Scholar] [CrossRef]
- Liao, Y.; Huang, J.L.; Qiu, M.X.; Ma, Z.W. Impact of serum vitamin D level on risk of bladder cancer: A systemic review and meta-analysis. Tumour. Biol. 2015, 36, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, Q.; Zhu, Z.; Zhang, J.; Chen, M.; Tang, P.; Li, K. Vitamin and multiple-vitamin supplement intake and incidence of colorectal cancer: A meta-analysis of cohort studies. Med. Oncol. 2015, 32, 434. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Sun, X.; Lu, S.; Liu, S. Vitamin intake and pancreatic cancer risk reduction: A meta-analysis of observational studies. Medicine 2018, 97, e0114. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Caleya, J.F.; Ortega-Valín, L.; Fernández-Villa, T.; Delgado-Rodríguez, M.; Martín-Sánchez, V.; Molina, A.J. The role of calcium and vitamin D dietary intake on risk of colorectal cancer: Systematic review and meta-analysis of case-control studies. Cancer Causes Control. 2022, 33, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Maalmi, H.; Ordóñez-Mena, J.M.; Schöttker, B.; Brenner, H. Serum 25-hydroxyvitamin D levels and survival in colorectal and breast cancer patients: Systematic review and meta-analysis of prospective cohort studies. Eur. J. Cancer 2014, 50, 1510–1521. [Google Scholar] [CrossRef]
- Maalmi, H.; Walter, V.; Jansen, L.; Boakye, D.; Schöttker, B.; Hoffmeister, M.; Brenner, H. Association between blood 25-hydroxyvitamin D levels and survival in colorectal cancer patients: An updated systematic review and meta-analysis. Nutrients 2018, 10, 896. [Google Scholar] [CrossRef]
- Pu, Y.; Zhu, G.; Xu, Y.; Zheng, S.; Tang, B.; Huang, H.; Wu, I.X.Y.; Huang, D.; Liu, Y.; Zhang, X. Association Between Vitamin D Exposure and Head and Neck Cancer: A Systematic Review with Meta-Analysis. Front. Immunol. 2021, 12, 627226. [Google Scholar] [CrossRef]
- Shahvazi, S.; Soltani, S.; Ahmadi, S.M.; de Souza, R.J.; Salehi-Abargouei, A. The Effect of Vitamin D Supplementation on Prostate Cancer: A Systematic Review and Meta-Analysis of Clinical Trials. Horm. Metab. Res. 2019, 51, 11–21. [Google Scholar] [CrossRef]
- Wei, H.; Jing, H.; Wei, Q.; Wei, G.; Heng, Z. Associations of the risk of lung cancer with serum 25-hydroxyvitamin D level and dietary vitamin D intake: A dose-response PRISMA meta-analysis. Medicine 2018, 97, e12282. [Google Scholar] [CrossRef]
- Xu, Y.; Qian, M.; Hong, J.; Ng, D.M.; Yang, T.; Xu, L.; Ye, X. The effect of vitamin D on the occurrence and development of colorectal cancer: A systematic review and meta-analysis. Int. J. Colorectal. Dis. 2021, 36, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, X.Z.; Chen, W.J.; Wu, J.; Chen, Y.; Wu, C.C.; Wang, Z.-N. Plasma 25-hydroxyvitamin D levels, vitamin D intake, and pancreatic cancer risk or mortality: A meta-analysis. Oncotarget 2017, 8, 64395–64406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Niu, W. Meta-analysis of randomized controlled trials on vitamin D supplement and cancer incidence and mortality. Biosci. Rep. 2019, 39, BSR20190369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, X.; Li, X.; Găman, M.A.; Kord-Varkaneh, H.; Rahmani, J.; Salehi-Sahlabadi, A.; Day, A.S.; Xu, Y. Serum Vitamin D Levels and Risk of Liver Cancer: A Systematic Review and Dose-Response Meta-Analysis of Cohort Studies. Nutr. Cancer 2021, 73, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, Y.; Liu, Z.; Pei, Y.; Xu, P.; Chong, W.; Hai, Y.; He, L.; He, Y.; Yu, J.; et al. Association between Vitamin D Supplementation and Cancer Mortality: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 3717. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, C.; Pan, W.; Gao, M.; He, W.; Mao, R.; Lin, T.; Huang, J. Comparative efficacy of vitamin D status in reducing the risk of bladder cancer: A systematic review and network meta-analysis. Nutrition 2016, 32, 515–523. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, B.; Sheng, L.; Turner, A. The effect of vitamin D supplementation on the risk of breast cancer: A trial sequential meta-analysis. Breast Cancer Res. Treat. 2020, 182, 1–8. [Google Scholar] [CrossRef]
| First Author/Year | Cancer Type | Characteristics of the Primary Studies | Vit-D Exposure | Total Number of Studies (n) | Total Sample Size (n) | Outcome | NoP Studies Included for Incidence (n) | NoP Studies Included for Mortality (n) | Effect Size (ES) and Confidence Interval (CI) for Incidence | Effect Size (ES) and Confidence Interval (CI) for Mortality |
|---|---|---|---|---|---|---|---|---|---|---|
| Boughanem 2022 (a) x [21] | Colorectal cancer | Case-control, prospective cohort | Vit-D intake | 31 | 926,237 | Incidence | 12 | N/A | OR = 0.75 (0.67–0.85) | N/A |
| Boughanem 2022 (b) y [21] | Colorectal cancer | Case-control, prospective cohort | Vit-D intake | 31 | 926,237 | Incidence | 6 | N/A | HR = 0.94 (0.79–1.11) | N/A |
| Cheema 2022 [19] | Total cancer | RCTs | Vit-D intake | 13 | 109,543 | Incidence, mortality | 12 | 7 | RR = 0.99 (0.94–1.04) | RR = 0.93 (0.84–1.03) |
| Chen 2022 (a) [36] | Gastric cancer | Case-control, prospective cohort | Serum 25(OH)D | 11 | N/A | Incidence | 11 | N/A | OR = 0.93 (0.77–1.11) | N/A |
| Chen 2022 (b) [36] | Gastric cancer | Case-control, prospective cohort | Vit-D intake | 11 | N/A | Incidence | 4 | N/A | OR = 1.00 (0.86–1.16) | N/A |
| Ekmekcioglu 2017 [12] | Colorectal cancer | Case-control, prospective cohort | Serum 25(OH)D | 14 | 12,110 | Incidence | 14 | N/A | RR = 0.62 (0.56–0.70) | N/A |
| Gao 2018 [37] | Prostate cancer | Case-control, prospective cohort | Serum 25(OH)D | 19 | 48,369 | Incidence | 19 | N/A | RR = 1.15 (1.06–1.24) | N/A |
| Goulão 2018 [18] | Total cancer | RCTs | Vit-D intake | 30 | 18,808 | Incidence | 24 | 7 | RR = 1.03 (0.91–1.15) | RR = 0.88 (0.70–1.09) |
| Guo 2020 [13] | Liver cancer | Case-control, prospective cohort | Serum 25(OH)D | 6 | 60,811 | Incidence | 6 | N/A | RR = 0.78 (0.63–0.95) | N/A |
| Guo 2022 [20] | Total cancer | RCTs | Vit-D intake | 26 | 121,529 | Incidence, mortality | 19 | 11 | RR = 0.98 (0.94–1.02) | RR = 0.88 (0.80–0.96) |
| Han 2019 [38] | Total cancer | Prospective cohort | Serum 25(OH)D | 23 | 170,618 | Incidence, mortality | 8 | 16 | RR = 0.86 (0.73–1.02) | RR = 0.81 (0.71–0.93) |
| Haykal 2019 [39] | Total cancer | RCTs | Vit-D intake | 10 | 79,055 | Incidence, mortality | 9 | 5 | RR = 0.96 (0.86–1.07) | RR = 0.87 (0.79–0.96) |
| Hernandez-Alonso 2023 (a) [14] | Colorectal cancer | Case-control | Serum 25(OH)D | 28 | 140,112 | Incidence | 11 | N/A | OR = 0.61 (0.52–0.71) | N/A |
| Hernandez-Alonso 2023 (b) [14] | Colorectal cancer | Prospective cohort | Serum 25(OH)D | 28 | 140,112 | Incidence | 6 | N/A | HR = 0.80 (0.66–0.97) | N/A |
| Huncharek 2009 [40] | Colorectal cancer | Case-control, cohort | Vit-D intake | 60 | N/R | Incidence | 10 | N/A | RR = 0.94 (0.83–1.06) | N/A |
| Keum 2014 [41] | Total cancer | RCTs | Vit-D intake | 4 | 45,151 | Incidence, mortality | 4 | 3 | RR = 1.00 (0.94–1.06) | RR = 0.88 (0.78–0.98) |
| Khayatzadeh 2015 (a) [42] | Gastric cancer | Case-control, cohort | Vit-D intake | 7 | 59,626 | Incidence | 4 | N/A | OR = 1.09 (0.94–1.25) | N/A |
| Khayatzadeh 2015 (b) [42] | Gastric cancer | Case-control, cohort | Serum 25(OH)D | 7 | 59,626 | Incidence | 3 | N/A | OR = 0.92 (0.74–1.14) | N/A |
| Kim 2014 (a) [43] | Breast cancer | Case-control, cohort | Vit-D intake | 30 | 762,859 | Incidence | 12 | N/A | RR = 0.95 (0.88–1.01) | N/A |
| Kim 2014 (b) [43] | Breast cancer | Case-control, cohort | Serum 25(OH)D | 30 | 762,859 | Incidence, mortality | 14 | 4 | RR = 0.92 (0.83–1.02) | RR = 0.58 (0.40–0.85) |
| Lee 2011 [44] | Colorectal cancer | Case-control, cohort | Serum 25(OH)D | 8 | N/A | Incidence | 8 | N/A | OR = 0.66 (0.54–0.81) | N/A |
| Liao 2015 [45] | Bladder cancer | Case-control, cohort | Serum 25(OH)D | 5 | 89,610 | Incidence | 5 | N/A | RR = 0.75 (0.65–0.87) | N/A |
| Liao 2020 [22] | Ovarian cancer | Case-control, cohort | Vit-D intake | 29 | 963,604 | Incidence | 6 | N/A | RR = 0.80 (0.67–0.95) | N/A |
| Liu 2015 [46] | Colorectal cancer | Cohort | Vit-D intake | 47 | 870,330 | Incidence | 17 | N/A | RR = 0.87 (0.77–0.99) | N/A |
| Liu 2017 (a) [23] | Lung cancer | Case-control, cohort | Vit-D intake | 22 | 813,801 | Incidence | 6 | N/A | OR = 0.89 (0.83–0.97) | N/A |
| Liu 2017 (b) [23] | Lung cancer | Case-control, cohort | Serum 25(OH)D | 22 | 813,801 | Incidence, mortality | 8 | 3 | OR = 0.72 (0.61–0.85) | OR = 0.39 (0.28–0.54) |
| Liu 2018 (a) * [47] | Pancreatic cancer | Case-control, cohort, RCTs | Vit-D intake | 25 | 1,213,821 | Incidence | 11 | N/A | RR = 0.90 (0.83–0.98) | N/A |
| Liu 2018 (b) ** [47] | Pancreatic cancer | Case-control, cohort, RCTs | Vit-D intake | 25 | 1,213,821 | Incidence | 14 | N/A | RR = 0.79 (0.73–0.85) | N/A |
| Lopez-Caleya 2022 [48] | Colorectal cancer | Case-control | Vit-D intake | 55 | 55,522 | Incidence | 23 | N/A | OR = 0.96 (0.93–0.98) | N/A |
| Maalmi 2014 *** [49] | Breast cancer | Cohort | Serum 25(OH)D | 5 | 4413 | Mortality | N/A | 3 | N/A | HR = 0.57 (0.38–0.84) |
| Maalmi 2018 *** [50] | Colorectal cancer | Cohort | Serum 25(OH)D | 11 | 7718 | Mortality | N/A | 6 | N/A | HR = 0.67 (0.57–0.78) |
| Pu 2021 (a) [51] | Head and neck cancer | Case-control, cohort | Vit-D intake | 16 | 81,908 | Incidence | 3 | N/A | OR = 0.77 (0.65–0.92) | N/A |
| Pu 2021 (b) [51] | Head and neck cancer | Case-control, cohort | Serum 25(OH)D | 16 | 81,908 | Incidence, mortality | 5 | 3 | OR = 0.68 (0.59–0.78) | OR = 0.75 (0.60–0.94) |
| Shahvazi 2019 [52] | Prostate cancer | Clinical trials | Vit-D intake | 22 | 1902 | Mortality | N/A | 3 | N/A | RR = 1.05 (0.81–1.36) |
| Sun 2021 (a) [24] | Lung cancer | Case-control, cohort, RCTs | Serum 25(OH)D | 40 | 1,566,662 | Incidence, mortality | 16 | 9 | RR = 0.91 (0.84–0.98) | RR = 0.71 (0.53–0.97) |
| Sun 2021 (b) [24] | Lung cancer | Case-control, cohort, RCTs | Vit-D intake | 40 | 1,566,662 | Incidence | 4 | N/A | RR = 0.90 (0.80–1.03) | N/A |
| Wei 2018 (a) [53] | Lung cancer | Case-control, cohort | Serum 25(OH)D | 16 | 280,127 | Incidence | 12 | N/A | RR = 1.04 (0.94–1.15) | N/A |
| Wei 2018 (b) [53] | Lung cancer | Case-control, cohort | Vit-D intake | 16 | 280,127 | Incidence | 5 | N/A | RR = 0.85 (0.74–0.98) | N/A |
| Xu 2021 [54] | Colorectal cancer | Case-control, cohort | Vit-D intake | 25 | 911,638 | Incidence | 21 | N/A | OR = 0.87 (0.82–0.92) | N/A |
| Zhang 2017 (a) [55] | Pancreatic cancer | Case-control, cohort | Serum 25(OH)D | 12 | 893,168 | Incidence, mortality | 5 | 5 | RR = 1.02 (0.66–1.57) | HR = 0.81 (0.68–0.96) |
| Zhang 2017 (b) [55] | Pancreatic cancer | Case-control, cohort | Vit-D intake | 12 | 893,168 | Incidence | 2 | N/A | RR = 1.11 (0.67–1.86) | N/A |
| Zhang 2019 [56] | Total cancer | RCTs | Vit-D intake | 10 | 81,362 | Incidence, mortality | 10 | 7 | RR = 0.99 (0.94–1.03) | RR = 0.87 (0.79–0.95) |
| Zhang 2021 [57] | Liver cancer | Cohort | Serum 25(OH)D | 6 | 6357 | Incidence | 6 | N/A | HR = 0.53 (0.41–0.68) | N/A |
| Zhang 2022 [58] | Total cancer | RCTs | Vit-D intake | 12 | 72,669 | Incidence, mortality | 11 | 6 | RR = 0.99 (0.93–1.06) | RR = 0.96 (0.80–1.16) |
| Zhao 2016 [59] | Bladder cancer | Case-control, cohort | Serum 25(OH)D | 7 | 90,757 | Incidence | 7 | N/A | OR = 0.76 (0.66–1.87) | N/A |
| Zhou 2020 [60] | Breast cancer | RCTs | Vit-D intake | 8 | 72,275 | Incidence | 6 | N/A | RR = 1.04 (0.85–1.29) | N/A |
| Analysis | Model | Number of Reports (n) | Effect Size (ES) (OR or RR) | 95% CI | p Value | I2 | p Value | Intercept | Tau (t) | p Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin D intake and cancer risk * | ||||||||||
| Total cancer | Fixed | 7 | 0.99 ** | 0.97–1.01 | 0.300 | 0.00 | 0.983 | 0.37 | 0.72 | 0.506 |
| Colorectal carcinoma | Random | 6 | 0.89 ** | 0.83–0.96 | 0.002 | 79.4 | <0.001 | −2.11 | −1.70 | 0.164 |
| Lung cancer | Fixed | 3 | 0.88 ** | 0.83–0.94 | <0.001 | 0.00 | 0.817 | −0.72 | −0.59 | 0.658 |
| RCTs *** | Fixed | 8 | 0.99 ** | 0.97–1.01 | 0.320 | 0.00 | 0.988 | 0.49 | 1.35 | 0.227 |
| Observational | Random | 14 | 0.90 ** | 0.86–0.95 | <0.001 | 68.43 | <0.001 | −1.09 | −1.51 | 0.156 |
| Serum 25 (OH)D levels and cancer risk * | ||||||||||
| Colorectal carcinoma | Fixed | 4 | 0.65 ** | 0.60–0.70 | <0.001 | 48.4 | 0.121 | 3.23 | 1.21 | 0.351 |
| Lung cancer | Random | 3 | 0.89 ** | 0.75–1.05 | 0.178 | 85.84 | 0.001 | −4.32 | −0.68 | 0.619 |
| Vitamin D intake and cancer related mortality * | ||||||||||
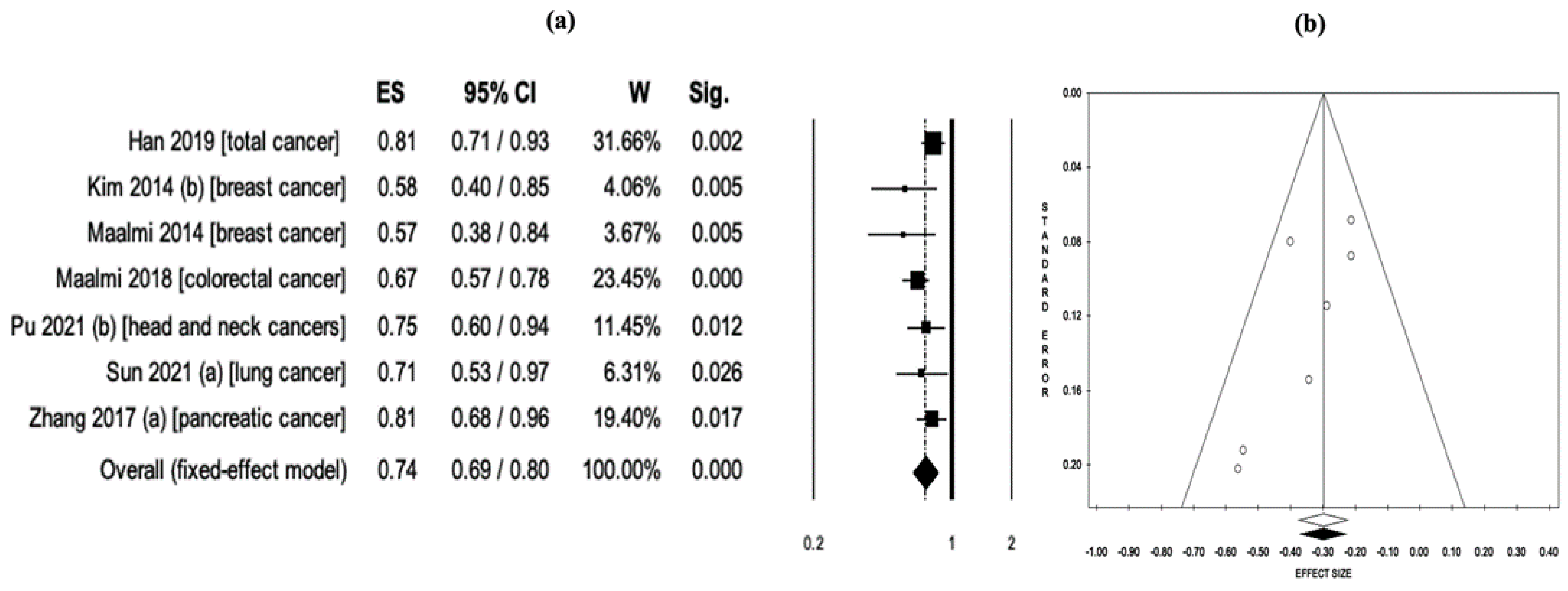
| Total cancer | Fixed | 7 | 0.89 **** | 0.85–0.93 | <0.001 | 0.00 | 0.929 | 0.77 | 0.98 | 0.372 |
| RCTs *** | Fixed | 7 | 0.89 **** | 0.85–0.93 | <0.001 | 0.00 | 0.929 | 0.77 | 0.98 | 0.372 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arayici, M.E.; Basbinar, Y.; Ellidokuz, H. Vitamin D Intake, Serum 25-Hydroxyvitamin-D (25(OH)D) Levels, and Cancer Risk: A Comprehensive Meta-Meta-Analysis Including Meta-Analyses of Randomized Controlled Trials and Observational Epidemiological Studies. Nutrients 2023, 15, 2722. https://doi.org/10.3390/nu15122722
Arayici ME, Basbinar Y, Ellidokuz H. Vitamin D Intake, Serum 25-Hydroxyvitamin-D (25(OH)D) Levels, and Cancer Risk: A Comprehensive Meta-Meta-Analysis Including Meta-Analyses of Randomized Controlled Trials and Observational Epidemiological Studies. Nutrients. 2023; 15(12):2722. https://doi.org/10.3390/nu15122722
Chicago/Turabian StyleArayici, Mehmet Emin, Yasemin Basbinar, and Hulya Ellidokuz. 2023. "Vitamin D Intake, Serum 25-Hydroxyvitamin-D (25(OH)D) Levels, and Cancer Risk: A Comprehensive Meta-Meta-Analysis Including Meta-Analyses of Randomized Controlled Trials and Observational Epidemiological Studies" Nutrients 15, no. 12: 2722. https://doi.org/10.3390/nu15122722
APA StyleArayici, M. E., Basbinar, Y., & Ellidokuz, H. (2023). Vitamin D Intake, Serum 25-Hydroxyvitamin-D (25(OH)D) Levels, and Cancer Risk: A Comprehensive Meta-Meta-Analysis Including Meta-Analyses of Randomized Controlled Trials and Observational Epidemiological Studies. Nutrients, 15(12), 2722. https://doi.org/10.3390/nu15122722







