The Causal Effect of Dietary Composition on the Risk of Breast Cancer: A Mendelian Randomization Study
Abstract
1. Introduction
2. Methods and Materials
2.1. Breast Cancer Data
2.2. Relative Intake of Macronutrients Data
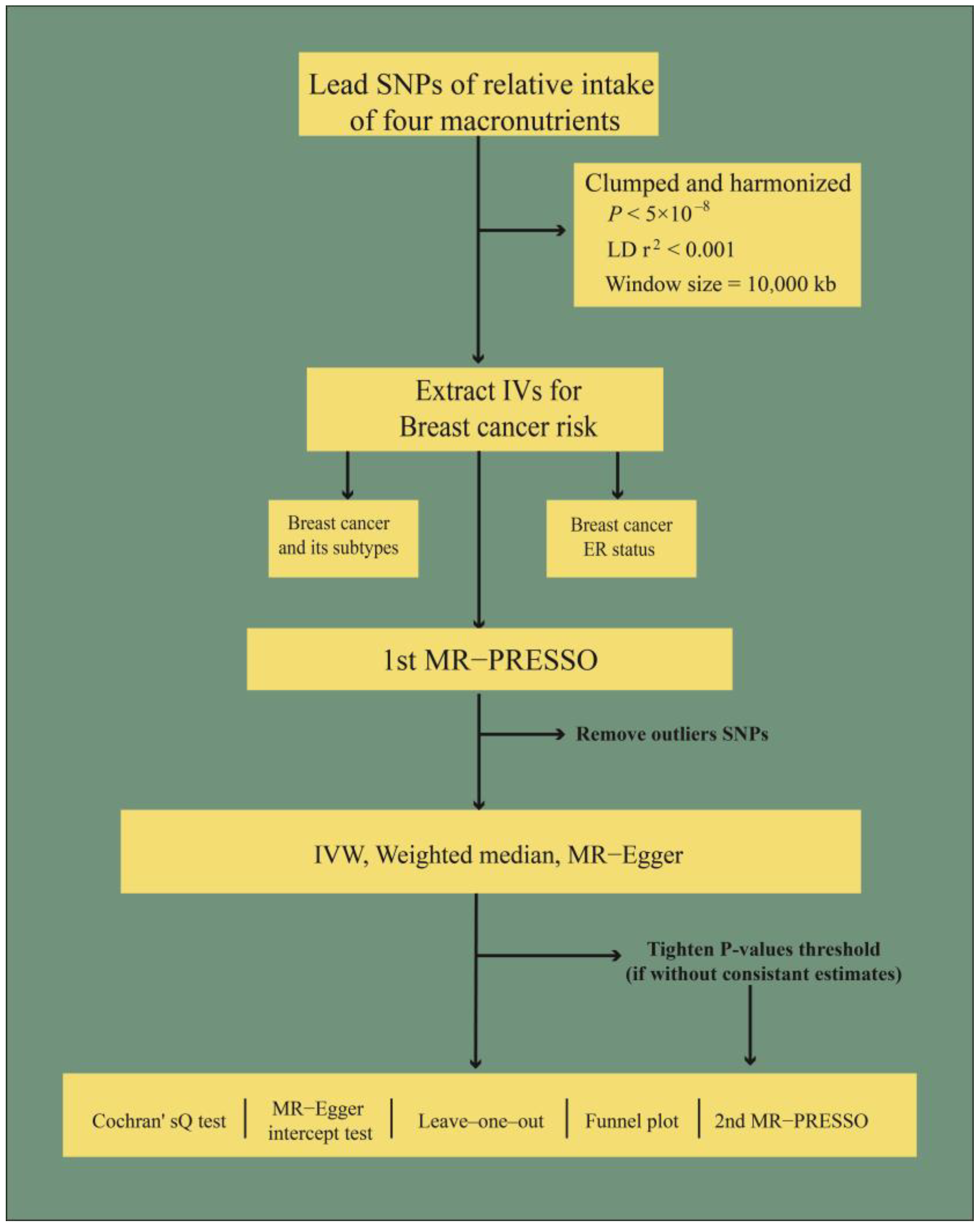
2.3. Selection of Instrumental Variables
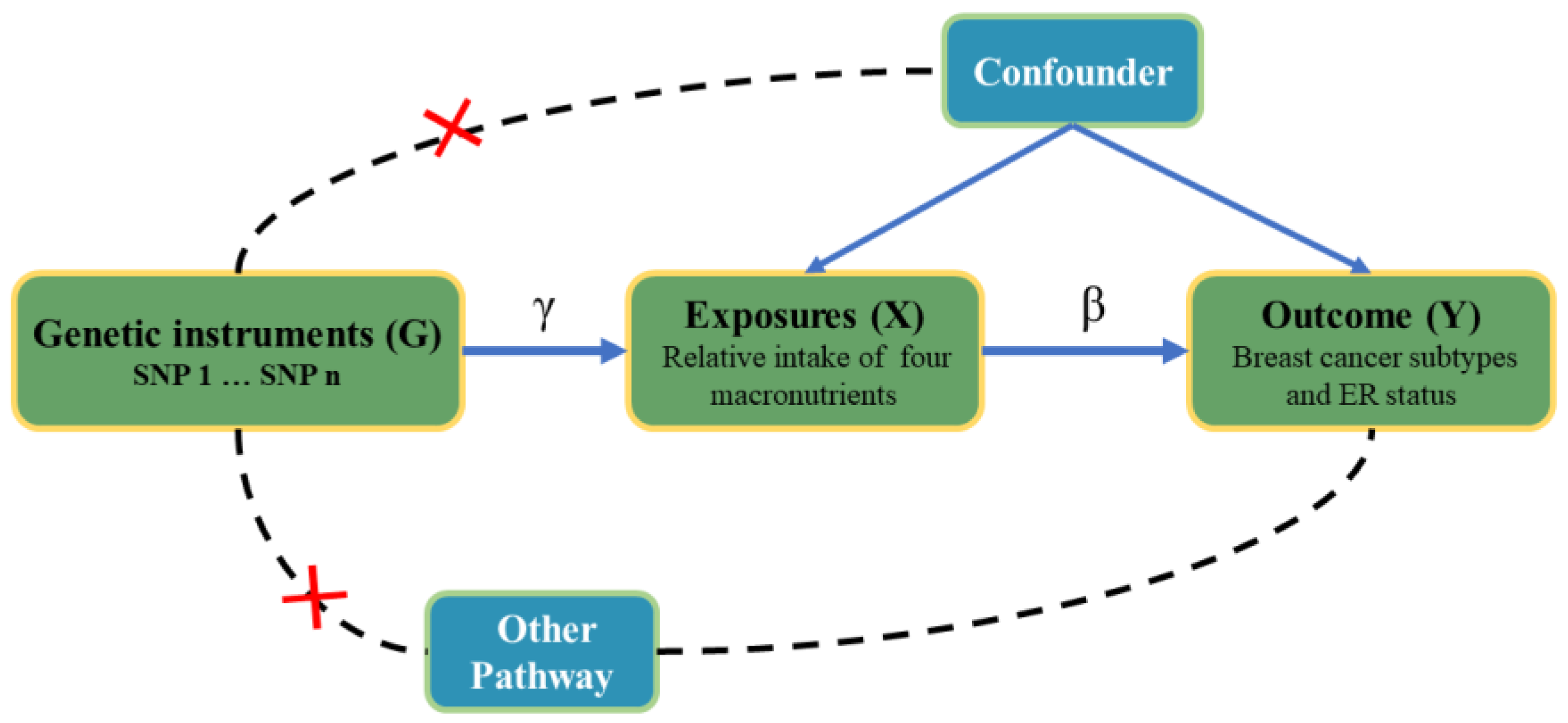
2.4. Mendelian Randomization Analysis
2.5. Sensitivity Analysis
2.6. Sample Overlap
3. Results
3.1. Causal Effects
3.2. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Heer, E.; Harper, A.; Escandor, N.; Sung, H.; McCormack, V.; Fidler-Benaoudia, M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob. Health 2020, 8, e1027–e1037. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; Abdulle, A.S.M.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Aragaki, A.K.; Anderson, G.L.; Pan, K.; Neuhouser, M.L.; Manson, J.E.; Thomson, C.A.; Mossavar-Rahmani, Y.; Lane, D.S.; Johnson, K.C.; et al. Dietary Modification and Breast Cancer Mortality: Long-Term Follow-Up of the Women’s Health Initiative Randomized Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Prentice, R.L.; Aragaki, A.K.; Howard, B.V.; Chlebowski, R.T.; Thomson, C.A.; Van Horn, L.; Tinker, L.F.; Manson, J.E.; Anderson, G.L.; Kuller, L.E.; et al. Low-Fat Dietary Pattern among Postmenopausal Women Influences Long-Term Cancer, Cardiovascular Disease, and Diabetes Outcomes. J. Nutr. 2019, 149, 1565–1574. [Google Scholar] [CrossRef]
- Tsilidis, K.K.; Cariolou, M.; Becerra-Tomás, N.; Balducci, K.; Vieira, R.; Abar, L.; Aune, D.; Markozannes, G.; Nanu, N.; Greenwood, D.C.; et al. Postdiagnosis body fatness, recreational physical activity, dietary factors and breast cancer prognosis: Global Cancer Update Programme (CUP Global) summary of evidence grading. Int. J. Cancer 2023, 152, 635–644. [Google Scholar] [CrossRef]
- Chen, M.; Li, S.; Arora, I.; Yi, N.; Sharma, M.; Li, Z.; Tollefsbol, T.O.; Li, Y. Maternal soybean diet on prevention of obesity-related breast cancer through early-life gut microbiome and epigenetic regulation. J. Nutr. Biochem. 2022, 110, 109119. [Google Scholar] [CrossRef]
- Kazemi, A.; Barati-Boldaji, R.; Soltani, S.; Mohammadipoor, N.; Esmaeilinezhad, Z.; Clark, C.C.T.; Babajafari, S.; Akbarzadeh, M. Intake of Various Food Groups and Risk of Breast Cancer: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Adv. Nutr. 2021, 12, 809–849. [Google Scholar] [CrossRef]
- Debras, C.; Chazelas, E.; Srour, B.; Kesse-Guyot, E.; Julia, C.; Zelek, L.; Agaësse, C.; Druesne-Pecollo, N.; Galan, P.; Hercberg, S.; et al. Total and added sugar intakes, sugar types, and cancer risk: Results from the prospective NutriNet-Santé cohort. Am. J. Clin. Nutr. 2020, 112, 1267–1279. [Google Scholar] [CrossRef]
- Wei, Y.; Lv, J.; Guo, Y.; Bian, Z.; Gao, M.; Du, H.; Yang, L.; Chen, Y.; Zhang, X.; Wang, T.; et al. Soy intake and breast cancer risk: A prospective study of 300,000 Chinese women and a dose-response meta-analysis. Eur. J. Epidemiol. 2020, 35, 567–578. [Google Scholar] [CrossRef]
- Toledo, E.; Salas-Salvadó, J.; Donat-Vargas, C.; Buil-Cosiales, P.; Estruch, R.; Ros, E.; Corella, D.; Fitó, M.; Hu, F.B.; Arós, F.; et al. Mediterranean Diet and Invasive Breast Cancer Risk among Women at High Cardiovascular Risk in the PREDIMED Trial: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1752–1760. [Google Scholar] [CrossRef]
- Castelló, A.; Pollán, M.; Buijsse, B.; Ruiz, A.; Casas, A.M.; Baena-Cañada, J.M.; Lope, V.; Antolín, S.; Ramos, M.; Muñoz, M.; et al. Spanish Mediterranean diet and other dietary patterns and breast cancer risk: Case-control EpiGEICAM study. Br. J. Cancer 2014, 111, 1454–1462. [Google Scholar] [CrossRef]
- Klungel, O.H.; Martens, E.P.; Psaty, B.M.; Grobbee, D.E.; Sullivan, S.D.; Stricker, B.H.; Leufkens, H.G.; de Boer, A. Methods to assess intended effects of drug treatment in observational studies are reviewed. J. Clin. Epidemiol. 2004, 57, 1223–1231. [Google Scholar] [CrossRef]
- Sekula, P.; Del Greco, M.F.; Pattaro, C.; Köttgen, A. Mendelian Randomization as an Approach to Assess Causality Using Observational Data. J. Am. Soc. Nephrol. JASN 2016, 27, 3253–3265. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.; Timpson, N.; Davey Smith, G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008, 27, 1133–1163. [Google Scholar] [CrossRef]
- Davey Smith, G. Capitalizing on Mendelian randomization to assess the effects of treatments. J. R. Soc. Med. 2007, 100, 432–435. [Google Scholar] [CrossRef]
- Ba, D.M.; Ssentongo, P.; Beelman, R.B.; Muscat, J.; Gao, X.; Richie, J.P. Higher Mushroom Consumption Is Associated with Lower Risk of Cancer: A Systematic Review and Meta-Analysis of Observational Studies. Adv. Nutr. 2021, 12, 1691–1704. [Google Scholar] [CrossRef]
- Wansink, B. Environmental factors that increase the food intake and consumption volume of unknowing consumers. Annu. Rev. Nutr. 2004, 24, 455–479. [Google Scholar] [CrossRef]
- Frayling, T.M.; Timpson, N.J.; Weedon, M.N.; Zeggini, E.; Freathy, R.M.; Lindgren, C.M.; Perry, J.R.; Elliott, K.S.; Lango, H.; Rayner, N.W.; et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007, 316, 889–894. [Google Scholar] [CrossRef]
- Chung, S.J.; Nagaraju, G.P.; Nagalingam, A.; Muniraj, N.; Kuppusamy, P.; Walker, A.; Woo, J.; Győrffy, B.; Gabrielson, E.; Saxena, N.K.; et al. ADIPOQ/adiponectin induces cytotoxic autophagy in breast cancer cells through STK11/LKB1-mediated activation of the AMPK-ULK1 axis. Autophagy 2017, 13, 1386–1403. [Google Scholar] [CrossRef]
- Muntean, C.; Sasaran, M.O.; Crisan, A.; Banescu, C. Effects of PPARG and PPARGC1A gene polymorphisms on obesity markers. Front. Public Health 2022, 10, 962852. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, J.; Zou, Y.; Wang, F.; Li, J.; Sun, F.; Luo, X.; Zhang, M.; Guo, Y.; Yu, Q.; et al. Kdm2a deficiency in macrophages enhances thermogenesis to protect mice against HFD-induced obesity by enhancing H3K36me2 at the Pparg locus. Cell Death Differ. 2021, 28, 1880–1899. [Google Scholar] [CrossRef] [PubMed]
- Clément, K.; van den Akker, E.; Argente, J.; Bahm, A.; Chung, W.K.; Connors, H.; De Waele, K.; Farooqi, I.S.; Gonneau-Lejeune, J.; Gordon, G.; et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: Single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 2020, 8, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Azzam, S.K.; Alsafar, H.; Sajini, A.A. FTO m6A Demethylase in Obesity and Cancer: Implications and Underlying Molecular Mechanisms. Int. J. Mol. Sci. 2022, 23, 3800. [Google Scholar] [CrossRef]
- Mosialou, I.; Shikhel, S.; Liu, J.M.; Maurizi, A.; Luo, N.; He, Z.; Huang, Y.; Zong, H.; Friedman, R.A.; Barasch, J.; et al. MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature 2017, 543, 385–390. [Google Scholar] [CrossRef]
- Li, Y.; Su, R.; Deng, X.; Chen, Y.; Chen, J. FTO in cancer: Functions, molecular mechanisms, and therapeutic implications. Trends Cancer 2022, 8, 598–614. [Google Scholar] [CrossRef]
- Xin, J.; Jiang, X.; Ben, S.; Yuan, Q.; Su, L.; Zhang, Z.; Christiani, D.C.; Du, M.; Wang, M. Association between circulating vitamin E and ten common cancers: Evidence from large-scale Mendelian randomization analysis and a longitudinal cohort study. BMC Med. 2022, 20, 168. [Google Scholar] [CrossRef]
- Fu, Y.; Xu, F.; Jiang, L.; Miao, Z.; Liang, X.; Yang, J.; Larsson, S.C.; Zheng, J.S. Circulating vitamin C concentration and risk of cancers: A Mendelian randomization study. BMC Med. 2021, 19, 171. [Google Scholar] [CrossRef]
- Dimitrakopoulou, V.I.; Tsilidis, K.K.; Haycock, P.C.; Dimou, N.L.; Al-Dabhani, K.; Martin, R.M.; Lewis, S.J.; Gunter, M.J.; Mondul, A.; Shui, I.M.; et al. Circulating vitamin D concentration and risk of seven cancers: Mendelian randomisation study. BMJ (Clin. Res. Ed.) 2017, 359, j4761. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Rosenberg, I.; Uauy, R. History of modern nutrition science-implications for current research, dietary guidelines, and food policy. BMJ (Clin. Res. Ed.) 2018, 361, k2392. [Google Scholar] [CrossRef]
- Zhang, H.; Ahearn, T.U.; Lecarpentier, J.; Barnes, D.; Beesley, J.; Qi, G.; Jiang, X.; O’Mara, T.A.; Zhao, N.; Bolla, M.K.; et al. Genome-wide association study identifies 32 novel breast cancer susceptibility loci from overall and subtype-specific analyses. Nat. Genet. 2020, 52, 572–581. [Google Scholar] [CrossRef]
- Michailidou, K.; Lindström, S.; Dennis, J.; Beesley, J.; Hui, S.; Kar, S.; Lemaçon, A.; Soucy, P.; Glubb, D.; Rostamianfar, A.; et al. Association analysis identifies 65 new breast cancer risk loci. Nature 2017, 551, 92–94. [Google Scholar] [CrossRef]
- Meddens, S.F.W.; de Vlaming, R.; Bowers, P.; Burik, C.A.P.; Linnér, R.K.; Lee, C.; Okbay, A.; Turley, P.; Rietveld, C.A.; Fontana, M.A.; et al. Genomic analysis of diet composition finds novel loci and associations with health and lifestyle. Mol. Psychiatry 2021, 26, 2056–2069. [Google Scholar] [CrossRef]
- Liu, B.; Young, H.; Crowe, F.L.; Benson, V.S.; Spencer, E.A.; Key, T.J.; Appleby, P.N.; Beral, V. Development and evaluation of the Oxford WebQ, a low-cost, web-based method for assessment of previous 24 h dietary intakes in large-scale prospective studies. Public Health Nutr. 2011, 14, 1998–2005. [Google Scholar] [CrossRef]
- Cade, J.E.; Burley, V.J.; Warm, D.L.; Thompson, R.L.; Margetts, B.M. Food-frequency questionnaires: A review of their design, validation and utilisation. Nutr. Res. Rev. 2004, 17, 5–22. [Google Scholar] [CrossRef]
- Boef, A.G.; Dekkers, O.M.; le Cessie, S. Mendelian randomization studies: A review of the approaches used and the quality of reporting. Int. J. Epidemiol. 2015, 44, 496–511. [Google Scholar] [CrossRef]
- Yavorska, O.O.; Burgess, S. MendelianRandomization: An R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 2017, 46, 1734–1739. [Google Scholar] [CrossRef]
- Hao, Y.; Xiao, J.; Liang, Y.; Wu, X.; Zhang, H.; Xiao, C.; Zhang, L.; Burgess, S.; Wang, N.; Zhao, X.; et al. Reassessing the causal role of obesity in breast cancer susceptibility: A comprehensive multivariable Mendelian randomization investigating the distribution and timing of exposure. Int. J. Epidemiol. 2023, 52, 58–70. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Improving bias and coverage in instrumental variable analysis with weak instruments for continuous and binary outcomes. Stat. Med. 2012, 31, 1582–1600. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Protani, M.; Coory, M.; Martin, J.H. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res. Treat. 2010, 123, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Freuer, D.; Meisinger, C.; Linseisen, J. Causal relationship between dietary macronutrient composition and anthropometric measures: A bidirectional two-sample Mendelian randomization analysis. Clin. Nutr. 2021, 40, 4120–4131. [Google Scholar] [CrossRef]
- Kerr, J.; Anderson, C.; Lippman, S.M. Physical activity, sedentary behaviour, diet, and cancer: An update and emerging new evidence. Lancet Oncol. 2017, 18, e457–e471. [Google Scholar] [CrossRef]
- Farvid, M.S.; Cho, E.; Chen, W.Y.; Eliassen, A.H.; Willett, W.C. Adolescent meat intake and breast cancer risk. Int. J. Cancer 2015, 136, 1909–1920. [Google Scholar] [CrossRef]
- Farvid, M.S.; Cho, E.; Chen, W.Y.; Eliassen, A.H.; Willett, W.C. Dietary protein sources in early adulthood and breast cancer incidence: Prospective cohort study. BMJ (Clin. Res. Ed.) 2014, 348, g3437. [Google Scholar] [CrossRef]
- Pouchieu, C.; Deschasaux, M.; Hercberg, S.; Druesne-Pecollo, N.; Latino-Martel, P.; Touvier, M. Prospective association between red and processed meat intakes and breast cancer risk: Modulation by an antioxidant supplementation in the SU.VI.MAX randomized controlled trial. Int. J. Epidemiol. 2014, 43, 1583–1592. [Google Scholar] [CrossRef]
- Turteltaub, K.W.; Dingley, K.H.; Curtis, K.D.; Malfatti, M.A.; Turesky, R.J.; Garner, R.C.; Felton, J.S.; Lang, N.P. Macromolecular adduct formation and metabolism of heterocyclic amines in humans and rodents at low doses. Cancer Lett. 1999, 143, 149–155. [Google Scholar] [CrossRef]
- Sinha, R.; Rothman, N.; Salmon, C.P.; Knize, M.G.; Brown, E.D.; Swanson, C.A.; Rhodes, D.; Rossi, S.; Felton, J.S.; Levander, O.A. Heterocyclic amine content in beef cooked by different methods to varying degrees of doneness and gravy made from meat drippings. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1998, 36, 279–287. [Google Scholar] [CrossRef]
- Hodgson, J.M.; Ward, N.C.; Burke, V.; Beilin, L.J.; Puddey, I.B. Increased lean red meat intake does not elevate markers of oxidative stress and inflammation in humans. J. Nutr. 2007, 137, 363–367. [Google Scholar] [CrossRef]
- Shin, S.; Fu, J.; Shin, W.K.; Huang, D.; Min, S.; Kang, D. Association of food groups and dietary pattern with breast cancer risk: A systematic review and meta-analysis. Clin. Nutr. 2023, 42, 282–297. [Google Scholar] [CrossRef]
- Wada, K.; Nakamura, K.; Tamai, Y.; Tsuji, M.; Kawachi, T.; Hori, A.; Takeyama, N.; Tanabashi, S.; Matsushita, S.; Tokimitsu, N.; et al. Soy isoflavone intake and breast cancer risk in Japan: From the Takayama study. Int. J. Cancer 2013, 133, 952–960. [Google Scholar] [CrossRef]
- Messina, M. Soy foods, isoflavones, and the health of postmenopausal women. Am. J. Clin. Nutr. 2014, 100 (Suppl. S1), 423s–430s. [Google Scholar] [CrossRef]
- Caffa, I.; Spagnolo, V.; Vernieri, C.; Valdemarin, F.; Becherini, P.; Wei, M.; Brandhorst, S.; Zucal, C.; Driehuis, E.; Ferrando, L.; et al. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature 2020, 583, 620–624. [Google Scholar] [CrossRef]
- Vernieri, C.; Fucà, G.; Ligorio, F.; Huber, V.; Vingiani, A.; Iannelli, F.; Raimondi, A.; Rinchai, D.; Frigè, G.; Belfiore, A.; et al. Fasting-Mimicking Diet Is Safe and Reshapes Metabolism and Antitumor Immunity in Patients with Cancer. Cancer Discov. 2022, 12, 90–107. [Google Scholar] [CrossRef]
- Hopkins, B.D.; Pauli, C.; Du, X.; Wang, D.G.; Li, X.; Wu, D.; Amadiume, S.C.; Goncalves, M.D.; Hodakoski, C.; Lundquist, M.R.; et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 2018, 560, 499–503. [Google Scholar] [CrossRef]
- Fung, T.T.; Hu, F.B.; Hankinson, S.E.; Willett, W.C.; Holmes, M.D. Low-carbohydrate diets, dietary approaches to stop hypertension-style diets, and the risk of postmenopausal breast cancer. Am. J. Epidemiol. 2011, 174, 652–660. [Google Scholar] [CrossRef]
- Sasanfar, B.; Toorang, F.; Zendehdel, K.; Salehi-Abargouei, A. Substitution of dietary macronutrients and their sources in association with breast cancer: Results from a large-scale case-control study. Eur. J. Nutr. 2022, 61, 2687–2695. [Google Scholar] [CrossRef]
- Sieri, S.; Chiodini, P.; Agnoli, C.; Pala, V.; Berrino, F.; Trichopoulou, A.; Benetou, V.; Vasilopoulou, E.; Sánchez, M.J.; Chirlaque, M.D.; et al. Dietary fat intake and development of specific breast cancer subtypes. J. Natl. Cancer Inst. 2014, 106, dju068. [Google Scholar] [CrossRef]
| Exposure | Outcome (Breast Cancer) | Number of IVs | p | OR (95%CI) | Cochran’s Q Test | MR-Egger Intercept Test |
|---|---|---|---|---|---|---|
| Relative intake of carbohydrate | Overall | 6 | 0.19 | 1.26 (0.89–1.80) | 0.16 | 0.41 |
| Luminal A | 5 | 1.79 × 10−2 | 1.61 (1.09–2.40) | 0.44 | 0.76 | |
| Luminal B | 7 | 0.14 | 2.07 (0.78–5.50) | 0.14 | 0.04 | |
| Luminal B HER2-negative | 8 | 0.87 | 0.95 (0.49–1.83) | 0.34 | 0.64 | |
| HER2-positive | 8 | 0.89 | 1.10 (0.27–4.55) | 0.07 | 0.91 | |
| Triple-negative | 8 | 0.61 | 0.83 (0.41–1.69) | 0.25 | 0.51 | |
| ER-negative | 6 | 0.38 | 0.79 (0.46–1.34) | 0.78 | 0.45 | |
| ER-positive | 7 | 0.85 | 1.04 (0.72–1.51) | 0.26 | 0.33 | |
| Relative intake of fat | Overall | 4 | 0.68 | 0.91 (0.57–1.44) | 0.13 | 0.13 |
| Luminal A | 4 | 0.54 | 0.86 (0.52–1.40) | 0.16 | 0.17 | |
| Luminal B | 4 | 0.83 | 1.17 (0.29–4.80) | 0.47 | 0.15 | |
| Luminal B HER2-negative | 4 | 0.52 | 0.74 (0.29–1.87) | 0.13 | 0.15 | |
| HER2-positive | 4 | 0.16 | 0.51 (0.20–1.30) | 0.76 | 0.94 | |
| Triple-negative | 4 | 0.15 | 1.50 (0.87–2.58) | 0.98 | 0.90 | |
| ER-negative | 4 | 0.12 | 1.40 (0.92–2.12) | 0.95 | 0.80 | |
| ER-positive | 4 | 0.67 | 0.89 (0.50–1.57) | 0.26 | 0.11 | |
| Relative intake of protein | Overall | 4 | 8.46 × 10−3 | 0.64 (0.45–0.89) | 0.43 | 0.42 |
| Luminal A | 4 | 2.21 × 10−3 | 0.50 (0.32–0.78) | 1.00 | 0.96 | |
| Luminal B | 5 | 0.09 | 0.48 (0.21–1.13) | 0.26 | 0.19 | |
| Luminal B HER2-negative | 6 | 0.99 | 1.00 (0.56–1.79) | 0.56 | 0.32 | |
| HER2-positive | 6 | 0.06 | 0.31 (0.09–1.07) | 0.16 | 0.42 | |
| Triple-negative | 5 | 0.87 | 0.94 (0.45–1.95) | 0.79 | 0.95 | |
| ER-negative | 5 | 0.07 | 0.60 (0.35–1.04) | 0.70 | 0.44 | |
| ER-positive | 4 | 7.91 × 10−4 | 0.49 (0.32–0.74) | 0.54 | 0.34 | |
| Relative intake of sugar | Overall | 3 | 0.27 | 1.36 (0.79–2.35) | 0.17 | 0.31 |
| Luminal A | 3 | 0.38 | 1.28 (0.74–2.18) | 0.52 | 0.55 | |
| Luminal B | 4 | 1.39 × 10−3 | 8.72 (2.31–32.88) | 0.06 | 1.00 | |
| Luminal B HER2-negative | 5 | 0.15 | 2.41 (0.72–8.03) | 0.01 | 0.24 | |
| HER2-positive | 5 | 9.19 × 10−3 | 4.40 (1.44–13.43) | 0.60 | 0.56 | |
| Triple-negative | 3 | 0.94 | 1.04 (0.37–2.89) | 0.95 | 0.84 | |
| ER-negative | 4 | 0.90 | 0.96 (0.55–1.68) | 0.73 | 0.62 | |
| ER-positive | 4 | 0.21 | 1.26 (0.88–1.82) | 0.70 | 0.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Kong, X.; Wang, X.; Liu, Q.; Fang, Y.; Wang, J. The Causal Effect of Dietary Composition on the Risk of Breast Cancer: A Mendelian Randomization Study. Nutrients 2023, 15, 2586. https://doi.org/10.3390/nu15112586
Dong H, Kong X, Wang X, Liu Q, Fang Y, Wang J. The Causal Effect of Dietary Composition on the Risk of Breast Cancer: A Mendelian Randomization Study. Nutrients. 2023; 15(11):2586. https://doi.org/10.3390/nu15112586
Chicago/Turabian StyleDong, Hao, Xiangyi Kong, Xiangyu Wang, Qiang Liu, Yi Fang, and Jing Wang. 2023. "The Causal Effect of Dietary Composition on the Risk of Breast Cancer: A Mendelian Randomization Study" Nutrients 15, no. 11: 2586. https://doi.org/10.3390/nu15112586
APA StyleDong, H., Kong, X., Wang, X., Liu, Q., Fang, Y., & Wang, J. (2023). The Causal Effect of Dietary Composition on the Risk of Breast Cancer: A Mendelian Randomization Study. Nutrients, 15(11), 2586. https://doi.org/10.3390/nu15112586








