Rice Germ Attenuates Chronic Unpredictable Mild Stress-Induced Muscle Atrophy
Abstract
1. Introduction
2. Materials and Methods
2.1. RG Preparation
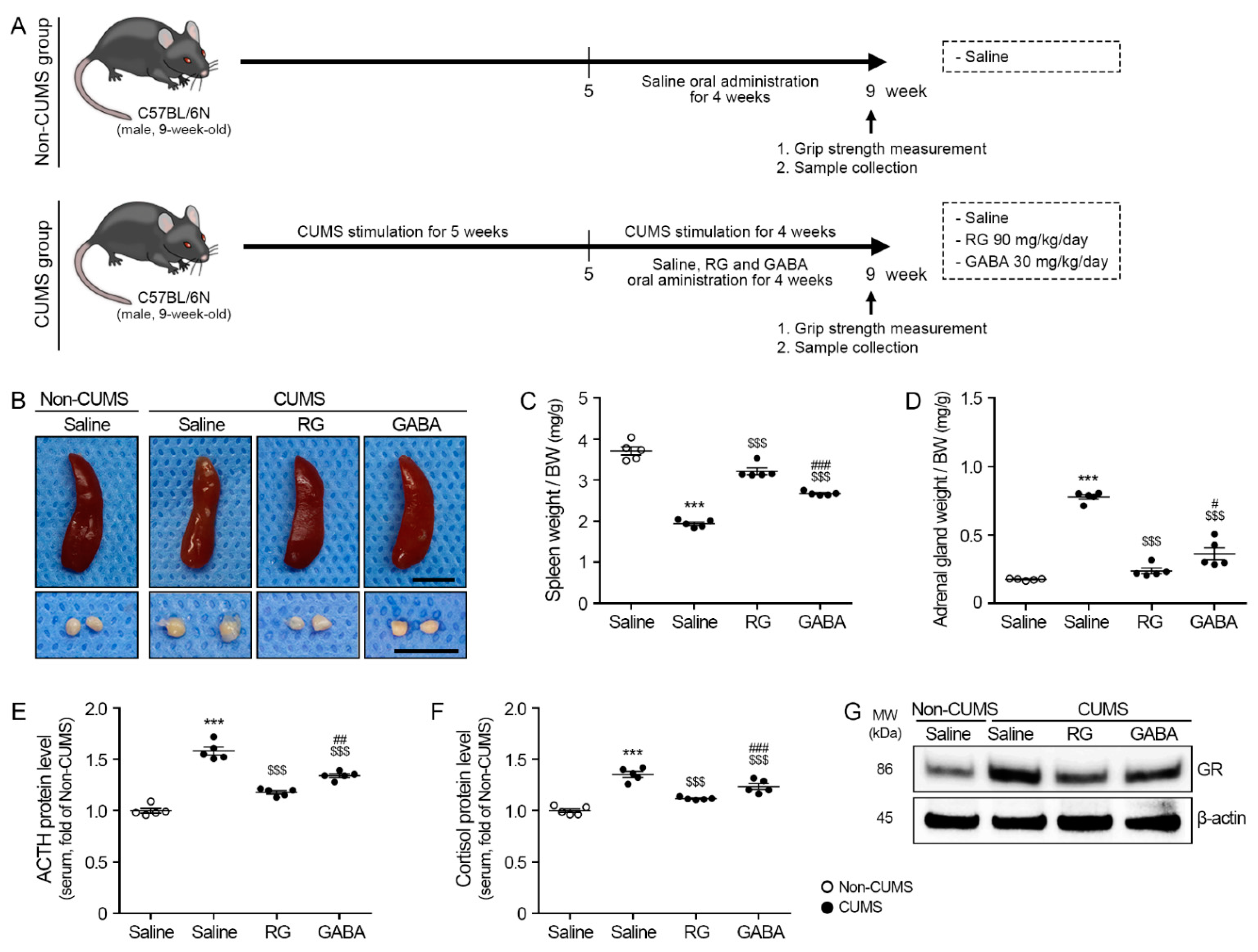
2.2. Induction of CUMS and Oral Administration of RG in Animals
- (1)
- Non-CUMS/Saline: Oral administration of saline at the same volume as the other group without stress.
- (2)
- CUMS/Saline: Oral administration of saline at the same volume as the other group with stress.
- (3)
- CUMS/RG 40: Oral administration of 40 mg/kg/day RG in saline with stress.
- (4)
- CUMS/RG 90: Oral administration of 90 mg/kg/day RG in saline with stress.
- (5)
- CUMS/RG 140: Oral administration of 140 mg/kg/day RG in saline with stress.
- (6)
- CUMS/GABA: Oral administration of 30 mg/kg/day GABA in saline with stress.
2.3. Grip Strength
2.4. Sample Collection
2.5. Sample Preparation
2.5.1. Serum Separation
2.5.2. Protein Isolation
2.5.3. RNA Extraction and cDNA Synthesis
2.5.4. Paraffin-Embedded Gastrocnemius Muscle Blocks
2.6. Indirect-Enzyme-Linked Immunosorbent Assay (Indirect-ELISA)
2.7. Sandwich-ELISA
2.8. Western Blotting
2.9. Quantitative Reverse Transcription–Polymerase Chain Reaction (qRT-PCR)
2.10. 3,3′-Diaminobenzidine (DAB) Staining
2.11. Hematoxylin and Eosin (H&E) Staining
2.12. Statistical Analysis
- *, Non-CUMS/Saline vs. CUMS/Saline
- $, CUMS/Saline vs. CUMS/RG or GABA
- #, CUMS/RG vs. CUMS/GABA
3. Results
3.1. RG Attenuated ACTH and Cortisol Levels
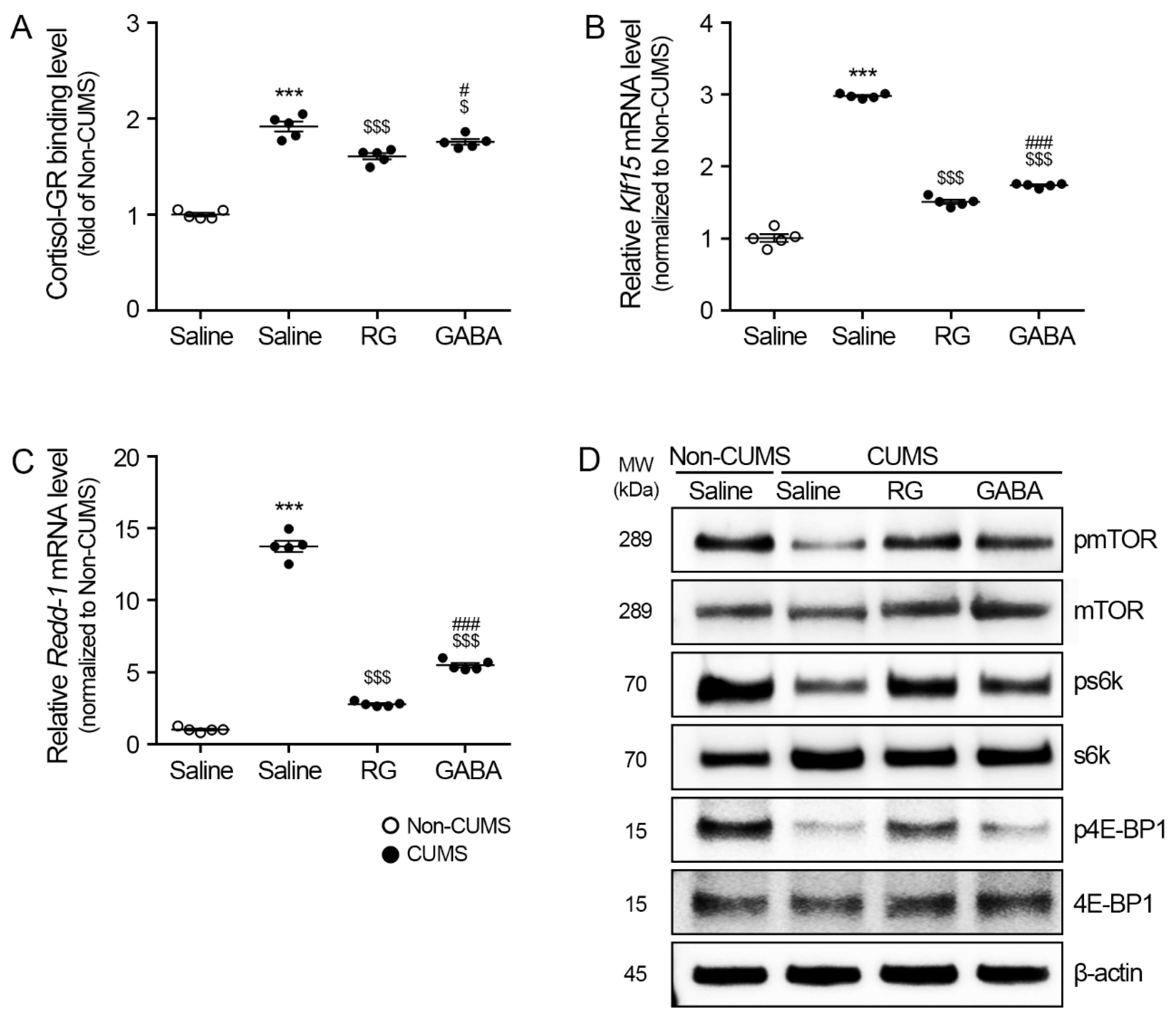
3.2. RG Attenuated Klf15/Redd-1 and Promoted mTOR/s6k and 4E-BP1 Levels
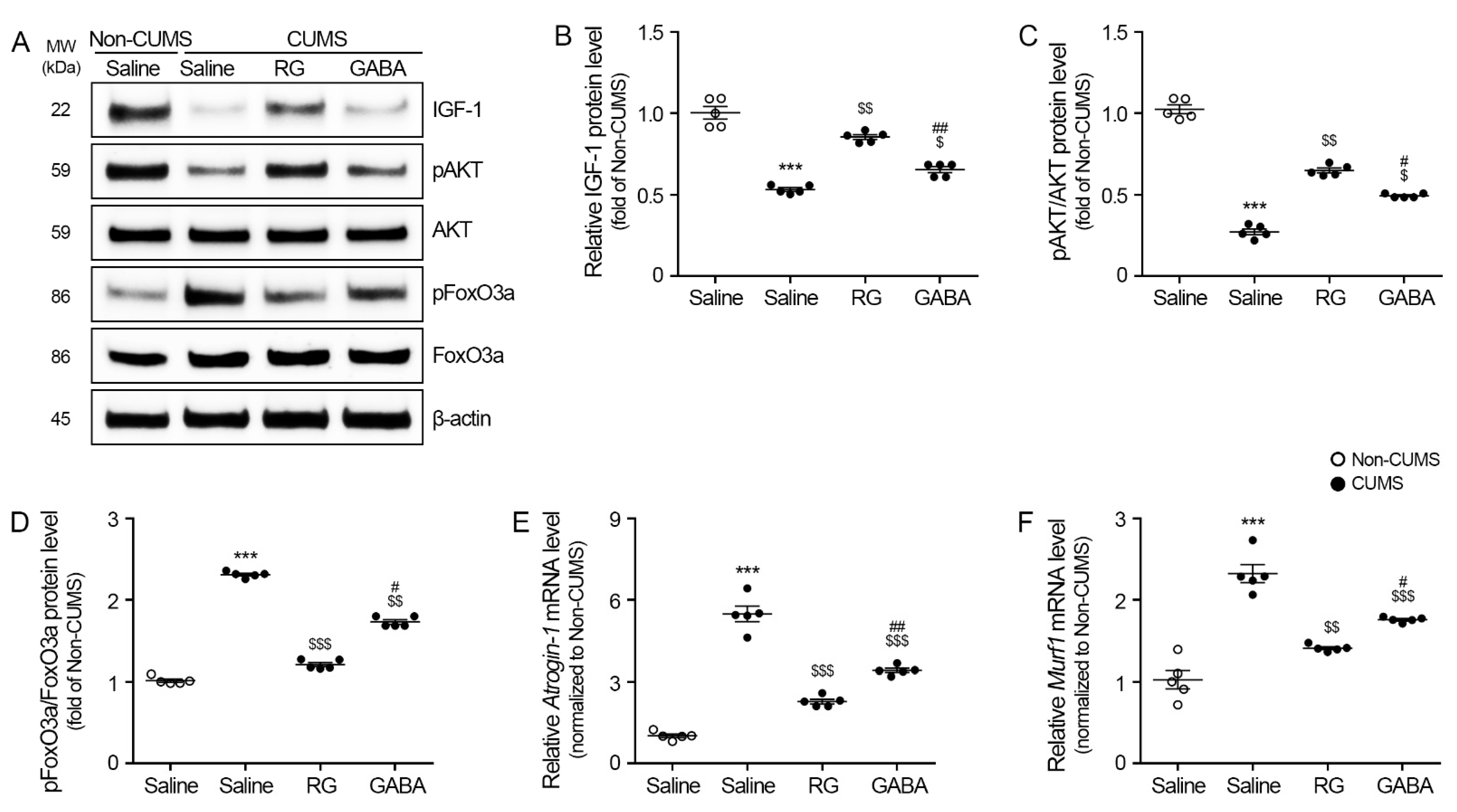
3.3. RG Promoted IGF-1/AKT and Attenuated FoxO3a, Atrogin-1, and MuRF1 Levels
3.4. RG Attenuated iNOS/p53 and Promoted Cyclin-Dependent Kinase 2 (CDK2)/Cyclin D1 Levels
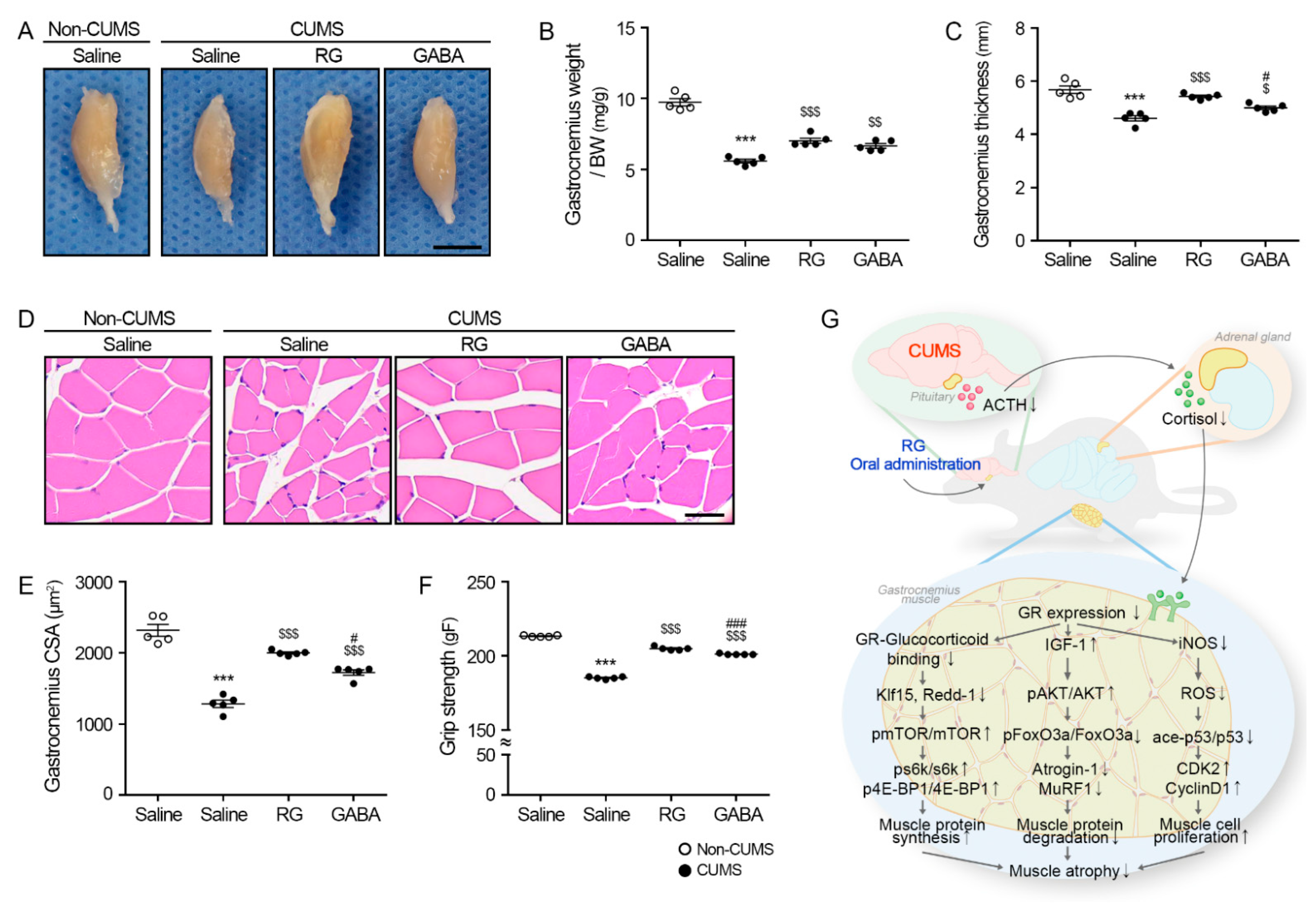
3.5. RG Attenuates Muscle Atrophy in CUMS Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pariante, C.M.; Lightman, S.L. The HPA axis in major depression: Classical theories and new developments. Trends Neurosci. 2008, 31, 464–468. [Google Scholar] [CrossRef]
- Cowen, P.J. Not fade away: The HPA axis and depression. Psychol. Med. 2010, 40, 1–4. [Google Scholar] [CrossRef]
- Doga, M.; Bonadonna, S.; Giustina, A. Glucocorticoids and bone: Cellular, metabolic and endocrine effects. Hormones 2004, 3, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Lamers, F.; Vogelzangs, N.; Merikangas, K.R.; de Jonge, P.; Beekman, A.T.; Penninx, B.W. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol. Psychiatry 2013, 18, 692–699. [Google Scholar] [CrossRef]
- Kaestner, F.; Hettich, M.; Peters, M.; Sibrowski, W.; Hetzel, G.; Ponath, G.; Arolt, V.; Cassens, U.; Rothermundt, M. Different activation patterns of proinflammatory cytokines in melancholic and non-melancholic major depression are associated with HPA axis activity. J. Affect. Disord. 2005, 87, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Harkness, K.L.; Monroe, S.M. Severe melancholic depression is more vulnerable than non-melancholic depression to minor precipitating life events. J. Affect. Disord. 2006, 91, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Sherin, J.E.; Nemeroff, C.B. Post-traumatic stress disorder: The neurobiological impact of psychological trauma. Dialogues Clin. Neurosci. 2011, 13, 263–278. [Google Scholar] [CrossRef]
- McEwen, B.S.; Sapolsky, R.M. Stress and cognitive function. Curr. Opin. Neurobiol. 1995, 5, 205–216. [Google Scholar] [CrossRef]
- Molina, P. Endocrine Physiology, 3rd ed.; McGraw-Hill Companies: New York, NY, USA, 2010; p. 301. [Google Scholar]
- Löfberg, E.; Gutierrez, A.; Wernerman, J.; Anderstam, B.; Mitch, W.; Price, S.R.; Bergström, J.; Alvestrand, A. Effects of high doses of glucocorticoids on free amino acids, ribosomes and protein turnover in human muscle. Eur. J. Clin. Investig. 2002, 32, 345–353. [Google Scholar] [CrossRef]
- Goldberg, A.L.; Tischler, M.; DeMartino, G.; Griffin, G. Hormonal regulation of protein degradation and synthesis in skeletal muscle. Fed. Proc. 1980, 39, 31–36. [Google Scholar]
- Landys, M.M.; Ramenofsky, M.; Wingfield, J.C. Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen. Comp. Endocrinol. 2006, 148, 132–149. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar]
- Mizoguchi, K.; Ishige, A.; Aburada, M.; Tabira, T. Chronic stress attenuates glucocorticoid negative feedback: Involvement of the prefrontal cortex and hippocampus. Neuroscience 2003, 119, 887–897. [Google Scholar] [CrossRef]
- Shimizu, N.; Yoshikawa, N.; Ito, N.; Maruyama, T.; Suzuki, Y.; Takeda, S.; Nakae, J.; Tagata, Y.; Nishitani, S.; Takehana, K.; et al. Crosstalk between glucocorticoid receptor and nutritional sensor mTOR in skeletal muscle. Cell Metab. 2011, 13, 170–182. [Google Scholar] [CrossRef]
- Kuo, T.; Lew, M.J.; Mayba, O.; Harris, C.A.; Speed, T.P.; Wang, J.C. Genome-wide analysis of glucocorticoid receptor-binding sites in myotubes identifies gene networks modulating insulin signaling. Proc. Natl. Acad. Sci. USA 2012, 109, 11160–11165. [Google Scholar] [CrossRef]
- Surjit, M.; Ganti, K.P.; Mukherji, A.; Ye, T.; Hua, G.; Metzger, D.; Li, M.; Chambon, P. Widespread negative response elements mediate direct repression by agonist-liganded glucocorticoid receptor. Cell 2011, 145, 224–241. [Google Scholar] [CrossRef]
- Seene, T.; Kaasik, P.; Pehme, A.; Alev, K.; Riso, E.M. The effect of glucocorticoids on the myosin heavy chain isoforms’ turnover in skeletal muscle. J. Steroid Biochem. Mol. Biol. 2003, 86, 201–206. [Google Scholar] [CrossRef]
- Amirouche, A.; Durieux, A.C.; Banzet, S.; Koulmann, N.; Bonnefoy, R.; Mouret, C.; Bigard, X.; Peinnequin, A.; Freyssenet, D. Down-regulation of Akt/mammalian target of rapamycin signaling pathway in response to myostatin overexpression in skeletal muscle. Endocrinology 2009, 150, 286–294. [Google Scholar] [CrossRef]
- Gilson, H.; Schakman, O.; Combaret, L.; Lause, P.; Grobet, L.; Attaix, D.; Thissen, J.P. Myostatin gene deletion prevents glucocorticoid-induced muscle atrophy. Endocrinology 2007, 148, 452–460. [Google Scholar] [CrossRef]
- Durieux, A.C.; Amirouche, A.; Banzet, S.; Koulmann, N.; Bonnefoy, R.; Pasdeloup, M.; Mouret, C.; Bigard, X.; Peinnequin, A.; Freyssenet, D. Ectopic expression of myostatin induces atrophy of adult skeletal muscle by decreasing muscle gene expression. Endocrinology 2007, 148, 3140–3147. [Google Scholar] [CrossRef]
- Bodine, S.C.; Furlow, J.D. Glucocorticoids and Skeletal Muscle. Adv. Exp. Med. Biol. 2015, 872, 145–176. [Google Scholar] [PubMed]
- Flaherty, R.L.; Owen, M.; Fagan-Murphy, A.; Intabli, H.; Healy, D.; Patel, A.; Allen, M.C.; Patel, B.A.; Flint, M.S. Glucocorticoids induce production of reactive oxygen species/reactive nitrogen species and DNA damage through an iNOS mediated pathway in breast cancer. Breast Cancer Res. 2017, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; You, Z.; Huang, X.; Dai, J.; Zhang, J.; Nie, S.; Xu, L.; Jiang, J.; Xu, J. Oxidative stress-induced premature senescence and aggravated denervated skeletal muscular atrophy by regulating progerin-p53 interaction. Skelet. Muscle 2022, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Vigneron, A.; Vousden, K.H. p53, ROS and senescence in the control of aging. Aging 2010, 2, 471–474. [Google Scholar] [CrossRef]
- Depke, M.; Fusch, G.; Domanska, G.; Geffers, R.; Völker, U.; Schuett, C.; Kiank, C. Hypermetabolic syndrome as a consequence of repeated psychological stress in mice. Endocrinology 2008, 149, 2714–2723. [Google Scholar] [CrossRef]
- Allen, D.L.; McCall, G.E.; Loh, A.S.; Madden, M.C.; Mehan, R.S. Acute daily psychological stress causes increased atrophic gene expression and myostatin-dependent muscle atrophy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R889–R898. [Google Scholar] [CrossRef]
- Engelbrecht, A.M.; Smith, C.; Neethling, I.; Thomas, M.; Ellis, B.; Mattheyse, M.; Myburgh, K.H. Daily brief restraint stress alters signaling pathways and induces atrophy and apoptosis in rat skeletal muscle. Stress 2010, 13, 132–141. [Google Scholar] [CrossRef]
- Batsukh, S.; Oh, S.; Rheu, K.; Lee, B.J.; Park, C.H.; Son, K.H.; Byun, K. Rice Germ Ameliorated Chronic Unpredictable Mild Stress-Induced Depressive-like Behavior by Reducing Neuroinflammation. Nutrients 2022, 14, 5382. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wu, H.R.; Zhang, S.S.; Xiao, H.L.; Yu, J.; Ma, Y.Y.; Zhang, Y.D.; Liu, Q. Catalpol ameliorates depressive-like behaviors in CUMS mice via oxidative stress-mediated NLRP3 inflammasome and neuroinflammation. Transl. Psychiatry 2021, 11, 353. [Google Scholar] [CrossRef]
- Yan, Z.; Jiao, H.; Ding, X.; Ma, Q.; Li, X.; Pan, Q.; Wang, T.; Hou, Y.; Jiang, Y.; Liu, Y.; et al. Xiaoyaosan Improves Depressive-Like Behaviors in Mice through Regulating Apelin-APJ System in Hypothalamus. Molecules 2018, 23, 1073. [Google Scholar] [CrossRef]
- Yun, B.; Yoo, J.Y.; Park, M.R.; Ryu, S.; Lee, W.J.; Choi, H.J.; Kang, M.K.; Kim, Y.; Oh, S. Ingestion of Gouda Cheese Ameliorates the Chronic Unpredictable Mild Stress in Mice. Food Sci. Anim. Resour. 2020, 40, 145–153. [Google Scholar] [CrossRef]
- Bonetto, A.; Andersson, D.C.; Waning, D.L. Assessment of muscle mass and strength in mice. BoneKEy Rep. 2015, 4, 732. [Google Scholar] [CrossRef]
- Ionescu, A.; Zahavi, E.E.; Gradus, T.; Ben-Yaakov, K.; Perlson, E. Compartmental microfluidic system for studying muscle-neuron communication and neuromuscular junction maintenance. Eur. J. Cell Biol. 2016, 95, 69–88. [Google Scholar] [CrossRef]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinforma Biomath. 2013, 3, 71–85. [Google Scholar]
- Kangralkar, V.A.; Patil, S.D.; Bandivadekar, R.M. Oxidative stress and diabetes: A review. Int. J. Pharm. Appl. 2010, 1, 38–45. [Google Scholar]
- Sears, R.C.; Nevins, J.R. Signaling networks that link cell proliferation and cell fate. J. Biol. Chem. 2002, 277, 11617–11620. [Google Scholar] [CrossRef]
- Dyson, N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998, 12, 2245–2262. [Google Scholar] [CrossRef]
- Pawlaczyk, B. The role of hormones in the regulation of human body homeostasis. Homines Hominibus 2010, 6, 7–20. [Google Scholar]
- Miklós, I.H.; Kovács, K.J. GABAergic innervation of corticotropin-releasing hormone (CRH)-secreting parvocellular neurons and its plasticity as demonstrated by quantitative immunoelectron microscopy. Neuroscience 2002, 113, 581–592. [Google Scholar] [CrossRef]
- Szosland-Fałtyn, A.; Królasik, J. Fermented dairy as a source of gamma aminobutyric acid. Food Ind. 2014, 68, 30–32. [Google Scholar]
- Lewicki, P.P. Sprouted seeds as a source of valuable nutrients. Food Sci. Technol. Qual. 2010, 6, 18–33. [Google Scholar]
- Yoshida, S.I.; Haramoto, M.; Fukuda, T.; Mizuno, H.; Tanaka, A.; Nishimura, M.; Nishihira, J. Optimization of a γ-aminobutyric acid (GABA) enrichment process for Hokkaido white rice and the effects of GABA-enriched white rice on stress relief in humans. Nippon. Shokuhin Kagaku Kogaku Kaishi J. Jpn. Soc. Food Sci. Technol. 2015, 62, 95–103. [Google Scholar] [CrossRef]
- Isingrini, E.; Camus, V.; Le Guisquet, A.M.; Pingaud, M.; Devers, S.; Belzung, C. Association between repeated unpredictable chronic mild stress (UCMS) procedures with a high fat diet: A model of fluoxetine resistance in mice. PLoS ONE 2010, 5, 10404. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Radhakrishnan, M.; Jindal, A.; Devadoss, T.; Dhar, A.K. Neuropharmacological evaluation of a novel 5-HT3 receptor antagonist (6g) on chronic unpredictable mild stress-induced changes in behavioural and brain oxidative stress parameters in mice. Indian J. Pharmacol. 2014, 46, 191–196. [Google Scholar] [CrossRef]
- Pesarico, A.P.; Sartori, G.; Brüning, C.A.; Mantovani, A.C.; Duarte, T.; Zeni, G.; Nogueira, C.W. A novel isoquinoline compound abolishes chronic unpredictable mild stress-induced depressive-like behavior in mice. Behav. Brain Res. 2016, 307, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Shepard, R.; Coutellier, L. Changes in the prefrontal glutamatergic and parvalbumin systems of mice exposed to unpredictable chronic stress. Mol. Neurobiol. 2018, 55, 2591–2602. [Google Scholar] [CrossRef] [PubMed]
- Gáll, Z.; Farkas, S.; Albert, Á.; Ferencz, E.; Vancea, S.; Urkon, M.; Kolcsár, M. Effects of chronic cannabidiol treatment in the rat chronic unpredictable mild stress model of depression. Biomolecules 2020, 10, 801. [Google Scholar] [CrossRef]
- O’Leary, O.F.; Cryan, J.F. Towards translational rodent models of depression. Cell Tissue Res. 2013, 354, 141–153. [Google Scholar] [CrossRef]
- Hill, M.N.; Hellemans, K.G.; Verma, P.; Gorzalka, B.B.; Weinberg, J. Neurobiology of chronic mild stress: Parallels to major depression. Neurosci. Biobehav. Rev. 2012, 36, 2085–2117. [Google Scholar] [CrossRef]
- Gokul, M.; Arun Kumar, N.; Kini, R.D.; Blossom, V.; Kodavanji, B.; Noojibail, A.; Murali, N.; Rai, S.P.V. Evaluation of biomarkers of stress in chronic stress-exposed comorbid depression model Wistar rats. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 30. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Myers, B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 2016, 6, 603. [Google Scholar]
- Sandri, M.; Lin, J.; Handschin, C.; Yang, W.; Arany, Z.P.; Lecker, S.H.; Goldberg, A.L.; Spiegelman, B.M. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc. Natl. Acad. Sci. USA 2006, 103, 16260–16265. [Google Scholar] [CrossRef]
- Dekhuijzen, P.N.; Gayan-Ramirez, G.; Bisschop, A.N.J.A.; De Bock, V.; Dom, R.E.N.É.; Decramer, M. Corticosteroid treatment and nutritional deprivation cause a different pattern of atrophy in rat diaphragm. J. Appl. Physiol. 1995, 78, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Kelly, F.J.; McGrath, J.A.; Goldspink, D.F.; Cullen, M.J. A morphological/biochemical study on the actions of corticosteroids on rat skeletal muscle. Muscle Nerve 1986, 9, 1–10. [Google Scholar] [CrossRef]
- Subramaniam, S.; Sabran, M.R.; Stanslas, J.; Kirby, B.P. Effect of aflatoxin B1 exposure on the progression of depressive-like behavior in rats. Front. Nutr. 2022, 9, 1032810. [Google Scholar] [CrossRef]
- Fulle, S.; Protasi, F.; Di Tano, G.; Pietrangelo, T.; Beltramin, A.; Boncompagni, S.; Fanò, G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp. Gerontol. 2004, 39, 17–24. [Google Scholar] [CrossRef]
- Jackson, M.J. Reactive oxygen species in sarcopenia: Should we focus on excess oxidative damage or defective redox signalling? Mol. Asp. Med. 2016, 50, 33–40. [Google Scholar] [CrossRef]
- Meng, S.J.; Yu, L.J. Oxidative stress, molecular inflammation and sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509–1526. [Google Scholar] [CrossRef]
- Fanzani, A.; Conraads, V.M.; Penna, F.; Martinet, W. Molecular and cellular mechanisms of skeletal muscle atrophy: An update. J. Cachexia Sarcopenia Muscle 2010, 3, 163–179. [Google Scholar] [CrossRef]
- Sakuma, K.; Yamaguchi, A. Molecular mechanisms controlling skeletal muscle mass. In Muscle Cell and Tissue; InTech: London, UK, 2015; Volume 484. [Google Scholar]
- Welle, S.; Brooks, A.I.; Delehanty, J.M.; Needler, N.; Thornton, C.A. Gene expression profile of aging in human muscle. Physiol. Genom. 2003, 14, 149–159. [Google Scholar] [CrossRef]
- Fox, D.K.; Ebert, S.M.; Bongers, K.S.; Dyle, M.C.; Bullard, S.A.; Dierdorff, J.M.; Kunkel, S.D.; Adams, C.M. p53 and ATF4 mediate distinct and additive pathways to skeletal muscle atrophy during limb immobilization. Am. J. Physiol. Endocrinol. Metab. 2014, 307, 245–261. [Google Scholar] [CrossRef]
- Reed, S.M.; Quelle, D.E. p53 Acetylation: Regulation and Consequences. Cancers 2014, 7, 30–69. [Google Scholar] [CrossRef]
- Luo, J.; Su, F.; Chen, D.; Shiloh, A.; Gu, W. Deacetylation of p53 modulates its effect on cell growth and apoptosis. Nature 2000, 408, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Lai, C.H.; Zhao, X.; Saito, S.; Hamilton, M.H.; Appella, E.; Yao, T.P. p300/CBP-mediated p53 acetylation is commonly induced by p53-activating agents and inhibited by MDM2. EMBO J. 2001, 20, 1331–1340. [Google Scholar] [CrossRef]
- Mankhong, S.; Kim, S.; Moon, S.; Kwak, H.B.; Park, D.H.; Kang, J.H. Experimental Models of Sarcopenia: Bridging Molecular Mechanism and Therapeutic Strategy. Cells 2020, 9, 1385. [Google Scholar] [CrossRef] [PubMed]
- Kelman, Z. PCNA: Structure, functions and interactions. Oncogene 1997, 14, 629–640. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.G.; da Silva Lauxen, I.; Chaves, A.C.; Rados, P.V.; Sant’Ana Filho, M. Immunohistochemical analysis of the patterns of p53 and PCNA expression in odontogenic cystic lesions. Med. Oral. Patol. Oral. Y Cir. Buccal 2008, 13, 275–280. [Google Scholar]
- Xie, Z.X.; Xia, S.F.; Qiao, Y.; Shi, Y.H.; Le, G.W. Effect of GABA on oxidative stress in the skeletal muscles and plasma free amino acids in mice fed high-fat diet. J. Anim. Physiol. Anim. Nutr. 2015, 99, 492–500. [Google Scholar] [CrossRef]
- Oh, S.; Choi, C.H.; Lee, B.J.; Park, J.H.; Son, K.H.; Byun, K. Fermented oyster extract attenuated dexamethasone-induced muscle atrophy by decreasing oxidative stress. Molecules 2021, 26, 7128. [Google Scholar] [CrossRef]
- Rondanelli, M.; Miccono, A.; Peroni, G.; Nichetti, M.; Infantino, V.; Spadaccini, D.; Alalwan, T.A.; Faliva, M.A.; Perna, S. Rice germ macro- and micronutrients: A new opportunity for the nutraceutics. Nat. Prod. Res. 2021, 35, 1532–1536. [Google Scholar] [CrossRef]
- Olaniyan, E.T.; O’Halloran, F.; McCarthy, A.L. Dietary protein considerations for muscle protein synthesis and muscle mass preservation in older adults. Nutr. Res. Rev. 2021, 34, 147–157. [Google Scholar] [CrossRef]
- Ohno, Y.; Ando, K.; Ito, T.; Suda, Y.; Matsui, Y.; Oyama, A.; Kaneko, H.; Yokoyama, S.; Egawa, T.; Goto, K. Lactate Stimulates a Potential for Hypertrophy and Regeneration of Mouse Skeletal Muscle. Nutrients 2019, 11, 869. [Google Scholar] [CrossRef]
- Senior, H.E.; Henwood, T.R.; Beller, E.M.; Mitchell, G.K.; Keogh, J.W. Prevalence and risk factors of sarcopenia among adults living in nursing homes. Maturitas 2015, 82, 418–423. [Google Scholar] [CrossRef]
- Ohara, D.G.; Pegorari, M.S.; Oliveira Dos Santos, N.L.; de Fátima Ribeiro Silva, C.; Monteiro, R.L.; Matos, A.P.; Jamami, M. Respiratory muscle strength as a discriminator of sarcopenia in community-dwelling elderly: A cross-sectional study. J. Nutr. Health Aging 2018, 22, 952–958. [Google Scholar] [CrossRef]
- Lecker, S.H.; Goldberg, A.L.; Mitch, W.E. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J. Am. Soc. Nephrol. 2006, 17, 1807–1819. [Google Scholar] [CrossRef]
- Jackman, R.W.; Kandarian, S.C. The molecular basis of skeletal muscle atrophy. Am. J. Physiol.-Cell Physiol. 2004, 287, C834–C843. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.L.; Shubert, T.; Doyle, J.; Soher, B.; Sakkas, G.K.; Kent-Braun, J.A. Muscle atrophy in patients receiving hemodialysis: Effects on muscle strength, muscle quality, and physical function. Kidney Int. 2003, 63, 291–297. [Google Scholar] [CrossRef]
- Sishi, B.; Loos, B.; Ellis, B.; Smith, W.; du Toit, E.F.; Engelbrecht, A.M. Diet-induced obesity alters signalling pathways and induces atrophy and apoptosis in skeletal muscle in a prediabetic rat model. Exp. Physiol. 2011, 96, 179–193. [Google Scholar] [CrossRef]
- Pasini, E.; Aquilani, R.; Gheorghiade, M.; Dioguardi, F.S. Malnutrition, muscle wasting and cachexia in chronic heart failure: The nutritional approach. Ital. Heart J. 2003, 4, 232–235. [Google Scholar] [PubMed]
- Haller, R.G.; Knochel, J.P. Skeletal muscle disease in alcoholism. Med. Clin. N. Am. 1984, 68, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Rom, O.; Kaisari, S.; Aizenbud, D.; Reznick, A.Z. Sarcopenia and smoking: A possible cellular model of cigarette smoke effects on muscle protein breakdown. Ann. N. Y. Acad. Sci. 2012, 1259, 47–53. [Google Scholar] [CrossRef]
- Papadopoulou, S.K. Sarcopenia: A Contemporary Health Problem among Older Adult Populations. Nutrients 2020, 12, 1293. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Bartolomucci, A.; Buwalda, B.; de Boer, S.F.; Flügge, G.; Korte, S.M.; Meerlo, P.; Murison, R.; Olivier, B.; Palanza, P.; et al. Stress revisited: A critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 2011, 35, 1291–1301. [Google Scholar] [CrossRef]
- Black, P.H. Stress and the inflammatory response: A review of neurogenic inflammation. Brain Behav. Immun. 2002, 16, 622–653. [Google Scholar] [CrossRef]
- Poornima, K.N.; Karthick, N.; Sitalakshmi, R. Study of the effect of stress on skeletal muscle function in geriatrics. J. Clin. Diagn. Res. 2014, 8, 8–9. [Google Scholar] [CrossRef]
- Branth, S.; Ronquist, G.; Stridsberg, M.; Hambraeus, L.; Kindgren, E.; Olsson, R.; Carlander, D.; Arnetz, B. Development of abdominal fat and incipient metabolic syndrome in young healthy men exposed to long-term stress. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 427–435. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Sheffield-Moore, M.; Cree, M.G.; Hewlings, S.J.; Aarsland, A.; Wolfe, R.R.; Ferrando, A.A. Atrophy and impaired muscle protein synthesis during prolonged inactivity and stress. J. Clin. Endocrinol. Metab. 2006, 91, 4836–4841. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batsukh, S.; Oh, S.; Rheu, K.; Lee, B.-J.; Choi, C.H.; Son, K.H.; Byun, K. Rice Germ Attenuates Chronic Unpredictable Mild Stress-Induced Muscle Atrophy. Nutrients 2023, 15, 2719. https://doi.org/10.3390/nu15122719
Batsukh S, Oh S, Rheu K, Lee B-J, Choi CH, Son KH, Byun K. Rice Germ Attenuates Chronic Unpredictable Mild Stress-Induced Muscle Atrophy. Nutrients. 2023; 15(12):2719. https://doi.org/10.3390/nu15122719
Chicago/Turabian StyleBatsukh, Sosorburam, Seyeon Oh, Kyoungmin Rheu, Bae-Jin Lee, Chang Hu Choi, Kuk Hui Son, and Kyunghee Byun. 2023. "Rice Germ Attenuates Chronic Unpredictable Mild Stress-Induced Muscle Atrophy" Nutrients 15, no. 12: 2719. https://doi.org/10.3390/nu15122719
APA StyleBatsukh, S., Oh, S., Rheu, K., Lee, B.-J., Choi, C. H., Son, K. H., & Byun, K. (2023). Rice Germ Attenuates Chronic Unpredictable Mild Stress-Induced Muscle Atrophy. Nutrients, 15(12), 2719. https://doi.org/10.3390/nu15122719





