Relationship between Whole-Blood Magnesium and Cognitive Performance among Chinese Adults
Abstract
1. Introduction
2. Method
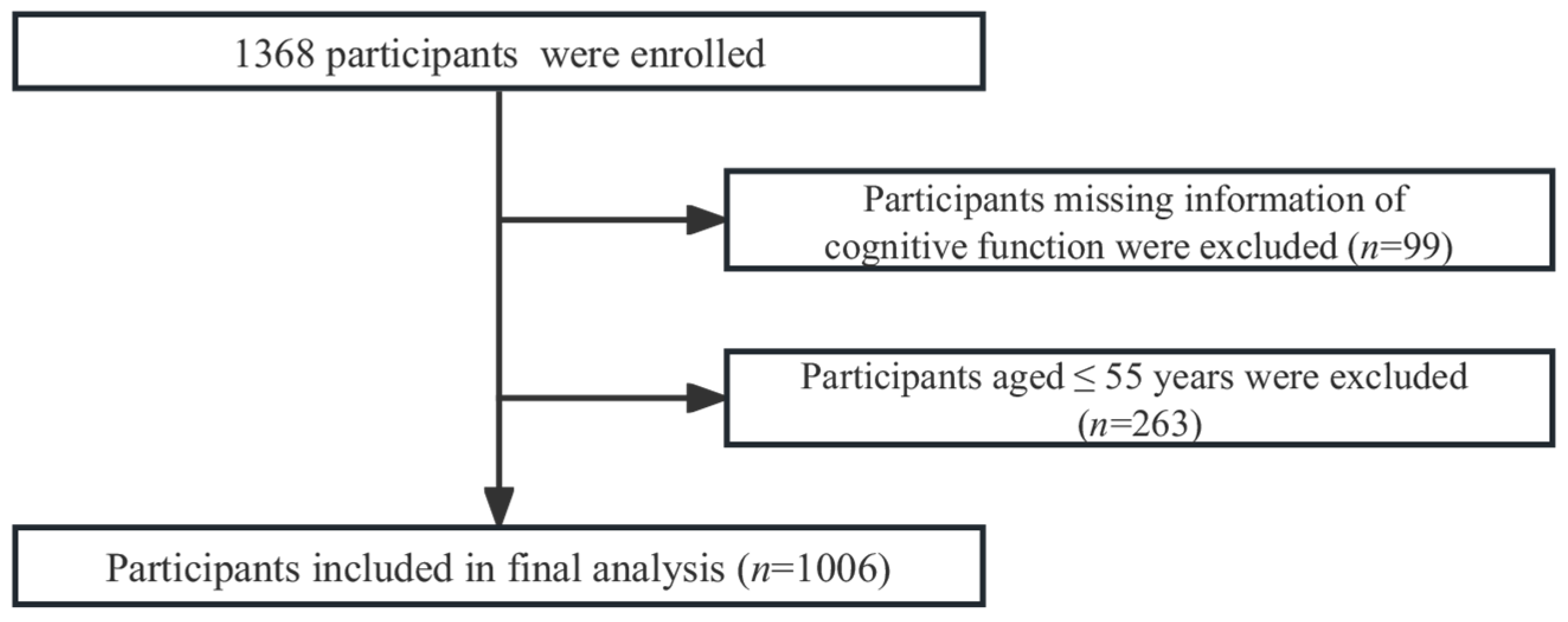
2.1. Study Population
2.2. Cognitive Function
2.3. Whole-Blood Magnesium Concentration
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Demographic Characterization
3.2. Association between Magnesium and Cognitive Function
3.2.1. Magnesium Level and Cognitive Function
3.2.2. Magnesium Concentrations and Multiple Cognitive Domains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nania, G. World Alzheimer Report 2021; Alzheimer’s Disease International: London, UK, 2021. [Google Scholar]
- Zhao, B.; Hu, L.; Dong, Y.; Xu, J.; Wei, Y.; Yu, D.; Xu, J.; Zhang, W. The Effect of Magnesium Intake on Stroke Incidence: A Systematic Review and Meta-Analysis With Trial Sequential Analysis. Front. Neurol. 2019, 10, 852. [Google Scholar] [CrossRef]
- Piuri, G.; Zocchi, M.; Della Porta, M.; Ficara, V.; Manoni, M.; Zuccotti, G.V.; Pinotti, L.; Maier, J.A.; Cazzola, R. Magnesium in Obesity, Metabolic Syndrome, and Type 2 Diabetes. Nutrients 2021, 13, 320. [Google Scholar] [CrossRef] [PubMed]
- Slutsky, I.; Abumaria, N.; Wu, L.-J.; Huang, C.; Zhang, L.; Li, B.; Zhao, X.; Govindarajan, A.; Zhao, M.-G.; Zhuo, M.; et al. Enhancement of Learning and Memory by Elevating Brain Magnesium. Neuron 2010, 65, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.H.; Liu, J.; Cervantes, D. Association between magnesium intake and cognition in US older adults: National Health and Nutrition Examination Survey (NHANES) 2011 to 2014. Alzheimer’s Dement. 2022, 8, e12250. [Google Scholar] [CrossRef]
- Huenges Wajer, I.M.C.; Dorhout Mees, S.M.; Van Den Bergh, W.M.; Algra, A.; Visser-Meily, J.M.A.; Rinkel, G.J.E.; van Zandvoort, M.J.E. Effect of magnesium on cognition after aneurysmal subarachnoid haemorrhage in a randomized trial. Eur. J. Neurol. 2018, 25, 1486–1489. [Google Scholar] [CrossRef]
- Al-Ghazali, K.; Eltayeb, S.; Musleh, A.; Al-Abdi, T.; Ganji, V.; Shi, Z. Serum Magnesium and Cognitive Function Among Qatari Adults. Front. Aging Neurosci. 2020, 12, 101. [Google Scholar] [CrossRef]
- Kieboom, B.C.T.; Licher, S.; Wolters, F.J.; Ikram, M.K.; Hoorn, E.J.; Zietse, R.; Stricker, B.H.; Ikram, M.A. Serum magnesium is associated with the risk of dementia. Neurology 2017, 89, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.B.; Lutsey, P.L.; Gottesman, R.F.; Tin, A.; Alonso, A. Low Serum Magnesium is Associated with Incident Dementia in the ARIC-NCS Cohort. Nutrients 2020, 12, 3074. [Google Scholar] [CrossRef]
- Chen, C.; Xun, P.; Unverzagt, F.; McClure, L.A.; Irvin, M.R.; Judd, S.; Cushman, M.; He, K. Serum magnesium concentration and incident cognitive impairment: The reasons for geographic and racial differences in stroke study. Eur. J. Nutr. 2021, 60, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Du, Y.; Chu, L.; Zhang, Z.; Li, F.; Lyu, D.; Li, Y.; Zhu, M.; Jiao, H.; Song, Y.; et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: A cross-sectional study. Lancet Public Health 2020, 5, e661–e671. [Google Scholar] [CrossRef]
- Cao, Y.; Zhen, S.; Taylor, A.W.; Appleton, S.; Atlantis, E.; Shi, Z. Magnesium Intake and Sleep Disorder Symptoms: Findings from the Jiangsu Nutrition Study of Chinese Adults at Five-Year Follow-Up. Nutrients 2018, 10, 1354. [Google Scholar] [CrossRef]
- Rc, P. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar]
- Lin, Y.T.B.; He, R.; Zhang, X.; Chen, J.; By-Health Co., Ltd., Assignee. The Invention Relates to a Method for Detecting Multiple Elements in Dry Blood Spots. China Patent CN201911081669.7, 7 November 2019. [Google Scholar]
- Okely, A.D.; Kontsevaya, A.; Ng, J.; Abdeta, C. 2020 WHO guidelines on physical activity and sedentary behavior. Sport. Med. Health Sci. 2021, 3, 115–118. [Google Scholar] [CrossRef]
- Kirkland, A.E.; Sarlo, G.L.; Holton, K.F. The Role of Magnesium in Neurological Disorders. Nutrients 2018, 10, 730. [Google Scholar] [CrossRef]
- Orhan, C.; Tuzcu, M.; Deeh Defo, P.B.; Sahin, N.; Ojalvo, S.P.; Sylla, S.; Komorowski, J.R.; Sahin, K. Effects of a Novel Magnesium Complex on Metabolic and Cognitive Functions and the Expression of Synapse-Associated Proteins in Rats Fed a High-Fat Diet. Biol. Trace Elem. Res. 2022, 200, 247–260. [Google Scholar] [CrossRef]
- Balmuș, I.M.; Strungaru, S.A.; Ciobica, A.; Nicoara, M.N.; Dobrin, R.; Plavan, G.; Ștefănescu, C. Preliminary Data on the Interaction between Some Biometals and Oxidative Stress Status in Mild Cognitive Impairment and Alzheimer’s Disease Patients. Oxidative Med. Cell. Longev. 2017, 2017, 7156928. [Google Scholar] [CrossRef]
- Barbagallo, M.; Belvedere, M.; Di Bella, G.; Dominguez, L.J. Altered ionized magnesium levels in mild-to-moderate Alzheimer’s disease. Magnes. Res. 2011, 24, S115–S121. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yu, J.; Liu, Y.; Huang, X.; Abumaria, N.; Zhu, Y.; Huang, X.; Xiong, W.; Ren, C.; Liu, X.G.; et al. Elevation of brain magnesium prevents synaptic loss and reverses cognitive deficits in Alzheimer’s disease mouse model. Mol. Brain 2014, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Won, Y.J.; Lim, B.G.; Min, T.J.; Kim, Y.H.; Lee, I.O. Neuroprotective effects of magnesium L-threonate in a hypoxic zebrafish model. BMC Neurosci. 2020, 21, 29. [Google Scholar] [CrossRef]
- Cherbuin, N.; Kumar, R.; Sachdev, P.S.; Anstey, K.J. Dietary Mineral Intake and Risk of Mild Cognitive Impairment: The PATH through Life Project. Front. Aging Neurosci. 2014, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Peeri, N.C.; Egan, K.M.; Chai, W.; Tao, M.H. Association of magnesium intake and vitamin D status with cognitive function in older adults: An analysis of US National Health and Nutrition Examination Survey (NHANES) 2011 to 2014. Eur. J. Nutr. 2021, 60, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Weinger, J.G.; Lu, Z.L.; Xue, F.; Sadeghpour, S. Efficacy and Safety of MMFS-01, a Synapse Density Enhancer, for Treating Cognitive Impairment in Older Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Alzheimers Dis. 2016, 49, 971–990. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hu, Q.; Li, S.; Dai, F.; Qian, W.; Hewlings, S.; Yan, T.; Wang, Y. A Magtein®, Magnesium L-Threonate, -Based Formula Improves Brain Cognitive Functions in Healthy Chinese Adults. Nutrients 2022, 14, 5235. [Google Scholar] [CrossRef] [PubMed]
- Gindler, E.H.D. Colorimetric determination with bound calmagite of magnesium in human blood serum. In Clinical Chemistry; American Association Clinical Chemistry: Washington, DC, USA, 1971; pp. 20037–21526. [Google Scholar]
- Dimai, H.P.; Wirnsberger, G.; Lau, K.H. Measurements of circulating ionized magnesium level: Fresh whole blood samples versus serum samples. Clin. Biochem. 2000, 33, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Andersen-Ranberg, K.; Hollergaard, M.; Nybo, M. Quantification of multiple elements in dried blood spot samples. Clin. Biochem. 2017, 50, 703–709. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, J.-H.; He, R.; Tang, B.; Jiang, L.; Zhang, X. A fully validated high-throughput liquid chromatography tandem mass spectrometry method for automatic extraction and quantitative determination of endogenous nutritional biomarkers in dried blood spot samples. J. Chromatogr. A 2020, 1622, 461092. [Google Scholar] [CrossRef]
- Fu, Y.; Zhu, Z.; Huang, Z.; He, R.; Zhang, Y.; Li, Y.; Tan, W.; Rong, S. Association between Vitamin B and Obesity in Middle-Aged and Older Chinese Adults. Nutrients 2023, 15, 483. [Google Scholar] [CrossRef]
- Jensen, B.P.; Saraf, R.; Ma, J.; Berry, S.; Grant, C.C.; Camargo, C.A., Jr.; Sies, C.W. Quantitation of 25-hydroxyvitamin D in dried blood spots by 2D LC-MS/MS without derivatization and correlation with serum in adult and pediatric studies. Clin. Chim. Acta 2018, 481, 61–68. [Google Scholar] [CrossRef]
- Olivares, D.; Deshpande, K.V.; Shi, Y.; Lahiri, K.D.; Greig, H.N.; Rogers, T.J.; Huang, X. N-methyl D-aspartate (NMDA) receptor antagonists and memantine treatment for Alzheimer’s disease, vascular dementia and Parkinson’s disease. Curr. Alzheimer Res. 2012, 9, 746–758. [Google Scholar] [CrossRef]
- Zipfel, G.J.; Babcock, D.J.; Lee, J.M.; Choi, D.W. Neuronal apoptosis after CNS injury: The roles of glutamate and calcium. J. Neurotrauma 2000, 17, 857–869. [Google Scholar] [CrossRef]
- Hynd, M.R.; Scott, H.L.; Dodd, P.R. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 2004, 45, 583–595. [Google Scholar] [CrossRef]
- Nowak, L.; Bregestovski, P.; Ascher, P.; Herbet, A.; Prochiantz, A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature 1984, 307, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H. Magnesium deficiency and increased inflammation: Current perspectives. J. Inflamm. Res. 2018, 11, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Morais, J.B.; Severo, J.S.; Santos, L.R.D.; de Sousa Melo, S.R.; de Oliveira Santos, R.; de Oliveira, A.R.S.; Cruz, K.J.C.; do Nascimento Marreiro, D. Role of Magnesium in Oxidative Stress in Individuals with Obesity. Biol. Trace Elem. Res. 2017, 176, 20–26. [Google Scholar] [CrossRef]
- Rakic, S.; Hung, Y.M.A.; Smith, M.; So, D.; Tayler, H.M.; Varney, W.; Wild, J.; Harris, S.; Holmes, C.; Love, S.; et al. Systemic infection modifies the neuroinflammatory response in late stage Alzheimer’s disease. Acta Neuropathol. Commun. 2018, 6, 88. [Google Scholar] [CrossRef]
- Yu, X.; Guan, P.-P.; Zhu, D.; Liang, Y.-Y.; Wang, T.; Wang, Z.-Y.; Wang, P. Magnesium Ions Inhibit the Expression of Tumor Necrosis Factor α and the Activity of γ-Secretase in a β-Amyloid Protein-Dependent Mechanism in APP/PS1 Transgenic Mice. Front. Mol. Neurosci. 2018, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Olinescu, R.M.; Kummerow, F.A. Influence of low magnesium concentrations in the medium on the antioxidant system in cultured human arterial endothelial cells. Magnes. Res. 1999, 12, 19–29. [Google Scholar] [PubMed]
- Sitzia, C.; Sterlicchio, M.; Crapanzano, C.; Dozio, E.; Vianello, E.; Romanelli, M.M.C. Intra-erythrocytes magnesium deficiency could reflect cognitive impairment status due to vascular disease: A pilot study. J. Transl. Med. 2020, 18, 458. [Google Scholar] [CrossRef]
- Hardwick, L.L.; Jones, M.R.; Brautbar, N.; Lee, D.B. Magnesium absorption: Mechanisms and the influence of vitamin D, calcium and phosphate. J. Nutr. 1991, 121, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Pointillart, A.; Denis, I.; Colin, C. Effects of dietary vitamin D on magnesium absorption and bone mineral contents in pigs on normal magnesium intakes. Magnes. Res. 1995, 8, 19–26. [Google Scholar]


| Total | Non-MCI | MCI | p-Value a | |
|---|---|---|---|---|
| N (n, %) | 1006 | 845 (84.0) | 161 (16.0) | |
| Age, years, mean (SD) | 63.7 (5.1) | 63.1 (4.9) | 66.5 (5.2) | <0.001 |
| Female (%) | 855 (85.0) | 718 (85.0) | 137 (85.1) | 0.968 |
| Living alone, n (%) | 71 (7.1) | 57 (6.8) | 14 (8.7) | 0.747 |
| Marital status, n (%) | ||||
| Married | 870 (86.5) | 734 (86.9) | 136 (84.5) | 0.416 |
| Single/widowed/divorced | 136 (13.5) | 111 (13.1) | 25 (15.5) | |
| Education level, n (%) | ||||
| Less than middle school | 107 (10.6) | 50 (5.9) | 57 (35.4) | <0.001 |
| Middle and high school | 762 (75.8) | 665 (78.7) | 97 (60.3) | |
| College or higher | 137 (13.6) | 130 (15.4) | 7 (4.4) | |
| Annual per capital income, n (%) | ||||
| <CNY 20,000 | 224 (22.3) | 177 (21.0) | 47 (29.2) | <0.001 |
| CNY 20,000–40,000 | 491 (48.8) | 403 (47.7) | 88 (54.7) | |
| >CNY 40,000 | 291 (28.9) | 265 (31.4) | 26 (16.2) | |
| BMI, n (%) | ||||
| <18.5 | 13 (1.3) | 9 (1.1) | 4 (2.5) | 0.056 |
| 18.5–23.9 | 454 (45.1) | 392 (46.4) | 62 (38.5) | |
| 24.0–27.9 | 424 (42.2) | 356 (42.1) | 68 (42.2) | |
| ≥28.0 | 103 (10.2) | 78 (9.2) | 25 (15.5) | |
| Smoking (current), n (%) | 59 (5.9) | 51 (6.0) | 8 (5.0) | 0.859 |
| Alcohol intake (current), n (%) | 201 (20.0) | 166 (19.6) | 35 (21.7) | 0.420 |
| Meeting physical activity recommendation, n (%) | 632 (62.8) | 550 (65.1) | 82 (50.9) | <0.001 |
| Retired, n (%) | 854 (84.9) | 729 (86.3) | 125 (77.6) | 0.005 |
| Hypertension, n (%) | 548 (54.5) | 447 (52.9) | 101 (62.7) | 0.022 |
| Diabetes, n (%) | 72 (7.2) | 61 (7.2) | 11 (6.8) | 0.862 |
| AVLT, score, mean (SD) | 28.8 (9.4) | 28.1 (9.6) | 18.9 (9.7) | <0.001 |
| VFT, score, mean (SD) | 16.6 (4.3) | 17.2 (4.1) | 13.0 (3.8) | <0.001 |
| DSST, score, mean (SD) | 30.6 (8.4) | 30.7 (8.4) | 18.9 (7.9) | <0.001 |
| TMT-B, seconds, mean (SD) | 56.3 (29.1) | 49.6 (19.4) | 91.6 (42.84) | <0.001 |
| Mg2+ (mg/L), mean (SD) | 36.4 (9.8) | 36.7 (9.7) | 34.7 (9.8) | 0.017 |
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p Trend | |
|---|---|---|---|---|---|
| Median (IQR) (mg/L) | 25.4 (14.0, 29.6) | 32.3 (29.6, 35.1) | 38.5 (35.1, 42.3) | 48.4 (42.4, 76.2) | |
| Model 1 | 1 (ref) | 0.49 (0.30, 0.81) | 0.54 (0.33, 0.87) | 0.53 (0.33, 0.86) | 0.009 |
| Model 2 | 1 (ref) | 0.44 (0.26, 0.74) | 0.51 (0.30, 0.85) | 0.55 (0.33, 0.92) | 0.008 |
| Model 3 | 1 (ref) | 0.44 (0.26, 0.75) | 0.52 (0.31, 0.87) | 0.53 (0.32, 0.90) | 0.009 |
| AVLT, Score | VFT, Score | DSST, Score | TMT-B, Score | Z-Score, Score | |
|---|---|---|---|---|---|
| Model 1 | 0.31 (−0.25, 0.98) | 0.40 (0.14, 0.66) | 0.58 (0.05, 1.12) | −1.90 (−3.59, −0.20) | 0.06 (0.02, 0.10) |
| Model 2 | 0.31 (−0.30, 0.91) | 0.37 (0.11, 0.62) | 0.49 (0.00, 0.98) | −1.75 (−3.41, −0.09) | 0.05 (0.02, 0.09) |
| Model 3 | 0.31 (−0.29, 0.91) | 0.37 (0.11, 0.62) | 0.50 (0.01, 0.98) | −1.73 (−3.40, −0.07) | 0.03 (0.02, 0.09) |
| OR (95%CI) | |||
|---|---|---|---|
| Total | <60 Years (n = 283) | ≥60 Years (n = 723) | |
| Mg (mg/L) | 0.79 (0.65, 0.95) | 0.74 (0.43, 1.28) | 0.82 (0.68, 0.98) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; He, R.; Zhang, Y.; Li, B.; Li, F.; Fu, Y.; Rong, S. Relationship between Whole-Blood Magnesium and Cognitive Performance among Chinese Adults. Nutrients 2023, 15, 2706. https://doi.org/10.3390/nu15122706
Lu Z, He R, Zhang Y, Li B, Li F, Fu Y, Rong S. Relationship between Whole-Blood Magnesium and Cognitive Performance among Chinese Adults. Nutrients. 2023; 15(12):2706. https://doi.org/10.3390/nu15122706
Chicago/Turabian StyleLu, Zijian, Ruikun He, Ying Zhang, Benchao Li, Fengping Li, Yu Fu, and Shuang Rong. 2023. "Relationship between Whole-Blood Magnesium and Cognitive Performance among Chinese Adults" Nutrients 15, no. 12: 2706. https://doi.org/10.3390/nu15122706
APA StyleLu, Z., He, R., Zhang, Y., Li, B., Li, F., Fu, Y., & Rong, S. (2023). Relationship between Whole-Blood Magnesium and Cognitive Performance among Chinese Adults. Nutrients, 15(12), 2706. https://doi.org/10.3390/nu15122706








