Pecans and Its Polyphenols Prevent Obesity, Hepatic Steatosis and Diabetes by Reducing Dysbiosis, Inflammation, and Increasing Energy Expenditure in Mice Fed a High-Fat Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Whole Pecans, Pecan Phenolic Extract Preparation and Phenolic Quantification
2.2. LC-MS Phenolic Profiling
2.3. Experimental Diets
2.4. Animal Study Design
2.5. Biochemical Parameters
2.6. Energy Expenditure and Body Composition Measurements
2.7. Intraperitoneal Insulin and Glucose Tolerance Test
2.8. Histopathologic Evaluation of Hepatic, Pancreatic and Adipose Tissues
2.9. Lipid Content in Liver and Skeletal Muscle
2.10. Mitochondrial Activity in Skeletal Muscle
2.11. Brown and White Adipose Tissues UCP-1 Content and F12/80 Abundance in Subcutaneous Adipose Tissue Macrophages by Immunohistochemistry
2.12. Immunoblotting
2.13. Gene Expression Analysis in Liver, Muscle and Adipose Tissue by Quantitative-Polymerase Chain Reaction (PCR)
2.14. DNA Extraction and Preparation of 16S rRNA Gene Amplicon Libraries
2.15. Sequencing and Data Analysis
2.16. Statistical Analysis
3. Results
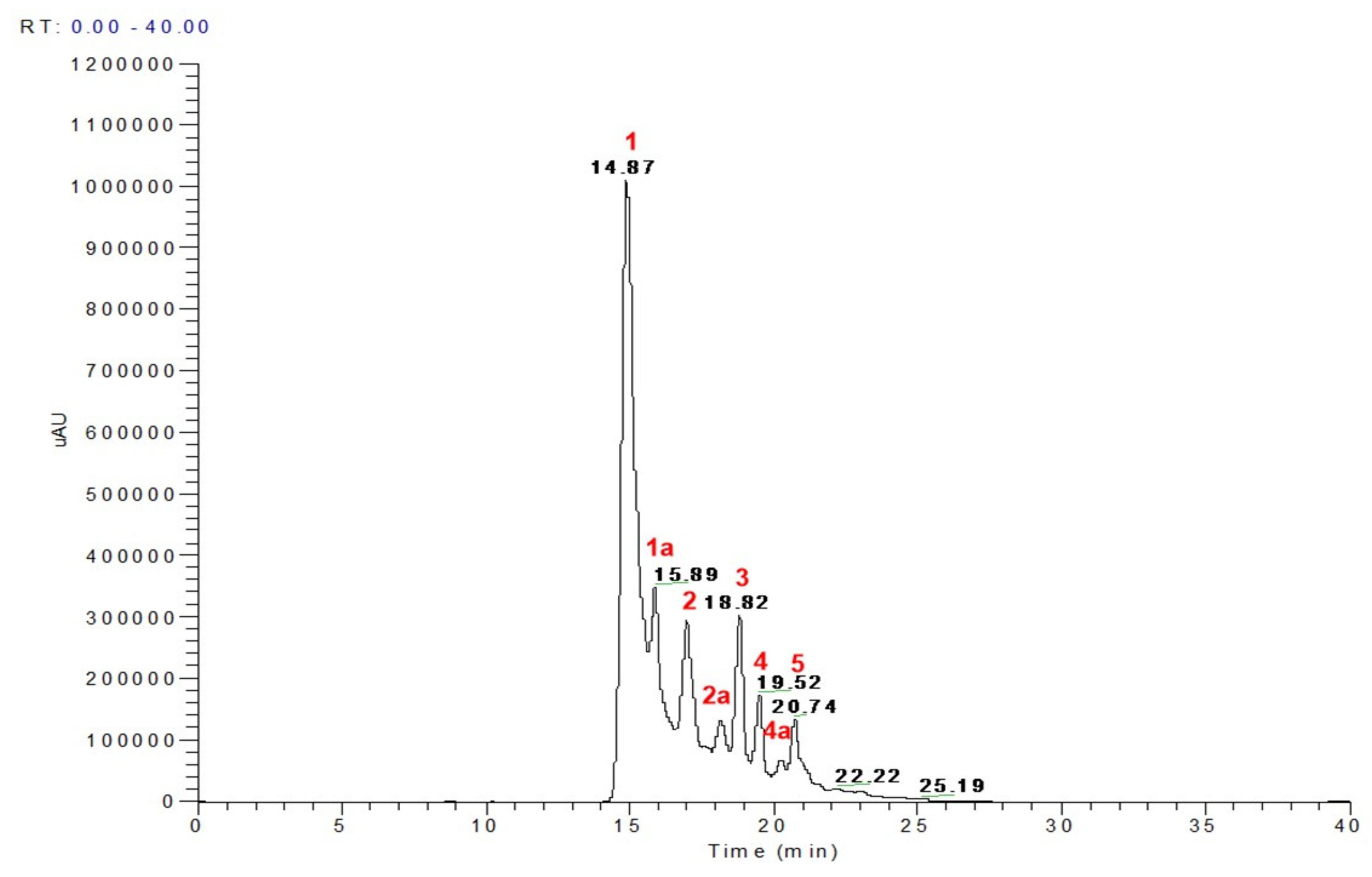
3.1. LC-MS Profiling of Phenolic Extracts
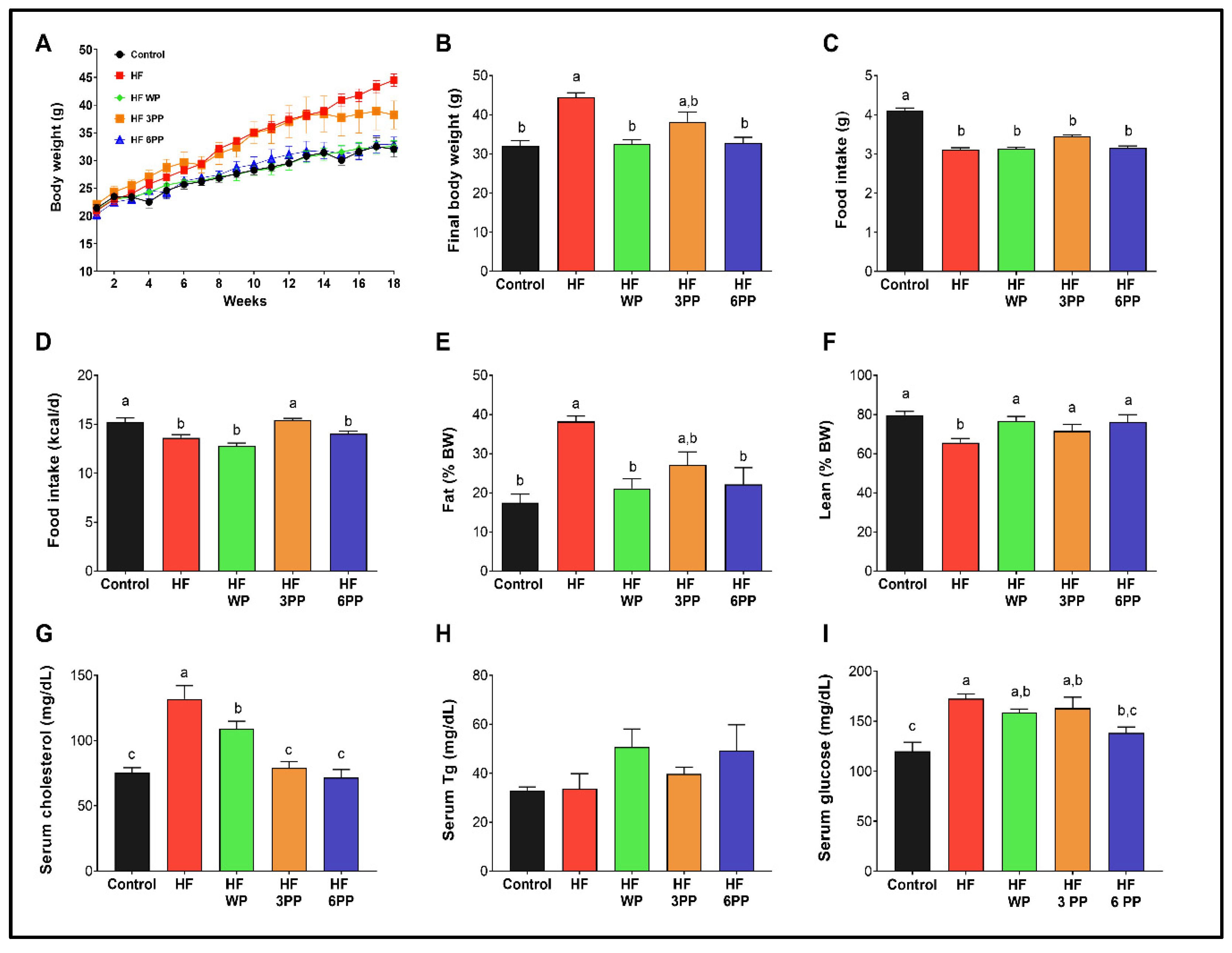
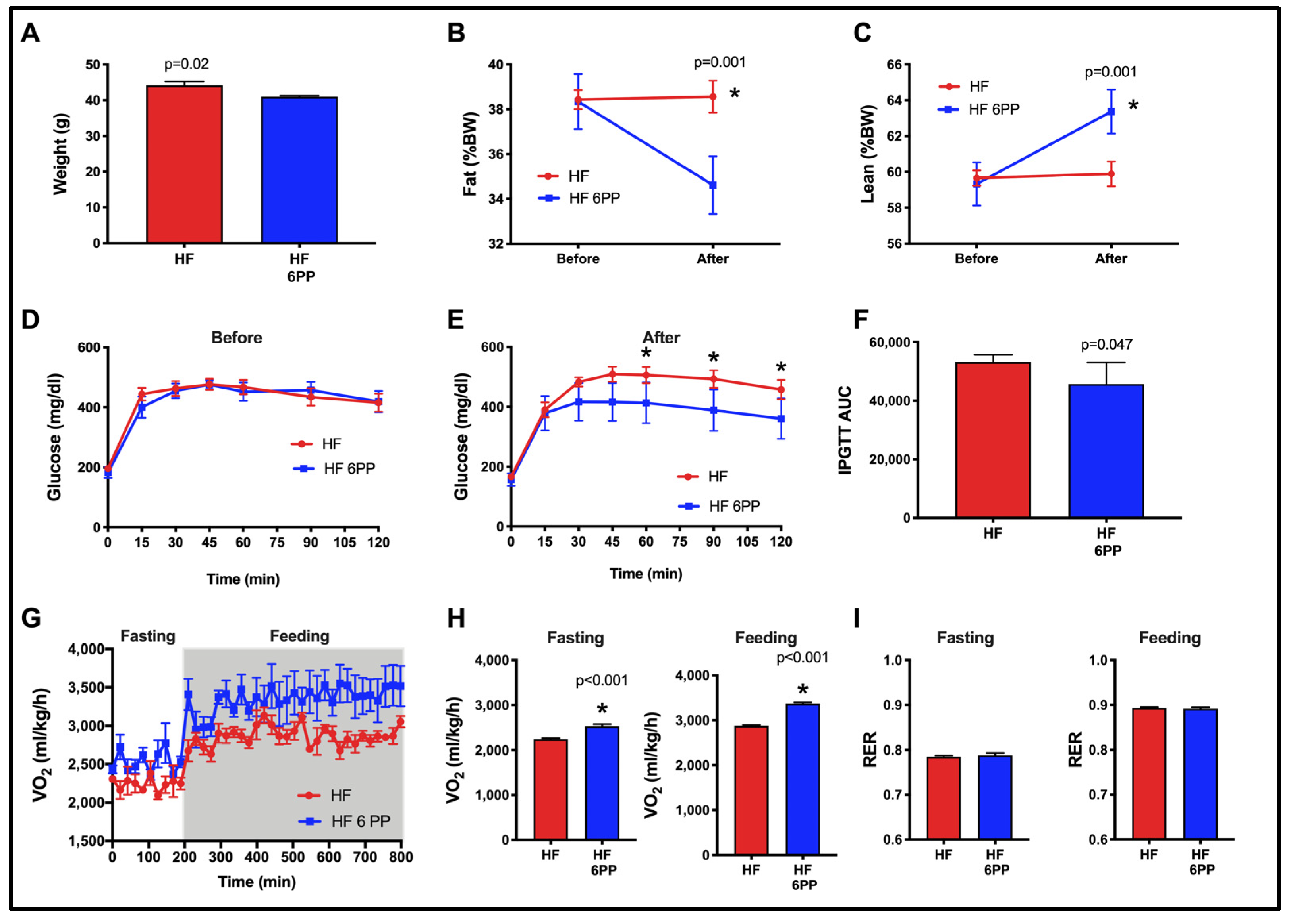
3.2. Consumption of Whole Pecans or a Phenolic Extract of Pecans Prevented Body Weight Gain and Maintained Body Composition of Mice Fed a High-Fat Diet
3.3. Whole Pecans or a Phenolic Extract of Pecans Attenuated Glucose Intolerance and Improved Insulin Sensitivity of Mice Fed a High-Fat Diet
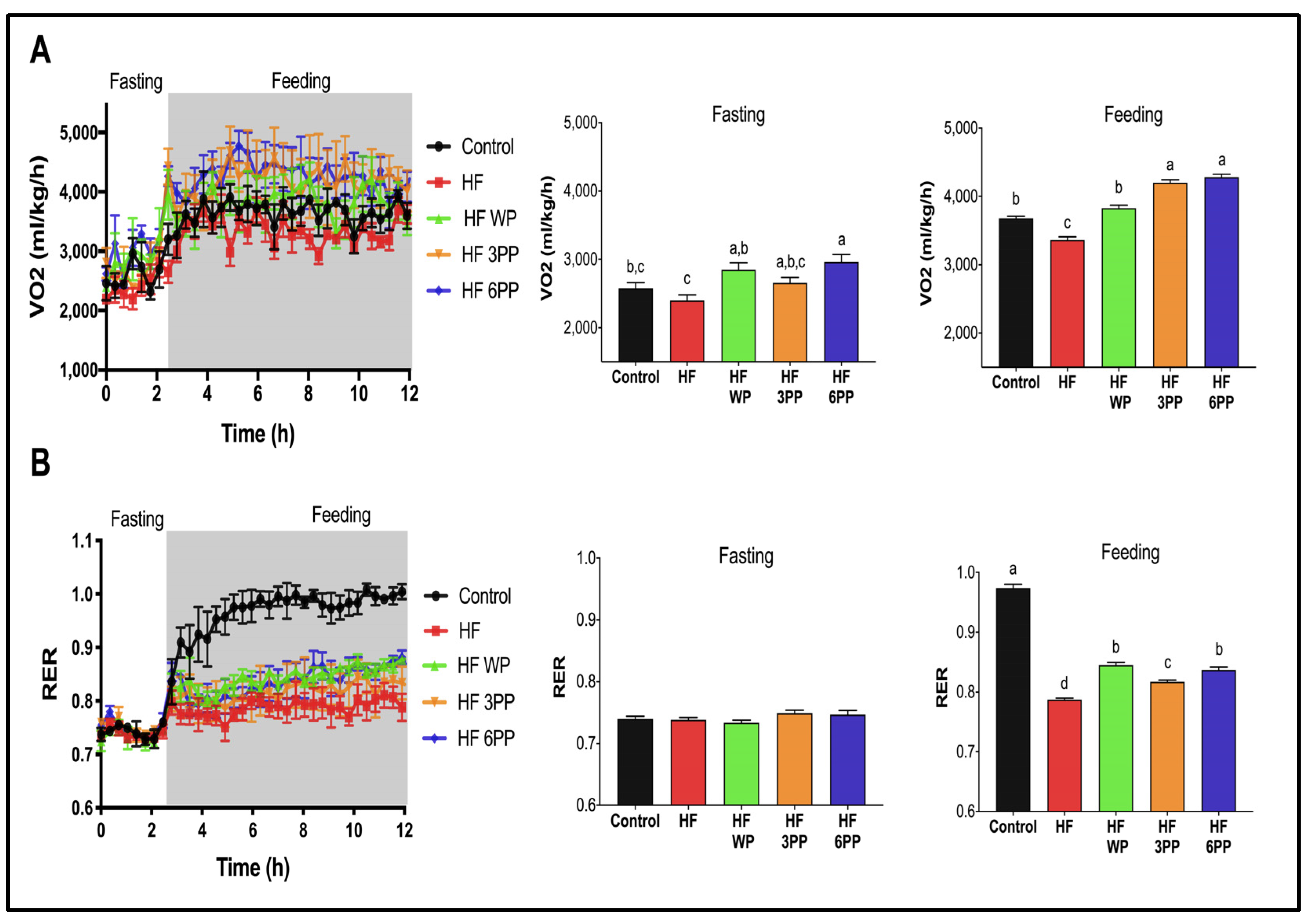
3.4. Consumption of Whole Pecans or a Phenolic Extract of Pecans Increase Energy Expenditure in Mice Fed a High-Fat Diet
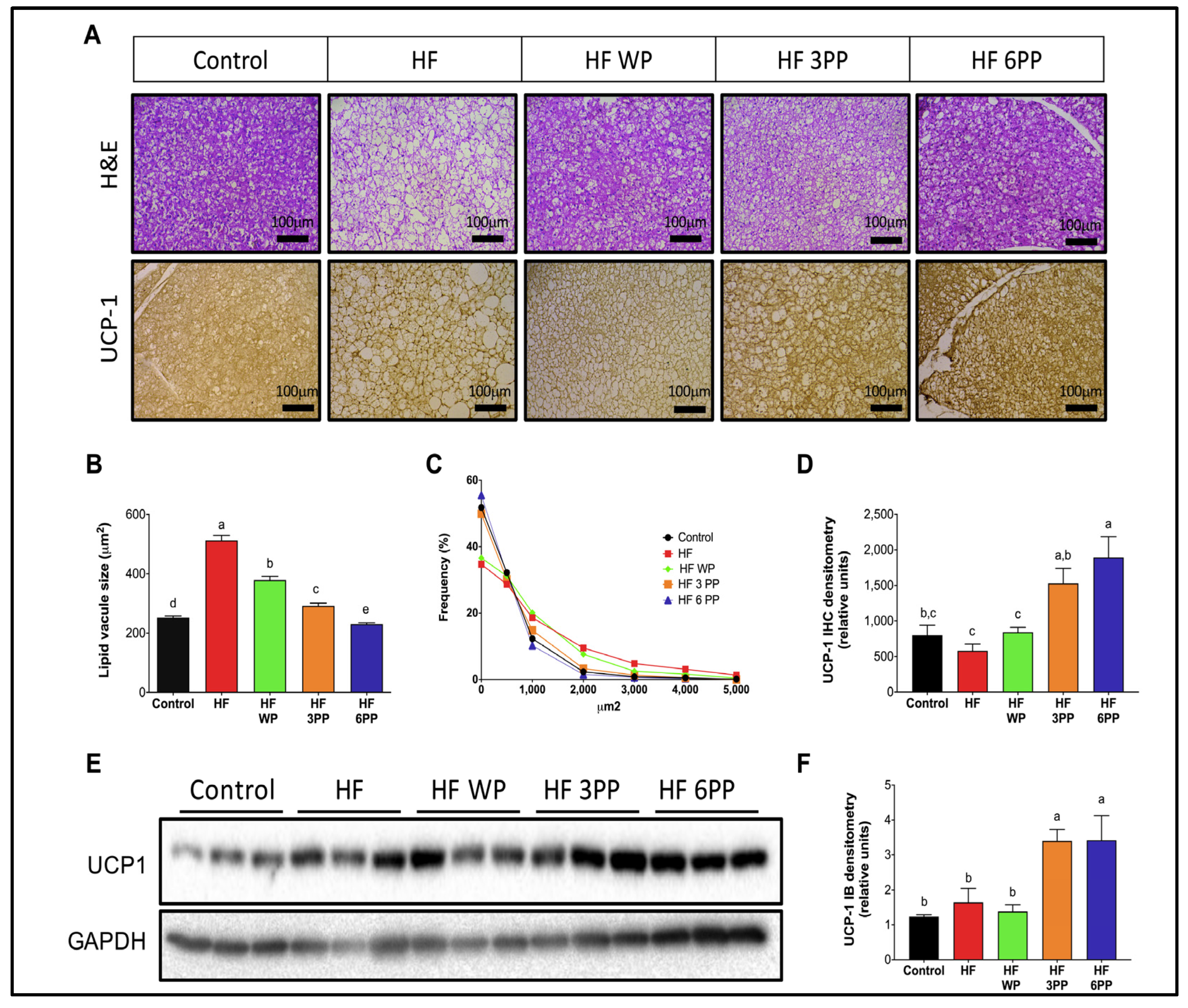
3.5. Increased Brown Adipose Tissue Thermogenic Activity in Mice Fed a High-Fat Diet Supplemented with Whole Pecans or a Pecan Phenolic Extract
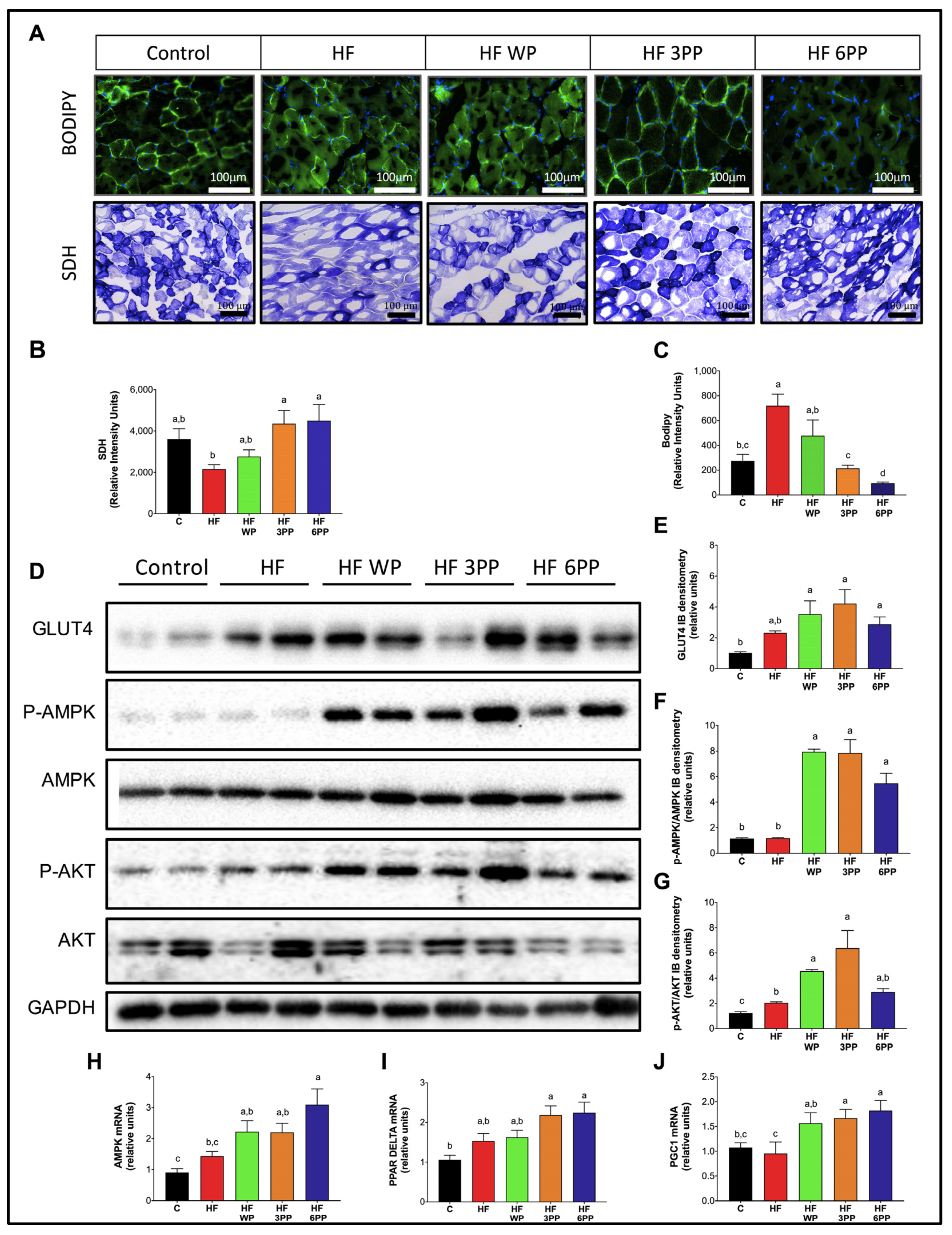
3.6. Whole Pecans or a Pecan Phenolic Extract Intake Increases Mitochondrial Content and Reduced Lipid Accumulation in Skeletal Muscle of Mice Fed a High-Fat Diet
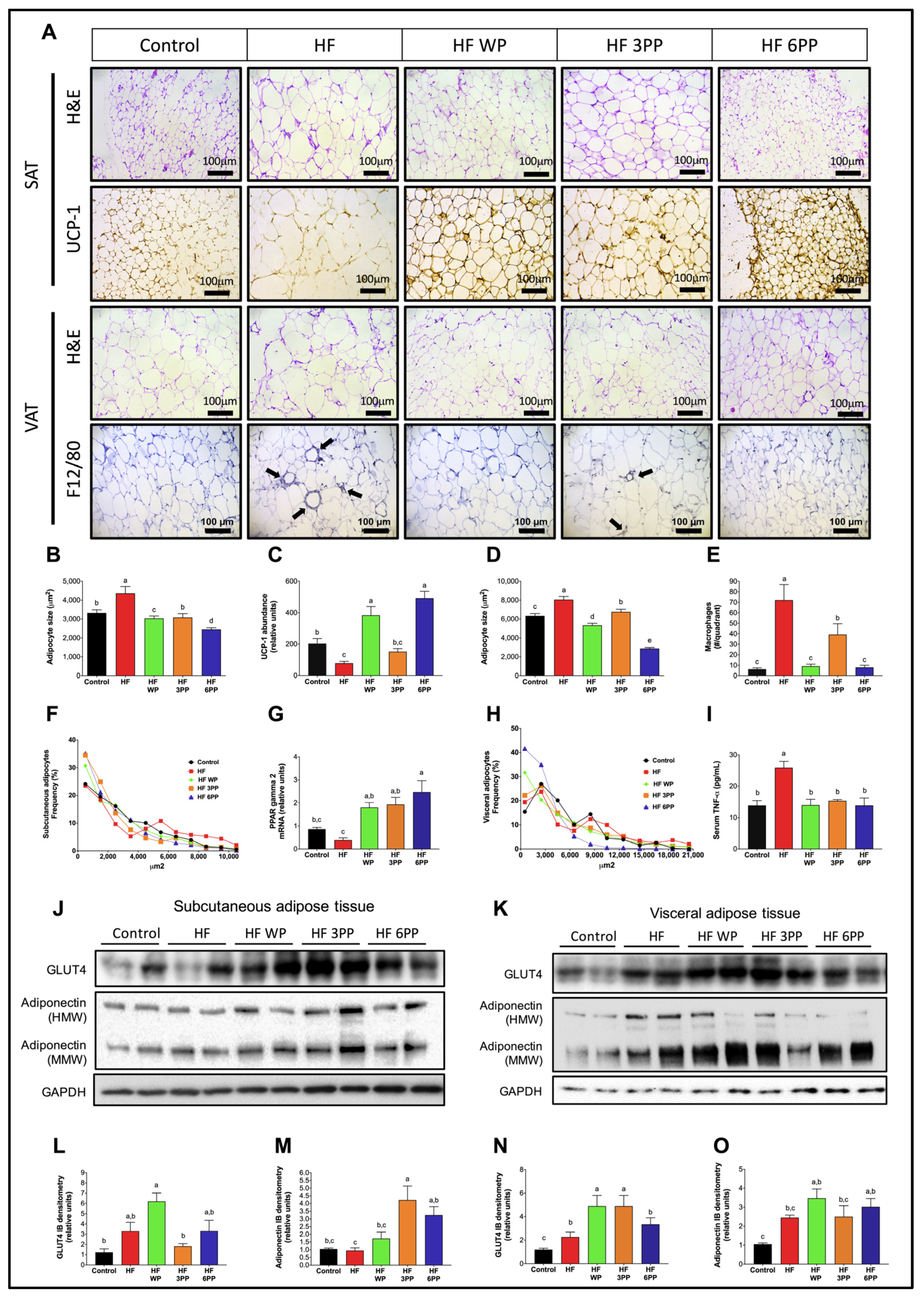
3.7. Whole Pecans or a Pecan Phenolic Extract Prevents Adipose Tissue Dysfunction in Mice Fed a High-Fat Diet
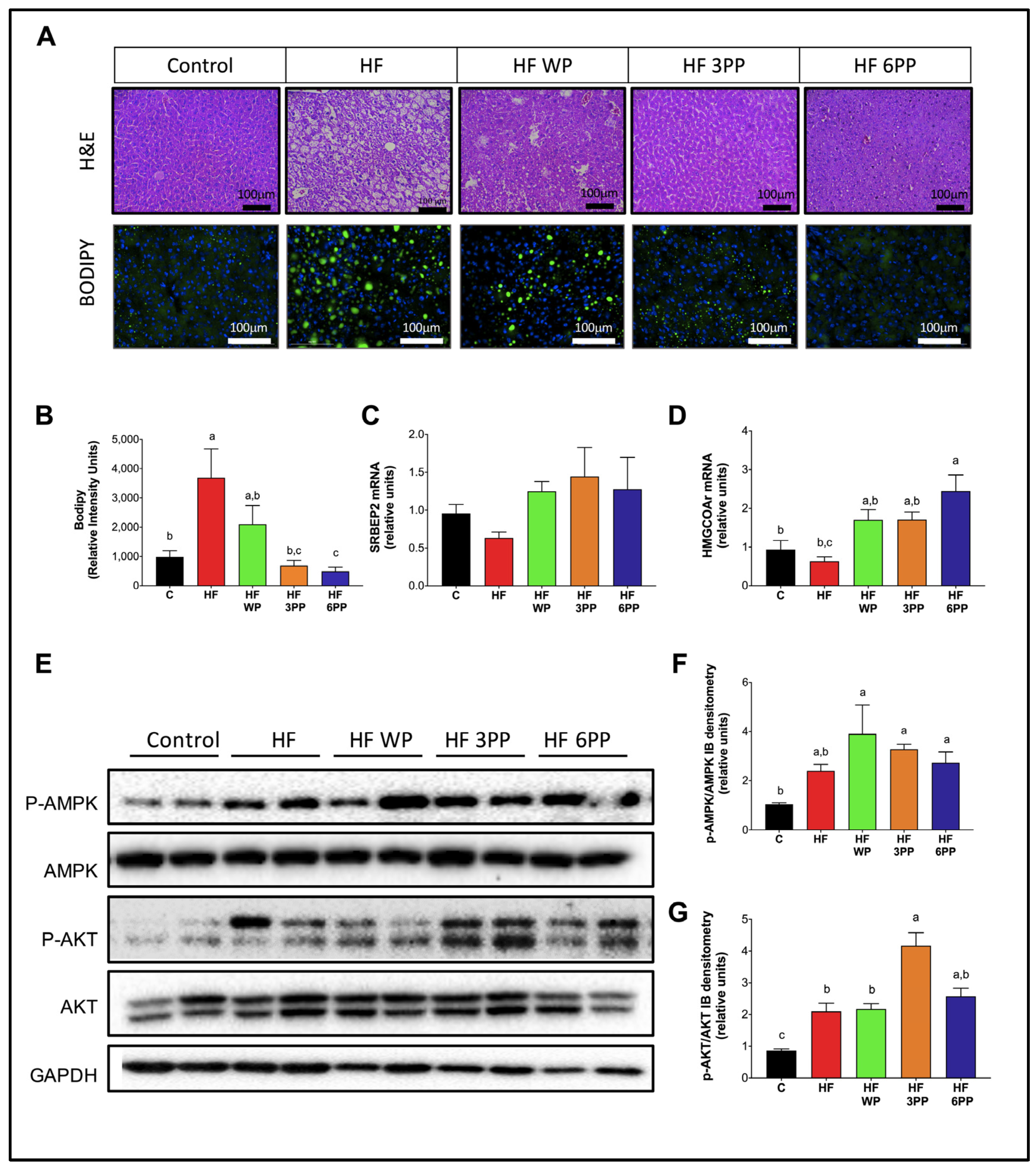
3.8. Consumption of Whole Pecans or a Phenolic Extract of Pecans Prevented Hepatic Steatosis and Metabolic Signaling Alterations in Liver of Mice Fed a High-Fat Diet
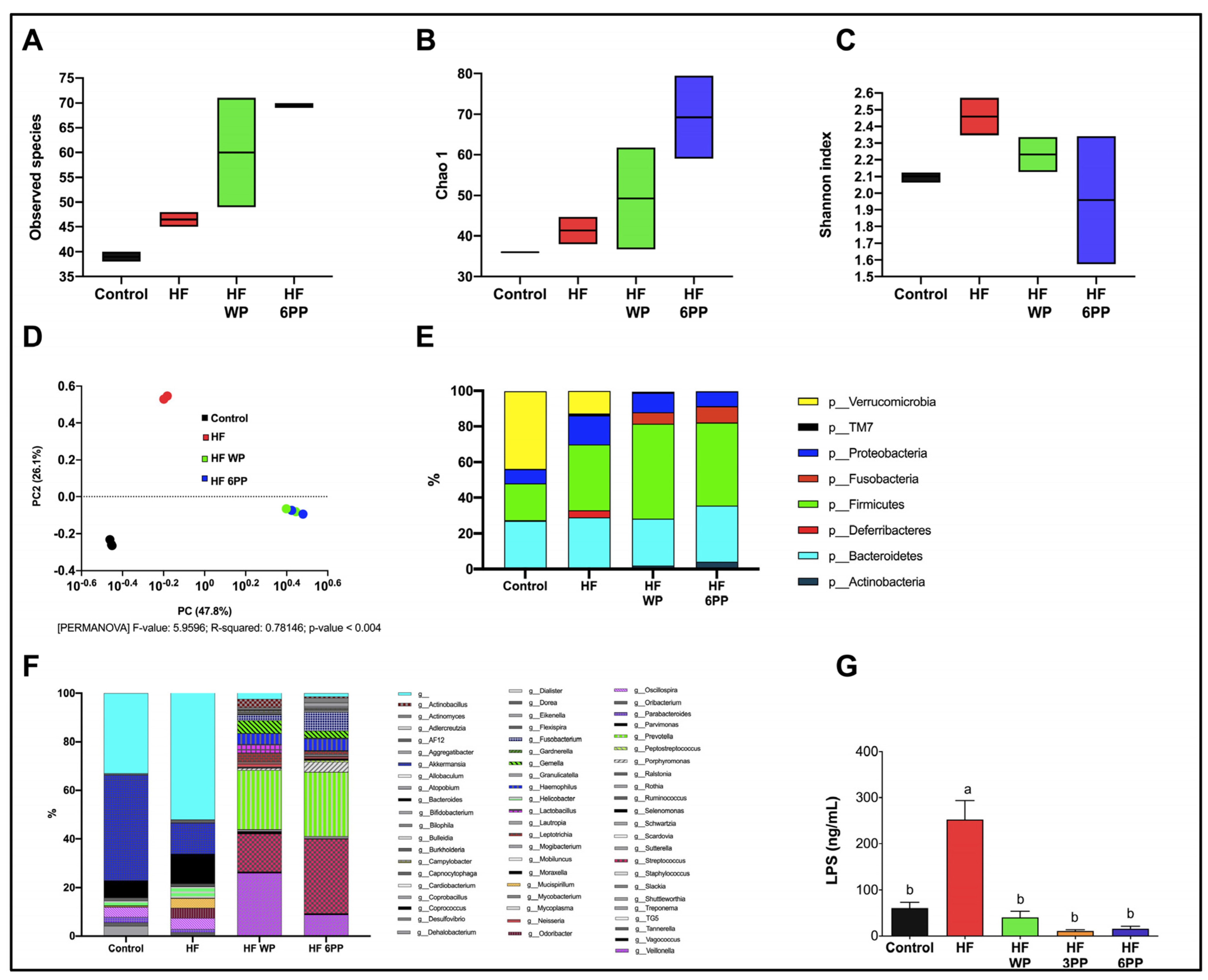
3.9. Whole Pecans and a Phenolic Extract of Pecans Improves the Gut Microbiota Induced by a High-Fat Diet in Mice
3.10. A Dietary Intervention with a Pecan Phenolic Extract Improves Metabolic Alterations of Obese Mice
4. Discussion
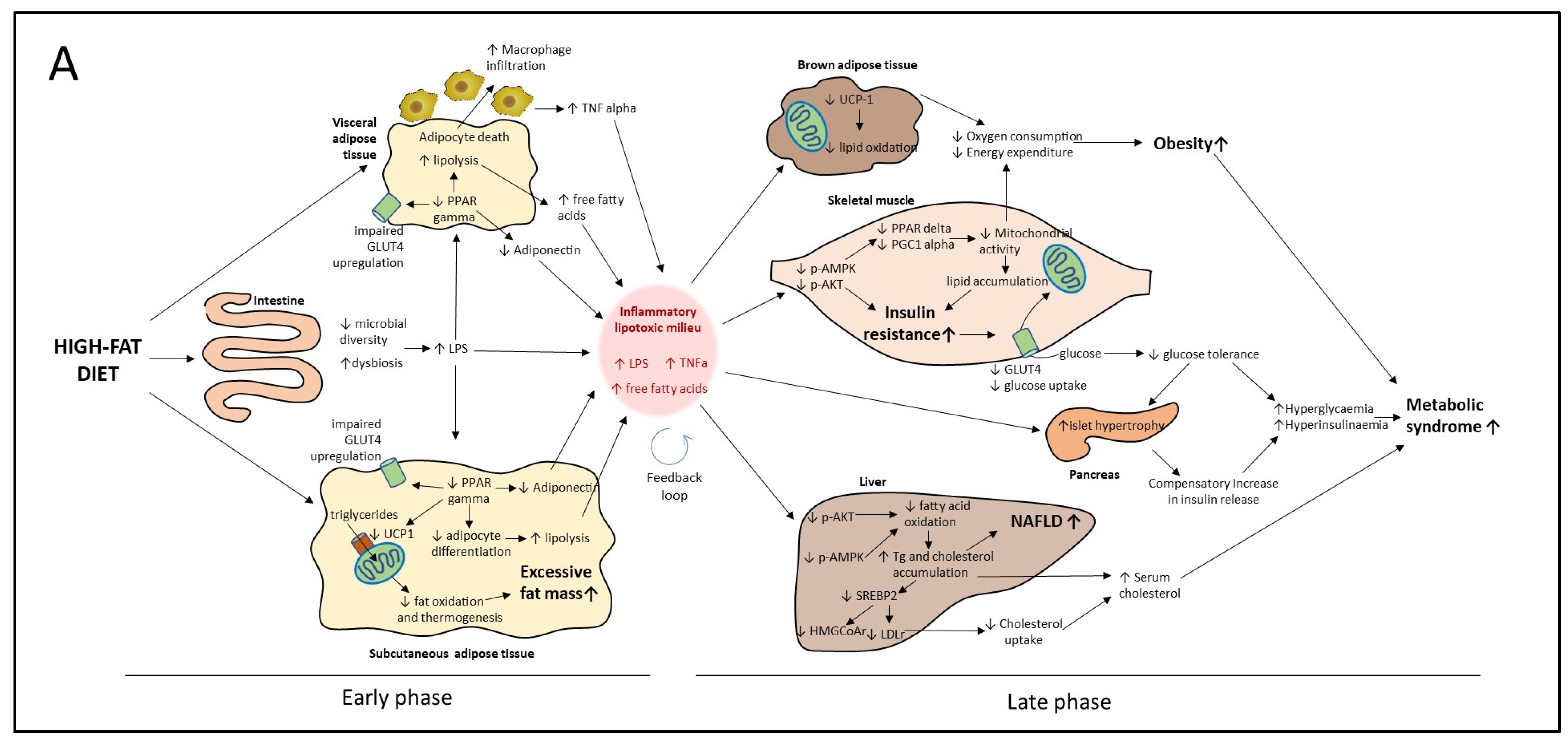
4.1. Proposed Model of Progression of HF Diet Mediated Metabolic Disorder Based on Early and Late Events
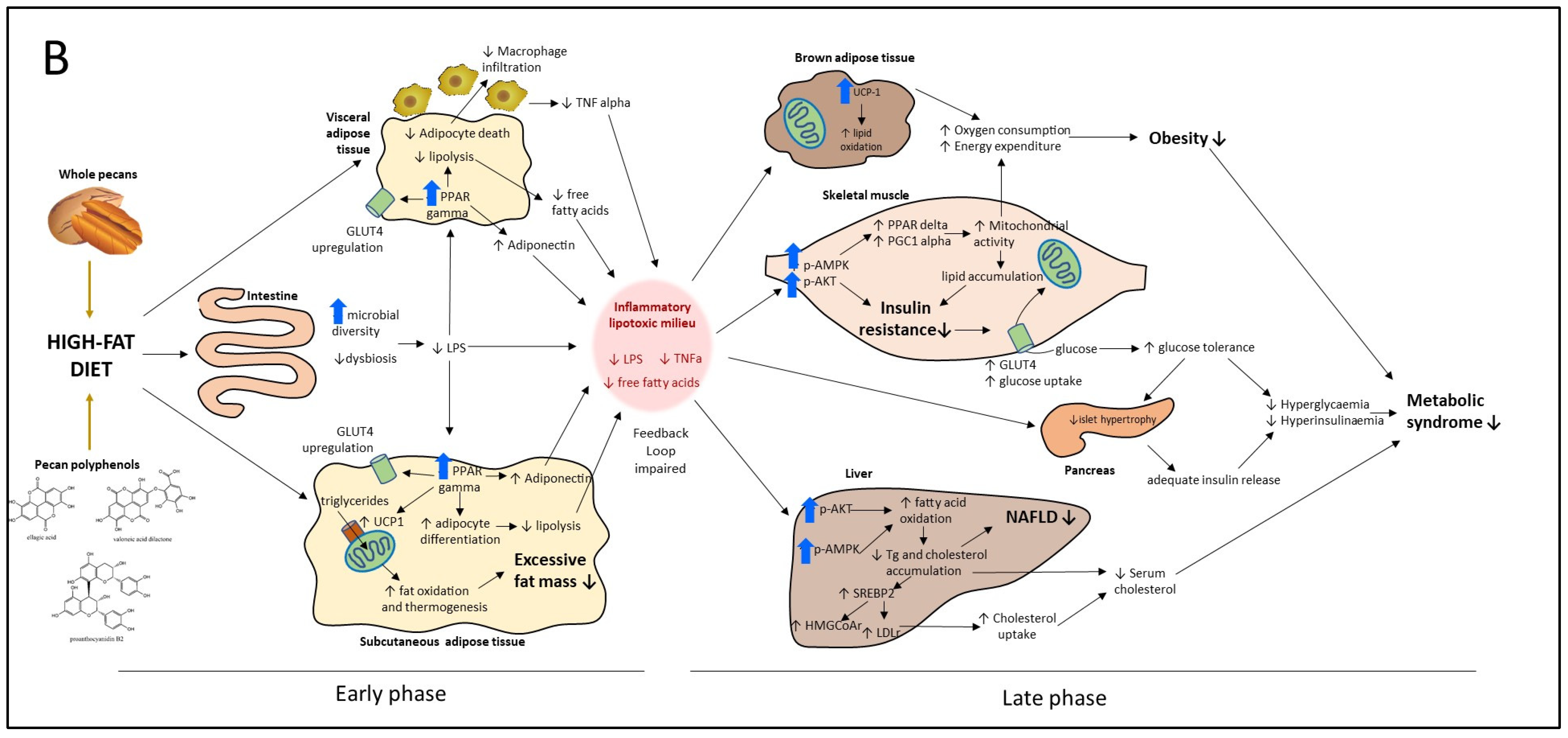
4.2. Molecular Targets of Whole Pecan and Pecan Phenolic Extracts in Preventive and Intervention Strategies
4.3. Whole Pecan and Pecan Phenolic Extracts and the Equivalent Dose for Future Clinical Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | Adenine monophosphate (AMP) activated protein kinase |
| BAT | Brown adipose tissue |
| BODIPY | 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene |
| CLA | Conjugated linoleic acid |
| DAPI | 4′,6-Diamidino-2-Phenylindole, Dihydrochloride |
| H&E | Hematoxylin & Eosin |
| HF | High-fat |
| HMGCOA | 5-hydroxy-3-methylglutaryl-coenzyme A) reductase |
| ipGTT | intraperitoneal Glucose Tolerance Test |
| ipITT | intraperitoneal Insulin Tolerance Test |
| mRNA | Messenger ribonucleic acid |
| PBS | Phosphate buffer saline |
| PCR | Polymerase chain reaction |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PPARδ | Peroxisome proliferator-activated receptor delta |
| RER | Respiratory Exchange Ratio |
| SAT | Subcutaneous adipose tissue |
| SDH | Succinate Dehydrogenase |
| SREBP-2 | Sterol regulatory element binding protein-2 |
| TNF-α | Tumor necrosis factor alpha |
| UCP-1 | Uncoupling protein one |
| VAT | Visceral adipose tissue |
| GLUT 4 | Glucose Transporter 4 |
| AKT | Serine/threonine-protein kinase |
| PGC-1 α | Peroxisome proliferator-activated receptor gamma coactivator 1 alpha |
References
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. In NCHS Data Brief; National Center for Health Statistics: Hyattsville, MD, USA, 2020; Volume 360. [Google Scholar]
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected U.S. state-level prevalence of adult obesity and severe obesity. N. Engl. J. Med. 2019, 381, 2440–2450. [Google Scholar] [CrossRef] [PubMed]
- DiBonaventura, M.D.; Meincke, H.; Le Lay, A.; Fournier, J.; Bakker, E.; Ehrenreich, A. Obesity in Mexico: Prevalence, comorbidities, associations with patient outcomes, and treatment experiences. Diabetes Metab. Syndr. Obes. Targets Ther. 2018, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, E.A.; Trogdon, J.G.; Cohen, J.W.; Dietz, W.H. Annual Medical Spending Attributable to Obesity: Payer-And Service-Specific Estimates. Health Aff. 2009, 28, w822–w831. [Google Scholar] [CrossRef]
- Finkelstein, E.A.; Khavjou, O.A.; Thompson, H.; Trogdon, J.G.; Pan, L.; Sherry, B.; Dietz, W. Obesity and Severe Obesity Forecasts Through 2030. Am. J. Prev. Med. 2012, 42, 563–570. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G. Antioxidant Phytochemicals in Fruits and Vegetables: Diet and Health Implications. Hortscience 2000, 35, 588–592. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res./Fund. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Wargovich, M.J. Anticancer Properties of Fruits and Vegetables. Hortscience 2000, 35, 573–575. [Google Scholar] [CrossRef]
- Cherniack, E.P. Polyphenols: Planting the seeds of treatment for the metabolic syndrome. Nutrition 2011, 27, 617–623. [Google Scholar] [CrossRef]
- Ortiz-Quezada, A.G.; Lombardini, L.; Cisneros-Zevallos, L. Antioxidants in Pecan Nut Cultivars [Carya illinoinensis (Wangenh.) K. Koch]. In Nuts and Seeds in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 881–889. [Google Scholar] [CrossRef]
- Villarreal-Lozoya, J.E.; Lombardini, L.; Cisneros-Zevallos, L. Phytochemical constituents and antioxidant capacity of different pecan [Carya illinoinensis (Wangenh.) K. Koch] cultivars. Food Chem. 2007, 102, 1241–1249. [Google Scholar] [CrossRef]
- Hudthagosol, C.; Haddad, E.H.; McCarthy, K.; Wang, P.; Oda, K.; Sabaté, J. Pecans Acutely Increase Plasma Postprandial Antioxidant Capacity and Catechins and Decrease LDL Oxidation in Humans. J. Nutr. 2011, 141, 56–62. [Google Scholar] [CrossRef]
- Morgan, W.A.; Clayshulte, B.J. Pecans Lower Low Density Lipoprotein Cholesterol in People with Normal Lipid Levels. J. Am. Diet. Assoc. 2000, 100, 312–318. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Eliasziw, M.; Chen, C.Y.O.; Blumberg, J.B. A Pecan-Rich Diet Improves Cardiometabolic Risk Factors in Overweight and Obese Adults: A Randomized Controlled Trial. Nutrients 2018, 10, 339. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA); Center for Food Safety and Applied Nutrition. Guidance for Industry: A Food Labeling Guide 2013, Docket No. 2002P-0505; Food and Drug Administration: College Park, MD, USA, 2013. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-food-labeling-guide (accessed on 31 December 2022).
- Taguchi, C.; Fukushima, Y.; Kishimoto, Y.; Suzuki-Sugihara, N.; Saita, E.; Takahashi, Y.; Kondo, K. Estimated Dietary Polyphenol Intake and Major Food and Beverage Sources among Elderly Japanese. Nutrients 2015, 7, 10269–10281. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Galarraga, M.; Campion, J.; Muñoz-Barrutia, A.; Boqué, N.; Moreno, H.; Martínez, J.A.; Milagro, F.; Ortiz-De-Solórzano, C. Adiposoft: Automated software for the analysis of white adipose tissue cellularity in histological sections. J. Lipid Res. 2012, 53, 2791–2796. [Google Scholar] [CrossRef] [PubMed]
- Blanco, C.E.; Sieck, G.C.; Edgerton, V.R. Quantitative histochemical determination of succinic dehydrogenase activity in skeletal muscle fibres. Histochem. J. 1988, 20, 230–243. [Google Scholar] [CrossRef]
- Leal-Díaz, A.M.; Noriega, L.G.; Torre-Villalvazo, I.; Torres, N.; Alemán-Escondrillas, G.; López-Romero, P.; Sánchez-Tapia, M.; Aguilar-López, M.; Furuzawa-Carballeda, J.; Velázquez-Villegas, L.A.; et al. Aguamiel concentrate from Agave salmiana and its extracted saponins attenuated obesity and hepatic steatosis and increased Akkermansia muciniphila in C57BL6 mice. Sci. Rep. 2016, 6, 34242. [Google Scholar] [CrossRef]
- Delgadillo-Puga, C.; Noriega, L.G.; Morales-Romero, A.M.; Nieto-Camacho, A.; Granados-Portillo, O.; Rodríguez-López, L.A.; Alemán, G.; Furuzawa-Carballeda, J.; Tovar, A.R.; Cisneros-Zevallos, L.; et al. Goat’s Milk Intake Prevents Obesity, Hepatic Steatosis and Insulin Resistance in Mice Fed A High-Fat Diet by Reducing Inflammatory Markers and Increasing Energy Expenditure and Mitochondrial Content in Skeletal Muscle. Int. J. Mol. Sci. 2020, 21, 5530. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L.; Bang, W.Y.; Delgadillo-Puga, C. Ellagic Acid and Urolithins A and B Differentially Regulate Fat Accumulation and Inflammation in 3T3-L1 Adipocytes While Not Affecting Adipogenesis and Insulin Sensitivity. Int. J. Mol. Sci. 2020, 21, 2086. [Google Scholar] [CrossRef]
- Shimizu, I.; Walsh, K. The Whitening of Brown Fat and Its Implications for Weight Management in Obesity. Curr. Obes. Rep. 2015, 4, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Lowell, B.B.; Shulman, G.I. Mitochondrial Dysfunction and Type 2 Diabetes. Science 2005, 307, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Villarroya, F.; Cereijo, R.; Gavaldà-Navarro, A.; Villarroya, J.; Giralt, M. Inflammation of brown/beige adipose tissues in obesity and metabolic disease. J. Intern. Med. 2018, 284, 492–504. [Google Scholar] [CrossRef]
- Ahechu, P.; Zozaya, G.; Martí, P.; Hernández-Lizoáin, J.L.; Baixauli, J.; Unamuno, X.; Frühbeck, G.; Catalán, V. NLRP3 Inflammasome: A Possible Link Between Obesity-Associated Low-Grade Chronic Inflammation and Colorectal Cancer Development. Front. Immunol. 2018, 9, 2918. [Google Scholar] [CrossRef]
- Klöting, N.; Blüher, M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev. Endocr. Metab. Disord. 2014, 15, 277–287. [Google Scholar] [CrossRef]
- Luo, J.; Yang, H.; Song, B.-L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef]
- Moorthy, M.; Sundralingam, U.; Palanisamy, U.D. Polyphenols as Prebiotics in the Management of High-Fat Diet-Induced Obesity: A Systematic Review of Animal Studies. Foods 2021, 10, 299. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Dhillon, J.; Li, Z.; Ortiz, R.M. Almond Snacking for 8 wk Increases Alpha-Diversity of the Gastrointestinal Microbiome and Decreases Bacteroides fragilis Abundance Compared with an Isocaloric Snack in College Freshmen. Curr. Dev. Nutr. 2019, 3, nzz079. [Google Scholar] [CrossRef]
- Bamberger, C.; Rossmeier, A.; Lechner, K.; Wu, L.; Waldmann, E.; Fischer, S.; Stark, R.G.; Altenhofer, J.; Henze, K.; Parhofer, K.G. A Walnut-Enriched Diet Affects Gut Microbiome in Healthy Caucasian Subjects: A Randomized, Controlled Trial. Nutrients 2018, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Ukhanova, M.; Wang, X.; Baer, D.J.; Novotny, J.A.; Fredborg, M.; Mai, V. Effects of almond and pistachio consumption on gut microbiota composition in a randomised cross-over human feeding study. Br. J. Nutr. 2014, 111, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial dysbiosis-induced obesity: Role of gut microbiota in homoeostasis of energy metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef]
- Medina-Gomez, G.; Gray, S.; Vidal-Puig, A. Adipogenesis and lipotoxicity: Role of peroxisome proliferator-activated receptor γ (PPARγ) and PPARγcoactivator-1 (PGC1). Public Health Nutr. 2007, 10, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Torre-Villalvazo, I.; Bunt, A.E.; Alemán, G.; Marquez-Mota, C.C.; Diaz-Villaseñor, A.; Noriega, L.G.; Estrada, I.; Figureueroa-Juárez, E.; Tovar-Palacio, C.; Rodriguez-López, L.A.; et al. Adiponectin synthesis and secretion by subcutaneous adipose tissue is impaired during obesity by endoplasmic reticulum stress. J. Cell. Biochem. 2018, 119, 5970–5984. [Google Scholar] [CrossRef]
- Zhou, M.; Wu, R.; Dong, W.; Jacob, A.; Wang, P. Endotoxin downregulates peroxisome proliferator-activated receptor-γ via the increase in TNF-α release. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R84–R92. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Tang, W.J.; Yin, J.J.; Zhou, B.J. Signal transductions and nonalcoholic fatty liver: A mini-review. Int. J. Clin. Exp. Med. 2014, 7, 1624–1631. [Google Scholar] [PubMed]
- Jia, L.; Song, N.; Yang, G.; Ma, Y.; Li, X.; Lu, R.; Cao, H.; Zhang, N.; Zhu, M.; Wang, J.; et al. Effects of Tanshinone IIA on the modulation of miR-33a and the SREBP-2/Pcsk9 signaling pathway in hyperlipidemic rats. Mol. Med. Rep. 2016, 13, 4627–4635. [Google Scholar] [CrossRef]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef]
- Oh, Y.S.; Bae, G.D.; Baek, D.J.; Park, E.-Y.; Jun, H.-S. Fatty Acid-Induced Lipotoxicity in Pancreatic Beta-Cells During Development of Type 2 Diabetes. Front. Endocrinol. 2018, 9, 384. [Google Scholar] [CrossRef]
- Christensen, A.A.; Gannon, M. The Beta Cell in Type 2 Diabetes. Curr. Diabetes Rep. 2019, 19, 81. [Google Scholar] [CrossRef]
- Pagel-Langenickel, I.; Bao, J.; Joseph, J.J.; Schwartz, D.R.; Mantell, B.S.; Xu, X.; Raghavachari, N.; Sack, M.N. PGC-1α Integrates Insulin Signaling, Mitochondrial Regulation, and Bioenergetic Function in Skeletal Muscle. J. Biol. Chem. 2008, 283, 22464–22472. [Google Scholar] [CrossRef] [PubMed]
- Corpeleijn, E.; Saris, W.H.M.; Blaak, E.E. Metabolic flexibility in the development of insulin resistance and type 2 diabetes: Effects of lifestyle. Obes. Rev. 2009, 10, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Brondani, L.D.A.; Assmann, T.S.; Duarte, G.C.K.; Gross, J.L.; Canani, L.H.; Crispim, D. The role of the uncoupling protein 1 (UCP1) on the development of obesity and type 2 diabetes mellitus. Arq. Bras. Endocrinol. Metabol. 2012, 56, 215–225. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2007, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Center for Drug Evaluation and Research; Center for Biologics Evaluation and Research. Estimating the Safe Starting Dose in Clinical Trials for Therapeutics in Adult Healthy Volunteers; U.S. Food and Drug Administration: Rockville, MD, USA, 2002. [Google Scholar]
- Reid, W.; Hunt, K.L. Pecan Production in the Northern United States. Horttechnology 2000, 10, 298–301. [Google Scholar] [CrossRef]
- Liu, A.G.; Ford, N.A.; Hu, F.B.; Zelman, K.M.; Mozaffarian, D.; Kris-Etherton, P.M. A healthy approach to dietary fats: Understanding the science and taking action to reduce consumer confusion. Nutr. J. 2017, 16, 53. [Google Scholar] [CrossRef]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet; A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef]
- Zhang, Y.; Lee, B.; Du, W.; Fan, Y.; Lyu, S.C.; Nadeau, K.C.; Grauke, L.J.; Zhang, Y.; Wang, S.; Mchugh, T.H. Identification and characterization of a new pecan [Carya illinoinensis (Wangenh.) K. Koch] allergen, Car i 2. J. Agric. Food Chem. 2016, 64, 4146–4151. [Google Scholar] [CrossRef]
- Clermont, K.; Graham, C.J.; Lloyd, S.W.; Grimm, C.C.; Randall, J.J.; Mattison, C.P. Proteomic Analysis of Pecan (Carya illinoinensis) Nut Development. Foods 2023, 12, 866. [Google Scholar] [CrossRef]

| Ingredients (g) | Control | HF | HF WP | HF 3PP | HF 6PP |
|---|---|---|---|---|---|
| Casein | 20 | 20 | 18.2 | 20 | 20 |
| Sucrose | 10 | 31.6 | 29.8 | 30.6 | 30.0 |
| Dextrose | 13.2 | 15 | 15 | 15 | 15 |
| Corn starch | 39.75 | 0 | 0 | 0 | 0 |
| Lard | 0 | 16.4 | 0 | 16.4 | 16.4 |
| Soy oil | 7 | 7 | 0 | 7 | 7 |
| Cellulose | 5 | 5 | 2 | 5 | 5 |
| Mineral mixture | 1 | 1 | 1 | 1 | 1 |
| Vitamin mixture | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 |
| Cystine | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Choline citrate | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Whole pecan | 0 | 0 | 30 | 0 | 0 |
| Pecan phenolic extract | 0 | 0 | 0 | 0.93 | 1.54 |
| Gene | Forward Sequence (5′-3′) | Reverse Sequence (3′-5′) |
|---|---|---|
| SREBP-2 | GATGATCACCCCGACGTTCA | GTCACGAGGCTTTGCACTTG |
| PPARδ | CTCTTCATCGCGGCCATCATTCT | TCTGCCATCTTCTGCAGCAGCTT |
| AMPK | ACCTGAGAACGTCCTGCTTG | GGCCTGCGTACAATCTTCCT |
| PPARγ2 | CTCCTGTTGACCCAGAGCAT | GAAGTTGGTGGGCCAGAA |
| PGC-1a | AAGTGTGGAACTCTCTGGAACTG | GGGTTATCTTGGTTGGCTTTATG |
| HMGCOA | GGGTATTGCTGGCCTCTTCA | GGATTGCCATTCCACGAGCT |
| Peak No | RT | M-H | * MS Fragments | Identification |
|---|---|---|---|---|
| 1 | 14.87–14.96 | 577 | 451, 425, 407, 289, 245 | Procyanidin B2 |
| 1a | 15.89–16.29 | 451 | 289 | Catechin hexoside |
| 2 | 17.00 | 433 | 301, 165 | Ellagic acid pentoside |
| 2a | 17.82–17.89 | 477 | 315, 301 | Methyl ellagic acid hexoside |
| 3 | 18.69–18.82 | 447 | 315, 301 | Methyl ellagic acid pentoside |
| 4 | 19.49–19.56 | 615 | 463, 301 | Di-galloyl ellagic acid |
| 4a | 19.89 | 585 | 433, 301 | Ellagic acid galloyl pentoside |
| 5 | 20.36 | 599 | 447, 315 | Methyl ellagic acid galloyl pentose |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgadillo-Puga, C.; Torre-Villalvazo, I.; Noriega, L.G.; Rodríguez-López, L.A.; Alemán, G.; Torre-Anaya, E.A.; Cariño-Cervantes, Y.Y.; Palacios-Gonzalez, B.; Furuzawa-Carballeda, J.; Tovar, A.R.; et al. Pecans and Its Polyphenols Prevent Obesity, Hepatic Steatosis and Diabetes by Reducing Dysbiosis, Inflammation, and Increasing Energy Expenditure in Mice Fed a High-Fat Diet. Nutrients 2023, 15, 2591. https://doi.org/10.3390/nu15112591
Delgadillo-Puga C, Torre-Villalvazo I, Noriega LG, Rodríguez-López LA, Alemán G, Torre-Anaya EA, Cariño-Cervantes YY, Palacios-Gonzalez B, Furuzawa-Carballeda J, Tovar AR, et al. Pecans and Its Polyphenols Prevent Obesity, Hepatic Steatosis and Diabetes by Reducing Dysbiosis, Inflammation, and Increasing Energy Expenditure in Mice Fed a High-Fat Diet. Nutrients. 2023; 15(11):2591. https://doi.org/10.3390/nu15112591
Chicago/Turabian StyleDelgadillo-Puga, Claudia, Ivan Torre-Villalvazo, Lilia G. Noriega, Leonardo A. Rodríguez-López, Gabriela Alemán, Erik A. Torre-Anaya, Yonatan Y. Cariño-Cervantes, Berenice Palacios-Gonzalez, Janette Furuzawa-Carballeda, Armando R. Tovar, and et al. 2023. "Pecans and Its Polyphenols Prevent Obesity, Hepatic Steatosis and Diabetes by Reducing Dysbiosis, Inflammation, and Increasing Energy Expenditure in Mice Fed a High-Fat Diet" Nutrients 15, no. 11: 2591. https://doi.org/10.3390/nu15112591
APA StyleDelgadillo-Puga, C., Torre-Villalvazo, I., Noriega, L. G., Rodríguez-López, L. A., Alemán, G., Torre-Anaya, E. A., Cariño-Cervantes, Y. Y., Palacios-Gonzalez, B., Furuzawa-Carballeda, J., Tovar, A. R., & Cisneros-Zevallos, L. (2023). Pecans and Its Polyphenols Prevent Obesity, Hepatic Steatosis and Diabetes by Reducing Dysbiosis, Inflammation, and Increasing Energy Expenditure in Mice Fed a High-Fat Diet. Nutrients, 15(11), 2591. https://doi.org/10.3390/nu15112591









