Highlights
What are the main findings?
- Colorectal cancer (CRC) patients with Vitamin D deficiency had notably poorer long-term survival outcomes compared to those with adequate vitamin D status.
- We observed a clear survival benefit from adequate Vitamin D status in GG carriers of the Cdx2 vitamin D receptor gene, compared to those with AA/AG genotypes.
What is the implication of the main finding?
- Vitamin D supplementation might offer a tailored therapeutic advantage in improving survival outcomes, particularly for CRC patients with Vitamin D deficiency and the GG Cdx2 genotype.
- Further research, including randomized trials, are needed to explore whether Vitamin D supplementation can be recommended as a supportive therapy for improving CRC prognosis in these patients.
Abstract
According to recent evidence, the prognostic value of Vitamin D (VitD) status for colorectal cancer (CRC) patients might be confined to patients with the GG genotype of Cdx2, a functional polymorphism of the VitD receptor gene. We aimed to validate these findings in a cohort of CRC patients. Post-operative serum 25-hydroxyvitamin D concentration was determined by mass spectrometry and Cdx2 genotyping was performed from blood or buccal swabs using standard methods. Joint associations of VitD status and Cdx2 with overall survival (OS), CRC-specific survival (CSS), recurrence-free survival (RFS), and disease-free survival (DFS) were assessed using Cox regression. For patients with GG genotype, adjusted hazard ratios (95% confidence interval) for the associations of sufficient compared with deficient VitD were 0.63 (0.50–0.78), 0.68 (0.50–0.90), 0.66 (0.51–0.86), and 0.62 (0.50–0.77) for OS, CSS, RFS, and DFS, respectively. These associations were weaker and not statistically significant for the AA/AG genotype. Interaction between VitD status and genotype did not reach statistical significance. VitD deficiency is an independent predictor of poorer survival, particularly for the GG Cdx2 carriers, suggesting a potential role of VitD supplementation according to VitD status and genotype, which should be evaluated in randomised trials.
1. Introduction
The global disease burden of colorectal cancer (CRC) is among the highest of all cancers, accounting for more than 900,000 deaths each year []. Prognosis strongly depends on stage at diagnosis. However, a number of factors beyond stage may also be crucial for prognosis and, to the extent they are modifiable, may offer opportunities of targeted tertiary prevention. Vitamin D (VitD) deficiency and insufficiency among CRC patients has been associated with a significantly worse prognosis of these patients compared to those with adequate VitD status even after thorough adjustment for potential confounders [,,,]. This observation has raised the hypothesis that VitD supplementation may enhance prognosis of CRC patients.
A systematic review and meta-analysis of randomised VitD supplementation trials found a significant 30% reduction in adverse CRC outcomes with supplementation, which could have tremendous clinical value []. This finding is the more remarkable, as it was derived from studies in which VitD supplementation was provided regardless of initial VitD status, and other potential determinants of effectiveness of VitD supplementation. One particularly important factor in this context could be genetically determined vitamin D receptor (VDR) function.
In two large cohorts of CRC patients from the UK, a strong inverse association between 25-hydroxyvitamin D (25(OH)D), the best-established biomarker of VitD status, and CRC-specific survival was observed among patients with the GG genotype of rs11568820 (also known as Cdx2), a functional polymorphism located in the promotor region of the VDR gene, whereas this association was not seen among other patients []. Identifying patients most likely to benefit from VitD supplementation could pave the way for more effective, personalised VitD supplementation of CRC patients. The objective of this study was to thoroughly assess the joint association of serum 25(OH)D levels and the Cdx2 polymorphisms with various survival outcomes in a cohort of CRC patients from Germany.
2. Materials and Methods
2.1. Study Details
We used data and serum samples of the DACHS-study, a population-based case-control study with long-term follow-up of patients with a first diagnosis of CRC recruited in south-west Germany between 2003 and 2021. The DACHS-study adheres to the guidelines of the Declaration of Helsinki. It was approved by the state medical boards of Baden-Württemberg and Rhineland-Palatinate, and the University of Heidelberg ethics committees (ethical code: 310/2001 approved on 6 December 2001). All participants provided written informed consent.
Details of the DACHS-study have been previously reported [,,,,]. In summary, patients who were eligible were identified from 22 participating clinics based on a first diagnosis of CRC (International Classification of Diseases, Tenth Revision [ICD-10] codes C18–C20). These patients were informed about the study shortly before or after surgery through clinicians or shortly after discharge by mail. Trained interviewers used standardised questionnaires to collect sociodemographic, lifestyle history, and medical information from study participants in personal interviews. Medical data on tumour stage, site, and therapy were obtained from hospital charts. Blood samples were collected after the personal interviews and serum aliquots were stored at −80 °C. Study participants were followed-up on therapy and health outcomes at 3-, 5-, and 10-year time points after diagnosis of CRC. Data on vital status were obtained from population registries and information on cause of death was obtained from health authorities, while details of recurrence and treatment were collected using standardised follow-up questionnaires. For the current study, 2819 patients with incident CRC and with both serum 25(OH)D measurements and Cdx2 genetic polymorphism information available were included, who were recruited from 2003 to 2010 and followed-up for a median time of about 10 years (Figure 1).
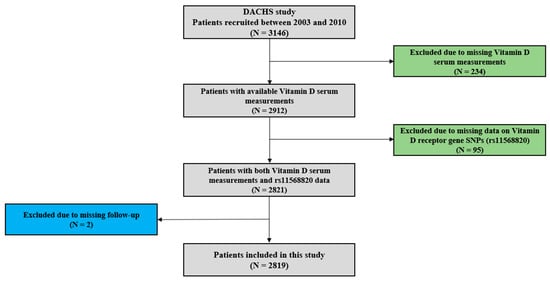
Figure 1.
Patient selection flow chart.
2.2. Serum Vitamin D Measurements
Serum 25(OH)D measurements were conducted at the German Cancer Research Centre using High Performance Liquid Chromatography–Electro Spray Ionisation–Mass Spectrometry (HPLC–ESI–MS). The HPLC–ESI–MS method was standardised using the Standard Reference Material (SRM) 972a developed by the National Institute of Standards and Technology (NIST) []. VitD status was defined by the serum 25(OH)D cut-offs according to the United States-American Institute of Medicine as follows: Deficient (<30 nmol/L), insufficient (30 to <50 nmol/L), sufficient (≥50 nmol/L) [].
2.3. Genotyping for Cdx2
Details for the determination of VDR gene single-nucleotide polymorphisms (SNPs) for this study have been reported elsewhere [,,]. In summary, DNA was extracted from blood samples or, in exceptional cases in which blood samples were not available, from buccal swab samples of participants using standard methods []. Genotyping was conducted using Illumina array technologies (San Diego, CA, USA) and PLINK software (version 1.9) was used to extract information about Cdx2 SNP genotypes AA, AG, and GG. For all analyses, Cdx2 genotypes were categorised into a binary variable, with the rarer variants AA + AG as one category and GG as the other category.
2.4. Outcomes
Survival outcomes of overall survival (OS), CRC-specific survival (CSS), recurrence-free survival (RFS), and disease-free survival (DFS) were defined as death from any cause, death from CRC, recurrence of or death from CRC, and recurrence of CRC or death from any cause, respectively. Times of follow-up for survival outcome endpoints were counted in days from the date of CRC diagnosis to the date of experiencing the event. Patients were censored at a date when they were last known to have been alive or free of recurrence if they did not reach a specific endpoint.
2.5. Statistical Analyses
Descriptive statistics were used to analyse population characteristics. Survival analysis was performed using Cox proportional hazard (PH) models to calculate hazard ratios (HRs) for the individual and joint associations of predictors [serum 25(OH)D and Cdx2 genetic variants] with survival outcomes (OS, CSS, RFS, and DFS). For joint associations, analyses were stratified by Cdx2 as a binary variable. Two different adjustment models were used to evaluate the predictor–outcome associations. Model 1 analyses were adjusted for sex (male/female), age (30–59/60–69/70–79/>80 years), and season of blood collection (winter, spring, summer, autumn). Model 2 analyses were further adjusted for tumour detection mode (screening/other), cancer site (colon/rectum) and stage (I–IV) at diagnosis, chemotherapy use (yes/no), surgery (yes/no), history of cardiovascular disease (CVD) (yes/no), diabetes (yes/no), hypertension (yes/no), lifetime smoking exposure (never/<10/10–19/20–29/≥30 pack-years), body mass index (BMI) (normal/overweight/obese), physical activity (quartiles of average lifetime Metabolic Equivalent of Task hours per week), and time between diagnosis and blood collection (<1 month/≥1 month). Interactions between 25(OH)D as a continuous variable and Cdx2 as a categorical variable with respect to survival were assessed by adding their product terms to model 2.
Cox PH model diagnostics were performed by evaluating interactions between time and covariates. Interactions between predictors and covariates were assessed by adding product terms to the regression models and evaluation of the corresponding Wald test statistics. Adjusted Kaplan–Meier (KM) survival curves were presented to assess survival outcomes according to serum 25(OH)D status and Cdx2 genotype. All statistical tests were performed using R-statistical software (version 4.2) and two-sided test significance levels were set at p-values < 0.05 for all analyses.
3. Results
3.1. Description of Patient Characteristics
A total of 2819 patients were included in our analyses (Table 1). Nearly 60% of patients were male, and the median age at diagnosis for the cohort was 69 years (interquartile range: 62–76 years). More than 50% of the patients were diagnosed in stages I or II, and about 14% in stage IV of CRC. A majority of 59% of patients had a serum 25(OH)D in the deficient range. Close to 65% of patients had a GG genotype for Cdx2. In addition, serum levels of 25(OH)D did not differ according to Cdx2 genotype. The proportion of patients with VitD deficiency was about 60% for all the three genotypes (GG, AG, and AA), while about 15% of patients had sufficient VitD status for all the genotypes (chi-square p-value = 0.64) (see Table 2). BMI interquartile range was 23.6–29.0 kg/m2 (median: 26.1 kg/m2) and about half of the patients were recruited within 30 days after primary diagnosis of CRC. After a median follow-up of 9.4 years, 1521 deaths were recorded and 798 of these were due to CRC.

Table 1.
Main characteristics of study population among 2819 colorectal cancer (CRC) patients.

Table 2.
Distribution of serum 25(OH)D level by Cdx2 genotype.
3.2. Vitamin D Status and Survival
Associations between categories of serum 25(OH)D levels and survival outcomes are shown in Table 3. After controlling for sex, age, and season of blood draw, significantly better survival was seen for both patients with VitD insufficiency and those with sufficient VitD compared to VitD deficient patients. Although the associations between VitD status and survival outcomes were attenuated after adjustment for all covariates of interest, they remained statistically significant. Fully adjusted HRs (95% CI) for sufficient versus deficient VitD status were 0.71 (0.59–0.84), 0.76 (0.60–0.95), 0.79 (0.64–0.98), and 0.69 (0.58–0.82) for OS, CSS, RFS, and DFS, respectively. No significant interactions were observed between VitD status and categorical covariates, and thus subgroup analyses for these variables were not conducted.

Table 3.
Individual associations of serum 25(OH)D concentration and Cdx2 genotype with the different survival outcomes.
3.3. VDR Cdx2 Locus Genotypes and Survival
Hazard ratios for the associations between Cdx2 genotypes and survival outcomes are also presented in Table 3. After controlling for sex, age, and season of blood draw, no significant associations were observed between VDR genotypes and any of the survival outcomes. Similar results were also observed after adjustment for all covariates of interest. Fully adjusted HRs (95% CI) for AA/AG genotype versus GG genotype were 0.99 (0.88–1.11), 0.93 (0.80–1.09), 0.97 (0.84–1.11), and 0.98 (0.88–1.10) for OS, CSS, RFS, and DFS, respectively.
3.4. Joint Associations of Vitamin D Status and VDR Cdx2 Locus Genotypes with Survival
Survival curves for the joint associations of VitD status and Cdx2 genotypes are shown in Figure 2. Among those with GG genotype, survival was consistently higher for all survival outcomes in both patients with sufficient and insufficient VitD status than in those with VitD deficiency. In contrast, no clear associations were seen between VitD status and the survival outcomes among patients with AA or AG genotype. These patterns were also confirmed in the multivariable analyses that are shown in Table 4. For patients with GG genotype, adjusted hazard ratios (95% CI) for those with sufficient VitD (25(OH)D > 50 nmol/L) or insufficient VitD (25(OH)D between 30 and 50 nmol/L) compared to those with deficient VitD (25(OH)D < 30 nmol/L) were 0.63 (0.50–0.78) and 0.69 (0.56–0.84) for OS, 0.68 (0.50–0.90) and 0.71 (0.55–0.92) for CSS, 0.66 (0.51–0.86) and 0.73 (0.58–0.91) for RFS, and 0.62 (0.50–0.77) and 0.68 (0.56–0.83) for DFS, respectively. Trend analyses for VitD status were also significant for all outcomes among patients with GG genotype (p-trend < 0.01). In contrast, no consistent patterns and no significant trends were seen among those with AA/AG genotype, except for the outcome of DFS (p-trend = 0.04). However, tests for interaction between VitD and genotype with respect to the survival outcomes did not reach statistical significance.
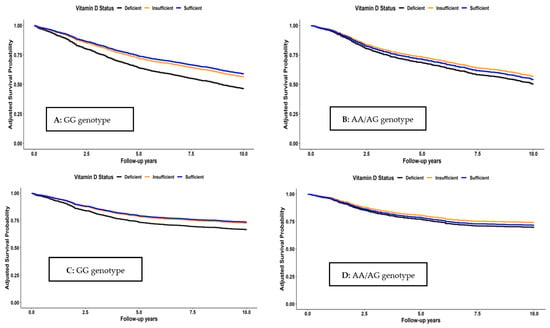
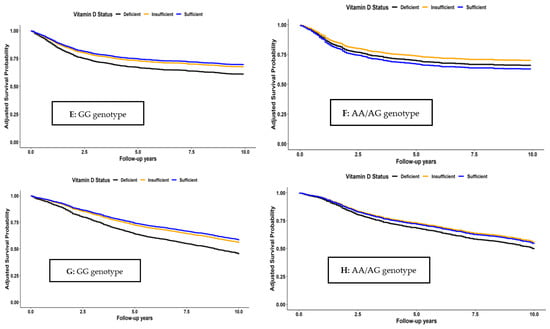
Figure 2.
Overall (A,B), CRC-specific (C,D), recurrence-free (E,F), and disease-free (G,H) survival according to vitamin D status and Cdx2 genotype.

Table 4.
Joint associations of serum 25(OH)D concentration and Cdx2 genotype with the different survival outcomes.
4. Discussion
In this large cohort of CRC patients, patients with deficient VitD status had significantly worse survival than patients with VitD in the insufficiency range or with sufficient VitD. Although this association was clearly seen in the majority of patients who carried the GG genotype of rs11568820 (Cdx2), this clear pattern was not seen among those with AA/AG genotype. However, tests of interaction between VitD status and genotype did not reach statistical significance.
4.1. Vitamin D Status and Survival
Our results showed significant associations for VitD status and survival outcomes independent of other established prognostic factors, such as stage at diagnosis. Our findings of substantially worse survival among patients with serum levels of 25(OH)D in the VitD deficiency range (<30 nmol/L) compared to those with higher concentrations are in agreement with previous observational studies [,,,]. In our study, these associations were evaluated and consistently observed for all of the four assessed major survival outcomes. Although these associations from observational studies cannot be taken as evidence for causality, they are highly consistent with findings of a recent meta-analysis of RCTs, in which a 30% lower risk for CSS and progression-free survival (PFS) outcomes by VitD supplementation among CRC patients has been found []. Although the exact mechanism by which VitD improves survival in CRC patients is not clear, mechanistic studies have reported that calcitriol (the active form of VitD) acts in various ways via VDRs expressed on human cells to regulate transcription of genes involved in metastasis [], cell proliferation [], angiogenesis, cell differentiation, apoptosis, and DNA repair []. Calcitriol may also play a key role in suppressing cancer development and progression through immune-inflammatory modulation [,].
4.2. VDR Cdx2 Locus Genotypes and Survival
Our findings of null associations between Cdx2 genotype and survival outcomes among CRC patients are consistent with previously reported null associations of VDR polymorphisms rs731236 (Taq1), rs2228570 (Fok1), Cdx2, and rs1989969 (VDR-5132) with OS and CSS in a significantly smaller (and partly overlapping) sample of CRC patients []. Whereas mixed results have been reported on the associations of Cdx2 with CRC incidence, studies on prognostic outcomes are rather limited [,,,,]. A meta-analysis published in 2016 found that the G-allele of the Cdx2 gene was associated with a 12% higher risk for CRC []. This protective role of the Cdx2 A-allele has previously been reported in a study that reported low risk of fractures among ethnic groups with higher A-allele frequencies. Higher frequency of the Cdx2 A-allele was observed among study subjects of African descent, followed by Asian and lastly Caucasian groups (74%, 43%, and 19%, respectively) []. These findings may also suggest a possible interference of Cdx2 with VitD status in the development and progression of cancer []. In another study by Ochs-Balcom et al., a strong and significant association between Cdx2 and risk of colon cancer was only observed for people with low BMI or waist circumference []. Therefore, the authors postulated a modifying effect of adiposity on this association. However, no effect on modification was observed in our study for the association between Cdx2 and survival outcomes by BMI.
The effects of VitD are mediated by the VDR, a member of the superfamily of nuclear receptors involved in regulation of a number of transcription genes. Consequently, cell response to VitD depends on the expression levels of the VDR []. CRC patients with low serum expression levels of VDR in a recent study have been reported to have poor prognosis compared to those with higher expression levels []. In addition, serum expression levels of VDR have been observed to be significantly lower for CRC patients than the general []. Future prognostic studies may need to consider both genotypes and serum expression levels of VDR.
4.3. Joint Associations of Vitamin D Status and Cdx2 Genotypes with Survival
Although tests for interaction between VitD status and Cdx2 genotype with respect to survival did not reach statistical significance in our cohort, the pattern of strong inverse associations between VitD and mortality among those with the GG genotype, and absence of these associations among those with the AA/AG genotype is highly consistent with observations from two somewhat smaller CRC patient cohorts from the UK (n = 1687 and n = 1848, respectively) []. The weaker association between VitD status and survival in the entire cohort and among those with the GG genotype in our study may be due to different categorisations of VitD status (commonly employed standard categories in our study, tertiles in the UK studies) and somewhat more comprehensive confounder adjustment in our study (adjustment for 10 covariates including chemotherapy use, smoking, and physical activity).
The Cdx2 SNP is located on the VDR gene at the 5′ end promoter region, and the polymorphism at the Cdx2 locus plays a key role in calcium regulation. In a previous study among 261 Japanese women, the G-allele has been reported to reduce VDR transcription through the elimination of the Cdx2 transcription binding site, while the A-allele was thought to upregulate VDR transcription []. In agreement with the results from the UK cohorts, results from our study, which, to the best of our knowledge, are the largest to investigate joint associations of VitD status and Cdx2 genotype with survival outcomes in CRC patients, do not seem to support advantages of those with the AA/AG genotype with respect to CRC survival in these Caucasian populations.
4.4. Strengths and Limitations
Strengths of our study include the large sample size of patients who were recruited from all (more than 20) clinics providing CRC surgery in a defined study region and comprehensively followed with respect to all of the four common survival outcomes, comprehensive ascertainment of clinical and lifestyle factors, and adjustment for potential confounding factors. Nevertheless, residual confounding by unmeasured or not perfectly measured covariates cannot be ruled out and causality cannot be established in this observational study. Finally, our study included almost exclusively patients of Caucasian origin, and results may not be generalised to populations with different ancestries.
5. Conclusions
Findings from this large cohort of CRC patients provide further evidence that post-surgery VitD status is a strong, independent, and potentially modifiable prognostic factor for CRC patients, the association being particularly strong among those with the GG Cdx2 genotype. Preliminary evidence from randomised trials supports suggestions that VitD supplementation may enhance prognosis of CRC patients. Given the dose-response relationship between VitD status and mortality outcomes, with worse survival being essentially restricted to those with VitD deficiency and (to a lesser extent) insufficiency, potential interventions, to be evaluated in further randomised trials, should focus on these groups of patients [,,]. If and to what extent VDR genotypes or other potential effect modifying factors deserve additional consideration for potential personalised tertiary prevention should be further explored in future research.
Author Contributions
Conceptualisation, T.G. and H.B.; methodology, T.G.; formal analysis, T.G.; writing—original draft preparation, T.G.; writing—review and editing, H.B., M.H., P.S.-K. and B.S.; supervision, H.B. All authors are responsible for the integrity and validity of the presented data. All authors have read and agreed to the published version of the manuscript.
Funding
The DACHS-study was supported by the German Research Council (BR 1704/6-1, BR 1704/6-3, BR 1704/6-4, CH 117/1-1, HO 5117/2-1, HE 5998/2-1, KL 2354/3-1, RO 2270/8-1, and BR 1704/17-1), the Interdisciplinary Research Program of the National Centre for Tumour Diseases (NCT), Germany, and the German Federal Ministry of Education and Research (01KH0404, 01ER0814, 01ER0815, 01ER1505A, 01ER1505B, and 01KD2104A). The funders had no role in study design and data analysis, publication decision, or manuscript preparation.
Institutional Review Board Statement
The study adheres to the standards of the Declaration of Helsinki. The DACHS-study was approved by the state medical boards of Baden-Württemberg and Rhineland-Palatinate, and the ethics committees of the University of Heidelberg.
Informed Consent Statement
All study participants gave their written and signed informed consent.
Data Availability Statement
For ethical reasons, the data are not publicly available but may be availed upon request. For more details about the DACHS-study, please refer to the official website: dachs.dkfz.org/dachs/, accessed on 1 July 2021.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zgaga, L.; Theodoratou, E.; Farrington, S.M.; Din, F.V.; Ooi, L.Y.; Glodzik, D.; Johnston, S.; Tenesa, A.; Campbell, H.; Dunlop, M.G. Plasma vitamin D concentration influences survival outcome after a diagnosis of colorectal cancer. J. Clin. Oncol. 2014, 32, 2430–2439. [Google Scholar] [CrossRef] [PubMed]
- Maalmi, H.; Walter, V.; Jansen, L.; Chang-Claude, J.; Owen, R.W.; Ulrich, A.; Schöttker, B.; Hoffmeister, M.; Brenner, H. Relationship of very low serum 25-hydroxyvitamin D(3) levels with long-term survival in a large cohort of colorectal cancer patients from Germany. Eur. J. Epidemiol. 2017, 32, 961–971. [Google Scholar] [CrossRef]
- Maalmi, H.; Walter, V.; Jansen, L.; Boakye, D.; Schöttker, B.; Hoffmeister, M.; Brenner, H. Association between Blood 25-Hydroxyvitamin D Levels and Survival in Colorectal Cancer Patients: An Updated Systematic Review and Meta-Analysis. Nutrients 2018, 10, 896. [Google Scholar] [CrossRef]
- Wu, G.; Xue, M.; Zhao, Y.; Han, Y.; Zhang, S.; Zhang, J.; Li, C.; Xu, J. Low circulating 25-hydroxyvitamin D level is associated with increased colorectal cancer mortality: A systematic review and dose-response meta-analysis. Biosci. Rep. 2020, 40, BSR20201008. [Google Scholar] [CrossRef] [PubMed]
- Vaughan-Shaw, P.G.; Buijs, L.F.; Blackmur, J.P.; Theodoratou, E.; Zgaga, L.; Din, F.V.N.; Farrington, S.M.; Dunlop, M.G. The effect of vitamin D supplementation on survival in patients with colorectal cancer: Systematic review and meta-analysis of randomised controlled trials. Br. J. Cancer 2020, 123, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Vaughan-Shaw, P.G.; Zgaga, L.; Ooi, L.Y.; Theodoratou, E.; Timofeeva, M.; Svinti, V.; Walker, M.; O’Sullivan, F.; Ewing, A.; Johnston, S.; et al. Low plasma vitamin D is associated with adverse colorectal cancer survival after surgical resection, independent of systemic inflammatory response. Gut 2020, 69, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Chang-Claude, J.; Seiler, C.M.; Rickert, A.; Hoffmeister, M. Protection from colorectal cancer after colonoscopy: A population-based, case-control study. Ann. Intern. Med. 2011, 154, 22–30. [Google Scholar] [CrossRef]
- Brenner, H.; Chang-Claude, J.; Jansen, L.; Knebel, P.; Stock, C.; Hoffmeister, M. Reduced risk of colorectal cancer up to 10 years after screening, surveillance, or diagnostic colonoscopy. Gastroenterology 2014, 146, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Carr, P.R.; Jansen, L.; Walter, V.; Kloor, M.; Roth, W.; Bläker, H.; Chang-Claude, J.; Brenner, H.; Hoffmeister, M. Associations of red and processed meat with survival after colorectal cancer and differences according to timing of dietary assessment. Am. J. Clin. Nutr. 2016, 103, 192–200. [Google Scholar] [CrossRef]
- Walter, V.; Jansen, L.; Ulrich, A.; Roth, W.; Bläker, H.; Chang-Claude, J.; Hoffmeister, M.; Brenner, H. Alcohol consumption and survival of colorectal cancer patients: A population-based study from Germany. Am. J. Clin. Nutr. 2016, 103, 1497–1506. [Google Scholar] [CrossRef]
- Phinney, K.W. Development of a standard reference material for vitamin D in serum. Am. J. Clin. Nutr. 2008, 88, 511s–512s. [Google Scholar] [CrossRef]
- Ross, A.C.; Taylor, C.L.; Yaktine, A.L.; Del Valle, H.B. Institute of Medicine Committee to Review Dietary Reference Intakes for Calcium and Vitamin D; National Academy of Sciences: Washington, DC, USA, 2011. [Google Scholar]
- Perna, L.; Hoffmeister, M.; Schöttker, B.; Arndt, V.; Haug, U.; Holleczek, B.; Burwinkel, B.; Ordóñez-Mena, J.M.; Brenner, H. Vitamin D receptor polymorphism and colorectal cancer-specific and all-cause mortality. Cancer Epidemiol. 2013, 37, 905–907. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Guo, F.; Hoffmeister, M.; Brenner, H. Alcohol consumption, polygenic risk score, and early- and late-onset colorectal cancer risk. EClinicalMedicine 2022, 49, 101460. [Google Scholar] [CrossRef]
- Guo, F.; Edelmann, D.; Cardoso, R.; Chen, X.; Carr, P.R.; Chang-Claude, J.; Hoffmeister, M.; Brenner, H. Polygenic Risk Score for Defining Personalized Surveillance Intervals After Adenoma Detection and Removal at Colonoscopy. Clin. Gastroenterol. Hepatol. 2023, 21, 210–219.e211. [Google Scholar] [CrossRef]
- Gupta, S.; Lieberman, D.; Anderson, J.C.; Burke, C.A.; Dominitz, J.A.; Kaltenbach, T.; Robertson, D.J.; Shaukat, A.; Syngal, S.; Rex, D.K. Recommendations for Follow-Up After Colonoscopy and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Am. J. Gastroenterol. 2020, 115, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Li, Y.; Gong, Y.; Huang, Q.; Cai, S.; Peng, J. Vitamin D Status and Survival in Stage II-III Colorectal Cancer. Front. Oncol. 2020, 10, 581597. [Google Scholar] [CrossRef]
- Zhou, J.; Ge, X.; Fan, X.; Wang, J.; Miao, L.; Hang, D. Associations of vitamin D status with colorectal cancer risk and survival. Int. J. Cancer 2021, 149, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Weng, Y.T.; Hsieh, N.T.; Li, P.C.; Lee, T.Y.; Li, C.I.; Liu, H.S.; Lee, M.F. Bioactive Vitamin D Attenuates MED28-Mediated Cell Growth and Epithelial-Mesenchymal Transition in Human Colorectal Cancer Cells. Biomed. Res. Int. 2022, 2022, 2268818. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Weng, Y.T.; Li, P.C.; Hsieh, N.T.; Li, C.I.; Liu, H.S.; Lee, M.F. Calcitriol Suppresses Warburg Effect and Cell Growth in Human Colorectal Cancer Cells. Life 2021, 11, 963. [Google Scholar] [CrossRef]
- Latacz, M.; Snarska, J.; Kostyra, E.; Fiedorowicz, E.; Savelkoul, H.F.; Grzybowski, R.; Cieślińska, A. Single Nucleotide Polymorphisms in 25-Hydroxyvitamin D3 1-Alpha-Hydroxylase (CYP27B1) Gene: The Risk of Malignant Tumors and Other Chronic Diseases. Nutrients 2020, 12, 801. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, L.; Xu, H.J.; Li, Y.; Hu, C.M.; Yang, J.Y.; Sun, M.Y. The Anti-Inflammatory Effects of Vitamin D in Tumorigenesis. Int. J. Mol. Sci. 2018, 19, 2736. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hou, J.; Xiao, Z.; Zhao, Y.; Du, F.; Wu, X.; Li, M.; Chen, Y.; Zhang, L.; Cho, C.H.; et al. The Role of Vitamin D in Gastrointestinal Diseases: Inflammation, Gastric Cancer, and Colorectal Cancer. Curr. Med. Chem. 2022, 29, 3836–3856. [Google Scholar] [CrossRef] [PubMed]
- Flügge, J.; Krusekopf, S.; Goldammer, M.; Osswald, E.; Terhalle, W.; Malzahn, U.; Roots, I. Vitamin D receptor haplotypes protect against development of colorectal cancer. Eur. J. Clin. Pharmacol. 2007, 63, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Ochs-Balcom, H.M.; Cicek, M.S.; Thompson, C.L.; Tucker, T.C.; Elston, R.C.; S, J.P.; Casey, G.; Li, L. Association of vitamin D receptor gene variants, adiposity and colon cancer. Carcinogenesis 2008, 29, 1788–1793. [Google Scholar] [CrossRef]
- Theodoratou, E.; Farrington, S.M.; Tenesa, A.; McNeill, G.; Cetnarskyj, R.; Barnetson, R.A.; Porteous, M.E.; Dunlop, M.G.; Campbell, H. Modification of the inverse association between dietary vitamin D intake and colorectal cancer risk by a FokI variant supports a chemoprotective action of Vitamin D intake mediated through VDR binding. Int. J. Cancer 2008, 123, 2170–2179. [Google Scholar] [CrossRef]
- Slattery, M.L.; Wolff, R.K.; Curtin, K.; Fitzpatrick, F.; Herrick, J.; Potter, J.D.; Caan, B.J.; Samowitz, W.S. Colon tumor mutations and epigenetic changes associated with genetic polymorphism: Insight into disease pathways. Mutat. Res. 2009, 660, 12–21. [Google Scholar] [CrossRef]
- Bentley, R.W.; Keown, D.A.; Gearry, R.B.; Cameron, V.A.; Keenan, J.; Roberts, R.L.; Day, A.S. Vitamin D receptor polymorphisms in colorectal cancer in New Zealand: An association study. N. Z. Med. J. 2012, 125, 47–51. [Google Scholar]
- Serrano, D.; Gnagnarella, P.; Raimondi, S.; Gandini, S. Meta-analysis on vitamin D receptor and cancer risk: Focus on the role of TaqI, ApaI, and Cdx2 polymorphisms. Eur. J. Cancer Prev. 2016, 25, 85–96. [Google Scholar] [CrossRef]
- Fang, Y.; van Meurs, J.B.; Bergink, A.P.; Hofman, A.; van Duijn, C.M.; van Leeuwen, J.P.; Pols, H.A.; Uitterlinden, A.G. Cdx-2 polymorphism in the promoter region of the human vitamin D receptor gene determines susceptibility to fracture in the elderly. J. Bone Miner. Res. 2003, 18, 1632–1641. [Google Scholar] [CrossRef]
- Gnagnarella, P.; Raimondi, S.; Aristarco, V.; Johansson, H.; Bellerba, F.; Corso, F.; De Angelis, S.P.; Belloni, P.; Caini, S.; Gandini, S. Ethnicity as modifier of risk for Vitamin D receptors polymorphisms: Comprehensive meta-analysis of all cancer sites. Crit. Rev. Oncol. Hematol. 2021, 158, 103202. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Mayorga, G.; Gómez-López, G.; Barbáchano, A.; Fernández-Barral, A.; Peña, C.; Pisano, D.G.; Cantero, R.; Rojo, F.; Muñoz, A.; Larriba, M.J. Vitamin D receptor expression and associated gene signature in tumour stromal fibroblasts predict clinical outcome in colorectal cancer. Gut 2017, 66, 1449–1462. [Google Scholar] [CrossRef]
- Shi, Q.; Han, X.P.; Yu, J.; Peng, H.; Chen, Y.Z.; Li, F.; Cui, X.B. Decreased vitamin D receptor protein expression is associated with progression and poor prognosis of colorectal cancer patients. Int. J. Clin. Exp. Pathol. 2020, 13, 746–755. [Google Scholar] [PubMed]
- Al-Ghafari, A.B.; Balamash, K.S.; Al Doghaither, H.A. Serum vitamin D receptor (VDR) levels as a potential diagnostic marker for colorectal cancer. Saudi. J. Biol. Sci. 2020, 27, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Miyamoto, K.I.; Yoshida, M.; Yamamoto, H.; Taketani, Y.; Morita, K.; Kubota, M.; Yoshida, S.; Ikeda, M.; Watabe, F.; et al. The polymorphism in the caudal-related homeodomain protein Cdx-2 binding element in the human vitamin D receptor gene. J. Bone Miner. Res. 2001, 16, 1256–1264. [Google Scholar] [CrossRef]
- Brenner, H.; Jansen, L.; Saum, K.U.; Holleczek, B.; Schöttker, B. Vitamin D Supplementation Trials Aimed at Reducing Mortality Have Much Higher Power When Focusing on People with Low Serum 25-Hydroxyvitamin D Concentrations. J. Nutr. 2017, 147, 1325–1333. [Google Scholar] [CrossRef]
- Grant, W.B.; Boucher, B.J.; Al Anouti, F.; Pilz, S. Comparing the Evidence from Observational Studies and Randomized Controlled Trials for Nonskeletal Health Effects of Vitamin D. Nutrients 2022, 14, 3811. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).